Abstract
Risk stratification in pulmonary arterial hypertension (PAH) is paramount to identifying individuals at highest risk of death. So far, there are only limited parameters for prognostication in patients with PAH.
95 patients with confirmed PAH were included in the present analysis and followed for a total of 4 years. Blood samples were analysed for serum levels of N-terminal pro-brain natriuretic peptide, high-sensitivity troponin T (hsTnT), pro-atrial natriuretic peptide (proANP), growth differentiation factor 15, soluble fms-like tyrosine kinase 1 and placental growth factor.
27 (28.4%) patients died during a follow-up of 4 years. Levels of all tested biomarkers, except for placental growth factor, were significantly elevated in nonsurvivors compared with survivors. Receiver operating characteristic analyses demonstrated that cardiac biomarkers had the highest power in predicting mortality. In particular, proANP exhibited the highest area under the curve, followed by N-terminal pro-brain natriuretic peptide and hsTnT. Furthermore, proANP and hsTnT added significant additive prognostic value to the established markers in categorical and continuous net reclassification index. Moreover, after Cox regression, proANP (hazard ratio (HR) 1.91), hsTnT (HR 1.41), echocardiographic right ventricular impairment (HR 1.30) and 6-min walk test (HR 0.97 per 10 m) remained the only significant parameters in prognostication of mortality.
Our data suggest benefits of the implementation of proANP and hsTnT as additive biomarkers for risk stratification in patients with PAH.
Short abstract
The cardiac biomarkers proANP and hsTnT may be of use in the assessment of risk stratification in PAH patients http://ow.ly/RJFtn
Introduction
Pulmonary arterial hypertension (PAH) is a devastating disease leading to a progressive increase in pulmonary vascular resistance (PVR), and subsequently resulting in right heart failure and death [1, 2]. Therefore, risk stratification is paramount in order to identify individuals at highest risk of death or progression of disease requiring more intense pharmacological treatment. So far, there are only few established parameters for follow-up and prognosis in patients with PAH. Recent international guidelines recommend using World Health Organization (WHO) functional class, 6-min walk test (6-MWT), cardiopulmonary exercise testing (CPX), brain natriuretic proteins (brain natriuretic peptide (BNP)/N-terminal proBNP (NT-proBNP)), specific echocardiographic parameters and haemodynamic findings as composite treatment goals and markers for follow-up and prognosis [3–5]. However, no single parameter can sufficiently fulfil the role of a reliable prognostic indicator by itself. Consequently, we aimed to enhance prognostication of mortality by evaluating novel functional or prognostic data by use of additional biomarkers as surrogates of angiogenesis, inflammation and heart failure. For that reason, pro-atrial natriuretic peptide (proANP) and high-sensitivity troponin T (hsTnT) were selected as markers reflecting cardiac injury, growth differentiation factor 15 (GDF-15) as a parameter of inflammation, and placental growth factor (PlGF) and soluble fms-like tyrosine kinase 1 (sFlt-1) as markers of angiogenesis [6–10].
Materials and methods
Patient population
Between January 2010 and May 2010, patients with confirmed PAH (Dana Point group 1) who provided written informed consent were prospectively enrolled in the outpatient department of the University Hospital of Heidelberg, Germany, which is a referral centre for PAH patients. Diagnosis of PAH was established according to European Society of Cardiology/European Respiratory Society guidelines for pulmonary hypertension [4]. The study was approved by the Heidelberg Medical Ethical Committee (application number 330/2003).
In total, 95 patients were included in the present analysis. All blood samples were taken during a visit to our pulmonary outpatient clinic and subsequently stored at −80°C in aliquots until samples were analysed after thawing once. Blood samples were analysed for all biomarkers (NT-proBNP, hsTnT, proANP, GDF-15, PlGF and sFlt-1) at the research laboratories of Roche (Penzberg, Germany). Due to insufficient sample volume, proANP could only be measured in 93 (97.9%) of the 95 patients, whereas all other biomarkers were measured in all 95 patients. Moreover, conclusive results from 17 (17.9%) 6-MWTs, two (2.2%) echocardiographies, 11 (12.0%) right heart catheterisations (RHCs) and 53 (55.8%) cardiopulmonary exercise tests were not available or inaccessible.
Diagnostic work-up
All patients received a questionnaire on history of disease and physical examination. Lung function testing, blood gas analysis, two-dimensional transthoracic echocardiography and a 6-min walk test were performed in all patients during their visit. Patients underwent cardiac catheterisation and CPX within a few days following their outpatient visit. Echocardiography was performed routinely in the echocardiography laboratory by an experienced echocardiographer. All patients were followed-up for a total of 4 years in terms of survival. Determination of end-point status was performed through electronic hospital records or telephone calls to patients' homes or their physicians continuously on a quarterly basis after the initial visit. No patient was lost to follow-up.
Biomarkers
hsTnT was measured using the High-Sensitivity Cardiac Troponin T assay (Roche Diagnostics Ltd, Rotkreuz, Switzerland), which is commercially available in Germany. The limit of blank (3 ng·L−1) and limit of detection (5 ng·L−1) were determined in accordance with Clinical and Laboratory Standards Institute guideline EP17-A. The inter-assay coefficient of variation was 8% at 10 ng·L−1 and 2.5% at 100 ng·L−1. The intra-assay coefficient of variation was 5% at 10 ng·L−1 and 1% at 100 ng·L−1. NT-proBNP was measured by electrochemiluminescence on an Elecsys 2010 analyser (Roche Diagnostics). Inter-assay coefficients of variation were 3.2% and 2.0% at mean values of 157 and 5125 ng·L−1. ProANP was measured by using ELISA (BI-20892; Biomedica Immunoassays, Vienna, Austria). The inter-assay coefficient of variation was 4% at a mean value of 0.88 nmol·L−1. PlGF and sFlt-1 were determined in plasma by enzyme-linked immunosorbent microlitre plate assay (cobas-PlGF; Roche Diagnostics, Mannheim, Germany). The measurement ranges for PlGF and sFlt-1 extend from 3 to 10 000 ng·L−1 (coefficient of variation 7.3%) and from 10 to 85 000 ng·L−1 (coefficient of variation 4.6%), respectively. GDF-15 was measured by an immunoradiometric assay with a limit of detection of 20 ng·L−1 and a linear range from 200 to 50 000 ng·L−1. The intra-assay imprecision of the assay ranges from 2.8% to 10.6% for samples containing 248–22 480 ng·L−1 GDF-15 and the inter-assay imprecision ranges from 4.0% to 12.2% for samples containing 232–39 370 ng·L−1 GDF-15.
Healthy subjects
In total, 200 clinically healthy subjects (71 men and 129 women) aged 18–56 years (mean 37.4 years, median 40 years) were screened to serve as a reference population. The inclusion criteria were healthy men and women with a body mass index between 20 and 35 kg·m−², who stated that they had been stably medicated for 4 weeks. The subjects' weight had to have been stable in the previous 4 weeks (a variance of ±2 kg was accepted). After physical examination and routine blood sampling, left and right ventricular pump function were assessed by echocardiography or cardiac magnetic resonance imaging (MRI). Healthy individuals had normal structural and functional cardiac parameters defined as a left ventricular ejection fraction ⩾55%, normal right ventricular function, normal valve function, normal dimensions and volumes of all heart chambers, and a normal stress test using either dynamic echocardiography, or adenosine or dobutamine stress MRI.
Statistical analysis
Comparisons between survivors and nonsurvivors were performed using descriptive statistics as well as the Mann–Whitney U-test for continuous and Chi-squared tests for categorical data. We further assessed the area under the receiver operating characteristic (ROC) curve and performed reclassification analyses (net reclassification index (NRI) for continuous and categorical variables, and integrated discrimination index (IDI)) on models based on the composite treatment goals according to the ESC/ERS guidelines and the proceedings of the 5th World Symposium on pulmonary hypertension consisting of WHO functional class, 6-MWT, tricuspid annular plane systolic excursion (TAPSE), peak oxygen uptake, NT-proBNP and cardiac index [4, 5, 11, 12]. In order to evaluate benefits of biomarkers in risk prediction, reclassification analyses were performed to quantify improvements in model performance (in terms of correct reclassification to respective risk categories) after addition of a new marker [13, 14]. Risk categories for mortality were set as 0–2% for low risk, 2–15% for intermediate risk and ⩾15% for high risk. Moreover, ROC curve analysis was used for determination of cut-off levels. Furthermore, the Youden index (sensitivity+specificity−1) was used to capture the diagnostic performance.
Cox regression analysis in combination with a variable selection procedure was used to evaluate a significant relationship between the survival time and functional parameters, patient characteristics, functional tests or laboratory values. A stepwise variable selection based on p-values was implemented with alternating steps between backward and forward selection using limits for p-values as 0.157 (for the backward selection steps) and 0.156 (for the forward selection steps), respectively. As we had to deal with missing data, we used multiple imputation via chained equations [15]. We generated 10 data sets and pooled the results using Rubin's rules [16]. For computation of p-values, a Wald test was applied [17].
A p-value <0.05 was considered to be significant. As this was an exploratory analysis, no adjustments for multiple testing were performed. Statistical analyses were performed with MedCalc Statistical Software version 14.12.0 (MedCalc Software bvba, Ostend, Belgium) and R version 3.0.2 (R Core Team, Vienna, Austria), as well as the R packages “PredictABEL” [18], “survival” (version 2.37-7) [19, 20], “mice” (version 2.22) [15] and “xtable” (version 1.7-1) [21].
Results
Patient population
In total, 95 PAH patients were recruited between January 2010 and May 2010. They had advanced PAH, as they were mainly in functional classes II and III, and had significantly impaired haemodynamics. Complete characteristics of the study population are presented in table 1 and online supplementary table 1. In total, 27 (28.4%) patients died during a follow-up of 4 years. No significant differences were found between sexes.
TABLE 1.
Clinical baseline characteristics
| Age years | 64.1±14.5 |
| Male sex % | 37.9 |
| BMI kg·m−2 | 26.6±5.3 |
| WHO functional class % | |
| I | 7.4 |
| II | 64.2 |
| III | 28.4 |
| IV | 0 |
| Diagnosis n (%) | |
| Idiopathic PAH | 65 (68.4) |
| PAH and connective tissue disease | 21 (22.1) |
| Other | 9 (9.5) |
| mPAP mmHg | 45.7±16.4 |
| PVR Wood units | 8.4±5.9 |
| PCWP mmHg | 12.0±5.1 |
| Cardiac index L·min−1·m−2 | 2.5±1.1 |
| 6-min walk distance m | 414±132 |
| LTOT % | 18.3 |
| Mortality n (%) | 27 (28.4) |
Data are presented as mean±sd unless otherwise stated. BMI: body mass index; WHO: World Health Organization; PAH: pulmonary arterial hypertension; mPAP: mean pulmonary artery pressure; PVR: pulmonary vascular resistance; PCWP: pulmonary capillary wedge pressure; LTOT: long-term oxygen therapy.
Univariate analysis of biomarkers and clinical parameters
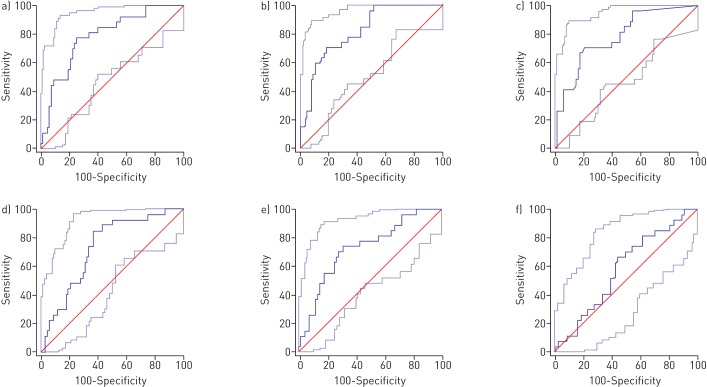
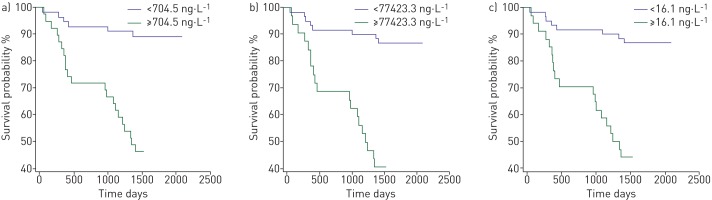
All tested biomarker levels (NT-proBNP, proANP, hsTnT, GDF-15 and sFlt-1) were significantly higher in patients with PAH compared to the healthy group (supplementary table 2) and all tested biomarker levels, except for PlGF, were significantly elevated in deceased patients compared to survivors (p<0.05 by Mann–Whitney U-test) (table 2). ROC analysis revealed that cardiac biomarkers, in the following order, proANP (area under the curve (AUC) 0.822), NT-proBNP (AUC 0.789) and hsTnT (AUC 0.782) had the highest AUC predicting mortality in our PAH population (figure 1 and table 3). However, comparison of ROC curves did not deliver a significant result (z-statistics) [22]. Cut-off values using the Youden index for the most relevant markers are shown in table 3. ROC curve analysis revealed that a proANP level >77 427.3 ng·L−1 was predictive of death with an AUC of 0.822, a sensitivity of 70.37% and a specificity of 79.41%. Optimum cut-off values for prediction of mortality were found for NT-proBNP and hsTnT serum levels >704.5 and >16.1 ng·L−1, respectively. Using the calculated cut-off values, we generated the Kaplan–Meier survival curves shown in figure 2.
TABLE 2.
Descriptive statistics on outcome
| Survivors | Nonsurvivors | p-value | AUC | |
| Sex | ||||
| Male | 22 (23.2) | 14 (14.7) | 0.125 | |
| Female | 46 (48.4) | 13 (13.7) | ||
| RV impairment | 45 (66.2) | 24 (96.0) | 0.0081 | |
| NYHA class III–IV | 14 (20.6) | 13 (48.1) | 0.0149 | |
| 6-min walking distance m | 461.5 (356.5–524.0) (n=60) | 345.0 (261.0–391.0) (n=18) | 0.0008 | 0.762 |
| TAPSE cm | 1.9 (1.5–2.2) (n=48) | 1.7 (1.3–1.9) (n=18) | 0.0715 | 0.645 |
| mPAP mmHg | 43.0 (33.0–54.3) (n=67) | 45.0 (36.0–49.5) (n=24) | 0.7287 | 0.524 |
| PVR WU | 6.9 (3.8–9.7) (n=61) | 8.6 (4.7–12.3) (n=23) | 0.2365 | 0.584 |
| Cardiac index L·min−1·m−2 | 2.3 (2.0–2.9) (n=55) | 2.3 (1.9–2.7) (n=20) | 0.4574 | 0.556 |
| Peak V′O2 mL·kg−1·min−1 | 14.6 (12.2–17.2) (n=34) | 12.3 (9.7–14.9) (n=8) | 0.1322 | 0.673 |
| PO2 mmHg | 68.7 (61.5–78.3) (n=67) | 62.2 (55.0–70.9) (n=25) | 0.0454 | 0.636 |
| PCO2 mmHg | 35.5 (32.5–37.5) (n=66) | 37.0 (32.6–40.4) (n=25) | 0.0955 | 0.614 |
| NT-proBNP ng·L−1 | 311.4 (134.9–721.9) (n=68) | 1273.5 (738.3–5705.5) (n=27) | <0.0001 | 0.789 |
| ProANP ng·L−1 | 38 959.5 (27 570.1–72 202.1) (n=66) | 100 687.2 (60 166.9–156 114.8) (n=27) | <0.0001 | 0.822 |
| hsTnT ng·L−1 | 6.7 (3.0–14.4) (n=68) | 23.3 (9.5–68.7) (n=27) | <0.0001 | 0.782 |
| GDF-15 ng·L−1 | 1481.1 (956.3–1987.2) (n=68) | 2292.7 (1686.6–3359.9) (n=27) | 0.0006 | 0.728 |
| PlGF ng·L−1 | 16.9 (13.6–21.3) (n=68) | 18.5 (15.4–21.9) (n=27) | 0.2283 | 0.580 |
| sFlt-1 ng·L−1 | 71.4 (64.0–87.80) (n=68) | 87.0 (79.4–111.8) (n=27) | 0.0004 | 0.733 |
| GFR mL·min−1 | 73.4 (58.9–90.1) (n=67) | 44.8 (34.5–72.4) (n=25) | 0.0013 | 0.719 |
Data are presented as n (%) or median (interquartile range), unless otherwise stated. Descriptive statistics with p-values of Mann–Whitney U-tests or Chi-squared tests for continuous or categorical data, respectively, and area under the curve (AUC) (receiver operating characteristic analysis). RV: right ventricular; NYHA: New York Heart Association; TAPSE: tricuspid annular plane systolic excursion; mPAP: mean pulmonary artery pressure; WU: Wood unit; V′O2: oxygen uptake; PO2: oxygen tension; PCO2: carbon dioxide tension; NT-proBNP: N-terminal pro-brain natriuretic peptide; proANP: pro-atrial natriuretic peptide; hsTnT: high-sensitivity troponin T; GDF-15: growth differentiation factor 15; PlGF: placental growth factor; sFlt-1: soluble fms-like tyrosine kinase 1; GFR: glomerular filtration rate.
FIGURE 1.
Receiver operating curves assessing the prognostic accuracy of a) N-terminal pro-brain natriuretic peptide, b) pro-atrial natriuretic peptide, c) high-sensitivity troponin T, d) soluble fms-like tyrosine kinase 1, e) growth differentiation factor 15 and f) placental growth factor for prediction of 4-year mortality.
TABLE 3.
Receiver operating characteristic analysis assessing the prognostic accuracy of N-terminal pro-brain natriuretic peptide (NT-proBNP), pro-atrial natriuretic peptide (proANP), high-sensitivity troponin T (hsTnT), soluble fms-like tyrosine kinase 1 (sFlt-1), growth differentiation factor 15 (GDF-15) and placental growth factor (PlGF) for prediction of 4-year mortality
| NT-proBNP | ProANP | hsTnT | sFlt-1 | GDF-15 | PlGF | |
| AUC | 0.789 | 0.822 | 0.782 | 0.733 | 0.728 | 0.580 |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | 0.0001 | 0.2066 |
| J (95% CI) | 0.5278 (0.3214–0.6830) | 0.4978 (0.3589–0.6347) | 0.4978 (0.3087–0.6378) | 0.4842 (0.2765–0.6242) | 0.4319 (0.2021–0.5800) | 0.4978 (0.1100–0.3442) |
| Cut-off value ng·L−1 (95% CI) | >704.52 (>251.46– >1142.51) | >77 427.3 (>37 793.36– >100 881.76) | >16.13 (>3– >18.37) | >75.9 (>73.25– >119.09) | >1759.2 (>741.6– >1955.99) | >17.15 (>13.59– >26.21) |
| Sensitivity % | 77.78 | 70.37 | 70.37 | 85.19 | 74.07 | 66.67 |
| Specificity % | 75.00 | 79.41 | 79.41 | 63.24 | 69.12 | 55.88 |
AUC: area under the curve; J: Youden index.
FIGURE 2.
Kaplan–Meier survival curves according to receiver operative characteristic-optimised cut-off values for a) N-terminal pro-brain natriuretic peptide, b) pro-atrial natriuretic peptide and c) high-sensitivity troponin T.
For clinical parameters, only 6-MWT distance was significantly higher in survivors and right ventricular impairment was observed significantly more frequently in nonsurvivors. No significant differences could be found in data from RHC or CPX (table 2). Cardiac biomarkers had higher AUCs in prognostication of mortality compared to clinical or functional data.
Improvement in prediction performance by addition of biomarkers using NRI and IDI
Next, we tested the value added by the novel biomarkers to the composite treatment goals according to recent guidelines [3–5] using categorical and continuous NRI and IDI, using risk categories of 0–2% for low risk, 2–15% for intermediate risk and ⩾15% for high risk, in terms of mortality. ProANP and hsTnT had significant additive value to the recommended treatment model in categorical and continuous NRI but not in IDI. Adding one or two of the other novel biomarkers did not show any significant improvement (table 4 and figure 3).
TABLE 4.
Reclassification analysis from biomarkers related to pulmonary arterial hypertension in multivariable-adjusted models
| Biomarker added to the composite treatment and prognosis goals# | NRI (95% CI) | IDI (95% CI) | |
| Categorical | Continuous | ||
| ProANP | 0.3235 (0.1285–0.5185) (p=0.00115) | 0.6993 (−0.0045–1.403) (p=0.05149) | 0.1262 (−0.0343–0.2867) (p=0.1233) |
| hsTnT | 0.2571 (0.0921–0.4222) (p=0.00226) | 0.7683 (0.0726–1.464) (p=0.03042) | 0.1429 (−0.0496–0.3354) (p=0.1456) |
| GDF-15 | 0.1143 (−0.0395–0.2681) (p=0.1453) | 0.654 (−0.0524–1.36) (p=0.06959) | 0.0814 (−0.0829–0.2457) (p=0.3315) |
| sFlt-1 | −0.0286 (−0.0838–0.0266) (p=0.3103) | 0.3683 (−0.3557–1.092) (p=0.3187) | 6e−04 (−0.0054–0.0065) (p=0.8554) |
| PlGF | −0.0286 (−0.0838–0.0266) (p=0.3103) | 0.3048 (−0.3946–1.004) (p=0.393) | 0.0137 (−0.0083–0.0356) (p=0.2226) |
| ProANP+hsTnT | 0.2941 (0.1206–0.4676) (p=0.00089) | 0.817 (0.1254–1.509) (p=0.02059) | 0.1582 (−0.0338–0.3502) (p=0.1064) |
| ProANP+GDF-15 | 0.2647 (0.0769–0.4525) (p=0.00574) | 0.7582 (0.0601–1.456) (p=0.03327) | 0.1312 (−0.0393–0.3018) (p=0.1315) |
| hsTnT+GDF-15 | 0.2 (0.0265–0.3735) (p=0.02387) | 0.7111 (0.0098−1.412) (p=0.04688) | 0.1482 (−0.044–0.3403) (p=0.1307) |
| ProANP+sFlt-1 | 0.3529 (0.1552–0.5507) (p=0.00047) | 0.6405 (−0.0686–1.35) (p=0.07667) | 0.1264 (−0.0339–0.2867) (p=0.1222) |
| hsTnT+sFlt-1 | 0.2857 (0.0988–0.4726) (p=0.00274) | 0.8825 (0.1999−1.565) (p=0.01128) | 0.1474 (−0.0453–0.3401) (p=0.1338) |
| ProANP+PlGF | 0.2941 (0.1024–0.4858) (p=0.00264) | 0.4641 (−0.2574–1.185) (p=0.2074) | 0.1545 (−0.0132–0.3221) (p=0.07091) |
| hsTnT+PlGF | 0.2857 (0.1164–0.455) (p=0.00094) | 0.4825 (−0.2359–1.201) (p=0.188) | 0.1588 (−0.0352–0.3529) (p=0.1086) |
Risk categories: low, 0–2%; intermediate, 2–15%; high, ⩾15%. NRI: net reclassification index; IDI: integrated discrimination index; proANP: pro-atrial natriuretic peptide; hsTnT: high-sensitivity troponin T; GDF-15: growth differentiation factor 15; sFlt-1: soluble fms-like tyrosine kinase 1; PlGF: placental growth factor. #: World Health Organization functional class, 6-min walk test, tricuspid annular plane systolic excursion, peak oxygen uptake, N-terminal pro-brain natriuretic peptide and cardiac index. Bold indicates statistical significance.
FIGURE 3.
Forest plots of a) categorical and b) continuous net reclassification index (NRI) and c) integrated discrimination index (IDI) from biomarkers related to pulmonary arterial hypertension. Error bars represent 95% confidence intervals. ProANP: pro-atrial natriuretic peptide; hsTnT: high-sensitivity troponin T; GDF-15: growth differentiation factor 15; sFlt-1: soluble fms-like tyrosine kinase 1; PlGF: placental growth factor.
Variable selection for prognosis of mortality
Cox regression, combined with a variable selection procedure, was calculated after multiple imputations. Therefore, considering NT-proBNP, hsTnT, proANP, New York Heart Association functional class, 6-MWT, right ventricular impairment, TAPSE, PVR and cardiac index only, the following variables were selected: proANP (hazard ratio (HR) 1.91), hsTnT (HR 1.4), echocardiographic right ventricular impairment (HR 1.30) and 6-MWT (HR 0.97 per 10 m) (table 5). Notably, NT-proBNP, hsTnT, proANP, PVR and cardiac index were logarithmically transformed to achieve normal distributions and reduce the significance of statistical outliers. Applying our model solely to the subgroup of patients without idiopathic PAH (n=30) did not retain significance for prognostication of mortality (data not shown).
TABLE 5.
Cox regression after variable selection procedure
| Variable | Hazard ratio (95% CI) | p-value |
| ProANP | 1.91 (1.03–3.53) | 0.039 |
| hsTnT | 1.41 (0.88–2.26) | 0.153 |
| RV impairment | 1.30 (0.94–1.80) | 0.107 |
| 6-min walk test | 0.97 (0.94–1.01) | 0.110 |
ProANP: pro-atrial natriuretic peptide; hsTnT: high-sensitivity troponin T; RV: right ventricular.
Discussion
Risk stratification and prediction of mortality remain critical challenges in PAH, as only limited diagnostic tools are available. Of all the clinical parameters, the 6-MWT performed best in predicting death in our patient population. This finding highlights its important role as a clinical test, as it stands out for its practicability in daily routine. With the exception of the registration trial for macitentan, a new dual endothelin antagonist, the 6-MWT has been used as the primary end-point in registration trials for all PAH medications [23–26]. However, recent publications have criticised the application of the 6-MWT as a primary end-point, indicating that changes in 6-MWT might not reflect benefits in outcome, and proposed new end-points such as time to clinical worsening [27–30]. Our results postulate that at least the initial absolute walking distance provides a meaningful prognostic implication for patients with PAH. This notion is supported by findings from a recent study by Fritz et al. [27]. However, no statement about the prognostic power of differences in 6-MWT can be derived from this study.
In view of the complex and multifactorial pathogenesis of PAH, consisting of right ventricular dysfunction, pulmonary vascular disease including endothelial dysfunction, in situ thrombosis, oxidative stress, and inflammatory processes, we hypothesised that changes in biomarker levels mirror pathophysiological mechanisms and may provide insights into dynamic processes or information on outcomes once the diagnosis has been established. Biomarkers are widely accepted diagnostic tools and are minimally invasive, without patient burden [31].
Primarily, our data demonstrate that all tested biomarkers were elevated in patients with PAH compared to a clinically healthy group. Moreover, all tested biomarkers except for PlGF were significantly elevated in nonsurvivors as compared to survivors and cardiac biomarkers performed better in risk assessment of mortality than any clinical parameter. Compared to NT-proBNP, the only established biomarker to date, hsTnT performed nearly equally well but proANP yielded an even higher AUC than NT-proBNP. Differences in AUC between biomarkers were not significant. However, comparison of models and evaluation of additive value by using novel markers, particularly in already satisfying models, remain a statistical challenge, as it was recently demonstrated that models with an AUC >0.75 cannot be improved by >0.05, even if the effect size is large [14]. The use of reclassification levels as a novel measure of added utility has been proposed [12]. The NRI was introduced by Pencina et al. [11] as a new statistical method to measure the improvement in prediction performance gained by adding additional marker. In line with this approach, our results suggest that proANP and hsTnT bear significant prognostic information value in classification, when added to the established clinical, biochemical and haemodynamic follow-up and prognostic parameters recommended by recent international guidelines [3–5]. Therefore, even though NT-proBNP and proANP derive from the same pathophysiological pathway, it is possible that proANP could even possess superior prognostic impact to NT-proBNP. Several studies already support the important role of hsTnT in PAH; however, hsTnT has not been included in the recent guidelines [5, 32, 33]. Taking all variables together, our data clearly demonstrate that indicators of cardiac function and injury play the key role in prediction of mortality in patients with PAH.
Here, we provide evidence for the following conclusions. 1) In univariate analysis, the cardiac biomarkers NT-proBNP, proANP and hsTnT perform better than any clinical parameter in prognostication of mortality in patients with PAH. 2) ProANP and hsTnT provide significant prognostic information when added to established follow-up and prognosis parameters recommended by current PAH guidelines. 3) Taking all prognostic parameters together, only proANP, hsTnT, 6-MWT and echocardiographic right ventricular impairment remained as significant predictive variables in our patient population, demonstrating the meaningful role of biomarkers in risk stratification in patients with PAH. Therefore, our results highlight the important role of the cardiac biomarkers proANP and hsTnT, and strongly suggest their implementation in the assessment of risk stratification in PAH patients.
Limitations of this study
There are some limitations that have to be highlighted. Both sample size and the number of events were limited, although sufficient to perform multivariate analysis. Therefore, a Type 2 error due to small numbers of patients has to be considered. Furthermore, due to the cross-sectional study design, we cannot exclude an impact on biomarker levels from different stages of disease, various pharmaceutical therapies or as response to treatment. Additionally, the various aetiologies and subgroups of PAH might differ in expression levels of biomarkers. However, due to the small sample size and limited, power this question cannot be answered thoroughly.
Moreover, this study represents an explorative study. Thus, the results cannot be interpreted in a confirmatory way.
Footnotes
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: Disclosures can be found alongside this article at openres.ersjournals.com
References
- 1.Humbert M, Morrell N, Archer S, et al. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol 2004; 43: Suppl. S, 13S–24S. [DOI] [PubMed] [Google Scholar]
- 2.Hassoun P, Mouthon L, Barberà J, et al. Inflammation, growth factors, and pulmonary vascular remodeling. J Am Coll Cardiol 2009; 54: Suppl., S10–S19. [DOI] [PubMed] [Google Scholar]
- 3.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 2009; 53: 1573–1619. [DOI] [PubMed] [Google Scholar]
- 4.Galiè N, Hoeper M, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J 2009; 30: 2493–2537. [DOI] [PubMed] [Google Scholar]
- 5.McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol 2013; 62: 25 Suppl, D73–D81. [DOI] [PubMed] [Google Scholar]
- 6.Autiero ML, Luttun A, Tjwa M, et al. Placental growth factor and its receptor, vascular endothelial growth factor receptor-1: novel targets for stimulation of ischemic tissue revascularization and inhibition of angiogenic and inflammatory disorders. J Thromb Haemost 2003; 1: 1356–1370. [DOI] [PubMed] [Google Scholar]
- 7.Inagami T. Atrial natriuretic factor as a volume regulator. J Clin Pharmacol 1994; 34: 424–426. [DOI] [PubMed] [Google Scholar]
- 8.Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci USA 1993; 90: 10705–10709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bootcov MR, Bauskin AR, Valenzuela SM, et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-β superfamily. Proc Natl Acad Sci USA 1997; 94: 11514–11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat Med 2001; 7: 575–583. [DOI] [PubMed] [Google Scholar]
- 11.Pencina MJ, D'Agostino RBS, D'Agostino RBJ, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008; 27: 157–172. [DOI] [PubMed] [Google Scholar]
- 12.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation 2007; 115: 928–935. [DOI] [PubMed] [Google Scholar]
- 13.Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, et al. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 2008; 27: 157–172. [DOI] [PubMed] [Google Scholar]
- 14.Pencina MJ, D'Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med 2010; 48: 1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Buuren S, Groothuis-Oudshoorn K. mice: Multivariate Imputation by Chained Equations in R. J Stat Softw 2011; 45. [Google Scholar]
- 16.Rubin D. Multiple Imputation for Nonresponse in Surveys. New York, John Wiley & Sons, 1987. [Google Scholar]
- 17.Li K, Meng X, Raghunathan T, et al. Significance levels from repeated p-values with multiply-imputed data. Statistica Sinica 1991; 1: 65–92. [Google Scholar]
- 18.Kundu S, Aulchenko YS, Duijn CM, et al. PredictABEL: an R package for the assessment of risk prediction models. 26: 261–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Therneau TM. A Package for Survival Analysis in S, R package version 2.37–7. http://CRAN.R-project.org/package=survival
- 20.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, Springer, 2000. [Google Scholar]
- 21.Dahl DB. xtable: Export tables to LaTeX or HTML, R package version 1.7–1. http://CRAN.R-project.org/package=xtable
- 22.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 23.Pulido T, Adzerikho I, Channick R, et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N Engl J Med 2013; 369: 809–818. [DOI] [PubMed] [Google Scholar]
- 24.Galiè N, Brundage BH, Ghofrani HA, et al. Tadalafil therapy for pulmonary arterial hypertension. Circulation 2009; 119: 2894–2903. [DOI] [PubMed] [Google Scholar]
- 25.Rubin LJ, Rubin LJ, Badesch D, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002; 346: 896–903. [DOI] [PubMed] [Google Scholar]
- 26.Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005; 353: 2148–2157. [DOI] [PubMed] [Google Scholar]
- 27.Fritz JS, Blair C, Oudiz RJ, et al. Baseline and follow-up 6-min walk distance and brain natriuretic peptide predict 2-year mortality in pulmonary arterial hypertension. Chest 2013; 143: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaine S, Simonneau G. The need to move from 6-minute walk distance to outcome trials in pulmonary arterial hypertension. Eur Respir Rev 2013; 22: 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin VV, McLaughlin VV, Badesch D, et al. End points and clinical trial design in pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54: S97–107. [DOI] [PubMed] [Google Scholar]
- 30.De Giorgi V, Grazzini M, Rossari S, et al. Is skin self-examination for cutaneous melanoma detection still adequate? A retrospective study. Dermatology 2012; 225: 31–36. [DOI] [PubMed] [Google Scholar]
- 31.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation 2006; 113: 2335–2362. [DOI] [PubMed] [Google Scholar]
- 32.Filusch A, Giannitsis E, Katus HA, et al. High-sensitive troponin T: a novel biomarker for prognosis and disease severity in patients with pulmonary arterial hypertension. Clin Sci 2010; 119: 207–213. [DOI] [PubMed] [Google Scholar]
- 33.Torbicki A, Kurzyna M, Kuca P, et al. Detectable serum cardiac troponin T as a marker of poor prognosis among patients with chronic precapillary pulmonary hypertension. Circulation 2003; 108: 844–848. [DOI] [PubMed] [Google Scholar]