Abstract
Background
Myonecrosis can rapidly develop in soft tissue necrotizing infections, often with initial sparing of the skin. Despite the improvements in management of necrotizing soft tissue infections, mortality remains high, according to the location, microbial agents and comorbidities, ranging between 17 and 46 %. A prompt diagnosis represents the greatest challenge for the emergency physician.
Case report
We describe the case of a patient with a history of hypertension and arrhythmia who developed nonclostridial necrotizing fasciitis with extensive myonecrosis, after articular infiltration procedure. A bedside focused ultrasonography (US) revealed disappearance of the regular fibrillar architecture of the long head of biceps muscle, with diffuse abnormal hyperechogenicity assembled in a “clod pattern”. Computed tomography (CT) of the right arm did not depict muscle involvement, but showed a small gas collection around the shoulder, spreading to the subclavian region behind the major pectoral muscle. Necrotizing fasciitis with wide myonecrosis was confirmed by surgical debridement. Microbiological results showed a Staphylococcus aureus infection, managed by a selected antibiotic therapy. The patient was discharged after a small period of mechanical ventilation.
Conclusion
This is the first report of a previously healthy patient developing a nonclostridial necrotizing fasciitis with extensive myonecrosis attributable to infiltrative procedure and detected early by bedside US in emergency department. The role of bedside US in the emergency setting may save time for the prompt management of life-threatening necrotizing infections.
Keywords: Bedside ultrasonography, Emergency ultrasound, Necrotizing fasciitis, Myonecrosis, Soft tissue infections
Riassunto
Introduzione
La mionecrosi può rapidamente svilupparsi come conseguenza di un’infezione necrotizzante dei tessuti molli superficiali. Nonostante i miglioramenti nelle cure delle infezioni necrotizzanti dei tessuti molli, la mortalità resta alta (tra il 17% ed il 46%), dipendendo da localizzazione dell’infezione, agenti responsabili e comorbidità. Una diagnosi precoce è il punto chiave e rappresenta una grande sfida per il medico d’urgenza.
Descrizione del caso
Descriviamo il caso di un paziente presentatosi in Pronto Soccorso per febbre e dolore al braccio dx, trattato in precedenza senza beneficio con antiinfiammatori ed infiltrazioni locali, e successivamente con antibiotici. In presenza di segni di infezione dei tessuti molli superficiali, veniva eseguita dal medico d’urgenza un’ecografia clinica (“bedside”), che documentava la scomparsa della regolare architettura fibrillare del muscolo bicipite del braccio destro, con una iperecogenicità anomala e diffusa disomogeneità della ecostruttura, che appariva assemblata a zolle; coesisteva una minima raccolta fluida lungo il piano fasciale distalmente. La TC del braccio destro non mostrava il coinvolgimento muscolare, ma evidenziava una piccola quota di gas intorno alla spalla. Una fascite necrotizzante con estesa mionecrosi veniva confermata dall’intervento chirurgico. L’esame microbiologico documentava infezione da Staphylococcus aureus, per cui si impostava terapia antibiotica mirata. Il paziente veniva dimesso dopo breve ciclo di ventilazione meccanica assistita e ricovero in rianimazione.
Conclusioni
E’il primo caso descritto di un paziente con fascite necrotizzante con mionecrosi non da clostridio, successiva a infiltrazione locale, diagnosticata precocemente tramite un’ecografia “bedside” in Pronto Soccorso. L’ecografia “bedside” si conferma presidio accurato e tempestivo nella gestione di condizioni potenzialmente fatali come le infezioni necrotizzanti dei tessuti molli.
Background
Soft tissue infections have a wide range of presentations, including common simple forms and severe life-threatening ones. Hence, according to the depth of soft tissue involvement, these infections imply superficial uncomplicated infections (impetigo, erysipelas and cellulitis) and necrotizing infections (fasciitis and myonecrosis), with or without gas production [1]. Tissue necrosis is a common feature in necrotizing infections, with wide spectrum of systemic symptoms. Moreover, the clinical findings of each soft tissue infections could evolve and overlap in the same patient [2].
Despite the improvements in management of necrotizing soft tissue infections, mortality remains high, according to the location, microbial agents and comorbidities, ranging between 17 and 46 % [3, 4]. Crucial in the management of these infections is a prompt diagnosis, sustained by a strong suspect based on disproportionate pain, swelling and discoloration of the skin, and systemic symptoms [5]. However, necrotizing infections commonly do not display specific symptoms, also because “the deeper soft tissue infection is the more normal the skin surface appears” [2].
Case report
We present the case of a 72-year-old man, complaining of right shoulder pain for over 2 weeks, for which he endured multiple medical examinations in the emergency department (ED), always being discharged after diagnosis of an impingement syndrome and receiving nonsteroidal anti-inflammatory drugs. After poor results with pharmacological therapy, he further received methylprednisolone and lidocaine local injection for 4 days without benefit. A day later, empirical antibiotic therapy (amoxicillin plus clavulanic acid and teicoplanin) was started for signs of superficial infections and orthopedic surgery was recommended. Nevertheless, worse pain and fever rapidly developed and he returned to the ED. He had a history of hypertension and arrhythmias, and was being treated with captopril, hydrochlorothiazide, propafenone, and omeprazole as medications.
On admission evaluation, the patient was tachycardiac, hemodynamically stable, febrile and tachypneic, without confusion. Physical examination revealed swelling, erythema, hardness, and severe pain in the lateral side of his right arm, and he was unable to move it. ECG documented a sinus tachycardia. Because of the clinical probability of pulmonary embolism based on common clinical decision rules (Wells and revised Geneva scores), arterial blood gas analysis was performed, which revealed a moderate hypossiemic-hypocapnic state. Routine laboratory test showed alteration in muscle enzymes and inflammation signs (Table 1).
Table 1.
Clinical data, routine laboratory tests, and blood gas analysis of patient with extensive myonecrosis in necrotizing fasciitis on admission to ED
| Clinical data | |
|---|---|
| Heart rate | 120 beats/min |
| Blood pressure | 105–80 mmHg |
| Respiratory rate | 30 breaths/min |
| Body temperature | 38.2 °C |
| Mental status (GCS) | 15 |
| Oxygen saturation | 90 % |
| Laboratory tests | ||
|---|---|---|
| Parameter | Normal values | Serum levels |
| Serum creatinine | 0.70–1.20 mg/dl | 1.30 |
| Sodium | 136–145 mmol/l | 128 |
| Potassium | 3.4–4.5 mmol/l | 3.7 |
| LDH | 248–480 IU/l | 499 |
| CK | 38–174 IU/l | 183 |
| ALT | <41 IU/l | 79 |
| AST | <38 IU/l | 123 |
| C-reactive protein | <5 mg/l | 504.3 |
| Myoglobin | 28–72 ng/ml | 312 |
| Troponin T HS | <0.014 ng/ml | <0.010 |
| Hemoglobin | 13.1–16.1 g/dl | 13.2 |
| Platelets | 140–450 × 109/l | 214 × 109/l |
| Leucocytes | 4.2–12.4 × 109/l | 4.7 × 109/l |
| Neutrophils | 1.9–8.2 × 109/l | 4.2 × 109/l |
| Arterial blood gas analysis | ||
|---|---|---|
| pH | 7.38–7.42 | 7.46 |
| pCO2 | 38–42 mmHg | 32.4 |
| Bicarbonates | 24–26 mmol/l | 20.6 |
| pO2 | 85–98 mmHg | 62.1 |
| Lactates | 0.5–1.6 mmol/l | 2.2 |
Abnormal values are marked in bold type
GCS Glasgow Coma Scale, LDH lactate dehydrogenase, CK creatine kinase, ALT alanine aminotransferase; AST aspartate aminotransferase, HS high sensitivity
A bedside chest US examination was performed by the emergency physician, according to previous report of integrated ultrasonographic approach in a peri-arrest setting [6]. A negative chest US picture (“dry lung”) was found, excluding interstitial syndrome as well as peripheral lung consolidations, pneumothorax, and pleural effusion. Cardiac US, performed using subcostal view, revealed normal global cardiac systolic function without pericardial effusion or right or left ventricular enlargement. A collapsed inferior caval vein was detected according to the reduced volume status. The compression US of the lower limbs excluded deep vein thrombosis. Finally, a focused US of the patient’s right arm, corresponding to the region of the maximum pain produced by pressure of the probe, revealed disappearance of the regular fibrillar architecture of the long head of the biceps muscle, with diffuse abnormal hyperechogenicity assembled in a “clod pattern”, extending to the overlying subcutaneous tissue and producing marked edge shadowing (Fig. 1). A small dependent fluid collection along the fascia plane was depicted in a more distal site (Fig. 2 and Clips 1 and 2). No typical comet tail artifacts (“gas sign”) were detected.
Fig. 1.
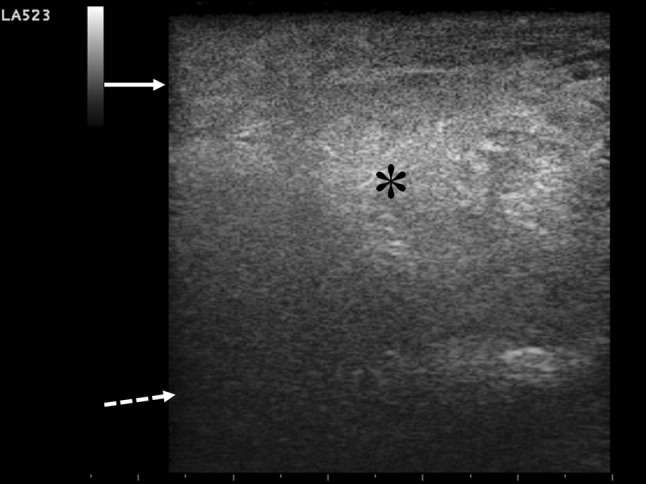
Bedside goal-directed ultrasonography performed by “small parts” probe on longitudinal scanning of affected right arm is shown. Myonecrosis of right long biceps muscle is revealed by the disappearance of the regular arrangement of muscular architecture, which is diffusely substituted by a hyperechoic “clod US pattern” (asterisk), due to disrupture of myocytes and inflammatory infiltrate, exceeding the fascial plane (full arrow). No intramuscular necrotic fluid collection is present. Deep hyperechoic humeral surface is marked by the broken arrow
Fig. 2.
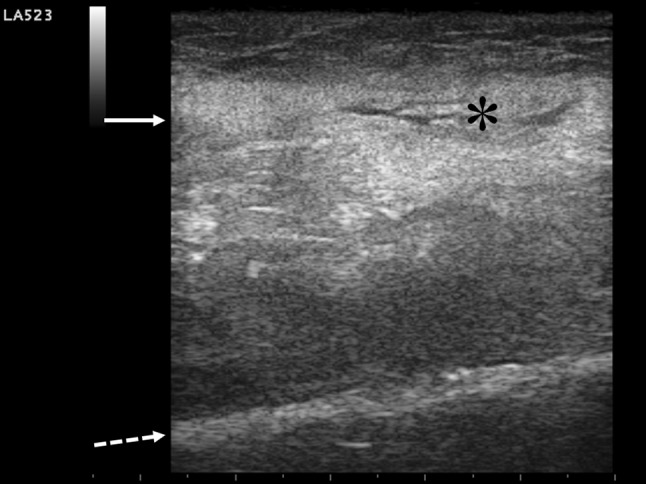
Abnormal diffuse thickening of long biceps fascia is evident (full arrow), with small fluid collection spreading along the fascial plane (asterisk). Overlying soft tissue and underlying muscle layers are hyperechoic and overthrown in their regular architecture due to inflammatory infiltration and necrosis involvement. Deep hyperechoic humeral surface is marked by the broken arrow
Computed tomography (CT) of the right arm did not depict muscle involvement, but showed a small gas collection around the shoulder, spreading to the subclavian region behind the major pectoral muscle (Fig. 3). Moreover, chest CT scan excluded pulmonary embolism and detected a postero-basal paramediastinal lung consolidation.
Fig. 3.

Reconstructed chest CT scan shows gas bubbles in soft tissue (arrow), medially to the right shoulder, in the subclavian region below the pectoral major muscle. This minimal deep gas collection was not revealed on physical examination, standard Rx, or bedside ultrasonography
A widespread antibiotic therapy (piperacillin plus tazobactam and metronidazole) was promptly started, and a surgical debridement was performed. The soft tissue necrosis and muscle ischemia were confirmed, and pus collection in the articular space was drained, whose cultures yielded Staphylococcus aureus. The patient was admitted to ICU for mechanical assisted ventilation and discharged after complete recovery.
Discussion
Necrotizing fasciitis usually affects the lower and upper limbs, perineum, and abdomen in decreased order of incidence and is often due to a mix of aerobic and anaerobic germs (type 1). The shoulder localization of necrotizing fasciitis is very rare and has poor diagnosis because of its potential to spread to the chest wall [7]. The most frequent agents involved are Gram-positive group A Streptococci and S. aureus, with the incubation period ranging from 1 to 4 days and the onset of the disease being estimated in 6–24 h [2–5]. Many patients have underlying causes of immunocompromise such as diabetes, advanced age, cancer, chronic renal disease, and congestive heart failure [4]. Cases of necrotizing fasciitis caused by Staphylococcus infection after acupuncture have been described in high-risk patients [8], and myonecrosis has been reported as an uncommon complication of diabetes mellitus [9]. This is the first report of a previously healthy patient developing a nonclostridial necrotizing fasciitis with extensive myonecrosis, attributable to infiltrative procedure and detected early by bedside US in ED.
Myonecrosis can rapidly develop in necrotizing fasciitis, often with initial sparing of the skin, so its early recognition is difficult prior to extensive tissue destruction and systemic evolution. Sometimes, differentiation from a less dangerous infection is not easy, based only on the physical examination. That is why bedside-focused US is useful to exclude alternative etiologies [10] and confirm the occurrence of necrotizing infection depicting diffuse thickening of fascia and abnormal fluid collection [11], so combining a cost-effective advantage with a bedside real-time focused assessment. An irregularly shaped, poorly shaped hypo-anechoic area exhibiting posterior acoustic shadowing, due to focal fat necrosis and inflammatory changes, may be the only finding detected in early phase by focused real-time US examination [12]. US appearance of calcific myonecrosis has been also recently described [13]. Moreover, gas production may be recognized by comet tail artifacts (“gas sign”) [7]. In traumatic injury, bedside US can also distinguish a gas-producing bacterial infection by air contamination of a penetrating wound in soft tissue, which displays typical distribution and movement like those of ‘‘sparkling-wine microbubbles’’ [14]. Currently, MR is considered the gold standard imaging technique, for its great soft tissue contrast. CT scan is less precise than RM in muscle study but RM is too expensive and often unavailable in ED. CT scan remains the main imaging modality to detect even small fluid and/or air collections in ED, although it is not efficient in assessing muscle involvement [15].
Conclusion
The role of US in the emergency setting for early diagnosis of necrotizing fasciitis has been previously reported in literature and its accuracy estimated as 92 % by Yen et al. [16] also before or without gas production. Its utilization should be stressed to save time for the prompt management of life-threatening necrotizing infections.
Conflict of interest
The authors, Americo Testa, Rosangela Giannuzzi, and Valeria De Blasio, declare no competing interest.
Informed consent
Patient informed consent was received according to ethical standards, and he was assured that all pictures would be anonymized.
References
- 1.Stevens DL, Bisno AL, Chambers H, et al. Practise guidelines for the diagnosis and management of skin and soft tissue infections. Clin Infect Dis. 2005;41:1373. doi: 10.1086/497143. [DOI] [PubMed] [Google Scholar]
- 2.Meislin HW, Guisto JA, et al. Soft tissue infections. In: Marx JA, Hockberger RS, Walls RM, et al., editors. Rosen’ s Emergency Medicine: Concepts and Clinical Practice. 7. Philadelphia: Mosby Elsevier; 2010. p. 1836. [Google Scholar]
- 3.Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis. 2007;44:705. doi: 10.1086/511638. [DOI] [PubMed] [Google Scholar]
- 4.Chen I-C, Li W-C, Hong Y-C, Shie S-S, Fann W-C, Hsiao C-T. The microbiological profile and presence of bloodstream infection influence mortality rates in necrotizing fasciitis. Crit Care. 2011;15:R152. doi: 10.1186/cc10278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Testa A, Giannuzzi R. Gangrene. In: Heymann W, Anderson B, Hivnor C, Lessin S, editors. Clinical Decision Support: Dermatology. Wilmington, DE: Decision Support in Medicine LLC; 2012. [Google Scholar]
- 6.Testa A, Cibinel GA, Portale G, et al. The proposal of an integrated ultrasonographic approach into the ALS algorithm for cardiac arrest: the PEA protocol. Eur Rev Med Pharmacol Sci. 2010;14:77. [PubMed] [Google Scholar]
- 7.Testa A, Giannuzzi R, De Gaetano Donati K, Gentiloni Silveri N. Fulminant endogenous gas gangrene: role of ultrasonography in the emergency setting. Am J Emerg Med. 2010;28:643.e1–643.e3. doi: 10.1016/j.ajem.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Hsieh RL, Huangh CH, Uen WC. Necrotizing fasciitis After acupuncture in a patient with aplastic anemia. J Altern Complement Med. 2011;17:871. doi: 10.1089/acm.2010.0617. [DOI] [PubMed] [Google Scholar]
- 9.Nagdev A, Murphy M, Sisson C. Bedside ultrasound for the detection of diabetic myonecrosis. Am J Emerg Med. 2008;26:969.e3–969.e4. doi: 10.1016/j.ajem.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 10.Iverson K, Haritos D, Thomas R, Kannikeswaran N. The effect of bedside ultrasound on diagnosis and management of soft tissue infections in a pediatric ED. Am J Emerg Med. 2012;30:1347–1351. doi: 10.1016/j.ajem.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 11.Wronski M, Slodkowski M, Cebulski W, Karkocha D, Krasnodebski IW. Necrotizing fasciitis: early sonographic diagnosis. J Clin Ultrasound. 2011;39:236–239. doi: 10.1002/jcu.20766. [DOI] [PubMed] [Google Scholar]
- 12.Testa A, Giannuzzi R, Gentiloni Silveri N. Bedside focus ultrasound in necrotizing fasciitis: the “black hole sign”. British Journal of Medicine & Medical Research. 2014;4(3):898–904. [Google Scholar]
- 13.Finlay K, Friedman L, Ainsworth K. Calcific myonecrosis and tenosynovitis: sonographic findings with correlative imaging. J Clin Ultrasound. 2007;35:48. doi: 10.1002/jcu.20281. [DOI] [PubMed] [Google Scholar]
- 14.Testa A, Giannuzzi R, Zirio G, La Greca A, Gentiloni Silveri N. Ultrasound detection of foreign body and gas contamination in a penetrating wound. J Ultrasound. 2009;12:38. doi: 10.1016/j.jus.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wysoki MG, Santora TA, Shah RM, Friedman AC. Necrotizing fasciitis: CT characteristics. Radiology. 1997;203:859. doi: 10.1148/radiology.203.3.9169717. [DOI] [PubMed] [Google Scholar]
- 16.Yen ZS, Wang HP, Ma HM, Chen SC, Chen WJ. Ultrasonographic screening of clinically-suspected necrotizing fasciitis. Acad Emerg Med. 2002;9:1448. doi: 10.1111/j.1553-2712.2002.tb01619.x. [DOI] [PubMed] [Google Scholar]


