Abstract
Many factors are involved in weight gain and metabolic disturbances associated with obesity. The gut microbiota has been of particular interest in recent years, since both human and animal studies have increased our understanding of the delicate symbiosis between the trillions of microbes that reside in the GI tract and the host. It has been suggested that disruption of this mutual tolerance may play a significant role in modulating host physiology during obesity. Environmental influences such as diet, exercise, and early life exposures can significantly impact the composition of the microbiota, and this dysbiosis can in turn lead to increased host adiposity via a number of different mechanisms. The ability of the microbiota to regulate host fat deposition, metabolism, and immune function makes it an attractive target for achieving sustained weight loss.
The growing epidemic of obesity (17) and its associated complications of cardiovascular disease, hyperlipidemia, non-alcoholic fatty liver disease, and Type 2 diabetes mellitus present an increasing burden on the healthcare system worldwide. Since 1980, the prevalence of obesity has increased 75% (57, 75), with estimates that in 2014 ∼375 million women and 266 million men were reported to be obese worldwide (17). Simplistically, obesity results from imbalance between energy consumption and expenditure. In reality, however, the development of obesity is due to a complex interaction of genetic, environmental, cultural, behavioral, and microbial factors.
The human gut microbiota is a diverse array of microorganisms, including bacteria, archaea, fungi, and viruses that colonize the surfaces of the gastrointestinal (GI) tract (69). Under normal homoeostatic conditions, a physicochemical barrier of mucus, epithelial tissue, and regulatory components of the innate and adaptive immune system maintain a constant bidirectional communication between microbes and the host that results in mutual benefit. Disturbances in the microbial community can result in a compromised intestinal barrier, dysregulated immune system, and altered tissue metabolism, mechanisms which have been implicated in development of obesity. In this review, we discuss the factors that determine gut microbial composition, proposed mechanisms linking the gut microbiota with obesity, and potential interventions to combat obesity through manipulation of gut microbial communities.
Early Life Modulators of the Gut Microbiota
The gut microbiota is a delicate balance of organisms influenced by several factors including age, mode of delivery, infant feeding mode (e.g., breast milk vs. formula) (60), and use of antibiotics (25). Age is particularly important, and the gut microbiota composition may differ at different time points within the same individual (46). Vertical transmission of the microbiota from pregnant mother to offspring establishes the infant gut microbiota, with colonization beginning at or just before birth, as the meconium of full-term neonates has been shown to contain bacteria. Vaginally delivered infants have microbiomes that resemble the mothers' vaginal tract, whereas infants delivered by Caesarean section have microbiotas consisting largely of skin microbes, including Staphylococcus, Corynebacterium, and Propionibacterium (16, 28, 46). These differences may lead to long-term health consequences, as evidenced by a 46% increase in obesity risk at 7 years of age for children who were born by Caesarean delivery (54).
Prenatal and postnatal exposures to microbial modulators, such as diet and antibiotics, may also affect the infant microbiome and the predisposition to obesity. For instance, maternal antibiotic usage was associated with an 84% higher risk in obesity in their children (54). Meanwhile, in primates, an obesity-inducing diet during pregnancy reduced the diversity of the intestinal microbiota of the offspring, even after a switch to a healthy diet at weaning (48). Postnatal antibiotic exposure can also have profound effects on gut microbial communities and predisposition to obesity. Epidemiological studies have shown that children who receive broad-spectrum antibiotics in infancy are more likely to be overweight and obese later in life (1, 5, 79).
Diet as a Modulator of Gut Microbiota
Diet has a major role in modulating the composition and metabolic output of the gut microbiota and represents an important therapeutic strategy in the management of obesity. The effect of diet on the microbiota and obesity predisposition can begin even before the introduction of solid foods, as breast-fed infants have a significantly reduced risk of obesity later in life compared with formula-fed infants (56). Although the exact mechanisms for such an effect remain unclear, human milk, comprised of indigestible glycans, oligosaccharides, glycolipids, and glycoproteins, appears to create an environment conducive to a healthy microbiota and body weight later in life (58, 66).
The effect of diet on the microbiota is also evident on a global scale. For instance, the high-fiber diet of Burkino Faso children was associated with greater microbial richness, higher amounts of Prevotella, and lower amounts of Bacteroidetes compared with the microbiotas of European children eating a diet much lower in fiber (21). Irrespective of age, Western diets, consisting of high amounts of processed simple sugars, have been linked to microbial dysbiosis and the obesity epidemic. Metagenomic studies have also found that obesity is associated with low bacterial gene richness (43), whereas energy-restriction increases richness (19).
Both short-term and long-term dietary influences have been shown to affect the gut microbial diversity and function (20, 86). A recent study supporting this concept shows that lack of microbiota-accessible carbohydrates, as often seen with Western diets, can lead to a potentially irreversible loss in microbial diversity over generations (73).
The assessment of microbial composition in response to diet is helpful, but, given the inter-individual variability and the redundancy in microbial functions, it becomes imperative to concurrently assess changes in microbial function. Wu and colleagues characterized the gut microbiota and metabolome of individuals consuming a vegan or omnivorous diet in Western society. They found little difference in microbial community composition, but significant differences in the microbial metabolome (87) highlight the importance of assessing changes in microbial function.
The interactions of diet and the microbiota during obesity are also shaped by host genetics. For example, mice lacking the TLR5 gene have an altered gut microbiota, which promotes hyperphagia and an obese phenotype (81). In humans, the gut microbiota appears to be highly individualized, although its composition and function can be used with other host parameters to predict acute dietary responses (91). These studies highlight the potential role of gut microbiota in mediating host response to diet and their therapeutic utility in regulating metabolic response.
Exercise as a Microbiota Modulator
Regular exercise can significantly alter the gut microbiota. Recent studies indicate that exercise can initiate changes in the microbiota in a healthy state or in unfavorable circumstances, including consumption of a Western diet (39), exposure to oncogenic substances (14), and experimental diabetes (42). In relation to obesity, we recently demonstrated that exercise training and a high-sugar, high-fat diet orthogonally alter the gut microbiota (37). Evans et al. also showed changes in the microbiota by exercise training, both alone and in combination with a high-sugar, high-fat diet (30). Notably, exercise training in mice reduced levels of Erysipelotrichaceae and Turicibacteraceae, families of bacteria that are associated with obesity, bile acid regulation, and gut inflammation (30). Changes in the microbiota by exercise training have also been associated with improvements in metabolic parameters, including the reduction of circulating leptin (62). Detailed mechanisms for how exercise-induced changes in the microbiota occur are still unclear but could involve interactions between host and microbial metabolism, including cross feeding of metabolites (18). Further research is needed to determine whether exercise affects the human microbiota during obesity and whether the magnitude of weight loss and metabolic changes are linked to these changes.
The Gastrointestinal Mucus Layer, Epithelial Barrier, and Immune System: Gatekeepers and Active Signalers of the Gut Microbiota During Obesity
The mucus layer of the GI tract provides the most distinct microbial-host interface, consisting of an outer, non-adherent mucus layer colonized by commensal microbes and an inner, adherent layer closest to the epithelium that remains largely sterile. In mice, diet-induced obesity results in degradation of the outer mucus layer and change in bacterial niches with translocation to the inner mucus layer or epithelium (31). Although the mucus layer and spatial organization of microbes is much more difficult to characterize in humans, there is strong evidence of a relationship between mucus-degrading bacteria, host adiposity, and obesity-related conditions. For instance, the mucin-degrader Akkermansia muciniphila is negatively associated with adiposity and metabolic dysfunction (70), whereas other mucus-associated bacteria, such as Ruminococcus gnavus, are positively correlated with similar outcomes (41). Functionally, A. muciniphila contains a large collection of enzymes capable of degrading O-linked oligosaccharides, whereas R. gnavus contains enzymes that allow it to both cleave and consume terminal sialic acids on the mucin structure (76). Downstream, A. muciniphila can improve host metabolism and the immune system through numerous mechanisms, including, but not limited to, SCFA production, host endocannabinoid release (31), and regulation of lipid metabolism (47). Despite colonizing the same area, R. gnavus has vastly different downstream effects on host physiology, as it has been strongly correlated with both gut inflammation and serum triglycerides in mice and humans (35, 41). These data suggest an important role of the mucus layer and its associated microbiota in obesity, and provide rationale for a potential therapeutic role for mucin-degrading bacteria in the management of obesity and associated metabolic disorders.
In addition to its effects on mucus layer, the gut microbiota also alters expression of host genes that impact nutrient absorption and metabolism (36). For instance, germ-free mice fed a Western diet were resistant to diet-induced obesity and demonstrated elevated levels of fasting-induced adipose factor (Fiaf), a circulating inhibitor of lipoprotein lipase. Meanwhile, germ-free mice colonized with microbiota from wild-type mice displayed suppression of Fiaf and greater deposition of triglycerides in adipose tissue (88). More research is necessary to determine which microbes carry the most influence on Fiaf expression and whether these are the same microbes that are typically present or absent in greater numbers in obese individuals. AMP-activated protein kinase (AMPK) represents another mechanism by which the microbiota regulates host metabolism. Germ-free mice have elevated levels of activated AMPK, increased mitochondrial fatty acid oxidation, decreased glycogen storage, and they remained lean despite being fed a high-calorie diet (4).
The microbiota may also be partially responsible for reduced barrier integrity and systemic endotoxemia during obesity. For instance, a high-fat diet in mice was shown to induce phosphorylation of the myosin light chain and localization of occludin into the cytoplasm of intestinal epithelial cells. This change in barrier function was correlated with the rise in endotoxin in the blood throughout the diet, suggesting that barrier disruption within the gut is at least partially responsible for diet-induced endotoxemia (22). Endotoxemia also occurs in obese humans and may contribute to metabolic disturbances. For example, it was shown that obese, insulin-resistant individuals had higher levels of circulating LPS than their more insulin-sensitive counterparts (10). In rats, diet-induced endotoxemia was shown to contribute to leptin-resistance in vagal nerves, indicating a possible mechanism for endotoxin-induced metabolic disturbances (22). Despite this intriguing data, more research is needed (especially in humans) to determine the mechanisms related to low-grade endotoxemia and whether this phenomenon contributes significantly to increased adiposity.
If the mucosal and epithelial barrier of the gut is compromised, commensal microbes are also able to act locally on the underlying GI immune system (11). Antigen-presenting cells (e.g., dendritic cells and macrophages) throughout the gut express pathogen recognition receptors (PRRs), including Toll-like receptors (TLRs) and nucleotide-binding oligomerization domains (NOD)-like receptors (NLRs), which continually respond to antigenic stimuli, including the microbiota (12). During obesity, intestinal immune activation and inflammatory processes induced by the commensal microbiota can lead to enhanced adiposity and altered metabolism. For example, in wild-type mice, ileal TNF-α expression increased significantly at weeks 6 and 16 during exposure to high-sugar, high-fat diet, and was strongly associated with weight gain and metabolic disturbances, including an increase in fasting glucose (27). However, in germ-free mice exposed to the same diet, there was attenuation of intestinal inflammation, adipose gain, and metabolic disturbances. In another study, intestinal epithelial-specific deletion of MyD88, a downstream component of TLR signaling, partially inhibited diet-induced obesity and associated metabolic disturbances in mice. Meanwhile, upon transfer of the microbiota from MyD88 KO animals to germ-free animals, the obese phenotype was attenuated (32).
Together, these data provide evidence that alterations in gut microbiota by obesity can cause gastrointestinal barrier disruption, GI immune system activation, and bacterial translocation, all of which may lead to altered metabolism within the host. Future studies are needed to elucidate the mechanistic underpinnings of these phenomena to further understand how they relate to obesity.
Microbial Mechanisms Regulating Energy Homeostasis and Inflammation
The metabolic activity of gut microbiota can regulate energy balance via mechanisms that affect energy harvest from diet as well as modulate genes that affect energy storage/expenditure and inflammation. In this section, we will highlight some of the metabolites produced or altered by the microbiota that have been implicated in obesity.
Short-Chain Fatty Acids
Short-chain fatty acids (SCFAs) are mainly produced by gut microbiota as fermentation products of complex polysaccharides, including dietary fibers such as inulin and pectin, and endogenous substrates like mucins (55). Overall, the nature of fermentation and the SCFA profile within the gut is dependent on the composition of the gut microbiota, interactions between microbes and the host (i.e., composition of mucin oligosaccharides), and amount/type of fermentable carbohydrate consumption. Following absorption, butyrate serves as a source of energy for the colonic epithelial cells, whereas propionate and acetate mainly act as substrates for gluconeogenesis and lipogenesis in the gut and the liver (24, 72). Prior to metabolism, SCFAs are also able to interact with free fatty acid receptors (FFARs) located in the intestine and various other tissues.
With regard to obesity, SCFAs came into light when seminal studies showed that the microbial community of genetically obese mice was enriched for genes capable of harvesting energy from complex plant-derived polysaccharides (80). Germ-free, FFAR3−/− mice colonized with Bacteroidetes theatiotaamicron and Methanobrevibacter smithii were leaner compared with conventionally raised wild-type littermates, implying a role of FFAR3 and the gut microbiota in the regulation of host energy harvest (67). Such effects may be traced to FFAR3-induced release of peptide YY, a hormone derived from the enteroendocrine cells that slows intestinal transit and hence contributes to increased caloric extraction from the diet (67). Meanwhile, microbiota transfer from obese mice to lean, germ-free mice led to increased hepatic triacylglycerol accumulation (72), possibly through increased fermentation to SCFAs and thus energy harvest. In humans, it has been shown by more than one group that SCFAs are elevated in the feces of obese individuals (33, 71, 77).
Despite these energy-harvesting capabilities, which may exacerbate adipogenesis, SCFAs appear to have a complex and pleiotropic role in obesity. SCFAs can also induce satiety, sensitize cells to insulin, and reduce inflammation in the pancreas, muscle, and adipose tissue, among others. One example is SCFA-induced leptin secretion via FFAR3, which regulates appetite and energy metabolism (90). Meanwhile, stimulation of the FFAR2 receptors in the gut mediates release of glucagon-like peptide (GLP-1) to improve insulin secretion (78), whereas signaling in neutrophils suppresses inflammation (49). SCFAs can also directly modulate the adaptive immune system independent of FFARs. Most notably, SCFAs can directly modulate the metabolic phenotype of T-cells by inhibiting histone deacetylases (3, 92). Butyrate and acetate can also stimulate goblet cells to release Muc2, an essential component of the mucus layer that helps maintain the intestinal barrier and tolerance to foreign antigens (9). In humans, metagenome-wide association studies have identified butyrate-producing bacteria that are negatively associated with obesity and subsequent insulin resistance. For instance, Qin et al. (61) and Karlsson et al. (38) report lower proportions of butyrate-producing Clostridials, including Roseburia and Faecalibacterium prausnitzii, in obese individuals.
These conflicting data present a paradox for delegating these SCFAs into “good” or “bad” regarding the pathogenesis of obesity. Dichotomously, SCFAs not only may enhance energy harvest and contribute to excess lipogenesis in the liver, but also concurrently reduce inflammation, sensitize tissues to insulin, and contribute to satiety. Discrepancies in these findings may be related to categorizing SCFAs under one umbrella term, when, in reality, each metabolite has distinct metabolic effects. For instance, acetate displays more obesogenic potential, since it acts as a primary substrate for hepatic and adipocyte lipogenesis, compared with propionate or butyrate, which primarily serve as substrates for other metabolic processes (13). Moreover, excess SCFA concentration in feces, alone, likely does not represent bodily turnover and metabolism of these molecules. Thus future research requires a more detailed understanding of the transport, metabolism, and signaling of SCFAs in obesity and other chronic diseases.
Bile Acids
Bile acids (BAs) are synthesized in the liver from cholesterol, stored in the gall bladder, and released into the small intestine to aid in digestion of triglycerides. Upon release, the gut microbiota further metabolize BAs through deconjugation, dehydroxylation, and dehydrogenation. BA transformation by the gut microbiota has been shown to initiate dramatic changes in the BA pool size with subsequent effects of host physiology (64). Meanwhile, BAs can initiate changes in the diversity and composition of the gut microbiota, and thus can contribute to microbe and host physiology (65).
BAs (and their regulation by the microbiota) can significantly alter glucose/lipid metabolism and inflammation in the host. Such action primarily occurs through BA activation of the nuclear farnesoid X receptor (FXR) and cytoplasmic G-protein-coupled BA receptor TGR5 (82). Within the gut, BAs can act through TGR5 on enteroendocrine cells and stimulate the production of GLP-1, an incretin hormone implicated in improved glucose metabolism and satiety (8). Stimulation of TGR5 by BAs has also been linked to other metabolic actions outside of the GI tract. In particular, activation of TGR5 by BAs initiates the browning of adipose tissue and increases skeletal muscle energy expenditure through cAMP-dependent type 2 iodothyronine deiodinase, an enzyme that activates thyroid hormone (85). Activation of FXR by BAs, meanwhile, can initiate the release of fibroblast growth factors FGF19 and FGF21, both of which have insulin-sensitizing and hypolipidemic effects (15). BA activation of FXRs can also modulate TLRs on intestinal myeloid cells, thus implicating BAs in the regulation of innate immunity toward an anti-inflammatory state (34).
There are also negative consequences of microbial transformation of BAs. For instance, the dehydroxylation from primary to secondary BAs by the microbiota can lead to secondary BA accumulation in the colon. Secondary BAs, which are particularly high in individuals on Western diets, can activate numerous downstream signals involved in disease states (63). In relation to obesity, Yoshimoto et al. recently found that the secondary bile acid deoxycholic acid (DCA) has a primary role in obesity-associated hepatocellular carcinoma (89).
Endocannabinoids
The endocannabinoid (eCB) system has been proposed to regulate gut barrier function during obesity, in addition to regulating feeding behavior and metabolism (53). The eCB lipids, AEA (anandamide or N-arachidonyl ethanolamine) and 2-AG (2-arachidonoylglycerol), are widely expressed in tissues that control energy balance (muscle, gut, liver, adipose tissue, pancreas, hypothalamus) (50). They exert most of their functions by activating CB1 and CB2, two G-protein-coupled receptors expressed throughout the GI tract. The eCB system tends to be activated during obesity in both mice and humans, with an increase in both eCBs and their receptors (7, 29). In vitro work with Caco-2 cells suggests that expression of tight-junction proteins and transepithelial electrical resistance are CB1-dependent. These findings are consistent with in vivo work, since leptin-deficient, genetically obese mice treated with CB1 antagonists exhibit improved barrier function, reduced adipose tissue mass, and reduced metabolic endotoxemia, independent of food intake (2, 53). Moreover, the gut microbiota has been shown to modulate colonic CB1 mRNA. Conventionally raised mice have significantly lower CB1 expression in the colon compared with germ-free mice, and genetically obese mice fed prebiotic fiber have reduced CB1 expression in the colon compared with genetically obese mice fed control diets (53). These results suggest that the eCB system might link the microbiota and low-grade, systemic inflammation.
Shaping the Microbiota to Combat Obesity
Gut microbial ecology can be reshaped by several targeted and non-targeted interventions, including prebiotics, probiotics, fecal microbiota transplant, and surgical interventions (FIGURE 1).
FIGURE 1.
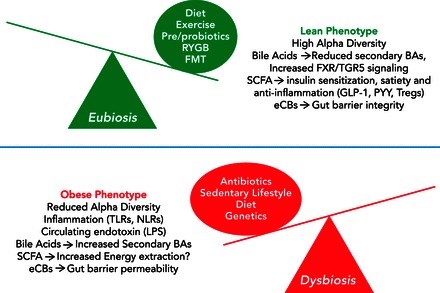
Genetic and environmental factors that can lead to eubiosis or dysbiosis of the gut microbiota and that are strongly linked to obesity
Bidirectionally, changes in the gut microbiota can alter the metabolism and immune function of the host, thus dramatically affecting the pathogenesis of obesity. TLRs, Toll-like receptors; NLRs, nucleotide-binding oligomerization domain like receptors; LPS, lipopolysaccharide; BA, bile acids; FXR, farnesoid X receptor; TGR5, G-protein-coupled bile acid receptor; GLP-1, glucagon-like peptide-1; PYY, peptide YY; Tregs, regulatory helper T-cells; eCB, endocannabinoid; FMT, fecal microbial transplant; RYGB, Roux-en-Y gastric bypass.
Prebiotics
Prebiotics have been defined as nondigestible compounds that, after metabolism by the gut microbiota, modulate the composition and metabolic activity of micro-organisms to confer physiological benefits to the host (6). Gut microbiota adapt to the available dietary and host nutrients either by shifting relative composition or changing microbial function (40). For instance, in adult humans, dietary polysaccharide and resistant starch increased levels of Ruminococcus bromii and Eubacterium rectale, two bacterial strains that are capable of metabolizing insoluble carbohydrates to SCFAs (84). The beneficial role of prebiotics in obesity remains to be elucidated, but the addition of inulin (a soluble, fermentable fiber) to diets of obese individuals has shown to decrease LPS in the blood, likely through changes in butyrate and butyrate-producing bacteria (44). In future studies, prebiotics targeting specific bacterial functions may have a role in management of obesity.
Live Bacterial Therapeutics
Live bacterial therapeutics (LBPs) have also shown potential for improving host physiology in obesity. Although it remains debatable whether they are able to permanently colonize the GI tract, transiently altering gut microbial community dynamics with LBPs has proven effective. For instance, various strains of Lactobacilli or Bifidobacteria have proven effective at promoting weight loss (26, 51). Lactobacillus plantarum has been shown to reduce adipocyte size and overall adiposity in mice (59), whereas Lactobacillus rhamnosus GG has been shown to increase weight loss in women in combination with a moderately energy-restricted diet (68). A 2015 study found that Bifidobacterium pseduocatenulatum reduces obesity-associated inflammation in mice with diet-induced obesity (52). Consumption of traditional fermented foods, which naturally contain large amounts of beneficial microbes, has also been shown to promote weight loss and improvement in metabolic disease parameters (39).
Roux-en-Y Gastric Bypass
Roux-en-Y gastric bypass (RYGB), an effective treatment for weight loss in morbidly obese individuals, has been associated with alterations in gut microbiota in both human and mouse models. The reported changes in gut microbiota include increased abundance of Gamma Proteobacteria (a facultative anaerobe) and decreased Firmicutes (93). When this altered microbial community is transplanted into germ-free mice, improvements in metabolic parameters are observed, suggesting that weight loss following surgery may be due in part to alteration in the gut microbiota (45). Specifically, there is an increase in Faecalibacterium prausnitzii in obese patients with Type 2 diabetes after surgery, and levels of this organism are negatively correlated with inflammatory markers. This again indicates that this species may contribute to the improvement in insulin sensitivity following gastric bypass, possibly through increased butyrate production (11, 38). Perhaps the largest contributor to improved health after RYGB is the alteration in bile acid flow, which can in turn dramatically alter signaling through downstream release of GLP-1 and FGF-19 (74).
Fecal Microbiota Transplant
Fecal microbiota transplantation (FMT) from lean donors to obese recipients with metabolic syndrome resulted in improved peripheral insulin sensitivity, enhanced gut microbial diversity, and increased numbers of butyrate-producing Eubacterium hallii (83). Although FMT is not currently accepted for the treatment of obesity, the ability to transfer the metabolic phenotype in mice and humans may support a future role of FMT in management of obesity and associated metabolic conditions in humans.
Conclusion and Future Perspectives
In this review, we outlined how the gut microbiota, directly or indirectly, influences the pathogenesis of obesity. The importance of the gut microbiota in host health is evidenced by the strong relationship of the gut microbiota to host adiposity and metabolism. Therefore, modulation of gut microbiota represents an important opportunity for management of obesity. We envision that advances in metagenomic analysis will unravel new details relative to the composition and functionality of gut microbiota, whereas targeted repletion of specific bacteria will become a more widely used therapeutic application to combat obesity. Based on the different microbiota compositions, there is also potential for development of specific biomarkers to identify individuals at risk for development of obesity and stratification to personalized treatment options, including diet and lifestyle modifications, prebiotics, probiotics, or bariatric surgery.
Footnotes
The authors are supported by Mayo Clinic, University of Illinois Alliance for Technology-Based Healthcare, and National Institute of Diabetes and Digestive and Kidney Diseases Grant K08 DK-100638 (P.C.K.).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: V.N., J.M.A., L.M., P.C.K., and J.A.W. drafted manuscript; V.N., P.C.K., and J.A.W. edited and revised manuscript; V.N. and J.A.W. approved final version of manuscript; J.M.A. prepared figures.
References
- 1.Ajslev TA, Andersen CS, Gamborg M, Sorensen TI, Jess T. Childhood overweight after establishment of the gut microbiota: the role of delivery mode, pre-pregnancy weight and early administration of antibiotics. Int J Obes (Lond) 35: 522–529, 2011. [DOI] [PubMed] [Google Scholar]
- 2.Alhouayek M, Muccioli GG. The endocannabinoid system in inflammatory bowel diseases: from pathophysiology to therapeutic opportunity. Trends Mol Med 18: 615–625, 2012. [DOI] [PubMed] [Google Scholar]
- 3.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504: 451–455, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA 104: 979–984, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey LC, Forrest CB, Zhang P, Richards TM, Livshits A, DeRusso PA. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr 168: 1063–1069, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Bindels LB, Delzenne NM, Cani PD, Walter J. Towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol 12: 303–310, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Bluher M, Engeli S, Kloting N, Berndt J, Fasshauer M, Batkai S, Pacher P, Schon MR, Jordan J, Stumvoll M. Dysregulation of the peripheral and adipose tissue endocannabinoid system in human abdominal obesity. Diabetes 55: 3053–3060, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brighton CA, Rievaj J, Kuhre RE, Glass LL, Schoonjans K, Holst JJ, Gribble FM, Reimann F. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein-coupled bile acid receptors. Endocrinology 156: 3961–3970, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burger-van Paassen N, Vincent A, Puiman PJ, van der Sluis M, Bouma J, Boehm G, van Goudoever JB, van Seuningen I, Renes IB. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J 420: 211–219, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmee E, Cousin B, Sulpice T, Chamontin B, Ferrieres J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56: 1761–1772, 2007. [DOI] [PubMed] [Google Scholar]
- 11.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57: 1470–1481, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Carotti S, Guarino MP, Vespasiani-Gentilucci U, Morini S. Starring role of toll-like receptor-4 activation in the gut-liver axis. World J Gastrointest Pathophysiol 6: 99–109, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chakraborti CK. New-found link between microbiota and obesity. World J Gastrointest Pathophysiol 6: 110–119, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi JJ, Eum SY, Rampersaud E, Daunert S, Abreu MT, Toborek M. Exercise attenuates PCB-induced changes in the mouse gut microbiome. Environ Health Perspect 121: 725–730, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cicione C, Degirolamo C, Moschetta A. Emerging role of fibroblast growth factors 15/19 and 21 as metabolic integrators in the liver. Hepatology 56: 2404–2411, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Claesson MJ, Cusack S, O'Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, Marchesi JR, Falush D, Dinan T, Fitzgerald G, Stanton C, van Sinderen D, O'Connor M, Harnedy N, O'Connor K, Henry C, O'Mahony D, Fitzgerald AP, Shanahan F, Twomey C, Hill C, Ross RP, O'Toole PW. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA 108, Suppl 1: 4586–4591, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collaboration NCDRF, Di Cesare M, Bentham J, Stevens GA, Zhou B, Danaei G, Lu Y, Bixby H, Cowan MJ, Riley LM, Hajifathalian K, Fortunato L, Taddei C, Bennett JE, Ikeda N, Khang YH, Kyobutungi C, Laxmaiah A, Li Y, Lin HH, Miranda JJ, Mostafa A, Turley ML, Paciorek CJ, Gunter M, Ezzati M. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 387: 1377–1396, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cook MD, Allen JM, Pence BD, Wallig MA, Gaskins HR, White BA, Woods JA. Exercise and gut immune function: evidence of alterations in colon immune cell homeostasis and microbiome characteristics with exercise training. Immunol Cell Biol 94: 158–163, 2016. [DOI] [PubMed] [Google Scholar]
- 19.Cotillard A, Kennedy SP, Kong LC, Prifti E, Pons N, Le Chatelier E, Almeida M, Quinquis B, Levenez F, Galleron N, Gougis S, Rizkalla S, Batto JM, Renault P, Dore J, Zucker JD, Clement K, Ehrlich SD. Dietary intervention impact on gut microbial gene richness. Nature 500: 585–588, 2013. [DOI] [PubMed] [Google Scholar]
- 20.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559–563, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA 107: 14691–14696, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de La Serre CB, Ellis CL, Lee J, Hartman AL, Rutledge JC, Raybould HE. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol 299: G440–G448, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A, Backhed F, Mithieux G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156: 84–96, 2014. [DOI] [PubMed] [Google Scholar]
- 25.Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 6: e280, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DiBaise JK, Zhang H, Crowell MD, Krajmalnik-Brown R, Decker GA, Rittmann BE. Gut microbiota and its possible relationship with obesity. Mayo Clin Proc 83: 460–469, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, Jobin C, Lund PK. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLos One 5: e12191, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dominguez-Bello MG, Blaser MJ, Ley RE, Knight R. Development of the human gastrointestinal microbiota and insights from high-throughput sequencing. Gastroenterology 140: 1713–1719, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engeli S, Bohnke J, Feldpausch M, Gorzelniak K, Janke J, Batkai S, Pacher P, Harvey-White J, Luft FC, Sharma AM, Jordan J. Activation of the peripheral endocannabinoid system in human obesity. Diabetes 54: 2838–2843, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans CC, LePard KJ, Kwak JW, Stancukas MC, Laskowski S, Dougherty J, Moulton L, Glawe A, Wang Y, Leone V, Antonopoulos DA, Smith D, Chang EB, Ciancio MJ. Exercise prevents weight gain and alters the gut microbiota in a mouse model of high fat diet-induced obesity. PLos One 9: e92193, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 110: 9066–9071, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Everard A, Geurts L, Caesar R, Van Hul M, Matamoros S, Duparc T, Denis RG, Cochez P, Pierard F, Castel J, Bindels LB, Plovier H, Robine S, Muccioli GG, Renauld JC, Dumoutier L, Delzenne NM, Luquet S, Backhed F, Cani PD. Intestinal epithelial MyD88 is a sensor switching host metabolism towards obesity according to nutritional status. Nat Commun 5: 5648, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes 4: e121, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haselow K, Bode JG, Wammers M, Ehlting C, Keitel V, Kleinebrecht L, Schupp AK, Haussinger D, Graf D. Bile acids PKA-dependently induce a switch of the IL-10/IL-12 ratio and reduce proinflammatory capability of human macrophages. J Leukoc Biol 94: 1253–1264, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann TW, Pham HP, Bridonneau C, Aubry C, Lamas B, Martin-Gallausiaux C, Moroldo M, Rainteau D, Lapaque N, Six A, Richard ML, Fargier E, Le Guern ME, Langella P, Sokol H. Microorganisms linked to inflammatory bowel disease-associated dysbiosis differentially impact host physiology in gnotobiotic mice. Isme J 10: 460–477, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hooper LV, Wong MH, Thelin A, Hansson L, Falk PG, Gordon JI. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291: 881–884, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Kang SS, Jeraldo PR, Kurti A, Miller ME, Cook MD, Whitlock K, Goldenfeld N, Woods JA, White BA, Chia N, Fryer JD. Diet and exercise orthogonally alter the gut microbiome and reveal independent associations with anxiety and cognition. Mol Neurodegener 9: 36, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fagerberg B, Nielsen J, Backhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498: 99–103, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Kim EK, An SY, Lee MS, Kim TH, Lee HK, Hwang WS, Choe SJ, Kim TY, Han SJ, Kim HJ, Kim DJ, Lee KW. Fermented kimchi reduces body weight and improves metabolic parameters in overweight and obese patients. Nutr Res 31: 436–443, 2011. [DOI] [PubMed] [Google Scholar]
- 40.Koropatkin NM, Cameron EA, Martens EC. How glycan metabolism shapes the human gut microbiota. Nat Rev Microbiol 10: 323–335, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lahti L, Salonen A, Kekkonen RA, Salojarvi J, Jalanka-Tuovinen J, Palva A, Oresic M, de Vos WM. Associations between the human intestinal microbiota, Lactobacillus rhamnosus GG and serum lipids indicated by integrated analysis of high-throughput profiling data. Peer J 1: e32, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lambert JE, Myslicki JP, Bomhof MR, Belke DD, Shearer J, Reimer RA. Exercise training modifies gut microbiota in normal and diabetic mice. Appl Physiol Nutr Metab 40: 749–752, 2015. [DOI] [PubMed] [Google Scholar]
- 43.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jorgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clement K, Dore J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T, Bork P, Wang J, Ehrlich SD, Pedersen O. Richness of human gut microbiome correlates with metabolic markers. Nature 500: 541–546, 2013. [DOI] [PubMed] [Google Scholar]
- 44.Lecerf JM, Depeint F, Clerc E, Dugenet Y, Niamba CN, Rhazi L, Cayzeele A, Abdelnour G, Jaruga A, Younes H, Jacobs H, Lambrey G, Abdelnour AM, Pouillart PR. Xylo-oligosaccharide (XOS) in combination with inulin modulates both the intestinal environment and immune status in healthy subjects, while XOS alone only shows prebiotic properties. Br J Nutr 108: 1847–1858, 2012. [DOI] [PubMed] [Google Scholar]
- 45.Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 5: 178ra141, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature 489: 220–230, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lukovac S, Belzer C, Pellis L, Keijser BJ, de Vos WM, Montijn RC, Roeselers G. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. MBio 5: 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma J, Prince AL, Bader D, Hu M, Ganu R, Baquero K, Blundell P, Alan Harris R, Frias AE, Grove KL, Aagaard KM. High-fat maternal diet during pregnancy persistently alters the offspring microbiome in a primate model. Nat Commun 5: 3889, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matias I, Gonthier MP, Petrosino S, Docimo L, Capasso R, Hoareau L, Monteleone P, Roche R, Izzo AA, Di Marzo V. Role and regulation of acylethanolamides in energy balance: focus on adipocytes and beta-cells. Br J Pharmacol 152: 676–690, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moreno-Indias I, Cardona F, Tinahones FJ, Queipo-Ortuno MI. Impact of the gut microbiota on the development of obesity and Type 2 diabetes mellitus. Front Microbiol 5: 190, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moya-Perez A, Neef A, Sanz Y. Bifidobacterium pseudocatenulatum CECT 7765 reduces obesity-associated inflammation by restoring the lymphocyte-macrophage balance and gut microbiota structure in high-fat diet-fed mice. PLos One 10: e0126976, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muccioli GG, Naslain D, Backhed F, Reigstad CS, Lambert DM, Delzenne NM, Cani PD. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol 6: 392, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mueller NT, Whyatt R, Hoepner L, Oberfield S, Dominguez-Bello MG, Widen EM, Hassoun A, Perera F, Rundle A. Prenatal exposure to antibiotics, cesarean section and risk of childhood obesity. Int J Obes (Lond) 39: 665–670, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nyman M. Fermentation and bulking capacity of indigestible carbohydrates: the case of inulin and oligofructose. Br J Nutr 87, Suppl 2: S163–S168, 2002. [DOI] [PubMed] [Google Scholar]
- 56.O'Sullivan A, Farver M, Smilowitz JT. The influence of early infant-feeding practices on the intestinal microbiome and body composition in infants. Nutr Metab Insights 8: 1–9, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ogden CL, Yanovski SZ, Carroll MD, Flegal KM. The epidemiology of obesity. Gastroenterology 132: 2087–2102, 2007. [DOI] [PubMed] [Google Scholar]
- 58.Parigi SM, Eldh M, Larssen P, Gabrielsson S, Villablanca EJ. Breast milk and solid food shaping intestinal immunity. Front Immunol 6: 415, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Park SY, Cho SA, Lee MK, Lim SD. Effect of Lactobacillus plantarum FH185 on the reduction of adipocyte size and gut microbial changes in mice with diet-induced obesity. Korean J Food Sci Anim Resour 35: 171–178, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Penders J, Vink C, Driessen C, London N, Thijs C, Stobberingh EE. Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiol Lett 243: 141–147, 2005. [DOI] [PubMed] [Google Scholar]
- 61.Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D, Peng Y, Zhang D, Jie Z, Wu W, Qin Y, Xue W, Li J, Han L, Lu D, Wu P, Dai Y, Sun X, Li Z, Tang A, Zhong S, Li X, Chen W, Xu R, Wang M, Feng Q, Gong M, Yu J, Zhang Y, Zhang M, Hansen T, Sanchez G, Raes J, Falony G, Okuda S, Almeida M, LeChatelier E, Renault P, Pons N, Batto JM, Zhang Z, Chen H, Yang R, Zheng W, Yang H, Wang J, Ehrlich SD, Nielsen R, Pedersen O, Kristiansen K. A metagenome-wide association study of gut microbiota in Type 2 diabetes. Nature 490: 55–60, 2012. [DOI] [PubMed] [Google Scholar]
- 62.Queipo-Ortuno MI, Seoane LM, Murri M, Pardo M, Gomez-Zumaquero JM, Cardona F, Casanueva F, Tinahones FJ. Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLos One 8: e65465, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ridlon JM, Alves JM, Hylemon PB, Bajaj JS. Cirrhosis, bile acids and gut microbiota: unraveling a complex relationship. Gut Microbes 4: 382–387, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Bile acids and the gut microbiome. Curr Opin Gastroenterol 30: 332–338, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ridlon JM, Kang DJ, Hylemon PB, Bajaj JS. Gut microbiota, cirrhosis, and alcohol regulate bile acid metabolism in the gut. Dig Dis 33: 338–345, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rogier EW, Frantz AL, Bruno ME, Wedlund L, Cohen DA, Stromberg AJ, Kaetzel CS. Lessons from mother: long-term impact of antibodies in breast milk on the gut microbiota and intestinal immune system of breastfed offspring. Gut Microbes 5: 663–668, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA 105: 16767–16772, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sanchez M, Darimont C, Drapeau V, Emady-Azar S, Lepage M, Rezzonico E, Ngom-Bru C, Berger B, Philippe L, Ammon-Zuffrey C, Leone P, Chevrier G, St-Amand E, Marette A, Dore J, Tremblay A. Effect of Lactobacillus rhamnosus CGMCC1.3724 supplementation on weight loss and maintenance in obese men and women. Br J Nutr 111: 1507–1519, 2014. [DOI] [PubMed] [Google Scholar]
- 69.Savage DC. Microbial ecology of the gastrointestinal tract. Annu Rev Microbiol 31: 107–133, 1977. [DOI] [PubMed] [Google Scholar]
- 70.Schneeberger M, Everard A, Gomez-Valades AG, Matamoros S, Ramirez S, Delzenne NM, Gomis R, Claret M, Cani PD. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci Rep 5: 16643, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C, Hardt PD. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 18: 190–195, 2010. [DOI] [PubMed] [Google Scholar]
- 72.Singh V, Chassaing B, Zhang L, San Yeoh B, Xiao X, Kumar M, Baker MT, Cai J, Walker R, Borkowski K, Harvatine KJ, Singh N, Shearer GC, Ntambi JM, Joe B, Patterson AD, Gewirtz AT, Vijay-Kumar M. Microbiota-dependent hepatic lipogenesis mediated by stearoyl CoA desaturase 1 (SCD1) promotes metabolic syndrome in TLR5-deficient mice. Cell Metab 22: 983–996, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. Diet-induced extinctions in the gut microbiota compound over generations. Nature 529: 212–208, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sweeney TE, Morton JM. Metabolic surgery: action via hormonal milieu changes, changes in bile acids or gut microbiota? A summary of the literature. Best Pract Res Clin Gastroenterol 28: 727–740, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, Gortmaker SL. The global obesity pandemic: shaped by global drivers and local environments. Lancet 378: 804–814, 2011. [DOI] [PubMed] [Google Scholar]
- 76.Tailford LE, Owen CD, Walshaw J, Crost EH, Hardy-Goddard J, Le Gall G, de Vos WM, Taylor GL, Juge N. Discovery of intramolecular trans-sialidases in human gut microbiota suggests novel mechanisms of mucosal adaptation. Nat Commun 6: 7624, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Teixeira TF, Grzeskowiak L, Franceschini SC, Bressan J, Ferreira CL, Peluzio MC. Higher level of faecal SCFA in women correlates with metabolic syndrome risk factors. Br J Nutr 109: 914–919, 2013. [DOI] [PubMed] [Google Scholar]
- 78.Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61: 364–371, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes (Lond) 37: 16–23, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027–1031, 2006. [DOI] [PubMed] [Google Scholar]
- 81.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328: 228–231, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vitek L, Haluzik M. The role of bile acids in the metabolic regulations. J Endocrinol 228: 85–96, 2016. [DOI] [PubMed] [Google Scholar]
- 83.Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, Dallinga-Thie GM, Ackermans MT, Serlie MJ, Oozeer R, Derrien M, Druesne A, Van Hylckama Vlieg JE, Bloks VW, Groen AK, Heilig HG, Zoetendal EG, Stroes ES, de Vos WM, Hoekstra JB, Nieuwdorp M. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 143: 913–916, e917, 2012. [DOI] [PubMed] [Google Scholar]
- 84.Walker AW, Ince J, Duncan SH, Webster LM, Holtrop G, Ze X, Brown D, Stares MD, Scott P, Bergerat A, Louis P, McIntosh F, Johnstone AM, Lobley GE, Parkhill J, Flint HJ. Dominant and diet-responsive groups of bacteria within the human colonic microbiota. Isme J 5: 220–230, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, Schoonjans K, Bianco AC, Auwerx J. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 439: 484–489, 2006. [DOI] [PubMed] [Google Scholar]
- 86.Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA, Bewtra M, Knights D, Walters WA, Knight R, Sinha R, Gilroy E, Gupta K, Baldassano R, Nessel L, Li H, Bushman FD, Lewis JD. Linking long-term dietary patterns with gut microbial enterotypes. Science 334: 105–108, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu GD, Compher C, Chen EZ, Smith SA, Shah RD, Bittinger K, Chehoud C, Albenberg LG, Nessel L, Gilroy E, Star J, Weljie AM, Flint HJ, Metz DC, Bennett MJ, Li H, Bushman FD, Lewis JD. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 65: 63–72, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, Friedman JM, Holmes WE, Spiegelman BM. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol Cell Biol 20: 5343–5349, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499: 97–101, 2013. [DOI] [PubMed] [Google Scholar]
- 90.Zaibi MS, Stocker CJ, O'Dowd J, Davies A, Bellahcene M, Cawthorne MA, Brown AJ, Smith DM, Arch JR. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett 584: 2381–2386, 2010. [DOI] [PubMed] [Google Scholar]
- 91.Zeevi D, Korem T, Zmora N, Israeli D, Rothschild D, Weinberger A, Ben-Yacov O, Lador D, Avnit-Sagi T, Lotan-Pompan M, Suez J, Mahdi JA, Matot E, Malka G, Kosower N, Rein M, Zilberman-Schapira G, Dohnalova L, Pevsner-Fischer M, Bikovsky R, Halpern Z, Elinav E, Segal E. Personalized nutrition by prediction of glycemic responses. Cell 163: 1079–1094, 2015. [DOI] [PubMed] [Google Scholar]
- 92.Zeng H, Chi H. Metabolic control of regulatory T cell development and function. Trends Immunol 36: 3–12, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, Parameswaran P, Crowell MD, Wing R, Rittmann BE, Krajmalnik-Brown R. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA 106: 2365–2370, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


