Abstract
In the airways of severe asthmatics, an increase of neutrophils and eosinophils is often observed despite high-dose corticosteroid therapy. We previously reported that interleukin-8-stimulated neutrophils induced trans-basement membrane migration (TBM) of eosinophils, suggesting the link between neutrophils and eosinophils. Concentrations of lipopolysaccharide (LPS) in the airway increase in severe asthma. As neutrophils express Toll-like receptor (TLR)4 and can release chemoattractants for eosinophils, we investigated whether LPS-stimulated neutrophils modify eosinophil TBM.
Neutrophils and eosinophils were isolated from peripheral blood of healthy volunteers and severe asthmatics. Eosinophil TBM was examined using a modified Boyden's chamber technique. Eosinophils were added to the upper compartment, and neutrophils and LPS were added to the lower compartment. Migrated eosinophils were measured by eosinophil peroxidase assays.
LPS-stimulated neutrophils induced eosinophil TBM (about 10-fold increase), although LPS or neutrophils alone did not. A leukotriene B4 receptor antagonist, a platelet-activating factor receptor antagonist or an anti-TLR4 antibody decreased eosinophil TBM enhanced by LPS-stimulated neutrophils by almost half. Neutrophils from severe asthmatics induced eosinophil TBM and lower concentrations of LPS augmented neutrophil-induced eosinophil TBM.
These results suggest that the combination of neutrophils and LPS leads eosinophils to accumulate in the airways, possibly involved the pathogenesis of severe asthma.
Short abstract
LPS-stimulated neutrophils induced eosinophil TBM, which may be involved in the pathogenesis of severe asthma http://ow.ly/UR4jP
Introduction
Bronchial asthma is a chronic disorder usually characterised by eosinophilic airway inflammation, mucus hypersecretion and an increase in airway hyperresponsiveness (AHR) [1]. However, in the case of severe asthma, neutrophilic inflammation in addition to eosinophilic inflammation may also play important role(s) in its pathogenesis [2–4]. For example, the European Network For Understanding Mechanisms of Severe Asthma study reported that patients with severe asthma have greater sputum neutrophil counts and greater release of eosinophil-derived mediators compared to patients with mild to moderate asthma [2]. Wenzel et al. [3] suggested that severe asthma could be divided into two inflammatory subtypes: the eosinophil-positive, neutrophil-positive group, and the eosinophil-negative, neutrophil-positive group. Furthermore, we reported a positive correlation between concentrations of neutrophils and eosinophils in induced sputum from patients with severe, corticosteroid-dependent asthma [4]. Therefore, both eosinophils and neutrophils are increased in the airways of patients with some phenotypes of severe asthma, and may contribute to the severity of asthma.
Interleukin (IL)-8 plays an important role in the accumulation of neutrophils in sites of inflammation and expression of IL-8 in the airway is upregulated in severe asthmatic patients [5, 6]. We also confirmed that the amount of IL-8 protein in induced sputum is higher in severe asthmatics than in mild asthmatics [7]. Furthermore, we reported that, even in the absence of chemoattractant for eosinophils, neutrophils stimulated by IL-8 are capable of inducing the trans-basement membrane migration (TBM) of eosinophils [8], suggesting that IL-8-stimulated neutrophils lead eosinophils to accumulate in the airways of asthmatic patients. The mechanism of upregulation of IL-8 in severe asthma remains to be elucidated.
Lipopolysaccharide (LPS) is an important candidate for inducing IL-8 or neutrophilic inflammation in the airway of severe asthmatics. For example, Hauk et al. [9] reported the correlation between LPS levels and airway neutrophils or IL-8 in bronchoalveolar lavage (BAL) fluid from children with asthma and persistent wheezing. Furthermore, Goleva et al. [10] reported that LPS and genes associated with activation of LPS signalling are higher in BAL fluid of corticosteroid-resistant asthma than in corticosteroid-sensitive asthma, and IL-8 mRNA expression by BAL cells positively correlates with the amount of LPS in BAL fluid. Although the reason why LPS is upregulated in the airway of severe asthma is unknown, several studies suggested the role of Gram-negative bacteria or of house dust in the upregulation of LPS in severe asthma [11–14]. For example, a relationship between Gram-negative bacterial colonisation in the airways and the severity of asthma has been reported [11, 12]. Furthermore, the frequency and severity of asthma correlated with LPS concentrations, not with house dust mite (HDM) concentrations, in house dust [13, 14].
As neutrophils express Toll-like receptor (TLR)4 [15, 16], the receptor for LPS [16], and produce a variety of inflammatory mediators including chemoattractants for eosinophils, such as leukotriene (LT)B4 and platelet-activating factor (PAF), on LPS stimulation [17–19], we hypothesised that LPS-stimulated neutrophils would play some role in the pathogenesis of eosinophilic inflammation observed with some cases of severe asthma, especially in neutrophil-dependent eosinophil accumulation in the airway. In the present study, we examined whether neutrophils stimulated with LPS modify the TBM of eosinophils. We found that LPS-stimulated neutrophils from healthy volunteers augment the TBM of eosinophils, while neither neutrophils nor LPS alone induced it. LTB4 and PAF are involved in the augmentation of eosinophil TBM by LPS-stimulated neutrophils. Furthermore, neutrophils from severe asthmatics induced eosinophil TBM even without LPS stimulation and lower concentrations of LPS augmented the neutrophil-mediated eosinophil TBM. These results provide a novel mechanism that could be a therapeutic target for severe asthma.
Materials and methods
Reagents
Anti-CD16 antibody (Ab)-coated magnetic beads were purchased from Miltenyi Biotec (Auburn, CA, USA). Hank's balanced salt solution (HBSS) was purchased from Gibco Brl (Grand Island, NY, USA). PBS was obtained from Wako (Osaka, Japan). Fetal bovine serum (FBS) was purchased from MP Biomedicals (Aurora, OH, USA). LPS (from Escherichia coli O111:B4) and bovine serum albumin (BSA) were purchased from Sigma-Aldrich (St Louis, MO, USA). Anti-TLR4 Ab and goat IgG (an isotype control for anti-TLR4 Ab) were purchased from R&D Systems (Minneapolis, MN, USA). BIIL260, a LTB4 receptor antagonist, and WEB2170, a PAF receptor antagonist, were purchased from Boehringer Ingelheim (Ridgefield, CT, USA).
Preparation of neutrophils and eosinophils
Neutrophils and eosinophils were prepared as previously described [8]. Both types of cells were isolated from the peripheral blood of the same donors, collected from nonatopic healthy volunteers whose eosinophil content was <5% of their peripheral leukocytes (14 subjects (nine males and five females, ranging in age from 24 to 40 years)) or from severe asthmatics (four patients (one male and three females, ranging in age from 42 to 87 years)), respectively. Healthy volunteers were defined as subjects with no history of allergic disease such as asthma or rhinitis and no symptoms such as cough or sneeze associated with allergic disease. We measured allergen-specific IgE Abs of some, but not all, subjects, and none had specific IgE Abs to allergens such as HDM, moulds or Japanese cedar pollen. None took concomitant medications or had a history of smoking. Severe asthma was defined according to the American Thoracic Society/European Respiratory Society statement [20]. We received approval for this study from the Ethics Committee of Saitama Medical University (Saitama, Japan) and informed consent was obtained from the study subjects before collection of each blood sample. 10 mL dextran were added to 40 mL heparinised blood and erythrocytes were removed as sediment. The Histopaque system (Sigma-Aldrich) was used for isolation according to the manufacturer's instructions. Separated lymphocytes were removed, and 2 mL of sterile distilled water and 2 mL of 1.8% salt solution were added, followed by 20 mL of sterile PBS, and the neutrophils (>95% purity and >99% viability) were separated by centrifuge. The neutrophils were suspended in HBSS containing 0.2% BSA (HBSS/BSA buffer). The remaining cells were washed with HBSS at 4°C supplemented with 5% FBS in PBS then incubated with anti-CD16 Ab-coated magnetic beads for 30 min at 4°C, and finally filtred with a column containing steel wool placed in a magnetic field (Miltenyi Biotec). Eosinophils (>98% purity and >99% viability), which passed through the column, were collected and washed, and the number of cells was adjusted to 5.0×105 cells·mL−1 with 0.2% HBSS/BSA buffer.
Trans-basement membrane migration
The TBM of neutrophils and eosinophils was examined using a modified Boyden's chamber method as previously described [8]. Eosinophils (1×105 cells) were placed into the upper compartment of a Matrigel-coated Transwell (3 μm; Becton Dickinson Labware, Franklin Lakes, NJ, USA). Neutrophils (2×104 cells) and LPS (10 µg·mL−1) were placed into the lower compartment of the chamber and incubated for 120 min in 5% CO2 at 37°C, according to the findings that eosinophil TBM by IL-8-stimulated neutrophils mainly occurred 15–60 min after the initiation of reaction and reached plateau at 90–120 min (data not shown) [8]. The peroxidase activity of migrated eosinophils in the lower compartment of the chamber was evaluated. To determine the peroxidase activity of eosinophils, the medium of the lower compartment was incubated with a substrate (1 mM o-phenylenediamine, 1 mM hydrogen peroxide and 0.1% Triton X-100 in Tris-HCI, pH 8.0) for 30 min at room temperature. The reaction was stopped by adding 100 μL of 4 N sulfuric acid and absorbance at 490 nm was measured using the Fluoromark (Bio-Rad Laboratories, Hercules, CA, USA) microplate fluorimeter. The number of migrated eosinophils was calculated from the eosinophil peroxidase activity of the standard media, which contained known numbers of eosinophils (1×103, 3×103, 1×104, 3×104 and 1×105), then percentage migration was calculated from the number of eosinophils (1×105) placed into the upper compartment. The viability of both eosinophils and neutrophils after migration exceeded 98% by trypan blue exclusion.
Blocking study
To examine the effect of antagonist for LTB4 or PAF, eosinophils (1×105 cells) were co-incubated with BIIL260 (10 μM) or WEB2170 (10 μM) for 20 min at 37°C with shaking. The media containing the cells were then transferred to the upper compartment of the chamber and the assay was performed as described above. To examine the effect of anti-TLR4 Ab, neutrophils (2×104 cells) were co-incubated with anti-TLR4 Ab (1 µg·mL−1) or an isotype-matched control goat IgG for 20 min at 4°C with shaking, then the media containing cells were transferred to the lower compartment and the assay was performed.
Statistical analysis
Statistical analysis was performed with SAS version 9.1.3 SP4 (SAS Institute Inc., Cary, NC, USA) and Graphpad Prism 6 (Graphpad Software Inc., San Diego, CA, USA). Values are expressed as mean±sem. After Shapiro-Wilk's normality test, according to the previous reports [8], data were compared using a one-way ANOVA followed by Tukey's multiple comparisons test when differences were significant or a paired t-test for analysis of the differences between two groups. As for experiments about the dose-dependent effect of LPS, we used the Jonckheere–Terpstra test for trend to analyse the monotonic increase of eosinophil TBM. A p<0.05 was considered statistically significant.
Results
LPS-stimulated neutrophils obtained from healthy volunteers induce the TBM of eosinophils
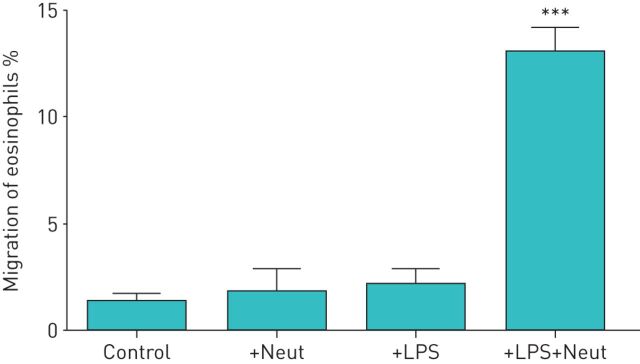
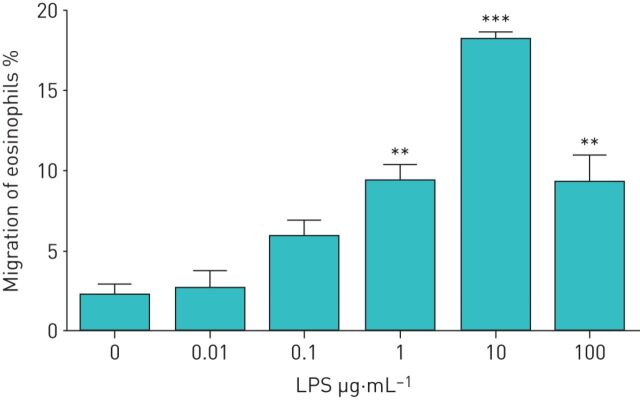
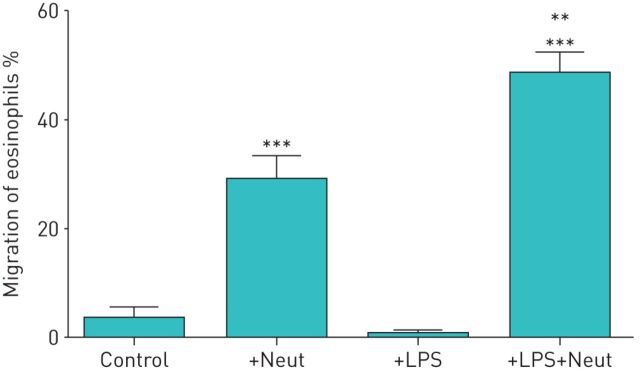
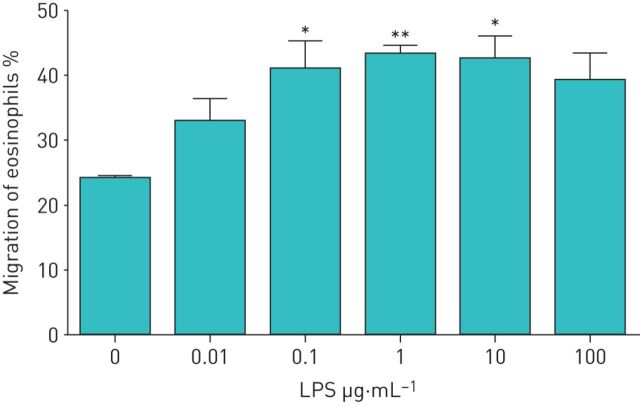
We investigated whether LPS-stimulated neutrophils affect the TBM of eosinophils. First, we obtained both eosinophils and neutrophils from healthy volunteers. Eosinophils were added to the upper compartment of a chamber and neutrophils in the presence or absence of LPS were placed in the lower compartment. After 120 min of incubation, migrated eosinophils in the lower chamber were measured by eosinophil peroxidase assays. In healthy volunteers, neither neutrophils nor LPS alone induced TBM of eosinophils (migrated eosinophils: 1.2±0.4% by medium control, 1.7±1.0% by neutrophils alone and 2.1±0.7% by LPS alone; n=5) (figure 1). However, when neutrophils were co-incubated with LPS in the lower compartment, a significant TBM of eosinophils was observed (migrated eosinophils: 13.0±1.1%, about 10-fold increase; p<0.001 versus the other three conditions, n=5) (figure 1). We next examined the concentrations of LPS required to induce neutrophil-dependent eosinophil TBM. The statistically significant monotonic increase in eosinophil TBM was observed in healthy volunteers when LPS concentrations increased from 0 to 1 µg·mL−1, and 10 µg·mL−1 LPS induced maximal response (migrated eosinophils: 2.2±0.7% by medium control, 9.4±1.0% by 1 µg·mL−1 LPS (p<0.001 by the Jonckheere–Terpstra test for trend) and 18.1±0.5% by 10 µg·mL−1 LPS (p<0.001); n=4) (figure 2).
FIGURE 1.
Lipopolysaccharide (LPS)-stimulated neutrophils from healthy volunteers induce the trans-basement membrane migration of eosinophils. Neutrophils and eosinophils were isolated from peripheral blood of healthy volunteers. Eosinophils (1×105 cells) were added to the upper compartment of a chamber with a Matrigel-coated Transwell insert. Hank's balanced salt solution (HBSS)/bovine serum albumin (BSA) (Control), neutrophils (2×104 cells) in HBSS/BSA (+Neut), LPS (10 µg·mL−1) in HBSS/BSA (+LPS) or the combination of LPS and neutrophils (+LPS+Neut) were placed into the lower compartment. After 120 min of incubation, migrated eosinophils in the lower chamber were measured by eosinophil peroxidase assays (n=5). Data are presented as mean±sem. ***: p<0.001 versus Control, +Neut, or +LPS.
FIGURE 2.
Dose-dependent effect of lipopolysaccharide (LPS) on neutrophil-induced trans-basement membrane migration of eosinophils in healthy volunteers. Neutrophils (2×104 cells) were stimulated with various concentrations of LPS (0.01–100 µg·mL−1) and then placed into the lower compartment. Eosinophils (1×105 cells) were added to the upper compartment of a chamber with a Matrigel-coated Transwell insert. After 120 min of incubation, migrated eosinophils in the lower chamber were measured by eosinophil peroxidase assays (n=4). Data are presented as mean±sem. **: p<0.01 versus spontaneous migration (0 µg·mL−1 LPS) by Tukey test; ***: p<0.001 versus spontaneous migration (0 µg·mL−1 LPS) by Tukey test.
LTB4 and PAF are involved in the augmentation of eosinophil TBM by LPS-stimulated neutrophils
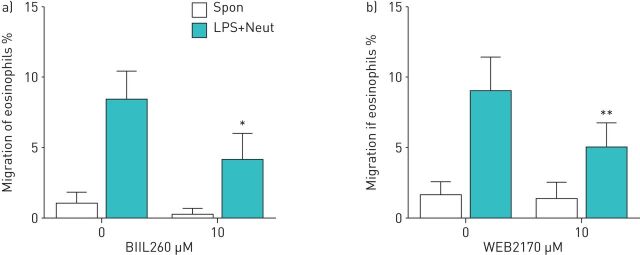
When activated, neutrophils can secrete molecules that are stimulatory for eosinophils. LTB4 and PAF are representative molecules secreted by neutrophils and are capable of inducing migration or activation of eosinophils [21–24]. Therefore, we examined whether inhibition of the activity of these molecules can modulate the neutrophil-dependent eosinophil TBM. The reagents used were BIIL260, a LTB4 receptor antagonist, and WEB2170, a PAF receptor antagonist. They were incubated with eosinophils and then placed in the upper compartment. Both reagents partially suppressed the TBM of eosinophils in the presence of LPS-activated neutrophils (migrated eosinophils 8.4±2.0% by LPS-stimulated neutrophils, and 4.1±1.9% by LPS-stimulated neutrophils and BIIL260 (51.3% inhibition; p<0.05, n=4 (figure 3a)); and migrated eosinophils 9.0±2.4% by LPS-stimulated neutrophils, and 4.9±1.8% by LPS-stimulated neutrophils and WEB2170 (45.4% inhibition; p<0.01, n=4 (figure 3b)), although there was no effect in the absence of LPS-stimulated neutrophils. BIIL260 and WEB2170 did not express a synergistic or additive effect (data not shown). These results indicate that the augmentation of eosinophil TBM due to LPS-stimulated neutrophils is partially mediated by LTB4 and PAF.
FIGURE 3.
a) Effect of a leukotriene B4 receptor antagonist on eosinophil trans-basement membrane migration (TBM). Eosinophils (1×105 cells) were treated with BIIL260 (10 μM) and then placed into the upper compartment of a chamber with a Matrigel-coated Transwell insert. Neutrophils (2×104 cells) and LPS (10 µg·mL−1) were placed into the lower compartment. After 120 min of incubation, migrated eosinophils in the lower chamber were measured (n=4). b) Effect of a platelet-activating factor receptor antagonist on eosinophil TBM. Eosinophils (1×105 cells) were treated with WEB2170 (10 μM) and placed into the upper compartment. Neutrophils (2×104 cells) and LPS (10 µg·mL−1) were placed into the lower compartment. After 120 min of incubation, migrated eosinophils were measured by eosinophil peroxidase assays (n=4). Data are presented as mean±sem. Spon: spontaneous migration. *: p<0.05 versus without BIIL260; **: p<0.01 versus without WEB2170.
The effect of anti-TLR4 Ab on eosinophil TBM induced by LPS-stimulated neutrophils
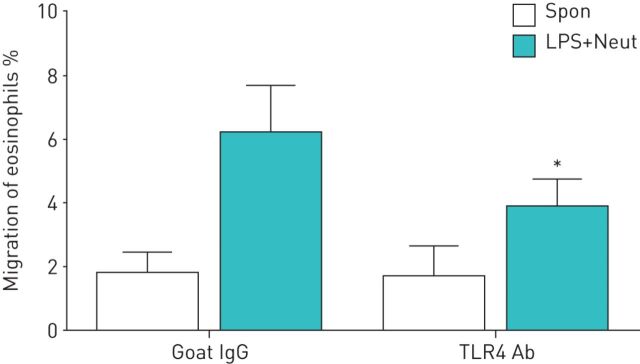
We next confirmed whether LPS/TLR4 is actually involved in neutrophil-dependent eosinophil TBM. Neutrophils were incubated with anti-TLR4 Ab or an isotype-matched control, placed in the lower compartment, and then stimulated with LPS. Anti-TLR4 Ab actually suppressed the augmentation of eosinophil TBM induced by LPS-stimulated neutrophils (migrated eosinophils: 6.2±1.4% by LPS-stimulated neutrophils, 3.9±0.9% by LPS-stimulated neutrophils and anti-TLR4 Ab, and 37.8% inhibition; p<0.05, n=4) (figure 4).
FIGURE 4.
Effect of an anti-Toll-like receptor (TLR)4 antibody (Ab) on the trans-basement membrane migration of eosinophils induced by lipopolysaccharide (LPS)-stimulated neutrophils (LPS+Neut). Neutrophils (2×104 cells) were treated with anti-TLR4 Ab (1 µg·mL−1) or isotype control goat IgG (1 µg·mL−1), placed into the lower compartment of a chamber with a Matrigel-coated Transwell insert, and then stimulated with LPS (10 µg·mL−1). Eosinophils (1×105 cells) were added to the upper compartment. After 120 min of incubation, migrated eosinophils in the lower chamber were measured by eosinophil peroxidase assays (n=4). Data are presented as mean±sem. Spon: spontaneous migration. *: p<0.05 versus without anti-TLR4 Ab.
The effect of neutrophils or LPS-stimulated neutrophils from severe asthmatics on eosinophil TBM
Finally, we examined whether LPS-stimulated neutrophils from severe asthmatics also induce eosinophil TBM. Both eosinophils and neutrophils were obtained from severe asthmatics. LPS alone did not induce TBM of eosinophils (migrated eosinophils: 3.4±2.0% by medium control, 0.7±0.6% by LPS alone; n=4), whereas neutrophils from severe asthma alone induced TBM of eosinophils in contrast to the case of healthy volunteers (migrated eosinophils: 28.8±4.6% by neutrophils alone; p<0.001 versus control, n=4) (figure 5). Furthermore, stimulation with LPS (10 µg·mL−1) increased neutrophil-mediated eosinophil TBM (migrated eosinophils: 48.4±3.8%, about 15-fold increase; p<0.001 versus control, p<0.01 versus neutrophils alone; n=4) (figure 5). We then examined the concentrations of LPS required to induce neutrophil-mediated eosinophil TBM. The statistically significant monotonic increase of eosinophil TBM was observed in severe asthmatics when LPS concentrations increased from 0 to 0.1 µg·mL−1 (migrated eosinophils: 23.8±0.3% by medium control, 40.6±4.6% by 0.1 µg·mL−1 LPS (p<0.05 by the Jonckheere–Terpstra test for trend), 42.9±1.5% by 1 µg·mL−1 LPS (p<0.01) and 42.2±3.7% by 10 µg·mL−1 LPS (p<0.01); n=4) (figure 6), which was lower than the case of healthy volunteers.
FIGURE 5.
Effect of neutrophils or lipopolysaccharide (LPS)-stimulated neutrophils from severe asthmatics on eosinophil trans-basement membrane migration. Neutrophils and eosinophils were isolated from the peripheral blood of severe asthmatics. Eosinophils (1×105 cells) were added to the upper compartment of a chamber with a Matrigel-coated Transwell insert. Hank's balanced salt solution (HBSS)/bovine serum albumin (BSA) (Control), neutrophils (2×104 cells) in HBSS/BSA (+Neut), LPS (10 µg·mL−1) in HBSS/BSA (+LPS) or the combination of LPS and neutrophils (+LPS+Neut) were placed into the lower compartment. After 120 min of incubation, migrated eosinophils in the lower chamber were measured by eosinophil peroxidase assays (n=4). Data are presented as mean±sem. **: p<0.01 versus +Neut; ***p<0.001 versus Control.
FIGURE 6.
Dose-dependent effect of lipopolysaccharide (LPS) on neutrophil-induced trans-basement membrane migration of eosinophils in severe asthmatics. Neutrophils (2×104 cells) from severe asthmatics were stimulated with various concentrations of LPS (0.01–100 µg·mL−1), and then placed into the lower compartment of a chamber with a Matrigel-coated Transwell insert. Eosinophils (1×105 cells) from severe asthmatics were added to the upper compartment. After 120 min of incubation, migrated eosinophils in the lower chamber were measured by eosinophil peroxidase assays (n=4). Data are presented as mean±sem. *: p<0.05 versus spontaneous migration (0 µg·mL−1 LPS) by Tukey test; **: p<0.01 versus spontaneous migration (0 µg·mL−1 LPS) by Tukey test.
Discussion
The present study demonstrated that LPS-stimulated neutrophils augmented the TBM of eosinophils in healthy volunteers. Neither neutrophils from healthy volunteers nor LPS alone induced the eosinophil TBM. A LTB4 receptor antagonist or a PAF receptor antagonist suppressed the augmentation of eosinophil TBM by LPS-stimulated neutrophils. Furthermore, neutrophils from severe asthma patients induced eosinophil TBM without LPS stimulation and lower concentrations of LPS augmented neutrophil-mediated eosinophil TBM in severe asthma. These findings suggest that interaction of LPS and neutrophils, both increased in the airway of severe asthmatics, play important roles in the development of eosinophilic inflammation in severe asthma. Furthermore, neutrophils of severe asthmatics are already activated in vivo and have a potential to migrate or activate eosinophils.
The TBM of eosinophils induced by LPS-stimulated neutrophils was inhibited by an anti-TLR4 Ab (figure 4). TLR4 is an established receptor for LPS and neutrophils constitutively express TLR4 [15, 16]. LPS binds to the serum LPS-binding protein (LBP) before subsequently binding to TLR4 [25] and then LBP catalyses the transfer of LPS to membrane-bound or soluble CD14. CD14 splits LPS aggregates into monomeric molecules and presents them to the TLR4–myeloid differentiation factor 2 complex [16]. Recently, a single-nucleotide polymorphism in the promoter region of CD14 has been studied in patients with allergy [26–31]. Early studies suggested that this variant, which affects the soluble CD14 level [27, 28], was associated with more severe atopy [27, 29, 30]. Although this finding was not uniformly replicated [31] and consideration of LPS exposure level is required to analyse the data [26], these findings indicated that interaction between LPS and CD14/TLR may be involved in the pathophysiology of some aspect of asthma.
Our results strongly support the significance of LPS and neutrophils in the pathogenesis of severe asthma. It is known that chronic exposure to low LPS concentrations can contribute to the development of obstructive lung disease including emphysema and asthma [32]. In fact, LPS inhalation has been shown to induce IL-8 production and neutrophilic inflammation in the airway [33]. Further, Hauk et al. [9] reported the correlation between LPS levels and airway neutrophils or IL-8 in BAL fluid of children with asthma. Moreover, Goleva et al. [10] reported that LPS and LPS-related gene products are higher in BAL fluid from patients with corticosteroid-resistant asthma and IL-8 mRNA expression by BAL cells positively correlates with the amount of LPS in BAL fluid.
The mechanism of the upregulation of LPS in the airway of patients with severe asthma has not been fully clarified. One possibility is the colonisation by Gram-negative bacteria of the lower airways, as LPS is found in the outer membrane of Gram-negative bacteria. Recently, the relationship between bacterial colonisation in the airways and the development or severity of asthma has been highlighted [11, 12, 34–36]. For example, Bisgaard et al. [34] reported that bacterial colonisation in the airway increased the risk for the development of asthma. Huang et al. [35] reported that airway microbiota composition and diversity were significantly correlated with AHR in asthma. Among these reports, several have emphasised the significance of Gram-negative bacteria [11, 12]. For example, Goleva et al. [11] reported the airway expansion of specific Gram-negative bacteria such as Haemophilus parainfluenzae in severe asthma. Moreover, Green et al. [12] reported that bacterial colonisation was associated with low forced expiratory volume in 1 s (FEV1), sputum neutrophil counts and disease duration, and that Moraxella catarrhalis was the bacterial species most associated with sputum neutrophilia and IL-8. Therefore, colonisation by Gram-negative bacteria may play a role in the pathogenesis of severe asthma through the upregulation of LPS and neutrophils in the airway.
Another possibility is the inhalation of LPS in environmental dust, especially house dust. Several reports have suggested that the frequency and severity of asthma correlated with LPS concentrations in house dust [13, 14]. For example, Michel et al. [13] reported that in subjects exposed to a high level of HDM allergen, the severity of asthma was related to concentrations of house dust LPS, not the concentration of HDM. Rizzo et al. [14] reported that clinical symptom scores in asthmatic children were correlated with the LPS level in house dust, not the concentrations of HDM. Therefore, a nonallergic mechanism involving LPS, including neutrophil-dependent eosinophil accumulation but not an allergic mechanism of HDM, might be highly involved in the pathogenesis of severe asthma.
The mechanisms of corticosteroid-dependent or -resistant asthma have not been fully elucidated. Inhaled corticosteroids are effective in the treatment of most patients with asthma. However, severe asthma and chronic obstructive pulmonary disease, which are both characterised by neutrophilic airway inflammation, are relatively resistant to the anti-inflammatory effects of corticosteroids. In fact, the functions of neutrophils are not effectively suppressed by corticosteroids [37]. Furthermore, recent studies have suggested that LPS or Gram-negative bacteria play important roles in the development of corticosteroid-dependent or -resistant asthma [10, 11, 38]. For example, Goleva et al. [11] reported that pre-incubation of asthmatic airway macrophages with H. parainfluenzae resulted in p38MAPK activation, increased IL-8 and mitogen-activated kinase phosphatase 1 mRNA expression, and inhibited corticosteroid responses. Furthermore, McSharry et al. [38] recently reported the significant inverse correlation between LPS concentration in sputum and improvement of FEV1 after oral corticosteroid therapy in asthmatics. Therefore, the combination of neutrophils and LPS may induce corticosteroid-dependent or -resistant airway inflammation through neutrophilic inflammation itself and neutrophil-dependent eosinophil accumulation by LPS.
In the present study, LPS alone did not induce eosinophil TBM (figure 1). In fact, whether LPS acts directly on eosinophils is still controversial. Takanaski et al. [39] reported that LPS induced survival and cytokine production by eosinophils. Furthermore, Plötz et al. [40] reported that eosinophils express CD14, and LPS was observed to induce production of tumour necrosis factor-α and eosinophil cationic protein from eosinophils through CD14. In contrast, other studies have reported that TLR4 and CD14 do not express on eosinophils [15, 41], which is consistent with the present study. Meerschaert et al. [41] reported that LPS-stimulated CD16-depleted eosinophils increased survival and produced granulocyte–macrophage colony-stimulating factor, whereas LPS-stimulated CD16/CD14-depleted eosinophils did not increase survival, even though eosinophils do not express CD14. Because monocytes express TLR4 and produce a variety of cytokines in response to LPS stimulation [16, 26], Meerschaert et al. [41] speculated that a small percentage of CD14+ monocytes would be involved in the activation of CD16-depleted eosinophils by LPS.
Furthermore, Sabroe et al. [15] reported that even in neutrophils, CD14-depleted neutrophils decrease the upregulation of L-selectin or CD11b expression by LPS stimulation, suggesting that a small percentage of CD14+ monocytes may also be involved in the activation of neutrophils by LPS. The present study did not use anti-CD14 Ab for preparation of neutrophils or eosinophils. Because the expression of TLR4 in monocytes is much higher than that in neutrophils [15], a very few CD14+ monocytes may have augmented the eosinophil TBM by LPS-stimulated neutrophils in the present study. This should be further examined in the future.
One limitation in interpreting the findings of our study is that LPS concentration used in this in vitro study is higher than that reported in the airway of asthmatics. For example, Dubin et al. [42] reported that LPS concentration in BAL fluid of mild asthmatics was 111±37 pg·mL−1, which is much lower than that used in this study. Although the actual concentration in the airway of asthmatics, especially of severe asthmatics, is more difficult to ascertain, stimulants other than LPS may be involved in the activation of neutrophils in severe asthmatics. We speculate that this is not an LPS-specific phenomenon although we have not examined the effect of other stimulants, such as concanavalin A or N-formylmethionyl-leucyl-phenylalanine. Rather, we would like to emphasise that neutrophils from healthy volunteers can induce eosinophil TBM when neutrophils were fully activated by stimulants such as LPS. Neutrophils from severe asthmatics can induce eosinophil TBM even without LPS (figure 5), suggesting they are already activated by a stimulant in vivo. However, the findings that neutrophils from severe asthmatics were more sensitive to LPS stimulation (figure 6) supported the importance of LPS in the pathogenesis of severe asthma.
Another limitation is that there is a little information about selectivity of BIIL260 and WEB2170 at such high concentrations. Birke et al. [43] reported that BIIL260 has high affinity to LTB4 receptor on isolated human neutrophil cell membranes with an inhibitory constant of 1.7 nM and BIIL260 at 100 nM almost completely suppressed LTB4 binding to their receptor in a competitive assay. It suppressed LTB4-induced intracellular calcium release and chemotaxis of neutrophils [43]. They also performed receptor binding assays using 27 different human non-LTB4 receptors and their specific ligands, and found that no relevant binding of BIIL260 was observed, suggesting high selectivity of the drug [43]. Furthermore, Heuer et al. [44] reported that WEB2170 inhibited PAF-induced aggregation of human platelets with an IC50 value of 0.32 µM whereas it did not affect the platelet aggregation by other agents such as adenosine diphosphate, adrenaline or collagen (IC50 > 1000 µM), suggesting high selectivity of the drug at 10 µM. They also reported that WEB2170 inhibited PAF-induced neutrophil aggregation, whereas it did not affect concanavalin A or N-formylmethionyl-leucyl-phenylalanine induced neutrophil aggregation [44]. In preliminary experiments, we found that treatment with BIIL260 (10 µM) abolished 1 µM LTB4-induced, but not 30 nM eotaxin-induced, eosinophil migration (data not shown). In addition, treatment with WEB2170 (10 µM) abolished 1 µM PAF-induced, but not 30 nM eotaxin-induced, eosinophil migration (data not shown). Furthermore, we have previously used the LTB4 antagonist or PAF antagonist at the same concentrations to examine the mechanisms of enhanced eosinophil TBM by IL-8-induced neutrophils [8]. However, we could not completely exclude the possibility of nonspecific effect of these antagonists. A further limitation is that synergistic or additive effects between a LTB4 antagonist and a PAF antagonist were not observed in this study. Several factors may be involved. LPS may induce more LTB4 and the LTB4 then induces PAF in neutrophils, or vice versa (more PAF, then more LTB4 in neutrophils), which can explain the lack of a synergistic or additive effect. Further, selectivity of the drug may affect the results although our preliminary experiments did not support this possibility.
We think that neutrophils or LPS-stimulated neutrophils could be a novel therapeutic target in severe asthma. In this study, we found neutrophils from severe asthmatics induced eosinophil TBM (figure 5). When we used eosinophils obtained from healthy volunteers, neutrophils from severe asthmatics also induced eosinophil TBM (data not shown). Therefore, neutrophils of severe asthmatics are already activated in vivo and have the potential to migrate or activate eosinophils, which may play roles in the development of airway inflammation of severe asthma. As for drugs, there is no beneficial drug for neutrophil-dominant asthma so far. In clinical practice, a LTB4 antagonist or a PAF antagonist had little effect on asthma [45–47]. However, if we select the patients with neutrophil-dominant asthma, these antagonists may have effects in a similar way to anti-IL-5 Ab in eosinophil-dominant asthma. Recent studies suggested that some drugs, including macrolide antibiotics and CXCR2 Ab, are expected to have effects [48–51]. The effect of these drugs on eosinophil TBM induced by neutrophils should be examined in the future.
In conclusion, the present study demonstrated that neutrophils stimulated with LPS induce the TBM of eosinophils. LTB4 and PAF are involved in the augmentation of eosinophil TBM by LPS-stimulated neutrophils. Therefore, these mechanisms could be a novel therapeutic target in patients with severe or corticosteroid-dependent asthma.
Acknowledgements
The authors thank Akemi Yokote (Saitama Medical University, Saitama, Japan) for her excellent technical assistance.
Footnotes
Support Statement: This study was funded by a grant from the Japanese Ministry of Education, Culture, Sports, Science and Technology (21790783). Funding information for this article has been deposited with FundRef.
Conflict of interest: None declared.
References
- 1.Bochner BS, Undem BJ, Lichtenstein LM. Immunological aspects of allergic asthma. Annu Rev Immunol 1994; 12: 295–335. [DOI] [PubMed] [Google Scholar]
- 2.The ENFUMOSA Study Group. The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. European Network for Understanding Mechanisms of Severe Asthma. Eur Respir J 2003; 22: 470–477. [DOI] [PubMed] [Google Scholar]
- 3.Wenzel SE, Schwartz LB, Langmack EL, et al. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med 1999; 160: 1001–1008. [DOI] [PubMed] [Google Scholar]
- 4.Kikuchi S, Nagata M, Kikuchi I, et al. Association between neutrophilic and eosinophilic inflammation in patients with severe persistent asthma. Int Arch Allergy Immunol 2005; 137(S): 7–11. [DOI] [PubMed] [Google Scholar]
- 5.Pepe C, Foley S, Shannon J, et al. Differences in airway remodeling between subjects with severe and moderate asthma. J Allergy Clin Immunol 2005; 116: 544–549. [DOI] [PubMed] [Google Scholar]
- 6.Shannon J, Ernst P, Yamauchi Y, et al. Differences in airway cytokine profile in severe asthma compared to moderate asthma. Chest 2008; 133: 420–426. [DOI] [PubMed] [Google Scholar]
- 7.Kikuchi S, Kikuchi I, Takaku Y, et al. Neutrophilic inflammation and CXC chemokines in patients with refractory asthma. Int Arch Allergy Immunol 2009; 149): 87–93. [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi I, Kikuchi S, Kobayashi T, et al. Eosinophil trans-basement membrane migration induced by interleukin-8 and neutrophils. Am J Respir Cell Mol Biol 2006; 34: 760–765. [DOI] [PubMed] [Google Scholar]
- 9.Hauk PJ, Krawiec M, Murphy J, et al. Neutrophilic airway inflammation and association with bacterial lipopolysaccharide in children with asthma and wheezing. Pediatr Pulmonol 2008; 43: 916–923. [DOI] [PubMed] [Google Scholar]
- 10.Goleva E, Hauk PJ, Hall CF, et al. Corticosteroid-resistant asthma is associated with classical antimicrobial activation of airway macrophages. J Allergy Clin Immunol 2008; 122: 550–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goleva E, Jackson LP, Harris JK, et al. The effects of airway microbiome on corticosteroid responsiveness in asthma. Am J Respir Crit Care Med 2013; 188: 1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green BJ, Wiriyachaiporn S, Grainge C, et al. Potentially pathogenic airway bacteria and neutrophilic inflammation in treatment resistant severe asthma. PLoS One 2014; 9: e100645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michel O, Kips J, Duchateau J, et al. Severity of asthma is related to endotoxin in house dust. Am J Respir Crit Care Med 1996; 154: 1641–1646. [DOI] [PubMed] [Google Scholar]
- 14.Rizzo MC, Naspitz CK, Fernández-Caldas E, et al. Endotoxin exposure and symptoms in asthmatic children. Pediatr Allergy Immunol 1997; 8: 121–126. [DOI] [PubMed] [Google Scholar]
- 15.Sabroe I, Jones EC, Usher LR, et al. Toll-like receptor (TLR)2 and TLR4 in human peripheral blood granulocytes: a critical role for monocytes in leukocyte lipopolysaccharide responses. J Immunol 2002; 168: 4701–4710. [DOI] [PubMed] [Google Scholar]
- 16.Akira S, Takeda K. Toll-like receptor signalling. Nature Rev Immunol 2004; 4: 499–511. [DOI] [PubMed] [Google Scholar]
- 17.Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today 1995; 16: 21–26. [DOI] [PubMed] [Google Scholar]
- 18.Kasama T, Miwa Y, Isozaki T, et al. Neutrophil-derived cytokines: potential therapeutic targets in inflammation. Curr Drug Targets Inflamm Allergy 2005; 4: 273–279. [DOI] [PubMed] [Google Scholar]
- 19.Shibata S, Takahashi G, Shioya N, et al. Suppressive effects of sivelestat on interleukin 8 and TNF-α production from LPS-stimulated granulocytes in whole blood culture. J Anesth 2010; 24: 901–907. [DOI] [PubMed] [Google Scholar]
- 20.Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. [DOI] [PubMed] [Google Scholar]
- 21.Bruijnzeel PL, Warringa RA, Kok PT, et al. Effects of nedocromil sodium on in vitro induced migration, activation, and mediator release from human granulocytes. J Allergy Clin Immunol 1993; 92: 159–164. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Zuurbier AE, Mul FP, et al. Triple role of platelet-activating factor in eosinophil migration across monolayers of lung epithelial cells: eosinophil chemoattractant and priming agent and epithelial cell activator. J Immunol 1998; 161: 3064–3070. [PubMed] [Google Scholar]
- 23.Okada S, Kita H, George TJ, et al. Transmigration of eosinophils through basement membrane components in vitro: synergistic effects of platelet-activating factor and eosinophil-active cytokines. Am J Respir Cell Mol Biol 1997; 16: 455–463. [DOI] [PubMed] [Google Scholar]
- 24.Schweizer RC, Welmers BA, Raaijmakers JA, et al. RANTES- and interleukin-8-induced responses in normal human eosinophils: effects of priming with interleukin-5. Blood 1994; 83: 3697–3704. [PubMed] [Google Scholar]
- 25.Schumann RR, Leong SR, Flaggs GW, et al. Structure and function of lipopolysaccharide binding protein. Science 1990; 249: 1429–1431. [DOI] [PubMed] [Google Scholar]
- 26.Simpson A, Martinez FD. The role of lipopolysaccharide in the development of atopy in humans. Clin Exp Allergy 2010; 40: 209–223. [DOI] [PubMed] [Google Scholar]
- 27.Baldini M, Lohman IC, Halonen M, et al. A polymorphism in the 5′ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Respir Cell Mol Biol 1999; 20: 976–983. [DOI] [PubMed] [Google Scholar]
- 28.Levan TD, Michel O, Dentener M, et al. Association between CD14 polymorphisms and serum soluble CD14 levels: effect of atopy and endotoxin inhalation. J Allergy Clin Immunol 2008; 121: 434–440. [DOI] [PubMed] [Google Scholar]
- 29.O'Donnell AR, Toelle BG, Marks GB, et al. Age-specific relationship between CD14 and atopy in a cohort assessed from age 8 to 25 years. Am J Respir Crit Care Med 2004; 169: 615–622. [DOI] [PubMed] [Google Scholar]
- 30.Koppelman GH, Reijmerink NE, Colin Stine O, et al. Association of a promoter polymorphism of the CD14 gene and atopy. Am J Respir Crit Care Med 2001; 163: 965–969. [DOI] [PubMed] [Google Scholar]
- 31.Kabesch M, Hasemann K, Schickinger V, et al. A promoter polymorphism in the CD14 gene is associated with elevated levels of soluble CD14 but not with IgE or atopic diseases. Allergy 2004; 59: 520–525. [DOI] [PubMed] [Google Scholar]
- 32.Reed CE, Milton DK. Endotoxin-stimulated innate immunity: A contributing factor for asthma. J Allergy Clin Immunol 2001; 108: 157–166. [DOI] [PubMed] [Google Scholar]
- 33.Nightingale JA, Rogers DF, Hart LA, et al. Effect of inhaled endotoxin on induced sputum in normal, atopic, and atopic asthmatic subjects. Thorax 1998; 53: 563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bisgaard H, Hermansen MN, Buchvald F, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 2007; 357: 1487–1495. [DOI] [PubMed] [Google Scholar]
- 35.Huang YJ, Nelson CE, Brodie EL, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol 2011; 127: 372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kloepfer KM, Lee WM, Pappas TE, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol 2014; 133: 1301–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cox G. Glucocorticoid treatment inhibits apoptosis in human neutrophils. Separation of survival and activation outcomes. J Immunol 1995; 154: 4719–4725. [PubMed] [Google Scholar]
- 38.McSharry C, Spears M, Chaudhuri R, et al. Increased sputum endotoxin levels are associated with an impaired lung function response to oral steroids in asthmatic patients. J Allergy Clin Immunol 2014; 134: 1068–1075. [DOI] [PubMed] [Google Scholar]
- 39.Takanaski S, Nonaka R, Xing Z, et al. Interleukin 10 inhibits lipopolysaccharide-induced survival and cytokine production by human peripheral blood eosinophils. J Exp Med 1994; 180: 711–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plötz SG, Lentschat A, Behrendt H, et al. The interaction of human peripheral blood eosinophils with bacterial lipopolysaccharide is CD14 dependent. Blood 2001; 97: 235–241. [DOI] [PubMed] [Google Scholar]
- 41.Meerschaert J, Busse WW, Bertics PJ, et al. CD14+ cells are necessary for increased survival of eosinophils in response to lipopolysaccharide. Am J Respir Cell Mol Biol 2000; 23: 780–787. [DOI] [PubMed] [Google Scholar]
- 42.Dubin W, Martin TR, Swoveland P, et al. Asthma and endotoxin: lipopolysaccharide-binding protein and soluble CD14 in bronchoalveolar compartment. Am J Physiol 1996; 270: L736–L744. [DOI] [PubMed] [Google Scholar]
- 43.Birke FW, Meade CJ, Anderskewitz R, et al. In vitro and in vivo pharmacological characterization of BIIL 284, a novel and potent leukotriene B4 receptor antagonist. J Pharmacol Exp Ther 2001; 297: 458–466. [PubMed] [Google Scholar]
- 44.Heuer HO, Casals-Stenzel J, Muacevic G, et al. Pharmacologic activity of bepafant (WEB 2170), a new and selective hetrazepinoic antagonist of platelet activating factor. J Pharmacol Exp Ther 1990; 255: 962–968. [PubMed] [Google Scholar]
- 45.Evans DJ, Barnes PJ, Spaethe SM, et al. Effect of a leukotriene B4 receptor antagonist, LY293111, on allergen induced responses in asthma. Thorax 1996; 51: 1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spence DP, Johnston SL, Calverley PM, et al. The effect of the orally active platelet-activating factor antagonist WEB 2086 in the treatment of asthma. Am J Respir Crit Care Med 1994; 149: 1142–1148. [DOI] [PubMed] [Google Scholar]
- 47.Hozawa S, Haruta Y, Ishioka S, et al. Effects of a PAF antagonist, Y-24180, on bronchial hyperresponsiveness in patients with asthma. Am J Respir Crit Care Med 1995; 152: 1198–1202. [DOI] [PubMed] [Google Scholar]
- 48.Simpson JL, Powell H, Boyle MJ, et al. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med 2008; 177: 148–155. [DOI] [PubMed] [Google Scholar]
- 49.Brusselle GG, Vanderstichele C, Jordens P, et al. Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax 2013; 68: 322–329. [DOI] [PubMed] [Google Scholar]
- 50.Holz O, Khalilieh S, Ludwig-Sengpiel A, et al. SCH527123, a novel CXCR2 antagonist, inhibits ozone-induced neutrophilia in healthy subjects. Eur Respir J 2010; 35: 564–570. [DOI] [PubMed] [Google Scholar]
- 51.Nair P, Gaga M, Zervas E, et al. Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin Exp Allergy 2012; 42: 1097–1103. [DOI] [PubMed] [Google Scholar]