Abstract
During vertebrate evolution, duplicated hemoglobin (Hb) genes diverged with respect to functional properties as well as the developmental timing of expression. For example, the subfamilies of genes that encode the different subunit chains of Hb are ontogenetically regulated such that functionally distinct Hb isoforms are expressed during different developmental stages. In some vertebrate taxa, functional differentiation between co-expressed Hb isoforms may also contribute to physiologically important divisions of labor.
Gene Duplication and the Evolution of Novel Protein Functions
Gene duplication is known to play an extremely important role in the evolution of new protein functions. Following the complete duplication of a protein-coding gene, functional redundancy between the two daughter copies will often entail a relaxation of selective constraints that permits the accumulation of degenerative mutations in one or both copies (52, 107). In the majority of cases, one of the two gene duplicates will be rendered functionless by inactivating mutations. However, in a small minority of cases, the fixation of previously forbidden mutations may lead to the acquisition of a novel function and/or expression pattern in one copy or the other. In such cases, both duplicate copies may be selectively retained in the genome, and they can then evolve new functions or divide up ancestral functions.
The diversification of the vertebrate globin gene family provides an excellent example of the role of gene duplication in promoting evolutionary innovation. In this review I highlight several important case studies. First, I describe how the proto hemoglobin (Hb) and myoglobin (Mb) genes originated via whole-genome duplication in the common ancestor of vertebrates. This duplication event facilitated a physiological division of labor between O2-binding proteins with distinct roles in respiratory gas transport. I then describe how repeated rounds of gene duplication and divergence promoted the functional diversification of the subfamilies of globin genes that encode the different subunit polypeptides of tetrameric Hb. These globin genes are ontogenetically regulated such that functionally distinct Hb isoforms (isoHbs) are expressed during different stages of prenatal development and postnatal life. I end by discussing the possible functional significance of Hb multiplicity in the definitive red blood cells of different vertebrate groups.
Phylogenetic Insights Into Gene Family Evolution
Phylogenetic reconstructions permit inferences about the branching relationships among homologous members of a multigene family that have diversified via successive rounds of duplication and divergence. In comparisons among different species, phylogenetic reconstructions provide a means of distinguishing different types of homology. Specifically, the congruence or lack of congruence between a species tree and the gene tree contained within it enables us to distinguish “paralogous” genes (which trace their common ancestry to duplication events) and “orthologous” genes (which trace their common ancestry to speciation events; that is, they descend from a common ancestral gene by phylogenetic splitting at the organsimal level) (FIGURE 1A).
FIGURE 1.
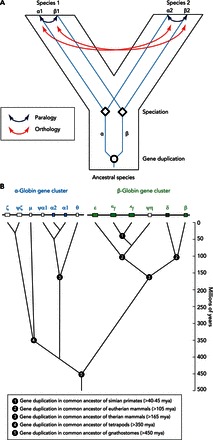
Phylogenetic reconstructions reveal the branching relationships among members of a multigene family that have diversified via successive rounds of duplication and divergence
A: paralogous genes trace their common ancestry to duplication events, whereas orthologous genes trace their common ancestry to speciation events. B: phylogenetic diversification of the α- and β-globin gene subfamilies. The human α- and β-globin gene clusters are shown at top. Pseudogenes are denoted by the Ψ symbol. In the human α-globin gene cluster, for example, Ψζ denotes an inactivated copy of the embryonic ζ-globin gene. The tree depicts phylogenetic relationships among the paralogous gene duplicates. The inferred timing of duplication events is indicated on the vertical axis. Note that the human μ-globin gene is orthologous to the αD-globin gene of other tetrapods, as discussed in the text.
The Role of Hemoglobin in Blood-Gas Transport
Hb is a red blood cell protein that plays an essential role in sustaining aerobic metabolism by transporting O2 from the respiratory exchange surfaces (e.g., lungs, gills, or skin) to the cells of respiring tissues. In jawed vertebrates (gnathostomes), Hb is a tetrameric protein composed of two α-chain subunits and two β-chain subunits. Each of these subunit polypeptides contains a heme group: an iron atom at the center of a poryphyrin ring, which reversibly binds a single O2 molecule in the ferrous state (Fe2+). The related myoglobin (Mb) protein stores O2 and facilitates intracellular O2 diffusion from the sarcolemma to the mitochondria of cardiac and skeletal muscle cells (35, 93). In contrast to the tetrameric Hb protein, Mb is a monomer and is therefore structurally similar to a single heme-bearing subunit of Hb. Mb and the individual Hb subunits have similar heme-coordination chemistries, but Mb has a much higher O2 affinity than Hb. This fulfills an important requirement of an efficient O2-transporting system, since the storage molecule (Mb) should have a higher O2 affinity than the carrier molecule (Hb) at the low Po2 that prevails in the cells of aerobically metabolizing tissues.
The evolution of Hb as a specialized O2-transport protein played a key role in the evolution of aerobic energy metabolism in early vertebrates. Without Hb to augment blood O2 content, the fluid convection of physically dissolved O2 in the blood plasma would not be generally sufficient to meet the cellular O2 demands of relatively large, mobile vertebrates. The one remarkable exception to this rule are the Notothenioid icefish that inhabit the freezing, ice-laden waters surrounding the continental shelf of Antarctica. Notothenioid fish in the family Channichthyidae do not express Hb, and many species do not express Mb either (76).
The O2-transport Hbs of ancestral vertebrates likely existed in a monomer-oligomer equilibrium as in modern-day lampreys and hagfish (see below), where cooperative O2-binding stemmed from association-dissociation dynamics. In modern gnathostomes, by contrast, the efficiency of Hb as a specialized O2-carrier molecule is chiefly attributable to its multisubunit quaternary structure. The interaction between unlike subunits gives rise to the cooperativity of Hb-O2 binding, whereby O2 binding of a given heme iron facilitates the binding of subsequent O2 molecules at the remaining unliganded hemes, and, conversely, O2 liberated by a heme iron facilitates the unloading of O2 molecules from the remaining liganded hemes. Thus Hb has a high O2 affinity at the sites of respiratory gas exchange (the alveoli of the lungs in humans and other mammals) where the Po2 is high, and a reduced affinity at the sites of O2 delivery in the tissue capillaries where the Po2 is substantially lower. The physiological significance of cooperativity is that it permits efficient O2 unloading over a relative narrow range of blood O2 tensions. In addition to cooperativity, which results from interactions between subunits, the O2 affinity of Hb is also modulated by the binding of allosteric cofactors at sites remote from the heme iron. These cofactors include H+, Cl−, CO2, and a variety of organic phosphates, all of which preferentially bind and stabilize deoxy-Hb, thereby shifting the allosteric equilibrium in favor of the low-affinity “tense-state” quaternary structure (92, 93).
In addition to Hb's familiar role as an O2 carrier, recent discoveries have revealed that Hb also plays a role in regulating blood flow in the arterial microcirculation. In this process, which may be important for matching tissue perfusion to local O2 demand, Hb functions as an O2 sensor and O2-responsive nitric oxide (NO) signal transducer, thereby contributing to red cell-dependent hypoxic vasodilation (47). This may be accomplished via enzymatic reduction of nitrite to NO by deoxy-Hb (18, 19, 31) and/or release of bioactive NO from S-nitrosylated Hb (2, 77, 108). Both of these proposed mechanisms of vasoregulation are governed by oxygenation-linked allosteric transitions in Hb quaternary structure. These findings suggest that vertebrate Hb has evolved physiologically important interactions with NO in addition to the more familiar interactions with CO, CO2, and O2.
Gene Duplication, Genome Duplication, and the Origin of Hemoglobin as an O2 Carrier
Two rounds of whole-genome duplication in the stem lineage of vertebrates played an important role in promoting the diversification of the globin gene superfamily (37, 38, 42, 60, 80, 81). The progenitors of the Hb and Mb gene lineages originated as products of one such genome duplication event (42). The retention of the proto Hb and Mb genes in the ancestor of gnathostomes set the stage for a physiological division of labor between O2-carrier and O2-storage functions. In the ancestor of gnathostomes, subsequent duplication of the proto Hb gene gave rise to the progenitors of the α- and β-type globins (FIGURE 1B). This duplication event occurred ∼450 million years ago, before the divergence between the ancestor of cartilaginous fish and the common ancestor of ray-finned fish and tetrapods (32, 42, 80, 81). Functional divergence of the proto α- and β-globin genes permitted the formation of multimeric Hbs composed of unlike subunits (α2β2). The evolution of this heteromeric quaternary structure was central to the emergence of Hb as a specialized O2-transport protein because it provided a mechanism for cooperative O2-binding and allosteric regulatory control. Both of these features require a coupling between the effects of ligand binding at individual subunits and the interactions between subunits in the quaternary structure (64).
The ancestral linkage arrangement of the proto α- and β-globin genes is still retained in the genomes of some modern-day amphibians and teleost fish (27, 56). In amniote vertebrates, by contrast, the α- and β-type globin genes are located on different chromosomes (34, 42, 44). In the human genome, the α-globin gene cluster is located on chromosome 16, and the β-globin gene cluster is located on chromosome 11 (FIGURE 2A). This reflects the fact that the ancestral β-globin gene was transposed to a new chromosomal location in the lineage leading to modern amniotes (34). Intriguingly, an “orphaned” β-type globin gene (ω-globin) is still found in association with the tandemly linked α-type globin genes in the genomes of monotremes and marsupials (41, 59, 105).
FIGURE 2.
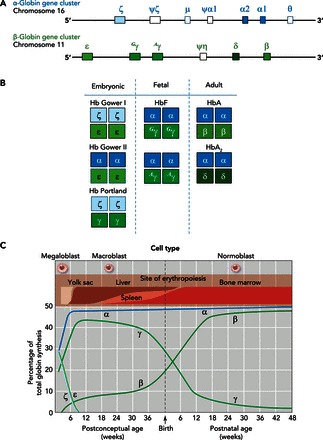
The expression of α- and β-type globin genes is developmentally regulated, resulting in the synthesis of functionally distinct isoHbs
A: structure of the human α- and β-globin gene clusters. B: the set of structurally distinct embryonic, fetal, and adult Hb isoHbs, with subunits encoded by each of the pre- and postnatally expressed α- and β-type genes. C: developmental timeline for changes in the expression levels of the various α- and β-type genes from the earliest stages of embryogenesis to the end of the first year of life. C was adapted from Ref. 106 with permission from British Medical Bulletin.
Phylogenetic evidence indicates that erythroid-specific, O2-transport Hbs evolved independently from different ancestral precursor proteins in the two deepest branches of the vertebrate family tree: gnathostomes and jawless fishes (cyclostomes, represented by lampreys and hagfish) (39, 75). The independent evolution of O2-transport Hbs in these two anciently diverged vertebrate lineages involved the convergent co-option of distinct globin precursors to perform similar respiratory functions in circulating red blood cells. In the Hbs of both gnathostomes and cyclostomes, multisubunit quaternary structures provide the basis for cooperative O2 binding and allosteric regulation, but differences in numerous structural details belie their independent origins. In the tertrameric Hbs of gnathostomes, cooperativity stems from an oxygenation-linked transition in quaternary structure between high- and low-affinity conformations (64). In the Hbs of cyclostomes, by contrast, cooperativity stems from an oxygenation-linked dissociation of low-affinity homo- and/or heterodimers into high-affinity monomers (11, 12, 22, 23, 66). Thus the O2-transport Hbs of gnathostomes and cyclostomes represent superficially similar but structurally distinct design solutions to the challenge of maintaining cellular O2 supply in support of aerobic metabolism (39).
Gene Duplication and the Developmental Regulation of Hemoglobin Synthesis
In mammals, the arrangements of tandemly linked genes in the α- and β-globin gene clusters are co-linear with the temporal order of expression during development (25, 73). For example, the human α-globin gene cluster is arranged: 5′-ζ (embryonic)-α2 (fetal and adult)-α1 (fetal and adult)-3′, and the human β-globin gene cluster is arranged: 5′-ε (embryonic)-Gγ (fetal)-Aγ (fetal)-δ (minor adult)-β (major adult)-3′ (FIGURE 2A). This same general arrangement also is seen in the α- and β-globin gene clusters of other amniotes, although the individual identities of early and late-expressed genes vary among taxa due to lineage-specific gene duplications and deletions (41, 44, 57, 58, 78).
Evolutionary changes in the developmental timing of isoHb expression are typically associated with changes in oxygenation properties, since the different isoHbs are adapted to perform distinct O2-scavenging/O2-transport tasks during different stages of development (7, 92, 106). Evolved changes in functional properties of differentially expressed isoHbs are attributable to amino acid substitutions in paralogous genes that encode the different α- and/or β-type subunits.
During human embryogenesis, O2 diffusion is sufficient to meet the metabolic demands of the developing embryo until day 15 postconception (7, 24). At that stage of development, the embryonic α- and β-type globin genes (ζ- and ε-globin, respectively) are transcriptionally activated to produce Hb Gower I (ζ2ε2), which serves as an O2 carrier (106). After 4 wk of gestation, the heart of the developing embryo becomes septated, the venous and arterial circulations are established, and the placenta begins to develop. During this phase, two additional embryonic isoHbs are synthesized: Hb Gower II (α2ε2) and Hb Portland (ζ2γ2). During the next 6 wk, the placental circulation is established, the yolk sac gradually disappears, and the liver becomes the major site for hematopoeisis, producing definitive, enucleated erythrocytes containing a mix of fetal Hb (HbF; α2γ2) and adult Hb (HbA; α2β2). After ∼20 wk of gestation, the bone marrow becomes established as a secondary site for hematopoesis, producing only HbA (106). At birth, the neonatal circulation consists of erythrocytes containing ∼70% HbF and ∼30% HbA. In 5-mo-old infants, the fraction of HbF in the blood falls to ∼3%, and by 2 years of age, circulating erythrocytes derived from the bone marrow contain ∼97% HbA and ∼3% HBA2 (α2δ2) (FIGURE 2B).
This same basic pattern of ontogenetic gene switching is observed in all other tetrapod vertebrates that have been examined to date (1, 78, 97, 104). In the α-globin gene cluster, the physiological division of labor between early and late-expressed genes was established in the common ancestor of tetrapod vertebrates, and it appears to have been retained in nearly all descendant lineages. The ancestral arrangement of the tetrapod α-globin gene cluster is 5′-αE-αD-αA-3′ (43, 44), where αE is orthologous to the embryonic ζ-globin gene in humans and αA is orthologous to the adult α-globin in humans. In the tetrapod common ancestor, the αA-globin gene and the (presumably embryonic) progenitor of the αE/αD genes originated via tandem duplication of an ancestral proto α-globin gene; the αE- and αD-globins originated via a subsequent tandem duplication (43). In modern tetrapods, the αE-globin gene appears to be expressed exclusively in larval/embryonic erythroid cells, and the αA-globin gene is expressed in definitive erythroid cells during later stages of prenatal development and postnatal life. In mammals, products of the αD-globin gene (annotated as “μ-globin” in the human genome assembly) do not appear to be incorporated into functional Hb tetramers. However, the αD-globin gene is expressed in both primitive and definitive erythroid cells of birds and non-archosaurian reptiles (1, 78).
In contrast to the ancient functional diversification of α-type globin genes (FIGURE 3A), the developmental regulation of gene expression in the β-globin gene cluster evolved independently in several different tetrapod lineages (44). For example, in mammals and birds, the β-type globin genes that are expressed during the earliest stages of embryogenesis were independently derived from lineage-specific duplications of the same proto-β-globin gene, that is, the embryonic β-globins of mammals and birds are not “1:1 orthologs” (FIGURE 3B). Even within mammals, embryonic β-type globin genes appear to have originated independently as the products of lineage-specific duplication events in monotremes (egg-laying mammals) and in the common ancestor of marsupials and eutherian mammals (59). Likewise, fetally expressed β-type globin genes originated independently in simian primates (New World monkeys, Old World monkeys, apes, and humans) and in bovid artiodactyls (cattle, antelope, and goats). In most eutherian mammals, the γ-globin gene encodes the β-chain subunit of embryonic isoHbs, but in simian primates, duplicated copies of γ-globin (Gγ and Aγ) have been co-opted for fetal expression (49, 50). In New World monkeys, Gγ-globin is expressed in nucleated erythroid cells derived from the embryonic yolk-sac (the ancestral condition), but Aγ-globin is expressed in enucleated erythroid cells derived from the fetal liver. In catarrhine primates (Old World monkeys, apes, and humans), both Gγ- and Aγ-globin are fetally expressed. This developmental switch was accompanied by a delay in the fetal expression of the β-globin gene, which is predominantly expressed during postnatal life in mammals. Goodman et al. (32) suggested that the acquisition of fetally expressed Hb may have played an important role in the life history evolution of simian primates because it facilitated an extended duration of fetal development.
FIGURE 3.
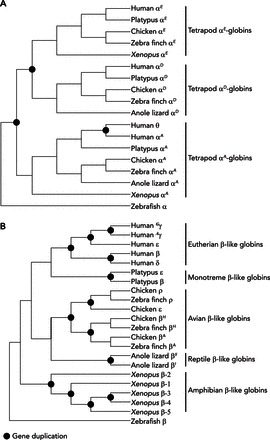
Diagrammatic phylogenies depicting the inferred relationships among members of the α- and β-globin gene subfamilies in tetrapods
In each tree, nodes depicted as filled symbols represent gene duplication events. The remaining nodes represent speciation events (phylogenetic splitting at the organismal level). A: phylogeny of α-type globin genes in representative tetrapod lineages. Note that the three paralogs (αE-, αD-, and αA-globin) are reciprocally monophyletic relative to one another. As discussed in the text, the αE- and αD-globin genes are products of a duplication event that occurred in the stem lineage of tetrapods. Orthologs of the embryonic αE-globin gene are known as αL-globin in amphibians, π-globin in birds, and ζ-globin in mammals. The human ortholog of the αD-globin gene is known as μ-globin. B: phylogeny of β-type globin genes in representative tetrapod lineages. Note that eutherian mammals, monotremes, birds, nonavian reptiles, and amphibians each inherited an ortholog of the same proto β-type gene, which then underwent one or more rounds of duplication and divergence to produce distinct repertoires of β-type globins in each descendent lineage. The depicted phylogenies are based on data reported in Refs. 43, 44, 59.
Whereas embryonic γ-globin genes were co-opted for fetal expression in simian primates, duplicate copies of the adult β-globin gene were co-opted for fetal expression in bovids (17, 74, 86). Thus the stage-specific expression of fetal isoHbs evolved twice independently from different ancestral states. In simian primates and bovids, the co-option of γ- or β-globin genes for fetal expression was likely facilitated by the fact that redundant or semi-redundant copies of other early or late-expressed β-type globin genes continued to perform their ancestral functions. The acquisition of fetally expressed isoHbs would not have been possible if the ancestor of simian primates had possessed only a single embryonic gene or if the ancestor of bovid artiodactyls had possessed only a single adult-expressed gene, as in contemporary monotremes and marsupials (58, 59).
In humans, the fetally expressed isoHb, HbF (α2γ2), exhibits a slightly lower intrinsic O2 affinity relative to adult Hb, HbA (α2β2). However, in the presence of physiological concentrations of allosteric cofactors that are present in the red blood cell, HbF exhibits a higher O2 affinity than HbA due to its reduced sensitivity to the organic phosphate 2,3-diphosphoglycerate (DPG), a metabolite of red cell glycolysis (85) (FIGURE 4). During pregnancy, the resultant O2-affinity difference between HbF in the fetal circulation and HbA in the maternal circulation facilitates O2 transfer across the placental barrier (7). Since HbF and HbA have identical α-type subunits, the different functional properties must be attributable to substitutions between the γ- and β-globin genes. The reduced DPG sensitivity of HbF relative to HbA appears to be mainly attributable to the amino acid substitution γ143His → Ser, which eliminates two DPG binding sites per tetramer (26), in combination with γ43Glu → Asp, which indirectly affects DPG binding by perturbing the allosteric α1β2 interface (15).
FIGURE 4.
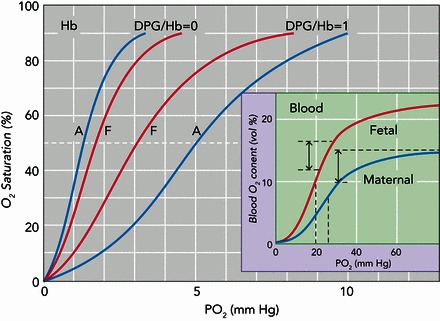
O2-equilibrium curves of human adult and fetal isoHbs
O2-equilibrium curves of human adult and fetal isoHbs (A and F, respectively). Data are shown for “stripped” Hbs (purified Hbs that are stripped of organic phosphates and other allosteric cofactors) in the absence and presence of equimolar concentrations of 2,3-diphosphoglycerate (DPG:Hb = 0 and DPG:Hb = 1, respectively) at 20°C and pH 7.2 (the approximate intraerythrocytic pH value). Inset: O2-equilibrium curves for maternal and fetal blood (solid and dashed lines, respectively) at 37°C and extracelluar pH 7.4 (corresponding to an intracellular pH of 7.2), illustrating the difference in arteriovenous O2 content (double-headed arrows), as well as the higher O2 affinity and higher O2-carrying capacity of fetal blood. Adapted, with permission, from Refs. 85, 90 and used with permissions from the Journal of Biological Chemistry and the Israel Journal of Zoology.
In other eutherian mammals, the requisite Po2 difference between the maternal and fetal circulations is accomplished via changes in red cell DPG concentrations that differentially modulate the O2 affinities of structurally identical Hbs. Viviparous vertebrates employ an astounding diversity of mechanisms for maintaining the Po2 differential between maternal and fetal circulations, only some of which involve genetically based differences in the oxygenation properties of isoHbs with stage-specific expression (17, 45, 90, 92, 98). One consistent pattern across all vertebrates is that, within a given species, isoHbs that are expressed during early embryogenesis have higher O2 affinities and lower cooperativities than isoHbs expressed later in prenatal development or in postnatal life (13, 45, 92, 97, 104). In humans, for example, the embryonic isoHbs (Hb Gower I, Hb Gower II, and Hb Portland) have uniformly higher O2 affinities and lower cooperativities than the later expressed HbF and HbA (7, 10).
Functional Differentiation of Co-Expressed Hb Isoforms
Most eutherian mammals possess multiple copies of α- and β-type globin genes that are co-expressed during postnatal life (30, 40, 41, 53, 58, 59, 61, 70, 82). Adult-expressed genes of the same subunit type typically have highly similar coding sequences and therefore encode identical or nearly identical polypeptides. Thus, in definitive red blood cells, isoHbs that incorporate the different α- and β-type subunits typically have very similar functional properties (14, 46, 51, 71, 83).
The situation is quite different in other tetrapods. The majority of birds, reptiles, and amphibians co-express multiple structurally and functionally distinct Hb isoforms during adult life (20, 33, 57, 78, 79). Crocodilians are a notable exception, since all species that have been examined to date express a single adult Hb (94, 100, 101). Birds typically express two main isoHbs in definitive red blood cells: HbA (the major isoHb, with α-chain subunits encoded by the αA-globin gene) and HbD (the minor isoHb, with α-chain subunits encoded by the αD-globin gene). Both isoHbs incorporate the same β-chain subunits. In all bird species that have been examined to date, the minor HbD exhibits a substantially higher O2 affinity than the major HbA in the presence of physiological concentrations of allosteric cofactors (16, 29, 33, 54, 57, 65). Turtles, lizards, and snakes also express homologous HbA and HbD isoHbs in definititive erythrocytes. [The α-type globin genes are orthologous to those in birds, but the β-type globins are not necessarily 1:1 orthologs (44, 78).] However, the reptilian pattern of isoHb differentiation is a mirror image of the avian pattern: In the few non-archosaurian reptiles that have been investigated, HbD is the major isoHb, and (at least in turtles and snakes) it has a lower O2 affinity than other isoHbs that incorporate products of the αA-globin gene (20, 78, 79).
Since the HbA and HbD isoHbs exhibit appreciable differences in O2-binding properties, regulatory changes in the HbA-to-HbD ratio could conceivably provide an effective mechanism for reversibly modulating blood-O2 affinity in response to changes in environmental O2 availability or changes in internal metabolic demands (33, 36, 95). Among sauropsid vertebrates, however, there is no evidence to suggest that isoHb switching plays an important role in acclimatization to environmental hypoxia. Birds that are native to different elevations in the Andes exhibit consistent differences in Hb-O2 affinity due to genetically based increases in the O2 affinities of HbA and HbD in highland taxa, but there are no detectable elevational differences in HbA-to-HbD ratios (16, 29, 54, 65). Likewise, in turtles, the HbA-to-HbD ratio does not change during acclimation to hypoxia (20).
The Root Effect and IsoHb Differentiation In Teleost Fish
To assess the possible physiological significance of Hb multiplicity, teleost fishes are an ideal group to study. First, teleosts exhibit the highest levels of functional isoHb diversity among vertebrates (28, 45, 48, 88, 91, 103). The extensive repertoire of α- and β-type globin genes in this group is partly attributable to a teleost-specific whole-genome duplication event (56). Second, teleosts inhabit aquatic environments that span an extraordinarily broad range of variation in O2 availability, salinity, ionic composition, pH, and temperature. In principle, the expression of multiple isoHbs with graded O2 affinities and allosteric regulatory capacities could broaden the permissible range of O2 tensions for efficient tissue O2 delivery (88, 89). The isoHb differentiation in some groups may be adaptive in this regard. Most notably, a number of taxa, including eels, catfish, and salmonids, express two electrophoretically distinct isoHb classes (designated as “anodic” and “cathodic”) that exhibit pronounced differences in intrinsic O2 affinity and buffer capacity, and sensitivity to pH, temperature, and organic phosphates (6, 48, 88, 89, 91, 99, 102, 103).
An important functional specialization of the anodic isoHbs involves an extreme form of pH sensitivity known as the Root effect, whereby the low-affinity “T-state” conformation of deoxyHb is strongly stabilized at low pH (3, 4, 8, 9, 62, 63). In tissues such as the retina and the gas gland of the swim bladder, reductions of blood pH in dense, counter-current capillary networks (rete) trigger the release of Hb-bound O2 via the Root effect, thereby promoting O2 secretion at high Po2. The evolution of the Root effect represents a key physiological innovation in teleosts, since O2 secretion in the ocular choroid rete increases the O2 diffusion gradient to highly aerobic cells in the avascular retina (which enhances high-acuity vision), and O2 secretion into the swim bladder provides a mechanism of buoyancy regulation (which facilitated the colonization of deep sea habitats). In addition to these well known functional specializations, recent in vitro and in vivo studies have demonstrated that Root effect Hbs, in conjunction with mechanisms for maintaining an arterial-venous pH difference, also play a significant role in general tissue O2 delivery (67-69).
The differentiation between (anodic) Root effect Hbs and cathodic isoHbs that have low to normal pH sensitivities may represent a physiologically significant division of labor for tissue O2 delivery, especially under hypoxic and/or hypercapnic stress (21, 48, 84, 88, 89, 103). Since the cathodic isoHbs typically have higher O2 affinities than the Root effect Hbs, they may help secure arterial O2 loading under conditions of severe hypoxia where the Root effect Hbs would not be fully saturated. Likewise, since the cathodic isoHbs typically have far lower pH sensitivities, they may help secure tissue O2 delivery during stress-induced acidosis if the red cell β-adrenergic response is not sufficient to safeguard intraerythrocytic pH.
Marine and freshwater fishes must often contend with extreme vicissitudes of O2 availability on a daily or seasonal basis. Given that adult-expressed isoHbs of teleost fish often exhibit physiologically significant differences in oxygenation properties, it seems plausible that regulatory adjustments in red cell isoHb composition could represent an important mechanism of phenotypic plasticity in blood-O2 transport. Experiments involving the African cichlid Haplochromis ishmaeli revealed that exposure to chronic hypoxia during postnatal development induced changes in the relative expression of functionally distinct isoHbs that increased blood-O2 affinity (72). However, as a mechanism of physiological plasticity during adulthood, there is not much evidence to suggest that regulatory changes in red cell isoHb composition make significant contributions to the acclimatization response to hypoxia (45, 103). In fishes, reversible changes in red cell pH and concentrations of allosteric effectors appear to represent far more important mechanisms for modulating blood-O2 affinity in response to changes in O2 availability (5, 48, 55, 87, 88, 91, 96, 103).
Conclusion
The duplication and functional divergence of globin genes has promoted a number of key physiological innovations in respiratory gas transport during vertebrate evolution. The physiological division of labor among developmentally regulated isoHbs has clear adaptive significance in viviparous and oviviparous vertebrates alike. Aside from isoHbs with unique specializations of function such as the Root effect Hbs of teleost fishes, the adaptive significance of Hb multiplicity in the definitive erythrocytes of vertebrates is generally unclear. It is also possible that Hb multiplicity confers physiological benefits that are not directly related to inherent oxygenation properties of the proteins. For example, Hb multiplicity may increase Hb solubility in the red blood cell, thereby increasing blood-O2 carrying capacity by raising the upper limit of intracellular Hb concentration (45, 89). An important line of future research is to elucidate the physiological significance and evolutionary origins of less well understood functions of Hb.
Acknowledgments
I thank C. J. Brauner, A. Fago, F. G. Hoffmann, J. C. Opazo, R. E. Weber, and two anonymous referees for helpful suggestions.
Footnotes
The author's research is supported by grants from the National Heart, Lung, and Blood Institute (HL-087216) and the National Science Foundation (IOS-1354390 and MCB-1517636).
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: J.F.S. conception and design of research; J.F.S. prepared figures; J.F.S. drafted manuscript; J.F.S. edited and revised manuscript; J.F.S. approved final version of manuscript.
References
- 1.Alev C, Shinmyozu K, McIntyre BAS, Sheng G. Genomic organization of zebra finch α and β globin genes and their expression in primitive and definitive blood in comparison with globins in chicken. Dev Genes Evol 219: 353–360, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Allen BW, Stamler JS, Piantadosi CA. Hemoglobin, nitric oxide and molecular mechanisms of hypoxic vasodilation. Trends Mol Med 15: 452–460, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berenbrink M. Historical reconstructions of evolving physiological complexity: O2 secretion in the eye and swimbladder of fishes. J Exp Biol 210: 1641–1652, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Berenbrink M, Koldkjaer P, Kepp O, Cossins AR. Evolution of oxygen secretion in fishes and the emergence of a complex physiological system. Science 307: 1752–1757, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Brauner CJ, Val AL. Oxygen transfer In: Fish Physiology: The Physiology of Tropical Fishes, edited by Val AL, Almeida-Val VMF. Amsterdam: Elsevier, 2006, p. 277–306. [Google Scholar]
- 6.Brauner CJ, Weber RE. Hydrogen ion titrations of the anodic and cathodic haemoglobin components of the European eel Anguilla anguilla. J Exp Biol 201: 2507–2514, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Brittain T. Molecular aspects of embryonic hemoglobin function. Mol Aspects Med 23: 293–342, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Brittain T. The Root effect. Comp Biochem Physiol B Biochem Mol Biol 86: 473–481, 1987. [DOI] [PubMed] [Google Scholar]
- 9.Brittain T. Root effect hemoglobins. J Inorg Biochem 99: 120–129, 2005. [DOI] [PubMed] [Google Scholar]
- 10.Brittain T, Hofmann OM, Watmough NJ, Greenwood C, Weber RE. A two-state analysis of co-operative oxygen binding in the three human embryonic haemoglobins. Biochem J 326: 299–303, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brittain T, Obrien AJ, Wells RMG, Baldwin J. A study of the role of subunit aggregation in the expression of cooperative ligand-binding in the hemoglobin of the lamprey Mordacia mordax. Comp Biochem Physiol B Biochem Mol Biol 93: 549–554, 1989. [Google Scholar]
- 12.Brittain T, Wells RMG. Characterization of the changes in the state of aggregation induced by ligand-binding in the hemoglobin system of a primitive vertebrate, the hagfish Eptatretus cirrhatus. Comp Biochem Physiol A Physiol 85: 785–790, 1986. [DOI] [PubMed] [Google Scholar]
- 13.Brittain T, Wells RMG. Oxygen transport in early mammalian development: molecular physiology of embryonic hemoglobins In: Development in Mammals Vol. 5, edited by Johnson MH. Amsterdam: Elsevier, 1983, p. 135–154. [Google Scholar]
- 14.Campbell KL, Storz JF, Signore AV, Moriyama H, Catania KC, Payson AP, Bonaventura J, Stetefeld J, Weber RE. Molecular basis of a novel adaptation to hypoxic-hypercapnia in a strictly fossorial mole. BMC Evol Biol 10: 214, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen WH, Dumoulin A, Li XF, Padovan JC, Chait BT, Buonopane R, Platt OS, Manning LR, Manning JM. Transposing sequences between fetal and adult hemoglobins indicates which subunits and regulatory molecule interfaces are functionally related. Biochem J 39: 3774–3781, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Cheviron ZA, Natarajan C, Projecto-Garcia J, Eddy DK, Jones J, Carling MD, Witt CC, Moriyama H, Weber RE, Fago A, Storz JF. Integrating evolutionary and functional tests of adaptive hypotheses: a case study of altitudinal differentiation in hemoglobin function in an Andean sparrow, Zonotrichia capensis. Mol Biol Evol 31: 2948–2962, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clementi ME, Scatena R, Mordente A, Condo SG, Castagnola M, Giardina B. Oxygen transport by fetal bovine hemoglobin. J Mol Biol 255: 229–234, 1996. [DOI] [PubMed] [Google Scholar]
- 18.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu XL, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med 9: 1498–1505, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood 107: 566–574, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Damsgaard C, Storz JF, Hoffmann FG, Fago A. Hemoglobin isoform differentiation and allosteric regulation of oxygen binding in the turtle, Trachemys scripta. Am J Physiol Regul Integr Comp Physiol 305: R961–R967, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fago A, Carratore V, Diprisco G, Feuerlein RJ, Sottrupjensen L, Weber RE. The cathodic hemoglobin of Anguilla anguilla: amino acid sequence and oxygen equilibria of a reverse Bohr effect hemoglobin with high oxygen-affinity and high phosphate sensitivity. J Biol Chem 270: 18897–18902, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Fago A, Giangiacomo L, D'Avino R, Carratore V, Romano M, Boffi A, Chiancone E. Hagfish hemoglobins: structure, function, and oxygen-linked association. J Biol Chem 276: 27415–27423, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Fago A, Weber RE. The hemoglobin system of the hagfish Myxine glutinosa: aggregation state and functional properties. BBA Protein Struct M 1249: 109–115, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Fantoni A, Farace MG, Gambari R. Embryonic hemoglobins in man and other mammals. Blood 57: 623–633, 1981. [PubMed] [Google Scholar]
- 25.Forget BG, Hardison RC. The normal structure and regulation of human globin gene clusters In: Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management (2nd ed), edited by Steinberg MH, Forget BG, Higgs DR, Weatherall DJ. Cambridge, UK: Cambridge Univ. Press, 2009. p. 46–61. [Google Scholar]
- 26.Frier JA, Perutz MF. Structure of human fetal deoxyhemoglobin. J Mol Biol 112: 97–112, 1977. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs C, Burmester T, Hankeln T. The amphibian globin gene repertoire as revealed by the Xenopus genome. Cytogenet Genome Res 112: 296–306, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Fyhn UEH, Fyhn HJ, Davis BJ, Powers DA, Fink WL, Garlick RL. Hemoglobin heterogeneity in Amazonian fishes. Comp Biochem Physiol A Physiol 62: 39–66, 1979. [Google Scholar]
- 29.Galen SC, Natarajan C, Moriyama H, Weber RE, Fago A, Benham PM, Chavez AN, Cheviron ZA, Storz JF, Witt CC. Contribution of a mutational hotspot to hemoglobin adaptation in high-altitude Andean house wrens. Proc Natl Acad Sci USA 112: 13958–13963, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gaudry MJ, Storz JF, Butts GT, Campbell KL, Hoffman FG. Repeated evolution of chimeric fusion genes in the β-globin gene family of laurasiatherian mammals. Genome Biol Evol 6: 1219–1233, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gladwin MT, Kim-Shapiro DB. The functional nitrite reductase activity of the heme-globins. Blood 112: 2636–2647, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodman M, Czelusniak J, Koop BF, Tagle DA, Slightom JL. Globins: a case-study in molecular phylogeny. Cold Spring Harb Sym 52: 875–890, 1987. [DOI] [PubMed] [Google Scholar]
- 33.Grispo MT, Natarajan C, Projecto-Garcia J, Moriyama H, Weber RE, Storz JF. Gene duplication and the evolution of hemoglobin isoform differentiation in birds. J Biol Chem 287: 12, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hardison RC. Evolution of hemoglobin and its genes. Cold Spring Harb Perspect Med 2: a011627, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helbo S, Weber RE, Fago A. Expression patterns and adaptive functional diversity of vertebrate myoglobins. BBA Proteins Proteom 1834: 1832–1839, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Hiebl I, Weber RE, Schneeganss D, Kosters J, Braunitzer G. High-altitude respiration of birds - structural adaptations in the major and minor hemoglobin components of adult Ruppells griffon (Gryps rueppellii): a new molecular pattern for hypoxia tolerance. Biol Chem Hoppe Seyler 369: 217–232, 1988. [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann FG, Opazo JC, Hoogewijs D, Hankeln T, Ebner B, Vinogradov SN, Bailly X, Storz JF. Evolution of the globin gene family in deuterostomes: lineage-specific patterns of diversification and attrition. Mol Biol Evol 29: 1735–1745, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann FG, Opazo JC, Storz JF. Differential loss and retention of cytoglobin, myoglobin, and globin-E during the radiation of vertebrates. Genome Biol Evol 3: 588–600, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann FG, Opazo JC, Storz JF. Gene cooption and convergent evolution of oxygen transport hemoglobins in jawed and jawless vertebrates. Proc Natl Acad Sci USA 107: 14274–14279, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hoffmann FG, Opazo JC, Storz JF. New genes originated via multiple recombinational pathways in the β-globin gene family of rodents. Mol Biol Evol 25: 2589–2600, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann FG, Opazo JC, Storz JF. Rapid rates of lineage-specific gene duplication and deletion in the α-globin gene family. Mol Biol Evol 25: 591–602, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Hoffmann FG, Opazo JC, Storz JF. Whole-genome duplications spurred the functional diversification of the globin gene superfamily in vertebrates. Mol Biol Evol 29: 303–312, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoffmann FG, Storz JF. The αD-globin gene originated via duplication of an embryonic α-like globin gene in the ancestor of tetrapod vertebrates. Mol Biol Evol 24: 1982–1990, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Hoffmann FG, Storz JF, Gorr TA, Opazo JC. Lineage-specific patterns of functional diversification in the α- and β-globin gene families of tetrapod vertebrates. Mol Biol Evol 27: 1126–1138, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ingermann RL. Vertebrate hemoglobins In: Handbook of Physiology. Comparative Physiology. Bethesda, MD: Am. Physiol. Soc, 1997, sect. 13, vol. I, chapt. 6, p. 357–408. [Google Scholar]
- 46.Janecka JE, Nielsen SSE, Andersen SD, Hoffmann FG, Weber RE, Anderson T, Storz JF, Fago A. Genetically based low oxygen affinities of felid hemoglobins: lack of biochemical adaptation to high-altitude hypoxia in the snow leopard. J Exp Biol 218: 2402–2409, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen FB. The dual roles of red blood cells in tissue oxygen delivery: oxygen carriers and regulators of local blood flow. J Exp Biol 212: 3387–3393, 2009. [DOI] [PubMed] [Google Scholar]
- 48.Jensen FB, Fago A, Weber RE. Hemoglobin structure and function. In: Fish Physiology: Fish Respiration, edited by Perry SF, Tufts BL. San Diego, CA: Academic, 1998, p. 1–40. [Google Scholar]
- 49.Johnson RM, Buck S, Chiu CH, Gage DA, Shen TL, Hendrickx AG, Gumucio DL, Goodman M. Humans and old world monkeys have similar patterns of fetal globin expression. J Exp Zool 288: 318–326, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Johnson RM, Buck S, Chiu CH, Schneider H, Sampaio I, Gage DA, Shen TL, Schneider MPC, Muniz JA, Gumucio DL, Goodman M. Fetal globin expression in new world monkeys. J Biol Chem 271: 14684–14691, 1996. [DOI] [PubMed] [Google Scholar]
- 51.Kleinschmidt T, Rucknagel KP, Weber RE, Koop BF, Braunitzer G. Primary structure and functional properties of the hemoglobin from the free-tailed bat Tadarida brasiliensis (Chiroptera). Small effect of carbon dioxide on oxygen affinity. Biol Chem Hoppe Seyler 368: 681–690, 1987. [DOI] [PubMed] [Google Scholar]
- 52.Lynch M, Katju V. The altered evolutionary trajectories of gene duplicates. Trends Genet 20: 544–549, 2004. [DOI] [PubMed] [Google Scholar]
- 53.Natarajan C, Hoffman FG, Lanier HC, Wolf CJ, Cheviron ZA, Spangler ML, Weber RE, Fago A, Storz JF. Intraspecific polymorphism, interspecific divergence, and the origins of function-altering mutations in deer mouse hemoglobin. Mol Biol Evol 32: 978–997, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Natarajan C, Projecto-Garcia J, Moriyama H, Weber RE, Munoz-Fuentes V, Green AJ, Kopuchian C, Tubaro PL, Alza L, Bulgarella M, Smith MM, Wilson RE, Fago A, McCracken KG, Storz JF. Convergent evolution of hemoglobin function in high-altitude Andean waterfowl involves limited parallelism at the molecular sequence level. PLoS Genet 11: e1005681, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nikinmaa M. Haemoglobin function in vertebrates: evolutionary changes in cellular regulation in hypoxia. Respir Physiol 128: 317–329, 2001. [DOI] [PubMed] [Google Scholar]
- 56.Opazo JC, Butts GT, Nery MF, Storz JF, Hoffmann FG. Whole-genome duplication and the functional diversification of teleost fish hemoglobins. Mol Biol Evol 30: 140–153, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Opazo JC, Hoffman FG, Natarajan C, Witt CC, Berenbrink M, Storz JF. Gene turnover in the avian globin gene family and evolutionary changes in hemoglobin isoform expression. Mol Biol Evol 32: 871–887, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Opazo JC, Hoffmann FG, Storz JF. Differential loss of embryonic globin genes during the radiation of placental mammals. Proc Natl Acad Sci USA 105: 12950–12955, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Opazo JC, Hoffmann FG, Storz JF. Genomic evidence for independent origins of β-like globin genes in monotremes and therian mammals. Proc Natl Acad Sci USA 105: 1590–1595, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Opazo JC, Lee AP, Hoffmann FG, Toloza-Villalobos J, Burmester T, Venkatesh B, Storz JF. Ancient duplications and expression divergence in the globin gene superfamily of vertebrates: insights from the elephant shark genome and transcriptome. Mol Biol Evol 32: 1684–1694, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Opazo JC, Sloan AM, Campbell KL, Storz JF. Origin and ascendancy of a chimeric fusion gene: the β/δ-globin gene of paenungulate mammals. Mol Biol Evol 26: 1469–1478, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pelster B, Randall DJ. The physiology of the Root effect. In Fish Physiology: Fish Respiration, edited by Perry SF, Tufts BL. New York: Academic, 1998, p. 113–140. [Google Scholar]
- 63.Pelster B, Weber RE. The physiology of the Root effect. Adv Comp Environ Physiol 8: 51–77, 1991. [Google Scholar]
- 64.Perutz MF, Fermi G, Luisi B, Shaanan B, Liddington RC. Stereochemistry of cooperative mechanisms in hemoglobin. Cold Spring Harb Sym 52: 555–565, 1987. [DOI] [PubMed] [Google Scholar]
- 65.Projecto-Garcia J, Natarajan C, Moriyama H, Weber RE, Fago A, Cheviron ZA, Dudley R, McGuire JA, Witt CC, Storz JF. Repeated elevational transitions in hemoglobin function during the evolution of Andean hummingbirds. Proc Natl Acad Sci USA 110: 20669–20674, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qiu Y, Maillett DH, Knapp J, Olson JS, Riggs AF. Lamprey hemoglobin: structural basis of the Bohr effect. J Biol Chem 275: 13517–13528, 2000. [DOI] [PubMed] [Google Scholar]
- 67.Rummer JL, Brauner CJ. Plasma-accessible carbonic anhydrase at the tissue of a teleost fish may greatly enhance oxygen delivery: in vitro evidence in a rainbow trout, Oncorynchus mykiss. J Exp Biol 214: 2319–2328, 2011. [DOI] [PubMed] [Google Scholar]
- 68.Rummer JL, Brauner CJ. Root effect haemoglobins in fish may greatly enhance general oxygen delivery relative to other vertebrates. PLos One 10: e0139477, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rummer JL, McKenzie DJ, Innocenti A, Supuran CT, Brauner CJ. Root effect hemoglobin may have evolved to enhance general tissue oxygen delivery. Science 340: 1327–1329, 2013. [DOI] [PubMed] [Google Scholar]
- 70.Runck AM, Moriyama H, Storz JF. Evolution of duplicated β-globin genes and the structural basis of hemoglobin isoform differentiation in Mus. Mol Biol Evol 26: 2521–2532, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Runck AM, Weber RE, Fago A, Storz JF. Evolutionary and functional properties of a two-locus β-globin polymorphism in Indian house mice. Genetics 184: 1121–1131, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rutjes HA, Nieveen MC, Weber RE, Witte F, van den Thillart GEEJM. Multiple strategies of Lake Victoria cichlids to cope with lifelong hypoxia include hemoglobin switching. Am J Physiol Regul Integr Comp Physiol 293: R1376–R1383, 2007. [DOI] [PubMed] [Google Scholar]
- 73.Schechter AN. Hemoglobin research and the origins of molecular medicine. Blood 112: 3927–3938, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schimenti JC, Duncan CH. Structure and organization of the bovine β-globin genes. Mol Biol Evol 2: 514–525, 1985. [DOI] [PubMed] [Google Scholar]
- 75.Schwarze K, Campbell KL, Hankeln T, Storz JF, Hoffman FG, Burmester T. The globin gene repertoire of lampreys: convergent evolution of hemoglobin and myoglobin in jawed and jawless vertebrates. Mol Biol Evol 31: 2708–2721, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sidell BD, O'Brien KM. When bad things happen to good fish: the loss of hemoglobin and myoglobin expression in Antarctic icefishes. J Exp Biol 209: 1791–1802, 2006. [DOI] [PubMed] [Google Scholar]
- 77.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol 67: 99–145, 2005. [DOI] [PubMed] [Google Scholar]
- 78.Storz JF, Hoffmann FG, Opazo JC, Sanger TJ, Moriyama H. Developmental regulation of hemoglobin synthesis in the green anole lizard Anolis carolinensis. J Exp Biol 214: 575–581, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Storz JF, Natarajan C, Moriyama H, Hoffmann FG, Wang T, Fago A, Malte H, Overgaard J, Weber RE. Oxygenation properties and isoform diversity of snake hemoglobins. Am J Physiol Regul Integr Comp Physiol 309: R1178–R1191, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Storz JF, Opazo JC, Hoffmann FG. Gene duplication, genome duplication, and the functional diversification of vertebrate globins. Mol Phylogenet Evol 66: 469–478, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Storz JF, Opazo JC, Hoffmann FG. Phylogenetic diversification of the globin gene superfamily in chordates. IUBMB Life 63: 313–322, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Storz JF, Runck AM, Moriyama H, Weber RE, Fago A. Genetic differences in hemoglobin function between highland and lowland deer mice. J Exp Biol 213: 2565–2574, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Storz JF, Weber RE, Fago A. Oxygenation properties and oxidation rates of mouse hemoglobins that differ in reactive cysteine content. Comp Biochem Physiol A Mol Integr Physiol 161: 265–270, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tamburrini M, Verde C, Olianas A, Giardina B, Corda M, Sanna MT, Fais A, Deiana AM, di Prisco G, Pellegrini M. The hemoglobin system of the brown moray Gymnothorax unicolor: structure/function relationships. Eur J Biochem 268: 4104–4111, 2001. [DOI] [PubMed] [Google Scholar]
- 85.Tomita S. Modulation of the oxygen equilibria of human-fetal and adult hemoglobins by 2,3-diphosphoglyceric acid. J Biol Chem 256: 9495–9500, 1981. [PubMed] [Google Scholar]
- 86.Townes TM, Fitzgerald MC, Lingrel JB. Triplication of a four-gene set during evolution of the goat β-globin locus produced three genes now expressed differentially during development. Proc Natl Acad Sci USA 81: 6589–6593, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Val AL. Organic phosphates in the red blood cells of fish. Comp Biochem Physiol A Mol Integr Physiol 125: 417–435, 2000. [DOI] [PubMed] [Google Scholar]
- 88.Weber RE. Adaptations for oxygen transport: Lessons from fish hemoglobins. In: Hemoglobin Function in Vertebrates Molecular Adaptation to Extreme and Temperate Environments, edited by Di P risco G, Giardina B, Weber RE. Berlin: Springer-Verlag, 2000, p. 23–37. [Google Scholar]
- 89.Weber RE. Functional significance and structural basis of multiple hemoglobins with special reference to ectothermic vertebrates. In: Animal Nutrition and Transport Processes, edited by Truchot J-P, Lahlou B. Basel, Switzerland: S. Karger, 1990, p. 58–75. [Google Scholar]
- 90.Weber RE. Hemoglobin-based O2 transfer in viviparous animals. Isr J Zool 40: 541–550, 1994. [Google Scholar]
- 91.Weber RE. Hemoglobin adaptations in Amazonian and temperate fish with special reference to hypoxia, allosteric effectors and functional heterogeneity. In: Physiology and Biochemistry of the fishes of the Amazon, edited by Val AL, Almeida-Val VMF, Randall DJ. Manaus, Brazil: INPA, 1996, p. 75–90. [Google Scholar]
- 92.Weber RE. Hemoglobin adaptations to hypoxia and altitude - the phylogenetic perspective. In: Hypoxia and the Brain, edited by Sutton JR, Houston CS, Coates G. Burlington, VT: Queen City Printers, 1995, p. 31–44. [Google Scholar]
- 93.Weber RE, Fago A. Functional adaptation and its molecular basis in vertebrate hemoglobins, neuroglobins and cytoglobins. Respir Physiol Neurobiol 144: 141–159, 2004. [DOI] [PubMed] [Google Scholar]
- 94.Weber RE, Fago A, Malte H, Storz JF, Gorr TA. Lack of conventional oxygen-linked proton and anion binding sites does not impair allosteric regulation of oxygen binding in dwarf caiman hemoglobin. Am J Physiol Regul Integr Comp Physiol 305: R300–R312, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weber RE, Hiebl I, Braunitzer G. High-altitude and hemoglobin function in the vultures Gyps rueppellii and Aegypius monachus. Biol Chem Hoppe Seyler 369: 233–240, 1988. [PubMed] [Google Scholar]
- 96.Weber RE, Jensen FB. Functional adaptations in hemoglobins from ectothermic vertebrates. Annu Rev Physiol 50: 161–179, 1988. [DOI] [PubMed] [Google Scholar]
- 97.Weber RE, Kleinschmidt T, Braunitzer G. Embryonic pig hemoglobins Gower-I (ζ2ε2), Gower-II (α2ε2), Heide-I(ζ2θ2) and Heide-II(α2θ2): oxygen-binding functions related to structure and embryonic oxygen-supply. Respir Physiol 69: 347–357, 1987. [DOI] [PubMed] [Google Scholar]
- 98.Weber RE, Lalthantluanga R, Braunitzer G. Functional characterization of fetal and adult yak hemoglobins: an oxygen binding cascade and its molecular basis. Arch Biochem Biophys 263: 199–203, 1988. [DOI] [PubMed] [Google Scholar]
- 99.Weber RE, Lykkeboe G, Johansen K. Physiological properties of eel hemoglobin: hypoxic acclimation, phosphate effects and multiplicity. J Exp Biol 64: 75–88, 1976. [DOI] [PubMed] [Google Scholar]
- 100.Weber RE, White FN. Chloride-dependent organic phosphate sensitivity of the oxygenation reaction in crocodilian hemoglobins. J Exp Biol 192: 1–11, 1994. [DOI] [PubMed] [Google Scholar]
- 101.Weber RE, White FN. Oxygen binding in alligator blood related to temperature, diving, and alkaline tide. Am J Physiol Regul Integr Comp Physiol 251: R901–R908, 1986. [DOI] [PubMed] [Google Scholar]
- 102.Weber RE, Wood SC, Lomholt JP. Temperature acclimation and oxygen-binding properties of blood and multiple hemoglobins of rainbow trout. J Exp Biol 65: 333–345, 1976. [DOI] [PubMed] [Google Scholar]
- 103.Wells RMG. Blood-gas transport and hemoglobin function: Adaptations for functional and environmental hypoxia. In: Fish Physiology: Hypoxia, edited by Richards JG, Farrell AP, Brauner CJ. Amsterdam: Elsevier, 2009, p. 255–299. [Google Scholar]
- 104.Wells RMG. Hemoglobin-oxygen affinity in developing embryonic erythroid cells of the mouse. J Comp Physiol A 129: 333–338, 1979. [Google Scholar]
- 105.Wheeler D, Hope RM, Cooper SJB, Gooley AA, Holland RAB. Linkage of the θ-like ω-globin gene to α-like globin genes in an Australian marsupial supports the chromosome duplication model for separation of globin gene clusters. J Mol Evol 58: 642–652, 2004. [DOI] [PubMed] [Google Scholar]
- 106.Wood WG. Hemoglobin synthesis during human fetal development. Br Med Bull 32: 282–287, 1976. [DOI] [PubMed] [Google Scholar]
- 107.Zhang JZ. Evolution by gene duplication: an update. Trends Ecol Evol 18: 292–298, 2003. [Google Scholar]
- 108.Zhang R, Hess DT, Qian Z, Hausladen A, Fonseca F, Chaube R, Reynolds JD, Stamler JS. Hemoglobin βCys93 is essential for cardiovascular function and integrated response to hypoxia. Proc Natl Acad Sci USA 112: 6425–6430, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]


