Abstract
Evolution has endowed vertebrates with the remarkable tactile ability to explore the world through the perception of physical force. Yet the sense of touch remains one of the least well understood senses at the cellular and molecular level. Vertebrates specializing in tactile perception can highlight general principles of mechanotransduction. Here, we review cellular and molecular adaptations that underlie the sense of touch in typical and acutely mechanosensitive vertebrates.
Anatomical organization of the somatosensory system is conserved among vertebrates. Environmental stimuli are detected by the peripheral nerve endings of pseudounipolar neurons housed in trigeminal (TG) or dorsal root ganglia (DRG). TGs occupy a cavity at the base of the skull and innervate the head, whereas DRGs lie along the vertebral column and innervate body segments. Tactile stimuli are detected and transmitted by afferent neurons, termed low-threshold mechanoreceptors (LTMRs). Two broad types of LTMRs exist: slow-conducting non-myelinated C-type LTMRs and fast-conducting myelinated Aδ- and Aβ-LTMRs (40). In rodents, most LTMRs terminate in the skin as lanceolate endings in hair follicles or as part of mechanoreceptive end-organs, such as Merkel cell-neurite complexes, Pacinian and Meissner corpuscles, Ruffini endings, and others. The exception is C-LTMRs, which terminate as free nerve endings. The various types of LTMRs specialize in the detection of stimuli of a particular duration, magnitude, frequency, and velocity, providing information about forces acting on the skin or internal organs (84).
Even though the spectrum of sensory cues perceived by each of the different end-organs is well defined, the exact mechanisms that underlie such specialization remain largely obscure. LTMRs, which are at the core of every mechanoreceptive end-organ, are intrinsically mechanosensitive, i.e., they can generate a mechano-evoked current (MA current) and a train of action potentials in vitro in the absence of the somatic components of the end-organs (56). Action potential discharges are subdivided into slowly and rapidly adapting (SA and RA, respectively), reflecting the ability of the afferent to signal slow/static or transient/vibratory stimuli. These properties have long been considered innate to LTMRs themselves, yet recent evidence suggests that somatic components play an equally vital role in shaping the firing pattern of the afferent response.
In Merkel cell-neurite complexes (the principal detectors of static stimuli), activation of Merkel cells alone is sufficient to evoke a slowly adapting train of action potentials in the underlying neuron (49). Similarly, lamellar cells in the Pacinian corpuscles (detectors of transient touch and high-frequency vibration) were proposed to influence excitability of the encapsulated afferent, which normally has a rapidly adapting firing pattern (60, 61). The multi-component organization of sensory afferent and end-organs provides room for evolutionary changes to fine-tune mechanosensitivity to the species' needs.
Most of our knowledge of the physiology and molecular basis of mechanosensation comes from studies with rodents, worms, and flies, and is extensively covered in recent reviews (3, 32, 38, 41, 59, 72, 78, 80, 82, 84). Here, we will focus on adaptations to tactile perception in acutely mechanosensitive vertebrates, which are not considered standard model organisms. Animals with extremely sensitive tactile perception not only reveal a diversity of sensory adaptations, behaviors, and neuronal specialization, but have the potential to highlight key physiological and molecular features underlying mechanosensation in general.
Specialization in Reptiles
Serpentes
Snakes are a diverse group of voracious predators who developed specialized organs to aid hunting in various conditions. These include pits for infrared sensation in the terrestrial rattlesnakes, boas, and pythons (27), and sensory appendages (tentacles) to detect water movement in the aquatic tentacled snake (Erpeton tentaculatum). Even though tentacled snakes are highly visual, they can catch fish, their only food, in complete darkness, relying solely on the sense of touch (6, 8, 10). Each tentacle is innervated by two branches of the trigeminal nerve. Electrophysiological recordings from trigeminal cell bodies in response to mechanical stimulation of the tentacles or surrounding water revealed rapidly adapting discharges at the onset and offset of the stimulus. The neurons responded best in the 50- to 150-Hz range and detect minute tentacle movements caused by forces as little as 0.008 g (80 μN). In other vertebrates, rapidly adapting LTMRs are invariably associated with complex mechanoreceptive end-organs, such as Meissner or Pacinian corpuscles, yet no similar structures have been detected in the tentacles (10). The tentacles are devoid of electro- or chemoreceptors, suggesting that their only role is the detection of tactile stimuli and that they could be categorized as a separate (and the largest) type of mechanoreceptive end-organs (FIGURE 1 and Table 1).
FIGURE 1.
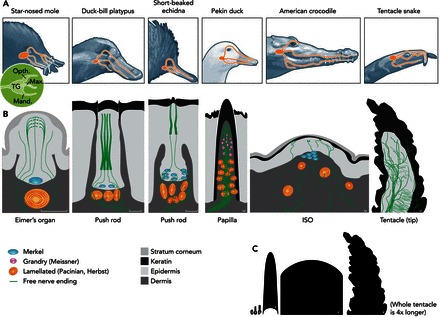
Mechanosensitive end-organs in tactile specialists
A: schematic of trigeminal innervation of mechanosensitive organs in tactile specialists. From left: star-nosed mole, duck-bill platypus, short-beaked echidna, Pekin duck, American crocodile, tentacle snake. Inset on left shows trigeminal ganglion (TG) with ophthalmic (Opth.), mandibular (Mand.), and maxillary (Max.) nerve branches. B: schematic of end-organs and arrangement of nerve fibers and corpuscles within end-organs, ordered by size (scale bar = 20 μm). ISO, integumentary sensory organ. See also Refs. 1, 4, 7, 10, 42, 51. C: relative scale of end-organs (scale bar = 20 μm).
Table 1.
Anatomical and functional properties of mechanoreceptors in tactile specialists
| Organism | Tactile Sensitive Structure | Where (How Many, Density, Etc.) | Size | Free Nerve Endings | Lanceolate Endings | Meissner Corpuscles | Merkel Cell-Neurite Complexes | Lamellated (Pacinian) Corpuscles | Other | Afferent Type(s) (% of All Mechano Responses) | Optimal Detection Frequency (RA Only)* | Force or deflection Threshold of Activation | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tentacled snake | Tentacles | Head (2) | 4–6 mm | + | − | − | − | − | − | RA | 50–150 Hz | ∼80 μN | 10 |
| Crocodile, alligator | Integumentary sensory organs (ISO) | Jaws, body scales (4,000–9,000 total, 0.2–2 ISO/mm2) | 0.2–1.2 mm Ø | + | + | − | + | + | Discoid cells | RA (51%) SA (49%) | 20–35 Hz | 0.08–14 mN | 42 |
| Tactile foraging waterfowl (ducks, geese) | Bill skin | Upper and lower bill | ∼30 cm2 (adult mallard) | + | − | + (Grandry†) up to 65/mm2 | − | + (Herbst) up to 140/mm2 | − | RA (80%) from Grandry/Herbst SA (20%) from unidentified | 50–150 Hz | 5 μm skin displacement at 100 μm/s velocity | 4, 23, 25, 26, 28, 43 |
| Sensory papillae | Maxillary & mandibular bill nails (45 ea.) | 975–1,050 μm (h) 100 μm Ø | + | − | 4–18 | − | 3–16 | − | RA (in duck) SA (in goose) | 50–1000 Hz | 1–20 μm | ||
| Platypus | Push rods | Upper and lower bill, 46,500 push rods total | 400 x 70 μm | − | − | − | + 12 per push rod | + 3–6 per push rod | Vesicle-chain receptors (7–13 chains per push rod) | RA SA | (150–250, up to 600 Hz) | 20 μm | 29, 51 |
| Short-beaked Echidna | Push rods | Glabrous skin of the rostral end of the bill, 20–40/mm2 | 300 μm (h) 50 μm Ø | − | − | − | + 18–26 per push rod | + 5–8 per push rod | Vesicle-chain receptors (4 neurons) | RA (25%) SA (75%) | (50–800 Hz) | 80 μm | 1, 37, 50 |
| Star-nosed mole | Eimer's organs | Star-shaped nose, 25,000 organs total | 60 μm (h) 30–50 μm Ø | + (4–7) | − | − | + (7–15) | + (1–2) | − | RA (80%) SA (20%) | 150–300 Hz | 10 μm displacement at 3 mm/s velocity | 9, 52, 53 |
| Big brown bat | Hairy skin of the wing | Leading edge, phalanges, dactylopatagium membrane | + | + >3/mm2 | − | + >3/mm2 | − | Diffuse endings (>0.4/mm2) | Unknown | Unknown | 0.4 mN | 54 |
Shown are optimal frequencies for the RA afferents only. Detectable frequency range is wider (see text and references). †In the literature, Grandry corpuscles are sometimes referred to as Merkel-like because of an apparent similarity. However, functional studies unequivocally indicate that they are RA, not SA; that they are corpuscles (Merkel are not corpuscles); and that they morphologically resemble Meissner, even though they very often have just two specialized cells (25, 75). +, Detected; -, not detected.
Crocodilia
Crocodilians can efficiently hunt at night, relying heavily on the sense of touch. Behavioral studies show that alligators can detect vibration from a water drop without any visual or auditory clues (73). All three families of Crocodilians have specialized miniature (0.2-1.2 mm) dome-like pigmented structures on their skin, called integumentary sensory organs (ISO). In Crocodylidae and Gavialidae, ISOs are distributed throughout the body, including the head, whereas in Alligatoridae ISOs are found only on the face near the mouth (42, 79). The dome of each ISO is covered with only a thin (5 μm) stratum corneum, allowing mechanical stimuli to easily reach the inside. The ISOs house unmyelinated free nerve endings that penetrate the upper level of epidermis, and a number of mechanoreptive end-organs: Merkel cell complexes, Pacinian-like lamellated corpuscles, and others, innervated by myelinated trigeminal afferents (79). Electrophysiological analysis of the trigeminal afferents from the American alligator (Alligator mississippiensis) revealed mechanosensory units with rapidly (51%) and slowly (49%) adapting responses (FIGURE 1 and Table 1). It has been proposed that ISOs play several important functions during prey capture: detection of water vibration and physical contact, as well as prey analysis after the capture (42).
Specialization in Birds
Birds live in nearly every habitat, facing diverse challenges for survival that have dramatically shaped their sensory abilities. Although all birds have some sense of touch of their epidermis, some species are capable of fine tactile discrimination using their bill or beak (rostum). Like their crocodilian ancestors, birds' rostrum is their primary tactile organ, homodynamic to the primate hand. Consistent with the rostrum's importance to avian behavior, more brain area is devoted to processing information from the bill in tactile-foraging species than nonspecialists across several orders (30, 55). Morphology of the beak varies dramatically between bird species, yet touch receptors of the beak and skin are highly conserved across avian taxa. In addition to free nerve endings, there are two major types of end-organs present in the bill of tactile foraging birds: Herbst and Grandry corpuscles. Herbst corpuscles (70) are fairly homologous to Pacinian or Pacinian-like lamellated corpuscles found in reptiles (42) and mammals (62), whereas Grandry corpuscles, found only in aquatic birds, are most homologous with mammalian Meissner corpuscles (25, 75). Therefore, it is likely that they first evolved in a more ancient common ancestor of modern birds. Examples of diverse tactile-foraging strategies exist in Paleognathae (kiwi) and the two major subgroups of Neognathae: Neoaves (parrots, ibises, and shorebirds) and Galloanserae (ducks, geese). Although morphology, foraging strategies, and environments differ, there are two things common to all tactile foraging birds: 1) a higher density of end-organs in the bill than in visual foragers (4, 16, 23) and 2) a larger percentage of their brain devoted to processing information from the trigeminal system (30, 83). In addition, tactile-foraging birds generally have smaller binocular visual fields than visually foraging birds (55).
Paleognathae
Kiwis have extremely small eyes and the smallest visual fields of any bird, making them ill-suited for visual foraging. Instead, kiwis forage nocturnally, relying on their sense of smell and touch (55, 81). They also use remote touch but are less effective foragers when relying on tactile cues alone than on smell alone (18). The morphology of their bill is consistent with a tactile foraging strategy, including >100 sensory pits in the distal bill containing Herbst corpuscles innervated by trigeminal afferents (19).
Neoaves
Parrots find food primarily using vision and use touch to skillfully extract edible parts of food, such as husking seeds (20). They palpate objects using specialized “bill-tip organs,” located inside the distal upper and lower beak. The bill-tip organ and tongue are densely populated with Herbst corpuscles. The geometry of the bill-tip organs suggests that parrots can only determine tactile information about objects held within the bill (20), consistent with a role in extractive foraging rather than prey detection.
Sandpipers (Scolopacidae) and Madagascar crested ibises (Lophotibis cristata) use remote touch to detect vibrations generated by motile prey buried in sand (17, 22). Red knots (Calidris canutis) can also detect sessile prey using remote touch. This ability was demonstrated elegantly using objects buried in wet sand as the conditioned stimulus in an operant conditioning experiment (63). Both molluscs and stones are effective conditioned stimuli, confirming that birds can detect their presence without smell, taste, or vibratory cues. Importantly, knots cannot do this task in dry sand, suggesting they detect differences in pressure resulting from water flow around the buried objects (63). The anatomical structures underlying shorebirds' remarkable sense of touch are sensory pits in the hard keratin of the bill filled with Herbst corpuscles.
Galloanserae
Anseriformes (ducks and geese) forage in aquatic environments, where mud and algae hide prey from sight, and also feed at night. Some species of ducks engage in a form of tactile foraging called “dabbling”: moving the bill rapidly back and forth in water (85, 86). Unlike tactile foragers from other orders, the bill of Anseriformes is mostly covered by leathery skin, except for the bill-tip organ, which is keratinaceous (4). The bill is extremely mechanosensitive, as both inner and outer surfaces of the dorsal and ventral bill are replete with trigeminal afferent-innervated Herbst and Grandry corpuscles (2, 4, 69, 70). The density of the corpuscles can reach 150 corpuscles/mm2 (4), which matches the density of the homologous corpuscles in primate finger pads (77). The bill-tip organ contains dermal papillae protruding through the keratin layer (FIGURE 1 and Table 1), reminiscent of the sensory pits found in shorebirds (4). The bill of geese is similar to ducks, although Grandry corpuscles outnumber Herbst by several fold in the distal bill (26).
More is known about the functional properties of mechanoreceptors in Anseriformes than in any other avian species. Single-unit recordings in both ducks and geese demonstrate sensitivity to vibrations between 50 and 1,000 Hz on the order of 1 μm, similar to mammalian Pacinian corpuscles. The majority of mechanosensitive units are rapidly adapting in both species, with the exception of the bill-tip organ in geese (23–26, 28, 67). Finally, MA currents from dissociated duck TG neurons are larger in amplitude, are longer in duration, and require less stimulus intensity than the same group of neurons from mice. Overall, duck TG neurons are innately more mechanosensitive than mouse cells, providing an explanation to the exceptional mechanosensitivity of this species at the neuronal level (71). The majority of trigeminal neurons in several tactile-foraging duck subspecies are LTMRs expressing Piezo2 (71), a known mechanotransducer discovered in the somatosensory system of mice (15). The numerical expansion of LTMRs in duck TG comes at the expense of nociceptors, suggesting that mechanosensory specialization occurs during the development of the somatosensory system (71).
Specialization in Monotremes
Platypus and its close relative echidna exhibit some anatomical and physiological features common to reptiles: features of the skull, the presence of a cloaca, and the ability to lay eggs, whereas others are similar to placental mammals: mammary glands, hairy skin, and a four-chambered heart. Platypus and echidna are the only terrestrial mammalian species that have developed electroreception, which they use together with mechanoreception to efficiently locate prey and avoid predators.
Duck-Billed Platypus (Ornithorhynchus Anatinus)
Platypi live in burrows on riverbanks. When a platypus dives to hunt underwater, it closes its eyes, ears, and nostrils, and completely relies on electro- and mechanosensation to locate its prey. The platypus is a champion in underwater mechanoreception, capable of detecting moving prey 50 cm away from its bill. The bill, especially its labial margins, contains over 40,000 mechanosensory appendages called push rods (51), which morphologically resemble Eimer's organs in the “star” of the star-nose mole (discussed below). The push rod organ is ∼400 μm long and 70 μm wide. The upper half of the rod is loosely attached to the epidermis, making its tip highly direction-sensitive, especially underwater. It has been suggested that platypi can adjust sensitivity and flexibility of the push rods by contracting the actin-rich sphincter localized at the base of the epidermal part of the structure (50). Push rods are densely innervated by myelinated trigeminal afferents and consist of keratinocytes with embedded mechanoreceptive end-organs: Merkel cell-neurite complexes, Pacinian-like corpuscles, and vesicle-chain receptors (FIGURE 1). Accordingly, electrophysiological recordings detected mechanosensory units with rapidly and slowly adapting discharges, and several intermediately adapting units (Table 1) (29).
Short-Beak Echidna (Tachyglossus Aculeatus)
Short-beak echidna, endemic to Australia and Tasmania, has an ∼7- to 8-cm-long beak covered with electro- and mechanoreceptors and innervated by trigeminal afferents. This antenna-like structure serves the purpose of detecting ants and termites underground. Similar to platypus, echidnas evolved push rod-like organs, which contain vesicle-chain receptors, Merkel cell-neurite complexes, and Pacinian-like corpuscles (FIGURE 1) (1). Ultrastructural studies showed that the push rods are flexible and not attached to the surrounding tissue. However, they are considered less sensitive than the push rods of platypus, because echidna push rods are tightly connected to the epidermis and thus have less freedom of movement during mechanical stimulation or shear stress (50). Moreover, the number of Pacinian corpuscles, vesicle chains, and Merkel cells are reduced compared with the push rods in platypus (64). Electrophysiological studies revealed that 75% of mechanosensitive units have slowly adapting discharges, whereas the rest are rapidly adapting with no intermediate discharges detected (Table 1) (36, 37).
Specialization in Placental Mammals
Star-Nosed Mole (Condylura Cristata)
The star-nosed mole forages in muddy soils using its “star” organ formed by two sets of 11 fleshy mechanosensory appendages around the nostrils, each containing 600-1,700 bulbous papillae on its surface, called Eimer's organs. Each Eimer's organ is innervated by myelinated trigeminal LTMRs terminating in Merkel cell-neurite complexes, in Pacinian-like corpuscles, or as free nerve endings (FIGURE 1), with an average of 6,110 fibers/ray, which together constitute the densest population of mechanoreceptive end-organs found in mammals (9, 68). This diversity of mechanoreceptors enables the Eimer's organ to recognize both static and dynamic stimuli with high precision and accuracy (52). The myelinated trigeminal LTMRs respond to mechanical stimulation with both rapidly and slowly adapting firing discharges (52). Histological and molecular data show that, similar to ducks (71), mole TG is enriched with LTMRs at the expense of nociceptors (21).
The 22 rays of the star are organized in two 11-ray sets and act together in a way similar to a primate's eye. Upon encountering an object, rays 1 through 10 perform saccade-like movements to direct the object to ray 11 in the center of the star. This ray has the greatest LTMR innervation and is represented in the somatosensory cortex by the largest area per afferent. Ray 11 performs the final detailed investigation of the object, which can be as small as 1 mm in diameter, functioning as the tactile fovea of the star organ. Such an elaborate somatosensory structure enables the mole to identify and eat its prey in as short as 100–300 ms, making it the fastest known mammalian predator (11).
Chiroptera
Bats are the only order of mammals capable of sustained flight. The remarkable agility of bats' flight requires precisely timed coordination of limb and body movements in response to changes in air flow and body position. In addition to hearing and vision, muscle movements controlling air maneuvers rely on the abundant, rapidly changing tactile information from the mechanosensory system in the wing (12). Histological analyses of the wing membrane of the big brown bat (Eptesicus fuscus) revealed that the hairs are situated in follicles. Almost 50% of the follicles are dually innervated by lanceolate nerve endings and Merkel cell-neurite complexes (54). In rodents, the inclusion of Merkel cells into hair follicles is rare and restricted only to guard hairs, which comprise ∼2% of all hairs (44). The two types of end-organs are known to detect rapid and slow hair deflection, respectively. In the context of flight, the presence of a multimodal mechanoreceptor could be crucial for precise monitoring of changes in the speed and direction of airflow. Indeed, depilation adversely alters flight behavior, suggesting that airflow is detected by hair deflection (74). Accordingly, air puffs activate neuronal responses in primary somatosensory cortex, demonstrating that airflow and tactile stimuli engage the same neuronal circuit (54). The expansion of this type of hairs and the elimination of the drag-inducing coat hairs could represent a unique evolutionary adaptation for flight at the level of the somatosensory system.
Cellular and Molecular Basis of Mechanoreception
The general principle of how touch is converted into excitation in LTMRs is well established. This involves a concerted action of two major functional groups of ion channels: cation nonselective mechanotransducers, which mediate initial cationic influx in response to touch (the so-called mechano-activated, or MA, current), and voltage-gated calcium- and sodium-selective channels, which generate and propagate action potentials. The understanding of molecules that mediate all these aspects of mechanosensation could be furthered by studying species specialized for tactile acuity.
Electrophysiological analyses of mechanically activated excitatory currents in cultured sensory neurons strongly suggest that more than one type of mechanotransducer should exist (14, 33, 35, 56, 66). Yet, despite years of research, their molecular identity remained a complete mystery until very recently. In 2010, Coste et al. reported the identification of Piezo2, a cation-selective ion channel that generates fast-inactivating MA current (15). In mouse TG and DRG, Piezo2-expressing neurons account for 20–40% of the total neuronal population (5, 15, 47, 65). In TG of tactile specialist ducks, however, such as the Pekin duck (Anas platyrhynchos domesticus), Mallard (Anas platyrhynchos), and Shoveler (Anas clypeata), the proportion of Piezo2-expressing neurons reaches 85% (71). Interestingly, the deletion of Piezo2 in mouse somatosensory neurons suppresses, but does not eliminate, light touch responses, strongly suggesting that Piezo2 is a major contributor to the perception of light touch, but not the only light touch receptor. Accordingly, the deletion of Piezo2 drastically decreases but does not eradicate fast-inactivating MA current in neurons in vitro (65). This leaves the possibility that a small subset of neurons with fast-inactivating MA current is mediated by an unknown mechanotransducer functionally similar to Piezo2. In mouse DRG, the deletion of Piezo2 has no significant effect on intermediately and slowly inactivating MA current, which together are present in almost 50% of mouse DRG neurons (65). Thus Piezo2 provides a molecular basis for the majority of fast-inactivating MA current, but the identity of mechanotransducers with slower kinetics of inactivation remains to be determined.
In addition to its role in LTMRs, Piezo2 was found to be important for innate mechanosensitivity of Merkel cells. Deletion of Piezo2 in Merkel cells shifts responses of associated LTMRs from slowly to rapidly adapting, suggesting that their typical slowly adapting discharge requires the presence of a Merkel cell. Accordingly, selective activation of Merkel cells alone triggers slowly adapting responses in the afferent (49). Thus the molecular mechanism that fine-tunes sensitivity of Merkel cell-neurite complex depends on the same mechanotransducer ion channel. Whether Piezo2 is similarly important for mechanosensitivity of the neural and somatic components of other types of mechanoreceptive end-organs is unclear. However, it has been established that somatic components play a crucial role in shaping mechanosensory specificity of LTMRs. For example, stimulation of Pacinian corpuscles evokes a fast-inactivating receptor potential in the associated rapidly adapting LTMR. However, stimulation of an LTMR devoid of most of the sheath forming the Pacinian capsule evokes a slowly inactivating receptor potential. It was proposed that the lamellae act as mechanical filters, allowing only transient responses to reach the neural core (46, 57). How the somatic and neural components communicate on the chemical or electrical level is unclear. Strong evidence suggests that, in the Merkel cell-neurite complex, the process relies on a synapse-like mechanism (31, 34, 48, 58), but the details are pending investigation. Similarly, a synapse-like communication between the neuron and lamellar cells also has been proposed to explain mechanosensitivity of Pacinian corpuscles (60, 61).
Electrophysiological properties of LTMRs, especially the Aδ and Aβ-type, are difficult to study, since these neurons are relatively rare in rodent DRG, where 60–70% of neurons are C-type nociceptors and thermoreceptors (39). For example, in mouse DRG, rapidly adapting LTMRs account for ∼6% of all DRG neurons (45), yet this type of afferent innervates several classes of mechanoreceptive end-organs: Pacinian corpuscles, Meissner corpuscles, and lanceolate endings surrounding guard and awl/auchene hairs. Recent studies utilizing microarray-based and single-cell PCR-based transcriptomic approaches further highlight the functional heterogeneity of rodent DRG neurons, which remain the main experimental model in sensory physiology (13, 76). These considerations catalyze the need to turn investigators' attention to tactile specialists who possess an increased proportion of LTMRs in their sensory ganglia. As mentioned above, star-nosed moles and tactile foraging ducks display a redistribution of neuronal subtypes in their trigeminal ganglia in favor of the Aβ-type LTMRs, as revealed by immunohistochemistry, in situ hybridization, and transcriptome analyses (21, 71). These animals could bring a fresh perspective to reveal both specialized and general molecular principles underlying tactile perception in vertebrates. The molecular basis of mechanosensation has only started to emerge. The discovery of Piezo2 marks a pivotal milestone, yet our understanding of this process is far from complete. Further studies combining the conventional rodent model system with non-standard organisms in which a sensory modality is taken to the extreme should yield novel and unexpected insights into these areas of sensory physiology.
Footnotes
This work was supported by a National Science Foundation grant 1453167 to S.N.B., by fellowships from the Beckman Foundation and Rita Allen Foundation, by National Institute of Health Grant 1R01 NS-091300-01A1 to E.O.G., and a fellowship from the Alfred P. Sloan Foundation to E.O.G. E.R.S. was supported by a training grant from National Institutes of Health (T32 HD-007094).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: E.R.S. prepared figures; E.R.S., E.O.G., and S.N.B. drafted manuscript; E.R.S., E.O.G., and S.N.B. edited and revised manuscript; E.O.G. and S.N.B. approved final version of manuscript.
References
- 1.Andres KH, von During M, Iggo A, Proske U. The anatomy and fine structure of the echidna Tachyglossus aculeatus snout with respect to its different trigeminal sensory receptors including the electroreceptors. Anat Embryol 184: 371–393, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Arends JJ, Dubbeldam JL. The subnuclei and primary afferents of the descending trigeminal system in the mallard (Anas platyrhynchos L.). Neuroscience 13: 781–795, 1984. [DOI] [PubMed] [Google Scholar]
- 3.Bagriantsev SN, Gracheva EO, Gallagher PG. Piezo proteins: regulators of mechanosensation and other cellular processes. J Biol Chem 289: 31673–31681, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkhoudt H. The morphology and distribution of cutaneous mechanoreceptors (Herbst and Grandry corpuscles) in bill and tongue of the Mallard (Anas platyrhynchos L.). Neth J Zool 30: 1–34, 1980. [Google Scholar]
- 5.Bron R, Wood RJ, Brock JA, Ivanusic JJ. Piezo2 expression in corneal afferent neurons. J Comp Neurol 522: 2967–2979, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Catania KC. Born knowing: tentacled snakes innately predict future prey behavior. PLos One 5: e10953, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catania KC. The sense of touch in the star-nosed mole: from mechanoreceptors to the brain. Philos Trans R Soc Lond B Biol Sci 366: 3016–3025, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catania KC. Tentacled snakes turn C-starts to their advantage and predict future prey behavior. Proc Natl Acad Sci USA 106: 11183–11187, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Catania KC, Kaas JH. Somatosensory fovea in the star-nosed mole: behavioral use of the star in relation to innervation patterns and cortical representation. J Comp Neurol 387: 215–233, 1997. [PubMed] [Google Scholar]
- 10.Catania KC, Leitch DB, Gauthier D. Function of the appendages in tentacled snakes (Erpeton tentaculatus). J Exp Biol 213: 359–367, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Catania KC, Remple FE. Asymptotic prey profitability drives star-nosed moles to the foraging speed limit. Nature 433: 519–522, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Chadha M, Moss CF, Sterbing-D'Angelo SJ. Organization of the primary somatosensory cortex and wing representation in the Big Brown Bat, Eptesicus fuscus. J Comp Physiol A 197: 89–96, 2011. [DOI] [PubMed] [Google Scholar]
- 13.Chiu IM, Barrett LB, Williams EK, Strochlic DE, Lee S, Weyer AD, Lou S, Bryman GS, Roberson DP, Ghasemlou N, Piccoli C, Ahat E, Wang V, Cobos EJ, Stucky CL, Ma Q, Liberles SD, Woolf CJ. Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. eLife 4: e06720, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coste B, Crest M, Delmas P. Pharmacological dissection and distribution of NaN/Nav1.9, T-type Ca2+ currents, and mechanically activated cation currents in different populations of DRG neurons. J Gen Physiol 129: 57–77, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE, Patapoutian A. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330: 55–60, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cunningham S, Castro I, Alley M. A new prey-detection mechanism for kiwi (Apteryx spp.) suggests convergent evolution between paleognathous and neognathous birds. J Anat 211: 493–502, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham SJ, Castro I, Jensen T, Potter MA. Remote touch prey-detection by Madagascar crested ibises Lophotibis cristata urschi. J Avian Biol 41: 350–353, 2010. [Google Scholar]
- 18.Cunningham SJ, Castro I, Potter MA. The relative importance of olfaction and remote touch in prey detection by North Island brown kiwis. Animal Behav 78: 899–905, 2009. [Google Scholar]
- 19.Cunningham SJ, Corfield JR, Iwaniuk AN, Castro I, Alley MR, Birkhead TR, Parsons S. The Anatomy of the bill Tip of Kiwi and Associated Somatosensory Regions of the Brain: Comparisons with Shorebirds. PLos One 8: e80036, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demery ZP, Chappell J, Martin GR. Vision, touch and object manipulation in Senegal parrots Poicephalus senegalus. Proc Biol Sci 278: 3687–3693, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerhold KA, Pellegrino M, Tsunozaki M, Morita T, Leitch DB, Tsuruda PR, Brem RB, Catania KC, Bautista DM. The star-nosed mole reveals clues to the molecular basis of mammalian touch. PLos One 8: e55001, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerritsen AFC, Meiboom A. The role of touch in prey density estimation by Calidris Alba. Netherlands J Zool 36: 530–561, 1985. [Google Scholar]
- 23.Gottschaldt KM. The physiological basis of tactile sensibility in the beak of geese. J Comp Physiol A 95: 29–47, 1974. [DOI] [PubMed] [Google Scholar]
- 24.Gottschaldt KM, Fruhstorfer H, Schmidt W, Kraft I. Thermosensitivity and its possible fine-structural basis in mechanoreceptors in the beak skin of geese. J Comp Neurol 205: 219–245, 1982. [DOI] [PubMed] [Google Scholar]
- 25.Gottschaldt KM, Lausmann S. Mechanoreceptors and their properties in the beak skin of geese (Anser anser). Brain Res 65: 510–515, 1974. [DOI] [PubMed] [Google Scholar]
- 26.Gottschaldt KM, Lausmann S. The peripheral morphological basis of tactile sensibility in the beak of geese. Cell Tissue Res 153: 477–496, 1974. [DOI] [PubMed] [Google Scholar]
- 27.Gracheva EO, Ingolia NT, Kelly YM, Cordero-Morales JF, Hollopeter G, Chesler AT, Sanchez EE, Perez JC, Weissman JS, Julius D. Molecular basis of infrared detection by snakes. Nature 464: 1006–1011, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gregory JE. An electrophysiological investigation of the receptor apparatus of the duck's bill. J Physiol 229: 151–164, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregory JE, Iggo A, McIntyre AK, Proske U. Receptors in the bill of the platypus. J Physiol 400: 349–366, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gutierrez-Ibanez C, Iwaniuk AN, Wylie DR. The independent evolution of the enlargement of the principal sensory nucleus of the trigeminal nerve in three different groups of birds. Brain Behav Evol 74: 280–294, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Haeberle H, Fujiwara M, Chuang J, Medina MM, Panditrao MV, Bechstedt S, Howard J, Lumpkin EA. Molecular profiling reveals synaptic release machinery in Merkel cells. Proc Natl Acad Sci USA 101: 14503–14508, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hao J, Bonnet C, Amsalem M, Ruel J, Delmas P. Transduction and encoding sensory information by skin mechanoreceptors. Pflügers Arch 467: 109–119, 2015. [DOI] [PubMed] [Google Scholar]
- 33.Hao J, Delmas P. Multiple desensitization mechanisms of mechanotransducer channels shape firing of mechanosensory neurons. J Neurosci 30: 13384–13395, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hitchcock IS, Genever PG, Cahusac PM. Essential components for a glutamatergic synapse between Merkel cell and nerve terminal in rats. Neurosci Lett 362: 196–199, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Hu J, Lewin GR. Mechanosensitive currents in the neurites of cultured mouse sensory neurones. J Physiol 577: 815–828, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iggo A, Gregory JE, Proske U. Studies of mechanoreceptors in skin of the snout of the echidna Tachyglossus aculeatus. Somatosensory Motor Res 13: 129–138, 1996. [DOI] [PubMed] [Google Scholar]
- 37.Iggo A, McIntyre AK, Proske U. Responses of mechanoreceptors and thermoreceptors in skin of the snout of the echidna Tachyglossus aculeatus. Proc R Soc Lond B 223: 261–277, 1985. [Google Scholar]
- 38.Katta S, Krieg M, Goodman MB. Feeling force: physical and physiological principles enabling sensory mechanotransduction. Annu Rev Cell Dev Biol 31: 347–371, 2015. [DOI] [PubMed] [Google Scholar]
- 39.Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol 493: 596–606, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Lawson SN, Perry MJ, Prabhakar E, McCarthy PW. Primary sensory neurones: neurofilament, neuropeptides, and conduction velocity. Brain Res Bull 30: 239–243, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Le Pichon CE, Chesler AT. The functional and anatomical dissection of somatosensory subpopulations using mouse genetics. Front Neuroanat 8: 21, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leitch DB, Catania KC. Structure, innervation and response properties of integumentary sensory organs in crocodilians. J Exp Biol 215: 4217–4230, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leitner LM, Roumy M. Mechanosensitive units in the upper bill and in the tongue of the domestic duck. Pflügers Arch 346: 141–150, 1974. [DOI] [PubMed] [Google Scholar]
- 44.Li L, Ginty DD. The structure and organization of lanceolate mechanosensory complexes at mouse hair follicles. eLife 3: e01901, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li L, Rutlin M, Abraira VE, Cassidy C, Kus L, Gong S, Jankowski MP, Luo W, Heintz N, Koerber HR, Woodbury CJ, Ginty DD. The functional organization of cutaneous low-threshold mechanosensory neurons. Cell 147: 1615–1627, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Loewenstein WR, Mendelson M. Components of receptor adaptation in a pacinian corpuscle. J Physiol 177: 377–397, 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lou S, Duan B, Vong L, Lowell BB, Ma Q. Runx1 controls terminal morphology and mechanosensitivity of VGLUT3-expressing C-mechanoreceptors. J Neurosci 33: 870–882, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maksimovic S, Baba Y, Lumpkin EA. Neurotransmitters and synaptic components in the Merkel cell-neurite complex, a gentle-touch receptor. Ann NY Acad Sci 1279: 13–21, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maksimovic S, Nakatani M, Baba Y, Nelson AM, Marshall KL, Wellnitz SA, Firozi P, Woo SH, Ranade S, Patapoutian A, Lumpkin EA. Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509: 617–621, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Manger PR, Hughes RL. Ultrastructure and distribution of epidermal sensory receptors in the beak of the echidna, Tachyglossus aculeatus. Brain Behav Evol 40: 287–296, 1992. [DOI] [PubMed] [Google Scholar]
- 51.Manger PR, Pettigrew JD. Ultrastructure, number, distribution and innervation of electroreceptors and mechanoreceptors in the bill skin of the platypus, Ornithorhynchus anatinus. Brain Behav Evol 48: 27–54, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Marasco PD, Catania KC. Response properties of primary afferents supplying Eimer's organ. J Exp Biol 210: 765–780, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Marasco PD, Tsuruda PR, Bautista DM, Catania KC. Fine structure of Eimer's organ in the coast mole (Scapanus orarius). Anat Rec 290: 437–448, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Marshall KL, Chadha M, deSouza LA, Sterbing-D'Angelo SJ, Moss CF, Lumpkin EA. Somatosensory substrates of flight control in bats. Cell Rep 11: 851–858, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin GR, Wilson KJ, Martin Wild J, Parsons S, Fabiana Kubke M, Corfield J. Kiwi forego vision in the guidance of their nocturnal activities. PLos One 2: e198, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCarter GC, Reichling DB, Levine JD. Mechanical transduction by rat dorsal root ganglion neurons in vitro. Neurosci Lett 273: 179–182, 1999. [DOI] [PubMed] [Google Scholar]
- 57.Mendelson M, Lowenstein WR. Mechanisms of receptor adaptation. Science 144: 554–555, 1964. [DOI] [PubMed] [Google Scholar]
- 58.Nunzi MG, Pisarek A, Mugnaini E. Merkel cells, corpuscular nerve endings and free nerve endings in the mouse palatine mucosa express three subtypes of vesicular glutamate transporters. J Neurocytol 33: 359–376, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Owens DM, Lumpkin EA. Diversification and specialization of touch receptors in skin. Cold Spring Harb Perspect Med 4: a013656, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pawson L, Pack AK, Bolanowski SJ. Possible glutaminergic interaction between the capsule and neurite of Pacinian corpuscles. Somatosensory Motor Res 24: 85–95, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Pawson L, Prestia LT, Mahoney GK, Guclu B, Cox PJ, Pack AK. GABAergic/glutamatergic-glial/neuronal interaction contributes to rapid adaptation in pacinian corpuscles. J Neurosci 29: 2695–2705, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pease DC, Quilliam TA. Electron microscopy of the pacinian corpuscle. J Biophys Biochem Cytol 3: 331–342, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piersma T, Aelst R, Kurk K, Berkhoudt H, Maas L. A new pressure sensory mechanism for prey detection in birds: the use of principles of seabed dynamics? Proc R Soc Lond B Biol Sci 265: 1377–1383, 1998. [Google Scholar]
- 64.Proske U, Gregory JE, Iggo A. Sensory receptors in monotremes. Philos Trans R Soc Lond B Biol Sci 353: 1187–1198, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ranade SS, Woo SH, Dubin AE, Moshourab RA, Wetzel C, Petrus M, Mathur J, Begay V, Coste B, Mainquist J, Wilson AJ, Francisco AG, Reddy K, Qiu Z, Wood JN, Lewin GR, Patapoutian A. Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516: 121–125, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rugiero F, Drew LJ, Wood JN. Kinetic properties of mechanically activated currents in spinal sensory neurons. J Physiol 588: 301–314, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sato M. Response of Pacinian corpuscles to sinusoidal vibration. J Physiol 159: 391–409, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sawyer EK, Leitch DB, Catania KC. Organization of the spinal trigeminal nucleus in star-nosed moles. J Comp Neurol 522: 3335–3350, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saxod R. Development of cutaneous sensory receptors in birds. In: Deveopment of Sensory System, edited by Bate CM. Berlin, Germany: Springer-Verlag, 1978, p. 337–417. [Google Scholar]
- 70.Saxod R. Ontogeny of the cutaneous sensory organs. Microsc Res Tech 34: 313–333, 1996. [DOI] [PubMed] [Google Scholar]
- 71.Schneider ER, Mastrotto M, Laursen WJ, Schulz VP, Goodman JB, Funk OH, Gallagher PG, Gracheva EO, Bagriantsev SN. Neuronal mechanism for acute mechanosensitivity in tactile-foraging waterfowl. Proc Natl Acad Sci USA 111: 14941–14946, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sharif-Naeini R. Contribution of mechanosensitive ion channels to somatosensation. Progr Mol Biol Transl Sci 131: 53–71, 2015. [DOI] [PubMed] [Google Scholar]
- 73.Soares D. Neurology: an ancient sensory organ in crocodilians. Nature 417: 241–242, 2002. [DOI] [PubMed] [Google Scholar]
- 74.Sterbing-D'Angelo S, Chadha M, Chiu C, Falk B, Xian W, Barcelo J, Zook JM, Moss CF. Bat wing sensors support flight control. Proc Natl Acad Sci USA 108: 11291–11296, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Toyoshima K. Are Merkel and Grandry cells two varieties of the same cell in birds? Arch Histol Cytol 56: 167–175, 1993. [DOI] [PubMed] [Google Scholar]
- 76.Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 18: 145–153, 2015. [DOI] [PubMed] [Google Scholar]
- 77.Verendeev A, Thomas C, McFarlin SC, Hopkins WD, Phillips KA, Sherwood CC. Comparative analysis of Meissner's corpuscles in the fingertips of primates. J Anat 227: 72–80, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Volkers L, Mechioukhi Y, Coste B. Piezo channels: from structure to function. Pflügers Arch 467: 95–99, 2015. [DOI] [PubMed] [Google Scholar]
- 79.von Düring M. The ultrastructure of cutaneous receptors in the skin of Caiman Crocodilus. In: Symposium Mechanoreception. Berlin, Germany: Springer VS, 1974, p. 123–134. [Google Scholar]
- 80.Walsh CM, Bautista DM, Lumpkin EA. Mammalian touch catches up. Curr Opin Neurobiol 34: 133–139, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wenzel BM. Olfactory prowess of the kiwi. Nature 220: 1133–1134, 1968. [DOI] [PubMed] [Google Scholar]
- 82.Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL, Patapoutian A. Piezo2 is required for Merkel-cell mechanotransduction. Nature 509: 622–626, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wylie DR, Gutierrez-Ibanez C, Iwaniuk AN. Integrating brain, behavior, and phylogeny to understand the evolution of sensory systems in birds. Front Neurosci 9: 281, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science 346: 950–954, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zweers GA. Mechanics of the Feeding of the Mallard (Anas Platyrhynchos, L; Aves, Anseriformes). Basel, Switzerland: S Karger Pub, 1977. [Google Scholar]
- 86.Zweers GA. Structure, movement, and myography of the feeding apparatus of the mallard (Anas Platyrhynchos L.) a study in functional anatomy. Netherlands J Zool 24: 323–467, 1973. [Google Scholar]


