Abstract
Prostatic artery embolization (PAE) is a promising, new, safe, minimally invasive procedure for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia. However, it can be a one of the most technically difficult interventional radiology procedures because of the challenging anatomy involved. To help achieve technical success and limit complications, the authors present here a series of tips and tricks that have been proven useful from prior PAE experience.
Keywords: prostatic artery embolization, technique, interventional radiology
Objectives: Upon completion of this article, the reader will be able to describe techniques that are necessary to help achieve technical success during difficult prostatic artery embolization and lower complication rates.
Accreditation: This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint providership of Tufts University School of Medicine (TUSM) and Thieme Medical Publishers, New York. TUSM is accredited by the ACCME to provide continuing medical education for physicians.
Credit: Tufts University School of Medicine designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
Prostatic artery embolization (PAE) is a technically challenging procedure with potentially prolonged fluoroscopy and procedure times and a significant risk of technical failure.1 2 The challenges are directly related to the following: target vessel recognition, small tortuous vasculature, atherosclerotic disease, and the rich collateral network. Herein, we discuss techniques that may alleviate the difficulty of PAE.
Target Vessel Recognition
Preoperative noninvasive imaging with computed tomography angiography (CTA) may allow for the identification of the prostatic arteries (PAs) prior to the procedure. The operator may plan ideal angle of imaging to identify the PA origin. This can also be utilized to create fused imaging with intraoperative noncontrast cone beam computed tomography (CBCT), and subsequent “road map” without repeated imaging. Rigorous technique must be utilized to generate high-quality images that may allow for manipulation and reconstruction. Magnetic resonance angiography (MRA) has been suggested as an alternative because of its lack of radiation and tissue characterization properties for the prostate itself. However, limitations of MRA include decreased spatial resolution, more difficult postacquisition manipulation, and increased expense and time.
PA identification can also be accomplished without preprocedural imaging. The conventional method is to use digital subtraction angiography (DSA) alone. Ipsilateral anterior obliquity with mild cranial angulation separates out the branches of the internal iliac artery, allowing for PA identification. For example, with a diagnostic catheter positioned in the left internal iliac artery, the image intensifier can be positioned at 35 degrees left anterior oblique with 10 degrees of cranial angulation. Injection of 5 mL/s for a total of 10 mL will allow for sufficient opacification of the branch vessels. The PAs will be seen coursing anteriorly and inferiorly from one of the branches of the anterior division. Alternatively, a contrasted CBCT can be acquired with the diagnostic catheter in the aorta above the bifurcation. Using contrast diluted to half strength, 4 mL/s is injected for 5 seconds. Imaging is then initiated and contrast injection continues for the remaining 6 seconds during acquisition. The resulting images can be used to identify the PAs, similar to a preprocedural CTA. Additionally, the three-dimensional, volume-rendered images can be overlaid onto the live fluoroscopic images and be used as a dynamic arterial roadmap.
Difficult Catheter Placement
PAs are tortuous with an average diameter of 1.6 mm.3 The origin of the PA often arises at acute angles with a short neck from its parent vessel, which can make wire selection and subsequent catheterization challenging. In this setting, pre-shaped microcatheters may be beneficial in advancing a guidewire into the PA (Fig. 1). Having both pre-shaped guidewires and microcatheters can result in a synergistic effect making catheterization of difficult vessels possible.
Fig. 1.

Left anterior oblique digital subtraction angiography image with pre-shaped microcatheter in the proximal vesiculo-prostatic (VP) trunk (black arrow), demonstrating the course of the anterior/lateral prostatic artery (white arrows). The VP trunk often arises sharply and courses anteriorly from the anterior division. The curved tip of a pre-shaped microcatheter makes advancing a guidewire through this angle easier.
Occasionally, because of the acute angles in which the PAs can arise, conventional catheters and guidewires will not provide enough support to advance a microcatheter into the appropriate position for embolization. This most commonly occurs when the anterior/lateral PA (ALPA) arises from a vesiculo-prostatic (VP) trunk. In these situations, a robotic catheter can be the difference between technical success and failure. After advancing the robotic catheter to the origin of the VP trunk, the tip can be deflected to provide the optimal angle to advance the guidewire into the ALPA (Fig. 2). Because the robotic catheter maintains its deflected shape even while the wire and catheter are advanced against the resistance of a tortuous PA, it provides the support needed to advance the microcatheter to achieve optimal positioning. While there are advantages of the robotic catheter including decreased radiation exposure to the operator4 and increased microcatheter support, the use of endovascular robotics is not necessary to achieve technical success in the majority of PAEs and the cost of the unit and the associated disposable accessories can be prohibitive.
Fig. 2.

Right anterior oblique digital subtraction angiography image demonstrating a robotic catheter in the origin of the anterior division of the right internal iliac artery with tip deflected toward an anterior/lateral prostatic artery (white arrows) arising from a vesiculo-prostatic trunk (black arrow).
Elderly male patients are prone to atherosclerotic disease, internal iliac artery tortuosity, and occasional complete PA occlusion. Internal iliac artery tortuosity (Fig. 3a) often results in unstable placement of the diagnostic catheter in the anterior division. Once the guidewire is removed, the catheter will spontaneously retract into the posterior division. Additionally, when positioning the catheter more proximally in the internal iliac artery, it tends to take on a posterolateral facing position, such that the microcatheter cannot be directed along the anteromedial wall of the anterior trunk, which is commonly where the origin of the VP trunk is located. The instability may be overcome with the use of more rigid support, such as a 6F sheath placed in the anterior division. If this fails as well, a “buddy wire” may be employed and advanced deep into the anterior division, such as in the distal inferior gluteal artery, to provide added stability and maintain sheath access to the anterior division (Fig. 3b). The microcatheter can then be placed parallel to the buddy wire for selective catheterization of the PA. Care should be taken with the buddy wire to avoid distal anterior division branch injury, especially in the internal pudendal artery.
Fig. 3.
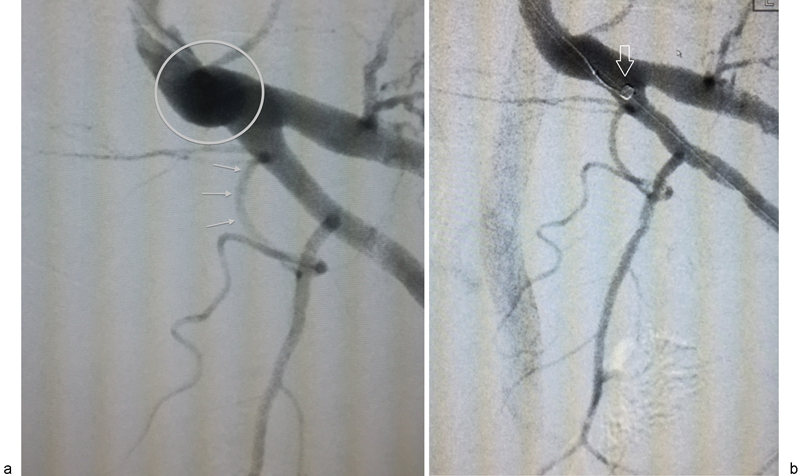
(a) Left anterior oblique digital subtraction angiography image demonstrating tortuosity of the distal left internal iliac artery (circle). Because of this “knuckle,” a catheter placed within the proximal anterior division will often spontaneously retract into the posterior division making catheterization of a prostatic artery (arrows) arising from a vesiculo-prostatic trunk difficult. (b) digital subtraction angiography image with similar obliquity from the same patient as 3A, demonstrating a sheath with tip (arrow) maintained in the proximal anterior division despite the internal iliac artery tortuosity by a “buddy wire” coursing deep into the gluteal-pudendal trunk.
Rarely the microcatheter cannot be placed into the target PA, as the wire preferentially selects a nontarget branch. In cases such as this, a coil can be deployed proximally in the nontarget artery, resulting in redirection of the wire and microcatheter to the target PA (Fig. 4). If the PA is completely occluded secondary to atherosclerotic disease, the microcatheter and a crossing wire may be used to cross the target occlusion and then perform subsequent prostatic parenchymal embolization.5
Fig. 4.
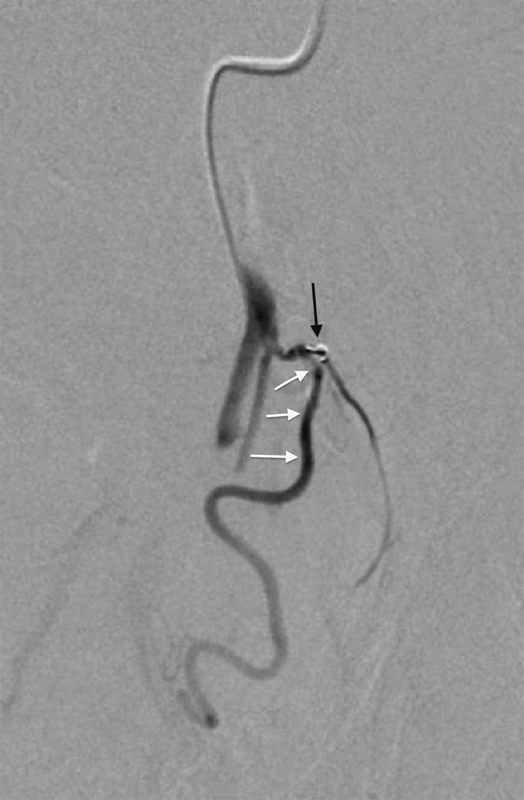
Digital subtraction angiography image with catheter tip in a vesiculo-prostatic trunk arising from the anterior division of the left internal iliac artery. Because the guidewire repetitively selected an anterior coursing vesicular branch, a coil was placed proximally within this artery (black arrow) resulting in the wire to be deflected into the prostatic artery (white arrows).
Confirming Correct Catheter Position Prior to Embolization
Once the microcatheter has been placed in the target vessel, additional steps are taken to prevent nontarget embolization. Initially, DSA imaging is obtained. If the catheter is located in the ALPA, the primary target vessel during PAE, parenchymal blush should be seen through the expected location of the prostate. If the catheter is located in the posterior/lateral PA (PLPA), only capsular branches will be seen with perhaps a small amount of blush at the inferior margin. If small branches supplying the inferior bladder wall are seen, either the catheter is too proximal and should be advanced distally prior to embolization or there is reflux into these vessels from too vigorous of an injection. Generally, to avoid reflux, contrast is hand injected with a 3-mL syringe slowly at a rate of approximately 0.2 to 0.5 mL/s using an imaging rate of 2 frames per second. The slow injection and decreased frames per second allows for minimal reflux and improved opacification of the prostate parenchyma while limiting the radiation exposure to the patient and operator.
If there is any doubt of the location of the microcatheter after DSA imaging, contrasted CBCT is a useful tool for confirmation.6 Prior to the acquisition, contrast is hand injected very slowly, again to avoid reflux. A CBCT is then performed with the patient holding as still as possible. If the catheter position is correct, resulting images should demonstrate enhancement of the hemiprostate on the same side as the catheter and no other organ enhancement. If the catheter is inadvertently placed in the PLPA instead of the ALPA, rectal enhancement is likely due to the frequency of rectal supply from that artery. Additionally, cavernosal enhancement can frequently be seen after injection into the ALPA due to the frequency of intra- and extraprostatic penile shunts arising from the ALPA. One common pitfall during contrasted CBCT of the prostate is injecting too vigorously prior to imaging, leading to reflux into parent vessels and resultant enhancement of nontarget organs.
Particle Redirection with Proximal Embolization
Intra- and extraprostatic PA anastomoses with vessels supplying other organs are relatively common.3 A commonly seen collateral pathway is an anastomosis between a branch of the ALPA and the internal pudendal artery. This collateral is particularly concerning because of the potential for nontarget embolization resulting in erectile dysfunction. These shunts can be managed in several ways. The optimal method is to advance the microcatheter beyond the branch coursing toward the penis and then embolizing very carefully to avoid particle reflux. However, this is not always possible due to the small size and tortuosity of these branches. Alternatively, the collateral pathway can be embolized proximally with either a coil or gelatin foam.7 One more option that can be useful when these anastomoses are particularly narrow is to use larger particles for embolization (300–500 vs. 100–300 µm) that will not pass through them.
Conclusion
PAE is a technically challenging procedure for multiple reasons: the difficulty in selecting the correct target artery among a multitude of internal iliac artery branches, the challenge of manipulating a catheter into position for embolization because of the tortuosity and small diameter of the vessels involved, and the potential for nontarget embolization due to commonly seen collaterals. Hopefully, the tips provided earlier will be useful in increasing the technical success rate and decreasing complications associated with PAE.
References
- 1.Laborda A, De Assis A M, Ioakeim I, Sánchez-Ballestín M, Carnevale F C, De Gregorio M A. Radiodermitis after prostatic artery embolization: case report and review of the literature. Cardiovasc Intervent Radiol. 2015;38(3):755–759. doi: 10.1007/s00270-015-1083-6. [DOI] [PubMed] [Google Scholar]
- 2.Schreuder S M, Scholtens A E, Reekers J A, Bipat S. The role of prostatic arterial embolization in patients with benign prostatic hyperplasia: a systematic review. Cardiovasc Intervent Radiol. 2014;37(5):1198–1219. doi: 10.1007/s00270-014-0948-4. [DOI] [PubMed] [Google Scholar]
- 3.Bilhim T, Pisco J M, Rio Tinto H. et al. Prostatic arterial supply: anatomic and imaging findings relevant for selective arterial embolization. J Vasc Interv Radiol. 2012;23(11):1403–1415. doi: 10.1016/j.jvir.2012.07.028. [DOI] [PubMed] [Google Scholar]
- 4.Rolls A E, Riga C V, Bicknell C D, Regan L, Cheshire N J, Hamady M S. Robot-assisted uterine artery embolization: a first-in-woman safety evaluation of the Magellan System. J Vasc Interv Radiol. 2014;25(12):1841–1848. doi: 10.1016/j.jvir.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Bagla S, Smirniotopolous J B, Vadlamudi V. Crossing a prostatic artery chronic total occlusion to perform prostatic arterial embolization. J Vasc Interv Radiol. 2016;27(2):295–297. doi: 10.1016/j.jvir.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Bagla S, Rholl K S, Sterling K M. et al. Utility of cone-beam CT imaging in prostatic artery embolization. J Vasc Interv Radiol. 2013;24(11):1603–1607. doi: 10.1016/j.jvir.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Isaacson A J, Bhalakia N, Burke C T. Coil embolization to redirect embolic flow during prostatic artery embolization. J Vasc Interv Radiol. 2015;26(5):768–770. doi: 10.1016/j.jvir.2014.12.615. [DOI] [PubMed] [Google Scholar]


