Abstract
Purpose
To quantify the relative biological effectiveness (RBE) of the distal edge of the proton Bragg peak, using an in vitro assay of DNA double-strand breaks (DSBs).
Methods and Materials
U2OS cells were irradiated within the plateau of a spread-out Bragg peak and at each millimeter position along the distal edge using a custom slide holder, allowing for simultaneous measurement of physical dose. A reference radiation signal was generated using photons. The DNA DSBs at 3 hours (to assess for early damage) and at 24 hours (to assess for residual damage and repair) after irradiation were measured using the γH2AX assay and quantified via flow cytometry. Results were confirmed with clonogenic survival assays. A detailed map of the RBE as a function of depth along the Bragg peak was generated using γH2AX measurements as a biological endpoint.
Results
At 3 hours after irradiation, DNA DSBs were higher with protons at every point along the distal edge compared with samples irradiated with photons to similar doses. This effect was even more pronounced after 24 hours, indicating that the impact of DNA repair is less after proton irradiation relative to photons. The RBE demonstrated an exponential increase as a function of depth and was measured to be as high as 4.0 after 3 hours and as high as 6.0 after 24 hours. When the RBE-corrected dose was plotted as a function of depth, the peak effective dose was extended 2-3 mm beyond what would be expected with physical measurement.
Conclusions
We generated a highly comprehensive map of the RBE of the distal edge of the Bragg peak, using a direct assay of DNA DSBs in vitro. Our data show that the RBE of the distal edge increases with depth and is significantly higher than previously reported estimates.
Introduction
Proton therapy allows for favorable dosimetry compared with photons by virtue of the higher mass and positive charge of the particle, resulting in a sharp rise in deposited dose followed by a rapid fall-off at the end of the particle's range. The shape of this dramatic dose deposition profile has been termed the Bragg peak. The relative biological effectiveness (RBE) of protons is assumed to be nearly equivalent to that of photons (1, 2). However, there is a growing body of evidence suggesting that the RBE of protons is significantly higher, especially along the fall-off portion of the Bragg peak, known as the distal edge. To date, a robust analysis of the RBE as a function of depth along the distal edge using a direct assay of biological effect has not been performed. The purpose of this study was to quantify the RBE of the distal edge of a proton Bragg peak, using a direct measurement of a surrogate marker of DNA double-strand breaks (DSBs) in vitro.
Methods and Materials
Sample and dosimeter setup
We built a custom slide holder to allow for simultaneous irradiation of a single-chamber microscope slide that could be cultured with adherent cells, and a parallel plate chamber (PPC). The slide holder was designed such that the surface of the microscope slide was coplanar with the effective point of measurement (proximal inner surface) of the PPC (Fig. 1A). This provided for simultaneous confirmation of physical dose delivered to the cultured cells.
Fig. 1.
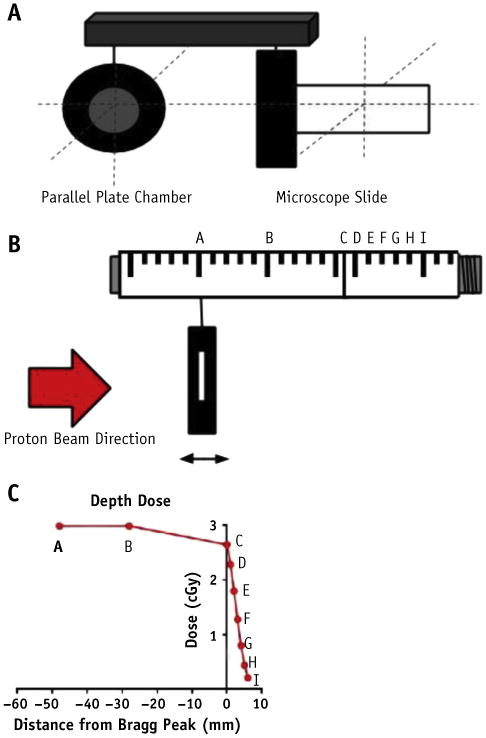
(A) Schematic of slide holder with microscope slide and parallel plate chamber in the same plane. (B) Schematic of worm drive scanner that allowed for distal and proximal shifts of slide holder in 1-mm increments. A-I represent positions of irradiation. (C) Nine positions of sample irradiation along distal edge.
Sample irradiation
Proton irradiation was performed using a calibrated uniform scanning proton beam with a maximum energy of 152 MeV, range of 16 cm in water, and a 10-cm spread-out Bragg peak (SOBP) (Procure Proton Therapy Center, Somerset, NJ). We chose U2OS cells for this study for their adherent properties and their ability to tolerate unfavorable culture conditions. Details of cell culture are detailed in Supplementary Methods, S1 (available online at www.redjournal.org). After overnight incubation, slides were maintained at 37°C, transported to the proton facility, and loaded onto the custom slide holder within a tank filled with sterile normal saline. The slide holder, slide, and PPC were then attached to an electronically controlled worm drive scanner that allowed all components to be shifted in 1-mm increments (Fig. 1B). In-room lasers were projected through the anterior surface of the slide and the reading surface of the PPC to confirm that both were in the same plane. A control slide was also placed in saline but not irradiated. After a slide was irradiated at a given point it was replaced by the subsequent slide, and the slide holder was advanced to the next position. The setup for proton irradiation is shown in Figure E1 (available online at www.redjournal.org). After irradiation, slide chambers were filled with 3 mL of fresh culture media and incubated at 37°C, 5% CO2, for either 3 hours to assess early DNA DSBs or for 24 hours to assess for DSB repair.
To generate a reference radiation signal, cells were irradiated to similar doses using photons at Memorial Sloan Kettering Cancer Center, using a 6-MV beam from a Varian TrueBeam linear accelerator (Varian Medical Systems, Palo Alto, CA). Culture and incubation methods were similar to those with protons. Solid water phantom blocks were placed inside a tank filled with normal saline to bring the surface of the cultured microscope slides to isocenter at a depth of 10 cm. The setup for photon irradiation is shown in Figure E2 (available online at www.redjournal.org). Calibration conditions and dosimetry for both photons and protons are described in Supplementary Methods, S2 (available online at www.redjournal.org). After irradiation, slide chambers were filled with 3 mL of fresh media and incubated for either 3 or 24 hours.
Quantification of DSBs via flow cytometry
To quantify DSBs, we used a γH2AX flow cytometric assay because of its sensitivity, specificity, and widespread use for this application. We have optimized a protocol using flow cytometry as a highly accurate and reproducible method of measuring DSBs in a large number of cells (Supplementary Methods, S3; available online at www.redjournal.org).
Clonogenic survival assay
We used a standard method for the clonogenic survival assay as detailed in the Supplementary Methods, S4 (available online at www.redjournal.org).
RBE estimates
The γH2AX assay yields a very linear dose–response curve for photons, allowing for generation of a standard curve. After photon irradiation, we performed a linear-quadratic least-squares regression analysis to generate an associated equation of the best fit curve based on the cytometric median fluorescent intensity of the irradiated samples. For each proton irradiation point along the distal edge, the photon equivalent dose was calculated by associating the median fluorescent intensity associated with that point to the photon best-fit curve. The RBE of each point on the Bragg peak and distal edge was then calculated by dividing the photon equivalent dose by the measured physical proton dose for each point.
Results
Verification of position
To map the level of DNA DSBs along the distal edge, we first verified physical position of the microscope slides using the following approach. The phantom was positioned such that the isocenter was at the center of the SOBP (ie, at 11-cm depth). The custom slide holder was inserted in the saline phantom and placed “at range,” defined as the point at which the dose was 90% of the dose delivered to the middle of the SOBP (ie, at 16-cm depth). This was considered to be the beginning of the distal edge. The slide holder was confirmed to be at the “at range” position by irradiating the PPC to 100 cGy (physical RBE) at a series of points at 1-mm intervals and confirming that the doses at positions displaced 1 mm proximally and 1 mm distally were 110% and 90% of the reference position dose. The reference position was then deemed to be “at range,” and further irradiations were carried out in relation to this point.
Early DNA damage measured by DSBs
Samples were irradiated at 9 points along the distal edge, shown in Figure 1C. Separate samples were irradiated with photons to 5 different doses, to build a dose–response curve encompassing the range of doses delivered to proton slides. The early level of DSBs after 3 hours of incubation was assessed by flow cytometry median fluorescent intensity of γH2AX immunostaining (Fig. 2A). Results are shown in Figure 2B. Within the plateau region of the SOBP (point A and B), levels of γH2AX intensity are comparable. However, at points along the distal edge, cells irradiated with protons demonstrated a higher signal of γH2AX signal as compared with cells irradiated with photons, suggesting that the biological dose of protons is higher than the RBE value of 1.1.
Fig. 2.
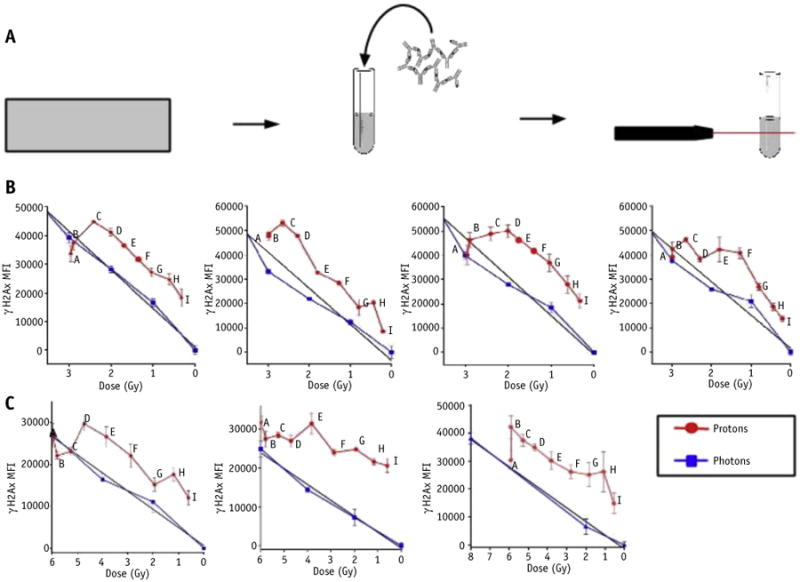
(A) Schematic of immunoassay with primary antibody against γH2AX and fluorescent secondary antibody measured via flow cytometry. (B) Double-strand breaks as assessed by γH2AX immunostaining after 3 hours and (C) after 24 hours of incubation. Replicates performed in quadruplicate for 3-hour time point and triplicate for 24-hour time point.
DSB repair
To assess the level of repair, cells were incubated for 24 hours after irradiation. Doses used for the 24-hour time point irradiations were double those used for the 3-hour time point, to account for the diminishing number of DSBs after repair. The level of residual DSBs (after a 24-hour incubation period), which reflects both early DNA damage and DNA repair, is shown in Figure 2C. The level of residual DSBs within the plateau region of the SOBP is comparable to the calculated physical dose. At points along the distal edge, protons induced a significantly higher number of residual DSBs as compared with photons, which is greater than observed at 3 hours, indicating that the impact of DNA repair is less after proton irradiation relative to photons. The implication is that the DNA lesions at the distal edge are more difficult to repair, as well as there being more DSBs induced.
Verification of decreased survival with colony survival assay
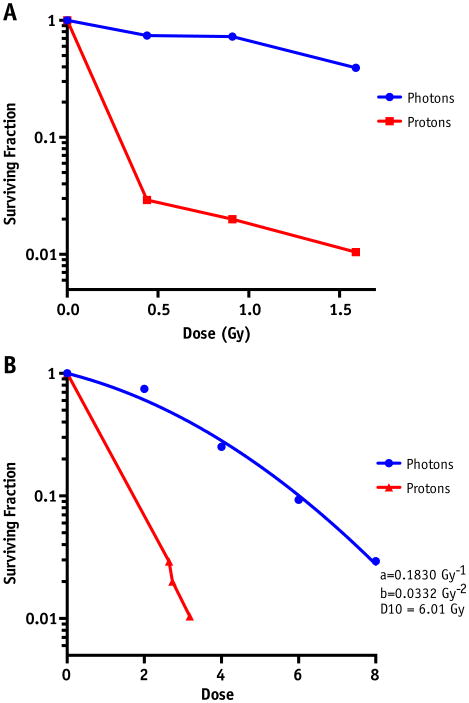
To evaluate and validate our results with γH2AX foci, we performed colony formation assays of cells irradiated at the most distal 3 points of the Bragg peak (points G, H, and I in Fig. 1C). When compared with photons, there is a significant decrease in absolute clonogenic survival in cells irradiated to equivalent doses along the distal edge (Fig. 3A). Figure 3B represents the same survival fractions plotted with RBE-corrected doses (ie, absolute physical dose delivered multiplied by RBE as determined by the γH2AX results), compared with a standard clonogenic survival curve of cells irradiated with photons from 0 to 8 Gy. Even with RBE corrections, the survival of the cells irradiated with protons is still significantly diminished compared with cells irradiated with photons. Although these results do not allow for the same quantitative determinations of RBE as seen with the γH2AX results, owing to the limited dose ranges and data points, it does confirm the enhancement of RBE at the most distal points of the Bragg peak and suggests that the RBE values as measured by γH2AX may be underestimated.
Fig. 3.
(A) Clonogenic survival assay of cells irradiated along 3 most distal points of the Bragg peak compared with cells irradiated with equivalent doses of photons. (B) Survival fractions from (A), plotted on a scale of corrected dose using relative biological effectiveness from γH2AX data (relative biological effectiveness of 2 for 1.5 Gy, 4 for 1 Gy, and 6 for 0.5 Gy), plotted simultaneously with a survival curve of photons irradiated from 0 to 8 Gy.
RBE as a function of depth
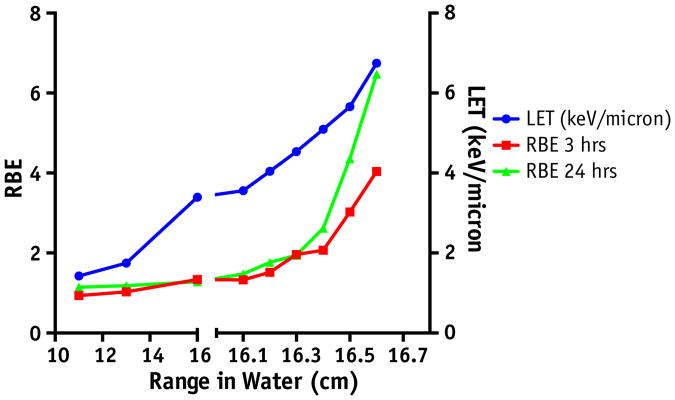
Using the methods for RBE estimates detailed above (Fig. 4A), the subsequent RBE as a function of depth was calculated for both the 3- and the 24-hour time points (Fig. 4B). As a result of the higher γH2AX signal with protons after 3 hours, the RBE is observed to be >2 within a few millimeters of the Bragg peak. By the final measured point, where the physical dose is low, the RBE is measured as >4.0. The difference in γH2AX signal is even more pronounced after 24 hours, and the resulting measured RBE is as high as 6.0 at the far end of the distal edge.
Fig. 4.
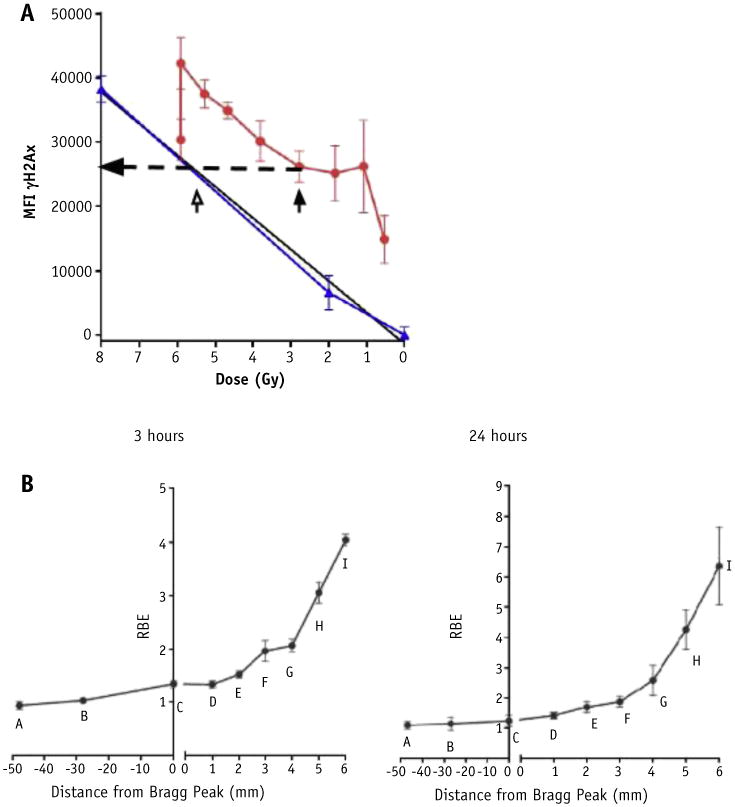
(A) Determination of relative biological effectiveness. For each point along distal edge, photon equivalent dose was calculated by associating proton median fluorescence intensity (MFI) (eg, closed arrowhead) to point on photon best-fit curve of equivalent median fluorescence intensity (open arrowhead). Relative biological effectiveness was calculated by dividing photon equivalent dose by measured physical proton dose for each point. (B) Relative biological effectiveness as a function of depth for each point along the Bragg peak after 3 and 24 hours.
Generation of biological depth dose curve
To illustrate the effect that RBE has on effective biological dose, physical dose measurements from the 3-hour and 24-hour time points were simultaneously plotted with (1) dose measurements assuming a uniform RBE of 1.1 throughout the Bragg peak; and (2) corrected dose measurements using the RBE as determined by γH2AX foci, shown in Figure 5. When physical dose is corrected for RBE from γH2AX foci (red curve), there is a clear extension of effective range and higher biological effective dose at all points along the distal edge after 3 hours when compared with the profile that assumes a uniform RBE of 1.1 (blue curve). Further, the effect seems even more pronounced after 24 hours, again suggesting that the more complex and higher number of DSBs contribute to an even more pronounced effective dose along the distal edge.
Fig. 5.
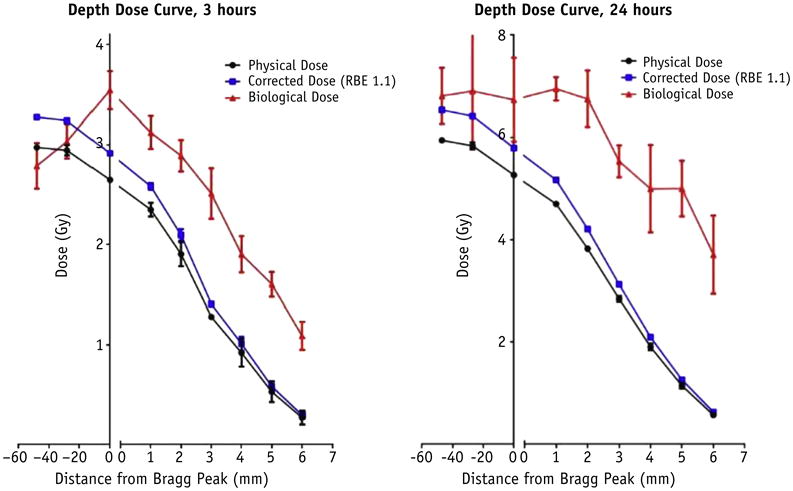
Biological dose as a function of depth along the distal edge, using calculated relative biological effectiveness measurements. Physical dose (as measured by parallel plate chamber) and corrected dose (assuming a uniform relative biological effectiveness of 1.1 from physical dose) were also plotted.
Discussion
Using a highly quantitative assay of DSBs utilizing flow cytometry to measure γH2AX foci, we were able to map in high resolution the level of DSBs at 3 and 24 hours after irradiation at each geographic position along the Bragg peak and generate a detailed map of the RBE of the distal edge. Surprisingly, we found that the RBE increases as a function of depth along the Bragg peak, reaching as high as 4.0 after 3 hours of incubation and as high as 6.0 after 24 hours. When we plotted physical dose and RBE-corrected dose as a function of depth, we found that the peak effective dose is extended 2-3 mm beyond what would be expected with physical calculations, or using a uniform RBE assumption of 1.1. The enhanced RBE effects of the most distal points of the Bragg peak were confirmed via clonogenic survival assay.
An RBE of 1.1 has been used when considering tumor control and normal tissue effects of proton therapy (PT). This presumed equivalence to photons has been driven by the lack of clinical data to suggest any significant difference. Baek et al (3) recently characterized 2 points (center and plateau) of SOBP in clinical use and showed an RBE of 1.1, further supporting the use of this value. However, early experiments investigating different points along a proton beam suggested an increased RBE at the end of range (4-8). More recent experiments have looked carefully at points along the distal edge and have continued to suggest a significantly higher RBE (9-12).
Although compelling, these experiments have several limitations. The endpoints used were biologically complex, with a significant separation of the ionizing radiation's interaction with matter and the ultimate effect on cells. Additionally, many previous reference radiation sources consisted of 60Co (with the exception of the modern studies). Finally, although these studies addressed the distal edge in some way, a detailed, robust, and systematic analysis of many points along the distal edge has not been previously performed.
The purpose of the present study was to address these limitations and to generate a detailed map of the distal edge, using an assay that directly measures the effect of ionizing radiation in vitro. γH2AX is a phosphorylated histone that recruits proteins to the sites of DNA DSBs, and immunohistochemistry can be used to directly assay the number and location of DNA DSBs in a variety of applications (13). By using this direct in vitro assay and comparing protons with a more modern form of photon irradiation, the present results show that the RBE of the distal edge is significantly higher than previously reported estimates. The RBE along the distal edge demonstrates an exponential increase with increasing depth. The increase in RBE and exponential shape as a function of depth is even more pronounced at the 24-hour time point compared with the 3-hour time point.
The more pronounced RBE at 24 hours is especially interesting, because this time point is more reflective of the status of unrepaired DNA damage between standard fractions of radiation therapy. The mechanism behind this observation may involve differences in the linear energy transfer (LET) of the 2 radiation species. Using Monte Carlo simulation, we verified that LET increases at the end of range of the proton beam, where the exponential increase in RBE was observed (Fig. 6). Previous data using Monte Carlo simulations have demonstrated that as compared with low-LET radiation, high-LET radiation induces more complex clusters of DNA damage (14, 15). Although these clusters contain base damages and single-strand breaks that would give a low signal in the γH2AX assay, these highly complex lesions can later be converted into DSBs as a result of attempted repair and replication (16, 17). Presumably, these inadvertent DSBs would be temporally separated from initial irradiation and would translate into a more persistent γH2AX signal and hence a higher RBE at 24 hours as compared with 3 hours. Additionally, protons and photons may require differential repair pathways for these more complex breaks (18), contributing to persistence of fluorescence and a higher RBE value. Other groups have demonstrated a normalization of differences in γH2AX after 6 hours in cells irradiated with X-rays and protons in the middle of an SOBP. The fact that the LET at this point on the Bragg peak is similar to that of photons may explain this conflicting result (19). Further study is warranted to help elucidate the mechanism behind this differential repair profile at the 24-hour time point.
Fig. 6.
Linear energy transfer (LET) and relative biological effectiveness (RBE) as a function of depth along the distal edge.
The implications of these findings affect all aspects of modern PT. The practice of assuming a uniform RBE across a proton beam for treatment planning purposes should be reconsidered for more accurate reporting of biological dose. Additionally, this analysis reinforces the importance of limiting stopping beams into critical structures. The full implications of the higher RBE values that only apply to a small volume of irradiation is hard to predict, but if that small volume were to be on a critical structure also of a small volume, then the consequences could be more significant. Finally, these findings highlight the need for careful and comprehensive reporting of acute and long-term efficacy and toxicity outcomes for patients treated with PT, to aid in defining the utility of PT as an effective modality for the treatment of malignancies.
The strengths of this study lie with the robust and systematic mapping of many points along the Bragg peak. An additional advantage is the direct relationship between the assay and the effect of ionizing radiation on cells. The flow cytometry assay provides a more objective and reproducible method for measuring γH2AX than manual or automated counting of nuclear foci. Finally, there was a significant amount of experimental design to ensure that doses delivered to the samples were accurately and precisely tracked with the use of a simultaneous dosimeter. This study also has several limitations, which we acknowledge. Although the γH2AX assay directly measures the amount of DNA DSBs in vitro, it is difficult to predict how this effect translates into either tumor cell kill or normal tissue toxicity in vivo. The ability to apply the absolute RBE values to other cell types is also limited, because the present analysis was only performed in U2OS cells. The challenges of having to culture cells at one institution and irradiate at another could have introduced confounding variables into the determination of the RBE, although these variables were adequately addressed with the careful use of control slides. Another source of uncertainty is the low dose level at the very distal edge. Because of the very low absolute dose, there could be subtle variations in the γH2AX signal that translate into large differences of RBE, especially after DSB repair. The effects of these uncertainties were addressed in part by conducting experiments with both biological and technical replicates.
Conclusions
The RBE of the proton distal edge is significantly higher than previously reported measurements, displaying an exponential increase as a function of depth. The RBE seems even higher when assessed after repair is maximized, at 24 hours. Exploitation of the dosimetric advantages of PT should be coupled with continued caution when using the distal edge for treatment planning purposes. Further analyses are needed from clinical and experimental settings to define the biological effectiveness and safety of the distal edge.
Supplementary Material
Summary.
The relative biological effectiveness (RBE) of protons may be underestimated. We generated a detailed RBE map of the distal edge of a proton Bragg peak, using an assay of DNA double-strand breaks. The RBE exponentially increased with depth and was significantly higher than previous estimates (as high as 4.0 after 3 hours and 6.0 after 24 hours). These results are further evidence of the complexity and underestimation of the RBE of the distal edge.
Acknowledgments
The authors thank Niek Schreuder, MSc, DABR, of the Provision Center for Proton Therapy, for his assistance in preparation of the manuscript.
Footnotes
Conflict of interest: none.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Paganetti H, Niemierko A, Ancukiewicz M, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53:407–421. doi: 10.1016/s0360-3016(02)02754-2. [DOI] [PubMed] [Google Scholar]
- 2.Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol. 2014;59:R419–R472. doi: 10.1088/0031-9155/59/22/R419. [DOI] [PubMed] [Google Scholar]
- 3.Baek HJ, Kim TH, Shin D, et al. Radiobiological characterization of proton beam at the National Cancer Center in Korea. J Radiat Res. 2008;49:509–515. doi: 10.1269/jrr.08017. [DOI] [PubMed] [Google Scholar]
- 4.Robertson JB, Williams JR, Schmidt RA, et al. Radiobiological studies of a high-energy modulated proton beam utilizing cultured mammalian cells. Cancer. 1975;35:1664–1677. doi: 10.1002/1097-0142(197506)35:6<1664::aid-cncr2820350628>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Tepper J, Verhey L, Goitein M, et al. In vivo determinations of RBE in a high energy modulated proton beam using normal tissue reactions and fractionated dose schedules. Int J Radiat Oncol Biol Phys. 1977;2:1115–1122. doi: 10.1016/0360-3016(77)90118-3. [DOI] [PubMed] [Google Scholar]
- 6.Hall EJ, Kellerer AM, Rossi HH, et al. The relative biological effectiveness of 160 MeV protons–II. Biological data and their interpretation in terms of microdosimetry. Int J Radiat Oncol Biol Phys. 1978;4:1009–1013. doi: 10.1016/0360-3016(78)90013-5. [DOI] [PubMed] [Google Scholar]
- 7.Raju MR, Amols HI, Bain E, et al. A heavy particle comparative study. Part III: OER and RBE. Br J Radiol. 1978;51:712–719. doi: 10.1259/0007-1285-51-609-712. [DOI] [PubMed] [Google Scholar]
- 8.Urano M, Goitein M, Verhey L, et al. Relative biological effectiveness of a high energy modulated proton beam using a spontaneous murine tumor in vivo. Int J Radiat Oncol Biol Phys. 1980;6:1187–1193. doi: 10.1016/0360-3016(80)90172-8. [DOI] [PubMed] [Google Scholar]
- 9.Gerweck LE, Kozin SV. Relative biological effectiveness of proton beams in clinical therapy. Radiother Oncol. 1999;50:135–142. doi: 10.1016/s0167-8140(98)00092-9. [DOI] [PubMed] [Google Scholar]
- 10.Britten RA, Nazaryan V, Davis LK, et al. Variations in the RBE for cell killing along the depth-dose profile of a modulated proton therapy beam. Radiat Res. 2013;179:21–28. doi: 10.1667/RR2737.1. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto Y, Matsuura T, Wada M, et al. Enhanced radiobiological effects at the distal end of a clinical proton beam: In vitro study. J Radiat Res. 2014;55:816–822. doi: 10.1093/jrr/rrt230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhary P, Marshall TI, Perozziello FM, et al. Relative biological effectiveness variation along monoenergetic and modulated Bragg peaks of a 62-MeV therapeutic proton beam: A preclinical assessment. Int J Radiat Oncol Biol Phys. 2014;90:27–35. doi: 10.1016/j.ijrobp.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 13.Bonner WM, Redon CE, Dickey JS, et al. GammaH2AX and cancer. Nat Rev Cancer. 2008;8:957–967. doi: 10.1038/nrc2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikjoo H, O'Neill P, Wilson WE, et al. Computational approach for determining the spectrum of DNA damage induced by ionizing radiation. Radiat Res. 2001;156:577–583. doi: 10.1667/0033-7587(2001)156[0577:cafdts]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 15.Georgakilas AG, O'Neill P, Stewart RD. Induction and repair of clustered DNA lesions: What do we know so far? Radiat Res. 2013;180:100–109. doi: 10.1667/RR3041.1. [DOI] [PubMed] [Google Scholar]
- 16.Singh SK, Wang M, Staudt C, et al. Post-irradiation chemical processing of DNA damage generates double-strand breaks in cells already engaged in repair. Nucleic Acids Res. 2011;39:8416–8429. doi: 10.1093/nar/gkr463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harper JV, Anderson JA, O'Neill P. Radiation induced DNA DSBs: Contribution from stalled replication forks? DNA Repair (Amst) 2010;9:907–913. doi: 10.1016/j.dnarep.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Grosse N, Fontana AO, Hug EB, et al. Deficiency in homologous recombination renders mammalian cells more sensitive to proton versus photon irradiation. Int J Radiat Oncol Biol Phys. 2014;88:175–181. doi: 10.1016/j.ijrobp.2013.09.041. [DOI] [PubMed] [Google Scholar]
- 19.Gerelchuluun A, Hong Z, Sun L, et al. Induction of in situ DNA double-strand breaks and apoptosis by 200 MeV protons and 10 MV X-rays in human tumour cell lines. Int J Radiat Biol. 2011;87:57–70. doi: 10.3109/09553002.2010.518201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.