Abstract
We developed a rodent crista slice to investigate regional variations in electrophysiological properties of vestibular afferent terminals. Thin transverse slices of the gerbil crista ampullaris were made and electrical properties of calyx terminals in central zones (CZ) and peripheral zones (PZ) compared with whole cell patch clamp. Spontaneous action potential firing was observed in 25% of current-clamp recordings and was either regular or irregular in both zones. Firing was abolished when extracellular choline replaced Na+ but persisted when hair cell mechanotransduction channels or calyx AMPA receptors were blocked. This suggests that ion channels intrinsic to the calyx can generate spontaneous firing. In response to depolarizing voltage steps, outward K+ currents were observed at potentials above −60 mV. K+ currents in PZ calyces showed significantly more inactivation than currents in CZ calyces. Underlying K+ channel populations contributing to these differences were investigated. The KCNQ channel blocker XE991 dihydrochloride blocked a slowly activating, sustained outward current in both PZ and CZ calyces, indicating the presence of KCNQ channels. Mean reduction was greatest in PZ calyces. XE991 also reduced action potential firing frequency in CZ and PZ calyces and broadened mean action potential width. The K+ channel blocker 4-aminopyridine (10–50 μM) blocked rapidly activating, moderately inactivating currents that were more prevalent in PZ calyces. α-Dendrotoxin, a selective blocker of KV1 channels, reduced outward currents in CZ calyces but not in PZ calyces. Regional variations in K+ conductances may contribute to different firing responses in calyx afferents.
Keywords: ganglion, hair cell, KCNQ, KV1, semicircular canal
the vestibular system senses acceleration and gives rise to the sense of balance. Each labyrinth contains a utricle, a saccule, and three semicircular canals. Together these end organs signal information to the central nervous system. Two types of mechanically sensitive sensory hair cell are present in mammalian vestibular neuroepithelia. Type I vestibular hair cells are almost completely engulfed by an afferent calyx nerve ending, whereas type II hair cells make synapses with smaller afferent boutons. Calyx-bearing afferents form two classes: pure calyx afferents that terminate on type I hair cells only and dimorphic afferents that branch to form both calyx and bouton endings. Type I hair cells and their calyx terminals are unique to amniote species (mammals, birds, and reptiles) and constitute about half of the total number of hair cells in the rodent crista (Desai et al. 2005; Lysakowski and Goldberg 1997). Type I and type II vestibular hair cells have different populations of K+ channels with different kinetic properties (reviewed in Eatock and Songer 2011). Sharp electrode recordings indicate that afferent fibers contacting vestibular hair cells fire spontaneously at rates ranging from 40 to 100 spikes/s and stimulus intensity drives the firing frequency (Goldberg 2000). Synaptic transmission at the hair cell synapse is mediated by glutamate, and highly specialized ribbon synapses signal graded stimulus intensity in a linear and inexhaustible fashion (Dulon et al. 2009; Fuchs 2005; Nouvian et al. 2006).
Afferent characteristics differ between central zones (CZ) and peripheral zones (PZ) of the crista and corresponding striolar (central) and extrastriolar (peripheral) zones of the utricle and saccule. Centrally located afferents typically have irregular firing and phasic response dynamics, whereas peripheral afferents have more regular firing patterns and tonic response properties (Eatock and Songer 2011; Goldberg 2000).
Recent immunohistochemical data show that calyx terminals in rat are richly endowed with several different types of ion channels, including voltage-gated K+ (KV) and Na+ (NaV) channels (Lysakowski et al. 2011). Intriguing differences in channel distribution exist across vestibular end organs and within individual calyx terminals. For example, immunostaining suggests that KCNQ4 K+ channels predominate in the central zone of the crista and utricle and appear to be highly expressed on the inner face of the terminals, facing the type I hair cell (Lysakowski et al. 2011; Spitzmaul et al. 2013). Labeling for KV1.1 and KV1.2 channels was also reported on the inner face of the calyx and at juxtaparaheminodal locations, near the first internode of the vestibular afferent neuron (Lysakowski et al. 2011). K+ channels are regulators of neuronal excitability, but the respective roles of different K+ channels in spike generation and firing in calyx terminals remain unclear. We previously probed afferent signal processing by identifying ionic conductances in isolated postsynaptic vestibular calyx terminals (Dhawan et al. 2010; Meredith et al. 2012; Rennie and Streeter 2006). In isolated calyx terminals, tetrodotoxin-sensitive Na+ currents and outward currents sensitive to the K+ channel blockers tetraethylammonium (TEA), 4-aminopyridine (4-AP; 0.25–1.25 mM), and apamin were identified (Dhawan et al. 2010; Meredith et al. 2011; Rennie and Streeter 2006). Macroscopic outward K+ currents varied considerably in their inactivation properties, but since calyx terminals were isolated from the entire crista, the original position of the cell within the crista epithelium was unknown (Dhawan et al. 2010). Hyperpolarization-activated cyclic nucleotide-sensitive (HCN) currents were also identified in calyces from gerbil crista (Meredith et al. 2012) and in mouse utricle (Horwitz et al. 2014). Although action potentials could be evoked with current steps, most isolated calyces lacked the ability to fire spontaneous action potentials. Here we describe a new rodent crista slice preparation for recording from calyces in situ. We investigated the expression of K+ currents in different zones and found a statistically significant difference in inactivation properties between CZ and PZ calyces. Underlying K+ channels were investigated by pharmacological approaches and their roles in firing assessed in different zones.
METHODS
Animal surgery.
Animal procedures followed protocols approved by the University of Colorado's Institutional Animal Care and Use Committee. Male and female Mongolian gerbils (Meriones unguiculatus) between the ages of postnatal day (P)17 and P33 were obtained from an in-house breeding colony. Gerbils were deeply anesthetized with an intraperitoneal injection of ketamine (70 mg/kg) and xylazine (3 mg/kg) mixed in normal saline. After decapitation, the brain was removed to expose the vestibular labyrinth and the semicircular canal sensory organs, the ampullae, were extracted from the bony canals.
Crista slices.
Ampullae were incubated for 10 min at 37°C in a high-Mg2+, low-Ca2+ solution containing (in mM) 135 NaCl, 5 KCl, 10 MgCl2, 0.02 CaCl2, 10 HEPES, and 3 d-glucose, pH 7.4 with NaOH and osmolality 300–305 mosmol/kgH2O. Ampullae were then transferred to Leibovitz's L-15 medium, pH 7.4–7.45, osmolality 300–305 mosmol/kgH2O (distilled water) with 0.5 mg/ml bovine serum albumin (BSA) for a minimum of 50 min at room temperature (21–24°C). Prior to slicing, each ampulla was trimmed and the membranous roof cut open before transfer to a 4% low-gelling-temperature agarose (2-hydroxyethylagarose, type VII; Sigma-Aldrich, St. Louis, MO) in Dulbecco's phosphate-buffered saline (in mM: 2.7 KCl, 1.5 KH2PO4, 137.9 NaCl, 8.1 Na2HPO4), still liquid at ∼40°C. A block containing the crista was cut and secured to the stage of a Vibratome 3000 EP (St. Louis, MO) specimen bath with Super Glue. The specimen bath was filled with L-15 (21–24°C), and transverse crista sections were cut at 100–120 μm, the blade (Personna reorder no. 74-0002) passing through the stroma first. Six slices were obtained per crista and stored in L-15-BSA before transfer to a recording dish filled with L-15. In some experiments, a HEPES solution (in mM: 140 NaCl, 5 KCl, 1.8 MgCl2, 1.3 CaCl2, 10 HEPES, and 3 glucose) replaced L-15 as the external solution. Slices were visualized with an Olympus upright microscope (BX50WI or BX51WI) equipped with water immersion objectives and differential interference contrast (DIC) optics. Images were captured with a digital camera (Rolera, QImaging) and QCapture Pro 6.0 software for subsequent image processing and determination of zonal location.
Cut peripheral end.
Ampullae were incubated in high-Mg2+, low-Ca2+ solution, transferred to L-15-BSA and then to L-15 as described above. Microscissors were used to separate the peripheral ends of the crista from the rest of the crista. The stroma is thinner at the peripheral end as it transitions into the planum semilunatum, and peripheral calyx terminals were therefore clearly visible under DIC optics. The cut end was anchored to the bottom of the recording dish with a fine stainless steel minuten pin (Fine Science Tools 26002-10). We recorded from PZ calyces only, which are situated between the widest point of the planum and the end of the planum (Desai et al. 2005).
Dissociated preparation.
Ampullae were incubated in high-Mg2+, low-Ca2+ solution for 20–32 min prior to transfer to L-15-BSA. Peripheral hair cells and their associated calyx endings were mechanically dissociated under a stereomicroscope by streaking a fine probe across the cut peripheral end of the crista as previously described for whole crista (Dhawan et al. 2010; Rennie and Streeter 2006). No exogenous enzymes were used, as these may alter ionic conductances of KCa, KV, and Ca2+ channels (Armstrong and Roberts 1998). Fifteen dissociated cells contributed to the data set examining K+ current inactivation in PZ calyces and one to the experiment testing the effect of 10–50 μM 4-AP on whole cell K+ currents. No differences were seen in the properties of the currents recorded from PZ calyx terminals in the slice, cut end, and dissociated preparations.
Electrophysiological recordings and solutions.
Micropipette glass capillary tubes (PG165T outer diameter 1.65 mm, inner diameter 1.28 mm; Warner Instrument, Hamden, CT), were pulled on a horizontal micropipette puller (Sutter Instruments, San Rafael, CA) and polished on a Narishige MF 830 microforge (Narishige International, East Meadow, NY). Patch pipettes were coated with silicone elastomer (Sylgard 184, Dow Corning, Midland, MI). The electrode solution contained (in mM) 115 KF, 10 KCl, 2 NaCl, 10 HEPES, 3 d-glucose, 2 MgCl2, and 10 EGTA, pH 7.4 with KOH (21–28 mM), osmolality 300–305 mosmol/kgH2O (adjusted with sucrose or mannitol). KF was included to maintain stable K+ currents (Kay 1992; Kostyuk et al. 1981). Conventional whole cell tight-seal voltage- and current-clamp experiments were carried out at room temperature (21–24°C). Signals were amplified with an Axopatch-1D or Axopatch 200B patch amplifier (Molecular Devices) connected to a PC through an AD converter (Digidata 1320A or 1440A, Molecular Devices, Sunnyvale, CA). Clampex software pCLAMP (v8 or 10) was used for data acquisition and analysis. Data were low-pass filtered online at 2 or 5 kHz and sampled between 5 and 20 kHz, depending on the protocol used. Open-tip patch pipette resistance ranged from 2 to 7 MΩ. A wide-tip cleaning pipette was used to siphon cells from the surface of the slice prior to advancing the patch pipette. Gigaohm seals were made onto the outer face of the calyx and electrode capacitance transients minimized before the whole cell configuration was achieved. Whole cell capacitance was measured off-line for a subset of cells by calculating the charge (Q) under the transient after attaining the whole cell configuration and deriving Cm from Q = Cm × ΔV, where ΔV is the size of the voltage step. Only calyces with zero-current membrane potential more hyperpolarized than −40 mV were included in the study. Liquid junction potential was calculated with the Clampex Junction Potential Calculator and corrected by −9 mV during data analysis. No leak subtraction was performed, and series resistance was not routinely compensated. Input resistance values were calculated by fitting a linear regression to a plot of voltage vs. current at potentials between −79 and −59 mV following a 40-ms test pulse to −129 mV.
4-AP, diclofenac sodium salt, and α-dendrotoxin (α-DTX) were obtained from Sigma-Aldrich. 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX) and 10,10-bis(pyridin-4-ylmethyl)anthracen-9-one (XE991) were obtained from Tocris Bioscience (Ellisville, MO). A 10 mM stock solution of 4-AP was made in L-15 and pH adjusted to 7.4 before storage at −20°C. Concentrated stock solutions of all other drugs were made in deionized water, stored at −20°C, and diluted in L-15 to a final concentration on the day of the experiment. Drugs were applied by local perfusion with a peristaltic pump (0.5–1.0 ml/min) in most experiments and by rapid replacement of bath solution with a transfer pipette in a few experiments. In most experiments, cells were perfused continuously with L-15 for a minimum of 5 min at a rate of 0.5–1 ml/min prior to drug application.
Data analysis.
Data were analyzed with pCLAMP 8 and 10 (Molecular Devices) and SigmaPlot 8 and 11 (Systat Software, San Jose, CA). Action potentials (magnitudes ≥40 mV, measured from peak of action potential to trough of afterhyperpolarization potential) were analyzed with Mini Analysis software (v 6.0.3, Synaptosoft, Decatur, GA). We defined cells as firing spontaneously if firing rate was ≥1 Hz over successive presentations of 3-s sweeps. Cells that fired action potentials at a rate ≥ 10 Hz were selected for further analysis of firing pattern, since very low resting rates will produce artificially high coefficient of variation (CV) values when not normalized to a mean interval (Lee et al. 2005). CV was used to classify cells as highly regular, highly irregular, or intermediate. CV was measured as the quotient of standard deviation (σ) of mean interspike interval (ISI) and mean ISI. Cells were classified as highly regular if CV was <0.1, highly irregular if CV was >0.5, and intermediate if CV was between 0.1 and 0.5 (Perachio and Correia 1983). Statistical significance was determined with Student's t-test (different populations), paired t-test (same population; before and after), Wilcoxon signed-rank test, or Mann-Whitney rank sum test (when data were not normally distributed). Values are presented as means ± SE or medians. For all tests of statistically significant change, a result was deemed significant when P < 0.05.
RESULTS
Crista slice preparation.
Transverse slices (100–120 μm) were made through the crista epithelium and secured in a recording chamber. The CZ (Fig. 1) was designated as the central third of the total area of the sensory epithelium, and the remaining slopes constituted the PZ (Desai et al. 2005; Lindeman 1969; Lysakowski and Goldberg 2008). Cup-shaped afferent calyx terminals contacted type I hair cells (Fig. 1), which had round bases, narrow necks, and wide cuticular plates. Type II hair cells were cylindrical and lacked calyx endings. Type I hair cells in the PZ are all innervated by dimorphic afferents. In the gerbil CZ approximately one-third of type I hair cells are contacted by pure calyx afferents and the rest by dimorphs (Desai et al. 2005). Slices cut from the midregion of the crista were preferentially selected to ensure that both CZ and PZ calyces were present. Alternatively, slices cut from the ends of the crista were selected to record from PZ calyces only.
Fig. 1.
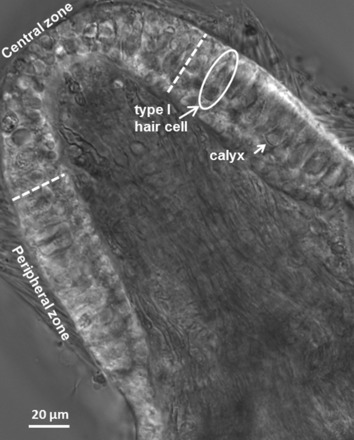
Differential interference contrast (DIC) image of a transverse slice cut from the midregion of a postnatal day (P)22 gerbil crista. White dashed lines show the boundary between the central zone (CZ) and peripheral zone (PZ). Long arrow indicates a typical type I hair cell and short arrow a cup-shaped calyx terminal. Patch electrodes targeted the unmyelinated basal surface of the outer face of calyx terminals. Hair cells are shorter and less densely packed in the CZ. Stereocilia extend from the cuticular plates of many hair cells, and myelinated axons can be seen coursing through the stroma.
Firing properties of calyx terminal.
Patch electrodes were placed at the base of calyx terminals to obtain seals, and whole cell recordings were made from CZ and PZ cells. In some recordings Alexa dye was included in the electrode solution to diffuse into and visualize afferent terminals as described previously for isolated calyces (Dhawan et al. 2010). In gerbil cristae, the majority of calyx afferents contact a single type I hair cell, but some calyces contain up to four type I hair cells (Desai et al. 2005). Accordingly, most of the recordings reported here were from single calyx terminals. Eighteen recordings were from verified double or triple calyces. In current clamp we found that 29 of 114 (25%) calyx terminals in the semi-intact preparations showed sustained firing of action potentials around the zero-current potential, which averaged between −55 and −60 mV (see below). As reported previously, isolated calyx terminals rarely demonstrated spontaneous firing (Dhawan et al. 2010; Meredith et al. 2012). Sustained, spontaneous firing was more prevalent in CZ (11/26 calyces or 42%) than in PZ (18/88 or 20%) terminals. In those cells that did not show spontaneous firing, action potentials could be evoked by a hyperpolarizing pulse prior to a depolarization.
Firing was abolished when choline replaced Na+ in the external solution in two cells, indicating that Na+ channels are required for action potential generation (Fig. 2A). However, action potentials persisted in streptomycin (Fig. 2B) at a concentration (0.5 mM) that blocks hair cell apical mechanotransduction channels (Marcotti et al. 2005). The persistence of firing in the presence of streptomycin suggests that calyceal ion channels play a role in generating action potentials. NBQX, an AMPA receptor antagonist, abolished excitatory postsynaptic currents (EPSCs) when applied to one CZ and two PZ calyces (Fig. 3A) but did not prevent firing (Fig. 3B), confirming that spontaneous firing can occur independently of synaptic transmission. Firing decreased from a mean of 24 ± 7.0 Hz in the control condition to 21.4 ± 6.7 Hz in the presence of 20 μM NBQX (n = 3, difference not significant).
Fig. 2.
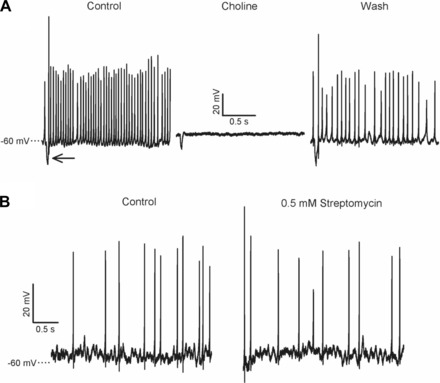
Spontaneous firing is Na+ dependent and not blocked by streptomycin. A: spontaneous action potentials recorded in current clamp in a CZ calyx terminal. A current injection (−100 pA, 20-ms duration) at the start of each recording elicited a hyperpolarizing voltage deflection (arrow) that served to monitor stability of the recording over time. Action potentials were abolished when choline chloride replaced NaCl in the external HEPES solution bathing the slice (center) and returned after a wash (right). B: action potentials recorded from a PZ calyx terminal persisted after extracellular application of 0.5 mM streptomycin sulfate.
Fig. 3.
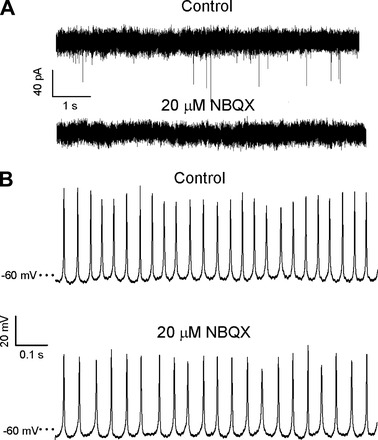
Action potentials persisted in the presence of NBQX. A: spontaneous excitatory postsynaptic currents (EPSCs) in a PZ calyx (top) were eliminated in the presence of 20 μM NBQX (bottom), which blocks postsynaptic AMPA receptors. Holding potential was −79 mV. B: NBQX did not abolish firing (different PZ calyx than in A).
Vestibular afferents have previously been classified according to their firing regularity (Goldberg 2000). We analyzed firing pattern(s) in 9 CZ and 11 PZ calyces selected from the 29 spontaneously firing cells and only included calyces that consistently produced 10 or more spikes/s in current clamp. Examples of regular and irregular firing patterns in two individual CZ calyx terminals are shown in Fig. 4, A and C, respectively. In Fig. 4A, the time between individual action potentials, the ISI, was more uniform, resulting in tighter ISI clustering (Fig. 4B). Firing pattern was more irregular in the second calyx (Fig. 4C), giving a greater ISI spread (Fig. 4D). The CV was calculated as CV = σ/(mean ISI) where σ is the standard deviation of the mean ISI (Fig. 4E). Although three PZ calyces had the most regular firing pattern and the smallest CV, calyces from both regions showed a similar range of firing patterns (Fig. 4E). Four calyx terminals (3 PZ and 1 CZ) had highly regular firing (CV < 0.1), 4 (3 PZ and 1 CZ) had a highly irregular firing pattern (CV > 0.5), and 12 (5 PZ and 7 CZ) were intermediate (CV from 0.1 to 0.5). Mean CV in PZ calyces was similar to the mean CV in CZ (Table 1).
Fig. 4.
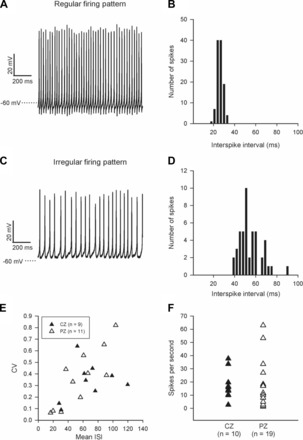
Firing properties of calyx terminals. A: firing pattern was regular in a CZ calyx terminal, resulting in tighter clustering of interspike intervals (ISIs) (B) and a coefficient of variation (CV) of 0.14. ISIs were more variable in a different CZ calyx terminal where firing pattern was irregular (C and D) and CV was 0.4. E: although 3 PZ calyces showed the most regular firing pattern and smallest CVs (open triangles, bottom left), both CZ and PZ calyx terminals showed a range of firing patterns. The frequency of action potential firing ranged from 1.3 to 63 Hz (F).
Table 1.
Zonal variations in calyx properties
| PZ | CZ | PPZ, CZ | |
|---|---|---|---|
| Mean CV | 0.36 ± 0.07 (n = 11) | 0.33 ± 0.05 (n = 9) | ns |
| Mean firing rate, spikes/s | 16.6 ± 4.0 (n = 19) | 17.4 ± 3.4 (n = 10) | ns |
| Median outward K+ current inactivation, % | 9.4 (n = 66) | 4.2 (n = 33) | <0.001 (Mann-Whitney) |
| Median peak K+ current amplitude, pA | 1,943 (n = 66) | 2,788 (n = 33) | <0.05 (Mann-Whitney) |
| Median peak Na+ current amplitude, pA | −1,303 (n = 66) | −1,618 (n = 33) | <0.05 (Mann-Whitney) |
| Whole cell capacitance, pF | 6.2 ± 0.7 (n = 13) | 8.1 ± 2.3 (n = 6) | ns |
| % Decrease in 20 μM XE991 | 31.4 ± 3.6 (n = 10) | 17.6 ± 3.3 (n = 8) | <0.05 (t-test) |
| % Increase in 100 μM diclofenac | 14.3 ± 5.8 (n = 6) | 23.3 ± 14 (n = 4) | ns |
| % Decrease in 10–50 μM 4-AP | 35 ± 4.2 (n = 7) | 21 ± 3.8 (n = 6) | <0.05 (t-test) |
| % Decrease in 100–250 nM α-DTX | 3.5 ± 0.1 (n = 6) | 12.8 ± 0.3 (n = 6) | <0.05 (t-test) |
Values are means ± SE. PZ, peripheral zone; CZ, central zone; CV, coefficient of variation; 4-AP, 4-aminopyridine; α-DTX, α-dendrotoxin; ns, not significant.
Firing frequency ranged from 1.3 to 63 Hz (Fig. 4F), with a mean of 17.4 ± 3.4 Hz (n = 10) in CZ and 16.6 ± 4.0 Hz (n = 19) in PZ terminals. When both zones were considered, firing rate averaged 16.8 ± 2.9 spikes/s (n = 29).
Inactivation of voltage-dependent outward K+ currents varies with zone.
We previously reported that outward K+ currents in isolated calyces varied considerably in their inactivation properties, but the original location of each calyx within the crista was unknown (Dhawan et al. 2010). To investigate zonal differences in outward K+ currents, whole cell voltage-clamp recordings were made from PZ and CZ calyx terminals in the slice (n = 65), cut end (n = 19), and dissociated PZ (n = 15) preparations. PZ recordings from the semi-intact and dissociated preparations were similar, and data were pooled. Zero-current potential was similar in PZ and CZ calyx terminals [−58.4 ± 2.3 mV in CZ (n = 10), −55.6 ± 1.1 mV in PZ (n = 38); difference not significant]. In response to a standard voltage protocol, which included a hyperpolarizing step to remove channel inactivation, depolarizing voltage steps activated rapid inward Na+ currents and sustained outward K+ currents (Fig. 5A). Longer hyperpolarizing voltage steps elicited slowly activating inward currents in ∼70% of calyx terminals tested (17/24 CZ calyces and 26/40 PZ calyces; data not shown) previously characterized as HCN currents in isolated calyces (Meredith et al. 2012). Outward currents developed at potentials positive to −60 mV (Fig. 5, A–C). Peak outward current ranged from 231 pA to 5,561 pA in PZ calyces (mean 2,233 ± 178 pA, n = 66) and from 1,090 pA to 8,240 pA in CZ calyces (mean 2,924 ± 231 pA, n = 33). Representative currents in a PZ and a CZ calyx from the crista slice indicate greater time-dependent inactivation of the outward current in the PZ calyx (Fig. 5A). Currents at peak and at the end of the 40-ms voltage step to +21 mV were measured to calculate percent inactivation (Fig. 5D). Median inactivation was significantly greater in PZ calyces (9.4%, n = 66) than in CZ calyces (4.2%, n = 33; P < 0.001, Mann-Whitney rank sum test; Table 1). The difference in inactivation suggests that there is zonal variation in the relative contribution of inactivating and sustained outward K+ currents. The median peak amplitude of the outward K+ current was significantly greater in CZ calyx terminals (2,788 pA; n = 33) compared with PZ terminals (1,943 pA; n = 66, P < 0.05, Mann-Whitney rank sum test). Median peak inward Na+ current amplitude was also significantly greater in CZ calyces (−1,618 pA, n = 33) compared with PZ calyces (−1,303 pA, n = 66, P < 0.05). In a subset of calyces whole cell capacitance was estimated at 8.1 ± 2.3 pF (n = 6) in CZ and 6.2 ± 0.7 pF in PZ (n = 13) cells, which may account for the larger mean current values in CZ calyces. This is also consistent with the greater number of complex calyces (i.e., calyces containing >1 type I hair cell) in crista CZ (Desai et al. 2005; Lysakowski and Goldberg 1997). Mean input resistance in randomly selected cells was 189 ± 31 MΩ in CZ calyces (n = 15), similar to the value of 177 ± 23 MΩ in PZ calyces (n = 14). However, when PZ terminals showing >10% inactivation were compared to CZ terminals, median input resistance in PZ was 277 MΩ (n = 14), significantly more than 166 MΩ in CZ terminals (n = 15; P < 0.05, Mann-Whitney rank sum test). Therefore currents active at zero-current potential may contribute less in more highly inactivating PZ terminals.
Fig. 5.
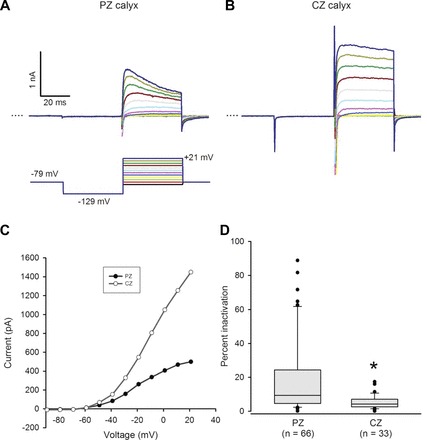
Inactivation of outward K+ currents varies with zone. A and B: representative outward currents in a PZ and a CZ calyx. Calyces were held at −79 mV, stepped to −129 mV, and then stepped from −89 to +21 mV in incrementing 10-mV steps of 40-ms duration (standard voltage protocol, shown below the PZ current traces). Dotted line indicates zero current (0 pA). Scale for B same as A. C: current amplitude measured at the end of each 40-ms voltage step is shown vs. voltage for the PZ and CZ calyces shown in A and B. Current activated above −60 mV. D: peak current and current at the end of the 40-ms voltage step to +21 mV (dark blue trace in A and B) were measured to calculate % inactivation [(Ipeak − Iend)/Ipeak × 100]. Box plots show % inactivation for PZ and CZ calyces. Median inactivation (line in box) was 9.4% in PZ calyx terminals (n = 66), significantly greater than the value of 4.2% in CZ calyx terminals (n = 33; P < 0.001, Mann-Whitney rank sum test).
Contribution of KCNQ channels to outward current.
We previously identified two voltage-dependent outward K+ currents in isolated calyx terminals, a slowly activating TEA-sensitive current that showed no inactivation during a 40-ms depolarizing test pulse and a rapidly activating and inactivating current blocked by 4-AP (Dhawan et al. 2010). The KCNQ channel blockers XE991 and linopirdine were also reported to reduce outward K+ current in isolated calyx terminals; however, the underlying current kinetics and regional differences in KCNQ subtypes were not investigated (Hurley et al. 2006; Rennie and Streeter 2006). We therefore used KCNQ channel modulators to study regional variations in KCNQ channels in calyces. XE991, which blocks KCNQ1–5, consistently reduced a portion of the outward current in calyces. Figure 6, A and B, show a reversible reduction of outward current in a central calyx in response to 20 μM XE991. The XE991-sensitive current was slow to activate and showed no inactivation during the 40-ms step. The current-voltage plot (Fig. 6B) shows the effect of XE991 on the same calyx at a range of voltages. Percent block by XE991 was significantly greater in PZ cells (31.4 ± 3.6%, n = 10) compared with CZ cells (17.6 ± 3.3%, n = 8, P < 0.05, t-test) (Fig. 6C).
Fig. 6.
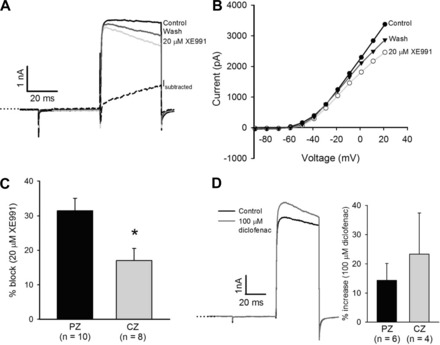
The KCNQ channel blocker XE991 decreases outward current. A: currents in response to a +21-mV voltage step for control current (black) and after extracellular application of 20 μM XE991 (light gray) in a CZ calyx. The XE991-sensitive current (Isubtracted) activated slowly and continued to increase throughout the 40-ms voltage pulse. B: current voltage (I-V) plot for cell in A, showing control currents, currents following application of 20 μM XE991, and recovery after XE991 was replaced with L-15 (wash). C: mean reduction in current measured at the end of a +21-mV voltage step was 31.4 ± 3.6% (n = 10) in PZ calyces, which was significantly greater than the value of 17.0 ± 3.5% seen in CZ calyces (n = 8; *P < 0.05, t-test). D: current in response to a +21-mV voltage step in a CZ calyx increased after application of 100 μM diclofenac (left; dark gray). Mean increase measured at the end of the voltage step was 14.3 ± 5.8% in PZ calyces (n = 6) and 23.3 ± 14 in CZ calyces (n = 4).
KCNQ channels have been localized to calyx terminals by immunocytochemistry (Hurley et al. 2006; Lysakowski et al. 2011; Rocha-Sanchez et al. 2007; Spitzmaul et al. 2013). KCNQ4 immunostaining was predominant in CZ calyces, whereas KCNQ5 staining, which was confined to dimorphic afferents (Lysakowski et al. 2011), prevailed in PZ calyces (Spitzmaul et al. 2013). We therefore used diclofenac, which activates KCNQ4 channels but inhibits KCNQ5 channels (Brueggemann et al. 2011), to investigate regional differences in expression of KCNQ channel subunits. Diclofenac (100 μM) increased outward current by a mean value of 14.3 ± 5.8% in PZ calyces (n = 6) and by 23.3 ± 14.0% in CZ calyces (n = 4; Fig. 6D), supporting a greater contribution by KCNQ4 channels in CZ calyces. Current decreased by 29.9% in one PZ calyx and by a mean value of 5.5% in three CZ calyces, suggesting a smaller contribution by KCNQ5 subunits in centrally located calyces.
To probe the role of KCNQ currents in shaping action potential firing, we recorded spontaneous action potentials in six calyx terminals, three CZ and three PZ, before and after application of 20 μM XE991. Firing frequency decreased markedly in the presence of XE991 in all cells, as shown for the example in Fig. 7A. Overall, XE991 decreased firing frequency in CZ and PZ terminals by 63% of the control mean (Fig. 7B). When KCNQ current was blocked with XE991, repolarization of the action potential was slowed (Fig. 7C). Spike half-width increased significantly from a median of 1.5 ms to 1.9 ms (n = 6, 3 CZ and 3 PZ; P < 0.05, Wilcoxon signed-rank test). KCNQ channels therefore contribute to outward K+ currents and enhance firing frequency in both CZ and PZ calyx terminals.
Fig. 7.
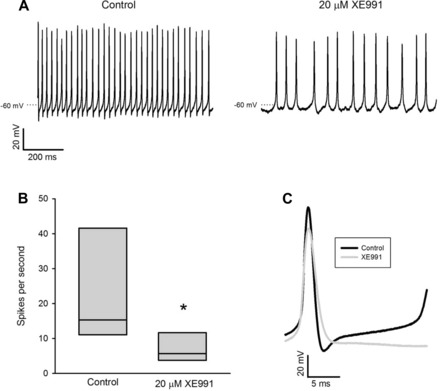
XE991 decreases firing frequency. A: action potentials in a CZ calyx terminal under control conditions and after application of 20 μM XE991. Firing decreased from 37.7 Hz to 16.7 Hz. B: median frequency decreased from 15.3 Hz to 5.6 Hz (n = 6, 3 CZ and 3 PZ; *P < 0.05, Wilcoxon signed-rank test). C: XE991 increased spike half-width from a mean of 1.3 ms to 1.6 ms, decreased the size of the afterhyperpolarization potential, and delayed the onset of the next action potential. Each trace is the average of 3 evoked spikes (same cell as in A).
Regional variation in 4-AP-sensitive K+ currents.
We investigated 4-AP-sensitive currents in CZ and PZ calyces using a range of 4-AP concentrations. Application of 4-AP reduced current in all cells tested, but the nature of the blocked current varied between cells. The effect of 10 μM 4-AP on a PZ calyx is shown in Fig. 8, A and B. The blocked current first activated above −50 mV and showed rapid activation and moderate inactivation (Fig. 8, A and B). At 10–50 μM, 4-AP blocked 35.0 ± 4.2% of outward current in PZ calyces at the end of a 40-ms pulse (n = 7), which was significantly greater than the block in CZ calyces (21.0 ± 3.8%, n = 6, P < 0.05, t-test) (Fig. 8C). In a subset of cells (4/7 PZ and 3/6 CZ calyces), 4-AP also reduced a rapidly activating, rapidly inactivating current at concentrations as low as 10 μM (not shown).
Fig. 8.
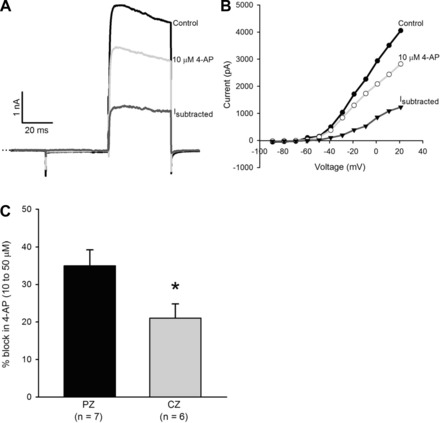
Sensitivity to 10–50 μM 4-aminopyridine (4-AP) varies with zone. A: currents in a PZ calyx in response to a +21-mV voltage step. Current was reduced by 29% in the presence of 10 μM 4-AP. The subtracted current (Isubtracted) activated rapidly and showed moderate inactivation. B: I-V plots show control currents, currents in the presence of 10 μM 4-AP, and the 4-AP-sensitive current (Isubtracted). Current amplitude was measured at the end of each 40-ms voltage pulse. C: 10–50 μM 4-AP reduced outward current by 35 ± 4.2% in PZ calyces (n = 7), which was significantly greater than the reduction of 21 ± 3.8% in CZ calyces (n = 6). *P < 0.05, t-test.
The effect of a higher concentration of 4-AP (0.25 mM) on a CZ calyx is shown in Fig. 9. In this cell 4-AP blocked a rapidly activating, rapidly inactivating current in addition to a slower current (Fig. 9A). Current measured at the end of the 40-ms step was reduced by 64%. Firing frequency and action potential amplitude were greatly reduced in the presence of 0.25 mM 4-AP (Fig. 9C). Action potential half-width increased significantly from 1.7 ms to 11.8 ms (mean of 3 evoked action potentials in control and after 0.25 mM 4-AP, P < 0.001, paired t-test, not shown).
Fig. 9.
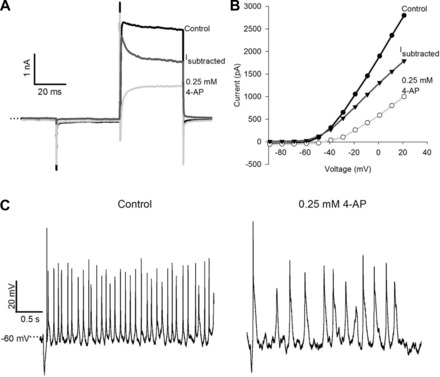
4-AP (0.25 mM) blocks a rapidly activating and inactivating current and reduces firing frequency. A: control current in response to a +21-mV voltage pulse and current in the presence of 0.25 mM 4-AP in a CZ calyx. The 4-AP-sensitive current (Isubtracted) showed a rapidly activating and inactivating component as well as a sustained component. B: I-V plot for the same cell as in A. Current amplitude was measured at the end of the 40-ms voltage pulse. C: action potentials under control conditions (left) and in the presence of 4-AP (right) (same cell as A). 4-AP increased action potential width and decreased firing frequency.
An α-DTX-sensitive current is present in CZ calyces.
The KV1 channel subunits KV1.1, 1.2, and 1.6 have been reported in rodent vestibular epithelia and ganglia (Iwasaki et al. 2012; Lysakowski et al. 2011). These channels mediate delayed rectifier currents that are blocked by relatively high concentrations of 4-AP (Coetzee et al. 1999). The snake venom peptide α-DTX is a selective blocker of KV1.1 and 1.2 channels at low nanomolar concentrations and has been shown to block a low-voltage-activated K+ current in vestibular ganglion cells (Chabbert et al. 2001; Iwasaki et al. 2008; Kalluri et al. 2010). In current clamp, evoked firing of action potentials in ganglion cells converted from phasic to tonic in response to α-DTX (Iwasaki et al. 2008; Kalluri et al. 2010). We applied α-DTX (100–250 nM) to calyx terminals in crista slices (Fig. 10). In CZ calyces, α-DTX reduced current amplitude by 12.8 ± 0.3% (n = 6). The blocked current was rapidly activating, did not inactivate (Fig. 10A), and activated at potentials above −60 mV (Fig. 10B). Of the six CZ calyx terminals tested, two fired spontaneously and two produced evoked action potentials in response to hyperpolarizing current pulses in most recordings. In the presence of 100 nM α-DTX, firing rate increased in both spontaneously firing calyces (from 1.3 Hz to 5 Hz and from 20.7 Hz to 22 Hz). In the two nonspontaneously firing calyces, evoked action potentials were often followed by a second or third action potential in the presence of 100 nM α-DTX (Fig. 10C). α-DTX blocked a much greater component of outward current in CZ calyces compared with PZ (Table 1), suggesting that α-DTX-sensitive KV1-containing channels are functionally relevant in CZ calyces only.
Fig. 10.
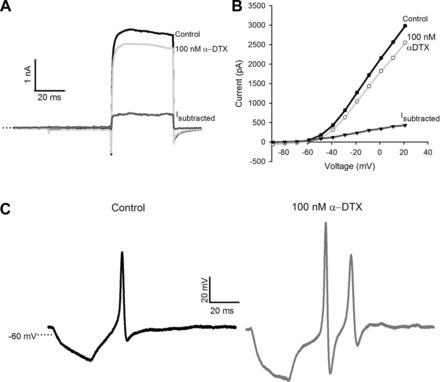
α-Dendrotoxin (α-DTX) blocks a noninactivating current in CZ calyces. A: 100 nM α-DTX blocked a component of the outward current in a CZ calyx. The blocked current (Isubtracted) activated rapidly and showed no inactivation. B: I-V plots show current amplitude measured at the end of each 40-ms pulse vs. voltage (same cell as A). The α-DTX-sensitive current activated at potentials more depolarized than −60 mV. C: a single action potential was evoked after injection of a brief hyperpolarizing current in a CZ calyx (left). α-DTX (100 nM) increased the amplitude of the evoked action potential, increased the afterhyperpolarization potential, and resulted in a second spike (right).
DISCUSSION
Crista slice.
We describe a new in vitro crista slice preparation that allows regional recordings from vestibular nerve calyx terminals of the mature rodent crista and could also be used to study zonal differences in hair cell properties and synaptic responses. We recorded voltage-dependent outward K+ currents in centrally and peripherally located calyces and found significant differences in inactivation kinetics between zones. Peak outward K+ currents and inward Na+ currents were on average larger in CZ calyces, which also had larger capacitance values than PZ calyces. This is likely because of the prevalence of complex multicalyces in central areas and dimorphic fibers with single calyx terminals in peripheral regions (Eatock and Songer 2011). Macroscopic currents were similar to those described previously in calyx terminals isolated together with their type I hair cells (Dhawan et al. 2010; Meredith et al. 2011, 2012). However, unlike dissociated calyx terminals, 25% of calyces in the semi-intact preparations showed spontaneous and sustained firing of action potentials, which allowed us to examine the role of individual K+ conductances in shaping firing patterns. The axon initial segment is intact in the slice preparation but may be lacking in dissociated calyx terminals, and its presence may facilitate spike initiation.
We showed that spontaneous action potentials in calyx terminals are Na+ driven. Recent data indicate that calyx afferent heminodes are mostly located above the basement membrane, whereas dimorphic afferent heminodes are located below it, perhaps reflecting different sites for spike initiation (Lysakowski and Price 2014). Interesting differences in Na+ channel distribution within vestibular afferents have been reported. NaV1.5 immunostaining was described on the inner face of calyx terminals (Wooltorton et al. 2007), and NaV1.6 was detected at the heminode of dimorphic afferents but not in pure calyx afferents (Lysakowski et al. 2011). The contribution of calyceal Na+ channel subunits to spike initiation and repetitive firing in different vestibular afferent populations remains to be established.
Comparison to previous observations in semi-intact vestibular epithelia.
Others have recorded from calyx-bearing afferents in turtle (Bonsacquet et al. 2006; Holt et al. 2007) and, more recently, mouse (Contini et al. 2012; Lasker et al. 2008; Lee et al. 2005; Lim et al. 2011; Tung et al. 2013) crista. Sharp electrode recordings from ampullary nerve in 3- to 4-wk-old mice yielded firing rates averaging 12 spikes/s in vitro in a modified Ringer solution and 27 spikes/s in vivo (Lee et al. 2005). Loose patch-clamp recordings from multicalyx afferents in the central hemicrista of turtle revealed a mean firing rate of 16.5 spikes/s (Bonsacquet et al. 2006). These firing rates are similar to those found in our whole cell recordings in CZ and PZ of the gerbil crista slice, which ranged from 1.3 to 63 spikes/s and averaged ∼17 spikes/s.
Whole cell recordings from calyx-containing terminals in excised otolith organs have been reported for early postnatal rat saccule (Songer and Eatock 2013) and mouse utricle (Horwitz et al. 2014). No differences in macroscopic Na+ or K+ currents in central versus peripheral zones of the mouse utricle (P8–P12), were reported, although the defined central and peripheral zones did not correspond to conventional striola and extrastriola regions (Horwitz et al. 2014). Outward currents at depolarized potentials were sustained and did not inactivate (Horwitz et al. 2014; Songer and Eatock 2013). Rat saccular striolar afferents averaged 6.3 spikes/s and extrastriola afferents averaged 15.3 spikes/s at 25–29°C (Songer and Eatock 2013). In mouse utricle, dimorphic afferents averaged 5.5 spikes/s in central areas and 11.3 spikes/s in a peripheral zone at room temperature (Horwitz et al. 2014).
Extracellular recordings from rat semicircular canal afferents indicated that firing rates increased during the first few postnatal weeks, and responses were considered mature at ∼3 wk after birth (Curthoys 1979). This is consistent with the morphological maturation of calyx afferent terminals, which are rarely seen at P0 but begin to engulf type I hair cells during the first few postnatal days (Rüsch et al. 1998). However, semicircular canal afferents in 4- to 7-wk-old mice lacked a class of highly irregular afferents that was present in older mice (10–24 wk) (Lasker et al. 2008), suggesting that electrophysiological maturation of afferents may be prolonged. Rodent type I hair cells also show postnatal electrophysiological maturation (Géléoc et al. 2004; Li et al. 2010; Rüsch et al. 1998). We recorded from calyces in slices prepared from juvenile gerbils at P17–P33, but further studies could address age-related changes.
Seventy-five percent of calyces in our semi-intact cristae did not fire spontaneous action potentials around rest. Such silent units have also been reported in calyx recordings from turtle crista (Bonsacquet et al. 2006; Brichta and Goldberg 2000) and rodent utricle and saccule (Horwitz et al. 2014; Songer and Eatock 2013). The physiological salience of these silent neurons remains unclear, but as described below KV1 channels may serve to inhibit spontaneous firing. Previous results suggested that spontaneous firing in turtle calyx afferents required functional mechanotransduction channels, since background activity was abolished by mechanotransduction channel blockers (Bonsacquet et al. 2006). However, recent recordings from mouse utricle calyx terminals showed that spontaneous firing persisted after tip links were disrupted in 5 mM EGTA (Horwitz et al. 2014). We found that neither streptomycin, a mechanotransduction channel blocker, nor NBQX, an AMPA receptor antagonist, blocked spontaneous firing in mammalian calyx terminals. This suggests that interplay of ionic channel conductances within the calyx terminal can sustain action potential activity in the absence of synaptic transmission. Voltage-gated K+ channels are important regulators of neuronal excitability, and we examined the roles of different K+ channel populations in firing.
Afferent firing and regularity.
Vestibular afferents convey action potentials to the brain stem, but signal coding is not well understood. Afferents that innervate the central zones of the crista and striolar regions of otolith organs are known to have thicker axons, faster conduction velocities, and more phasic responses than afferents that innervate peripheral and extrastriola regions (Baird et al. 1988; Hullar et al. 2005; Lysakowski et al. 1995). In the adult chinchilla crista, the firing pattern was predominantly irregular in fibers terminating in the CZ and regular in fibers supplying the PZ (Baird et al. 1988). Calyx-only afferents had more irregular firing patterns than dimorphic afferents, although intermediate and irregular firing patterns were seen in dimorphic vestibular nerves supplying both zones (Baird et al. 1988). We observed a range of firing patterns in calyx terminal recordings. Irregular and regular firing was seen in both zones, although the most regularly firing terminals were located in the PZ. We did not routinely distinguish dimorphic afferents from pure calyx afferents, but based on previous morphological characterization all PZ calyces and approximately two-thirds of CZ calyces form dimorphic terminal endings onto type I and type II hair cells (Desai et al. 2005).
Zonal differences.
Type I hair cells outnumber type II hair cells in the CZ, and calyx-only afferents in CZ of the gerbil crista selectively express the calcium-binding protein calretinin (Desai et al. 2005; Leonard and Kevetter 2002). Projections of efferent neurons also vary in the gerbil crista: contralateral efferents innervate PZ, whereas ipsilateral efferents innervate both PZ and CZ (Purcell and Perachio 1997). Ribbon synapses are more numerous in CZ type I hair cells (Lysakowski and Goldberg 1997). Topographic differences in type II cell hair cell membrane currents have been reported in birds and turtles, with outward currents in peripheral regions of the crista and utricle showing a greater degree of inactivation than those in central locations (Brichta et al. 2002; Masetto et al. 2000; Masetto and Correia 1997; Weng and Correia 1999). A rapidly activating and inactivating “A-type” current prevailed in PZ hair cells, whereas an outwardly rectifying K+ current with slower activation and inactivation kinetics showed a greater expression in CZ type II hair cells. CZ hair cells showed evoked membrane potential oscillations in current clamp that were lower in frequency than PZ cells, suggesting presynaptic zonal differences in filtering properties (Weng and Correia 1999). Similarly, we found a greater inactivation of outward K+ current in PZ calyces. A rapidly activating and moderately inactivating 4-AP-sensitive current was more prominent in PZ calyces and may contribute to the greater inactivation of the macroscopic current in PZ calyces. 4-AP increased action potential width and reduced firing frequency. Although not directly assessed, the 4-AP-sensitive inactivating current could contribute to tonic firing in PZ afferents. 4-AP blocks KV1, 3, and 4 channels (Coetzee et al. 1999), and the precise identity of the channel underlying the rapidly activating and moderately inactivating current remains to be determined.
KV1 channels.
KV1 channels are a diverse group of voltage-gated K+ channels named KV1.1 to KV1.7. KV1.1 and KV1.2 channel subunits were recently localized to the inner face and juxtaparaheminode of rat calyx terminals (Lysakowski et al. 2011). KV1.1, KV 1.2, and KV1.6 were also detected in rat vestibular ganglion cells, and KV1.6 was downregulated after P7, suggesting that changes in this subunit may be associated with postnatal developmental changes in firing patterns (Iwasaki et al. 2012). KV1 channels are important determinants of firing in spiral ganglion neurons (SGNs), which carry auditory information from inner hair cells to the brain. Recent data suggest that KV1 and HCN channels contribute to heterogeneity in action potential threshold and membrane potential in SGN (Liu et al. 2014; Wang et al. 2013).
KV1.1, KV 1.2, and KV1.6 are blocked by nanomolar concentrations of α-DTX. The presence of α-DTX-sensitive KV1 channels active above −60 mV in CZ but not PZ calyces may contribute to the increased irregularity of firing and phasic responses in CZ calyx afferents. A low-voltage-activated noninactivating K+ current (ILV), which activated above −50 mV and was sensitive to 4-AP and α-DTX, was described in phasic cells of young rat vestibular ganglion (Iwasaki et al. 2008). Blocking ILV enabled repetitive firing and converted phasic firing to tonic firing in ganglion cells, suggesting that KV1- and KCNQ-mediated currents contribute to the irregular firing of CZ afferents (Iwasaki et al. 2008; Kalluri et al. 2010). A large ILV has also been described in early postnatal striolar calyces, most of which did not show spontaneous firing, perhaps because of the young age of the preparation (P1–P9) and the presence of a large ILV (Songer and Eatock 2013). We did not observe a prominent ILV in our calyx recordings, but an α-DTX-sensitive current was found in CZ cells, supporting the presence of KV1 channels. We also observed an increase in firing in CZ calyces after α-DTX application, suggesting that there may be a class of phasic CZ calyces that express a sustained ILV and do not fire continuously.
KCNQ channels.
KCNQ channels exist as homo- or heterotetramers of KCNQ1–5 subunits. Voltage-dependent currents mediated by KCNQ channels are typically slow to activate and show little or no inactivation (Brown and Passmore 2009). KCNQ currents are present in vestibular ganglion cells, and KCNQ3-containing channels may contribute to ILV (Kalluri et al. 2010; Perez et al. 2009). Here we describe XE991-sensitive KCNQ currents in calyx terminals that activate at potentials above approximately −50 mV. Blocking KCNQ channels decreased firing frequency and increased the half-width of calyx action potentials in both zones. This differs from observations in vestibular ganglion cells, where conversion of phasic to tonic firing was observed in the presence of KCNQ channel blockers (Kalluri et al. 2010; Perez et al. 2009). Low-voltage-activated K+ channels minimize repetitive firing and therefore are not expected to be prevalent in spontaneously firing terminals. We suggest that KCNQ channel subunits and their activation ranges differ between the calyx afferent terminal and cell body in the ganglion and impact firing differently. Immunostaining for KCNQ (KV7) channels has been reported in calyx terminals (Hurley et al. 2006; Lysakowski et al. 2011; Rocha-Sanchez et al. 2007; Sousa et al. 2009; Spitzmaul et al. 2013). KCNQ4 was found on the inner face of calyces in both zones, but staining was heaviest in CZ calyces. KCNQ4 was present at lower levels on the outer face of the calyx and at the heminode of calyx-only terminals. Inner face KCNQ5 staining was found in calyces that formed dimorphic afferents but was lacking in central calretinin-containing afferents (Lysakowski et al. 2011). KCNQ5 staining was further reported to be predominant in calyces in extrastriola regions of the utricle and peripheral zones of the crista (Spitzmaul et al. 2013). KCNQ2 staining was also found on the inner face of calyces but did not vary with zone or afferent type, whereas KCNQ3 staining was found only on the calyx outer face and at the heminodes of dimorphic fibers (Lysakowski et al. 2011).
We investigated regional differences in KCNQ-mediated currents with diclofenac, which enhances KCNQ2/3 and KCNQ4 currents but inhibits KCNQ5 currents (Brueggemann et al. 2011; Peretz et al. 2005). Diclofenac produced a greater increase in outward current in CZ calyces compared with PZ calyces, supporting a greater contribution by KCNQ4 channels in CZ. Altered vestibuloocular reflexes were also reported in KCNQ4-knockout mice (Spitzmaul et al. 2013), suggesting that signals from CZ calyces may be important for driving this rapid reflex.
Other conductances.
Several voltage-dependent and calcium-activated K+ (KCa) currents have been reported in vestibular ganglion cells (Chabbert et al. 2001; Iwasaki et al. 2008; Limon et al. 2005; Risner and Holt 2006). Large-conductance (BK) channels were identified in vestibular ganglion and hair cells but were not detected in calyx terminals (Limon et al. 2005; Schweizer et al. 2009). We previously described apamin-sensitive small-conductance (SK) currents, which played a role in the afterhyperpolarization of evoked spikes in isolated calyx terminals (Meredith et al. 2011), but it remains to be determined whether expression of KCa channels varies with afferent type and location on the crista. The association of centrally located calretinin-containing calyx terminals with hair cells expressing KCa channels also begs further investigation. A rapidly activating and rapidly inactivating current was blocked by 4-AP in a subpopulation of PZ and CZ calyces in this study. We did not investigate this current further, but based on its rapid kinetics it may be a KV3-mediated current sensitive to the sea anemone toxin blood depressing substance I (BDS-I) as found previously (Chabbert et al. 2001; Dhawan et al. 2010).
In summary, we found that KV1 and KCNQ channels mediate outward currents in vestibular calyx terminals. An additional 4-AP-sensitive calyceal K+ current was found whose molecular identity awaits further determination. Differences in expression levels of these conductances contribute to regional variations in K+ current kinetics. The newly developed rodent crista slice will allow further zonal evaluation of the roles of different types of Na+ and K+ channels in determining spike regularity in vestibular afferent terminals. Identifying channel subtypes is important for future clinical interventions and vestibular therapies. K+ channel modulators that target, for example, KCNQ channels have significant potential for clinical management of neurological disorders, including those that affect the inner ear (Leitner et al. 2012; Soto and Vega 2010).
GRANTS
This work was supported by the National Space Biomedical Research Institute through NCC 9-58 to F. L. Meredith and an American Hearing Research Foundation Grant to K. J. Rennie.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: F.L.M. and K.J.R. conception and design of research; F.L.M. and K.J.R. performed experiments; F.L.M. and K.J.R. analyzed data; F.L.M. and K.J.R. interpreted results of experiments; F.L.M. prepared figures; F.L.M. and K.J.R. drafted manuscript; F.L.M. and K.J.R. edited and revised manuscript; F.L.M. and K.J.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Daniel Jagger for helpful comments on developing the slice technique and Yen Vu and Ikem Uge for technical assistance.
REFERENCES
- Armstrong CE, Roberts WM. Electrical properties of frog saccular hair cells: distortion by enzymatic dissociation. J Neurosci 18: 2962–2973, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird RA, Desmadryl G, Fernandez C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol 60: 182–203, 1988. [DOI] [PubMed] [Google Scholar]
- Bonsacquet J, Brugeaud A, Compan V, Desmadryl G, Chabbert C. AMPA type glutamate receptor mediates neurotransmission at turtle vestibular calyx synapse. J Physiol 576: 63–71, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichta AM, Aubert A, Eatock RA, Goldberg JM. Regional analysis of whole cell currents from hair cells of the turtle posterior crista. J Neurophysiol 88: 3259–3278, 2002. [DOI] [PubMed] [Google Scholar]
- Brichta AM, Goldberg JM. Morphological identification of physiologically characterized afferents innervating the turtle posterior crista. J Neurophysiol 83: 1202–1223, 2000. [DOI] [PubMed] [Google Scholar]
- Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol 156: 1185–1195, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann LI, Mackie AR, Martin JL, Cribbs LL, Byron KL. Diclofenac distinguishes among homomeric and heteromeric potassium channels composed of KCNQ4 and KCNQ5 subunits. Mol Pharmacol 79: 10–23, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabbert C, Chambard JM, Sans A, Desmadryl G. Three types of depolarization-activated potassium currents in acutely isolated mouse vestibular neurons. J Neurophysiol 85: 1017–1026, 2001. [DOI] [PubMed] [Google Scholar]
- Coetzee WA, Amarillo Y, Chiu J, Chow A, Lau D, McCormack T, Moreno H, Nadal MS, Ozaita A, Pountney D, Saganich M, Vega-Saenz de Miera E, Rudy B. Molecular diversity of K+ channels. Ann NY Acad Sci 868: 233–285, 1999. [DOI] [PubMed] [Google Scholar]
- Contini D, Zampini V, Tavazzani E, Magistretti J, Russo G, Prigioni I, Masetto S. Intercellular K+ accumulation depolarizes Type I vestibular hair cells and their associated afferent nerve calyx. Neuroscience 227: 232–246, 2012. [DOI] [PubMed] [Google Scholar]
- Curthoys IS. The development of function of horizontal semicircular canal primary neurons in the rat. Brain Res 167: 41–52, 1979. [DOI] [PubMed] [Google Scholar]
- Desai SS, Ali H, Lysakowski A. Comparative morphology of rodent vestibular periphery. II. Cristae ampullares. J Neurophysiol 93: 267–280, 2005. [DOI] [PubMed] [Google Scholar]
- Dhawan R, Mann SE, Meredith FL, Rennie KJ. K+ currents in isolated vestibular afferent calyx terminals. J Assoc Res Otolaryngol 11: 463–476, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulon D, Safieddine S, Jones SM, Petit C. Otoferlin is critical for a highly sensitive and linear calcium-dependent exocytosis at vestibular hair cell ribbon synapses. J Neurosci 29: 10474–10487, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eatock RA, Songer JE. Vestibular hair cells and afferents: two channels for head motion signals. Annu Rev Neurosci 34: 501–534, 2011. [DOI] [PubMed] [Google Scholar]
- Fuchs PA. Time and intensity coding at the hair cell's ribbon synapse. J Physiol 566: 7–12, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Géléoc GS, Risner JR, Holt JR. Developmental acquisition of voltage-dependent conductances and sensory signaling in hair cells of the embryonic mouse inner ear. J Neurosci 24: 11148–11159, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JM. Afferent diversity and the organization of central vestibular pathways. Exp Brain Res 130: 277–297, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JC, Chatlani S, Lysakowski A, Goldberg JM. Quantal and nonquantal transmission in calyx-bearing fibers of the turtle posterior crista. J Neurophysiol 98: 1083–1101, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz GC, Risner-Janiczek JR, Holt JR. Mechanotransduction and hyperpolarization-activated currents contribute to spontaneous activity in mouse vestibular ganglion neurons. J Gen Physiol 143: 481–497, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hullar TE, Della Santina CC, Hirvonen T, Lasker DM, Carey JP, Minor LB. Responses of irregularly discharging chinchilla semicircular canal vestibular-nerve afferents during high-frequency head rotations. J Neurophysiol 93: 2777–2786, 2005. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Gaboyard S, Zhong M, Price SD, Wooltorton JR, Lysakowski A, Eatock RA. M-like K+ currents in type I hair cells and calyx afferent endings of the developing rat utricle. J Neurosci 26: 10253–10269, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki S, Chihara Y, Komuta Y, Ito K, Sahara Y. Low-voltage-activated potassium channels underlie the regulation of intrinsic firing properties of rat vestibular ganglion cells. J Neurophysiol 100: 2192–2204, 2008. [DOI] [PubMed] [Google Scholar]
- Iwasaki S, Nakajima T, Chihara Y, Inoue A, Fujimoto C, Yamasoba T. Developmental changes in the expression of Kv1 potassium channels in rat vestibular ganglion cells. Brain Res 1429: 29–35, 2012. [DOI] [PubMed] [Google Scholar]
- Kalluri R, Xue J, Eatock RA. Ion channels set spike timing regularity of mammalian vestibular afferent neurons. J Neurophysiol 104: 2034–2051, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AR. An intracellular medium formulary. J Neurosci Methods 44: 91–100, 1992. [DOI] [PubMed] [Google Scholar]
- Kostyuk PG, Krishtal OA, Pidoplichko VI. Intracellular perfusion. J Neurosci Methods 4: 201–210, 1981. [DOI] [PubMed] [Google Scholar]
- Lasker DM, Han GC, Park HJ, Minor LB. Rotational responses of vestibular-nerve afferents innervating the semicircular canals in the C57BL/6 mouse. J Assoc Res Otolaryngol 9: 334–348, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Camp AJ, Callister RJ, Brichta AM. Vestibular primary afferent activity in an in vitro preparation of the mouse inner ear. J Neurosci Methods 145: 73–87, 2005. [DOI] [PubMed] [Google Scholar]
- Leitner MG, Feuer A, Ebers O, Schreiber DN, Halaszovich CR, Oliver D. Restoration of ion channel function in deafness-causing KCNQ4 mutants by synthetic channel openers. Br J Pharmacol 165: 2244–2259, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard RB, Kevetter GA. Molecular probes of the vestibular nerve. I. Peripheral termination patterns of calretinin, calbindin and peripherin containing fibers. Brain Res 928: 8–17, 2002. [DOI] [PubMed] [Google Scholar]
- Li GQ, Meredith FL, Rennie KJ. Development of K+ and Na+ conductances in rodent postnatal semicircular canal type I hair cells. Am J Physiol Regul Integr Comp Physiol 298: R351–R358, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim R, Kindig AE, Donne SW, Callister RJ, Brichta AM. Potassium accumulation between type I hair cells and calyx terminals in mouse crista. Exp Brain Res 210: 607–621, 2011. [DOI] [PubMed] [Google Scholar]
- Limon A, Perez C, Vega R, Soto E. Ca2+-activated K+-current density is correlated with soma size in rat vestibular-afferent neurons in culture. J Neurophysiol 94: 3751–3761, 2005. [DOI] [PubMed] [Google Scholar]
- Lindeman HH. Studies on the morphology of the sensory regions of the vestibular apparatus with 45 figures. Ergeb Anat Entwicklungsgesch 42: 1–113, 1969. [PubMed] [Google Scholar]
- Liu Q, Lee E, Davis RL. Heterogeneous intrinsic excitability of murine spiral ganglion neurons is determined by Kv1 and HCN channels. Neuroscience 257: 96–110, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Gaboyard-Niay S, Calin-Jageman I, Chatlani S, Price SD, Eatock RA. Molecular microdomains in a sensory terminal, the vestibular calyx ending. J Neurosci 31: 10101–10114, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Goldberg JM. A regional ultrastructural analysis of the cellular and synaptic architecture in the chinchilla cristae ampullares. J Comp Neurol 389: 419–443, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Goldberg JM. Ultrastructural analysis of the cristae ampullares in the squirrel monkey (Saimiri sciureus). J Comp Neurol 511: 47–64, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski A, Minor LB, Fernandez C, Goldberg JM. Physiological identification of morphologically distinct afferent classes innervating the cristae ampullares of the squirrel monkey. J Neurophysiol 73: 1270–1281, 1995. [DOI] [PubMed] [Google Scholar]
- Lysakowski A, Price SD. Sodium channel distribution in vestibular afferents—an update (Abstract). Assoc Res Otolaryngol 37th Annual Midwinter Meeting, Abstract 049, 2014. [Google Scholar]
- Marcotti W, van Netten SM, Kros CJ. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J Physiol 567: 505–521, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masetto S, Correia MJ. Electrophysiological properties of vestibular sensory and supporting cells in the labyrinth slice before and during regeneration. J Neurophysiol 78: 1913–1927, 1997. [DOI] [PubMed] [Google Scholar]
- Masetto S, Perin P, Malusa A, Zucca G, Valli P. Membrane properties of chick semicircular canal hair cells in situ during embryonic development. J Neurophysiol 83: 2740–2756, 2000. [DOI] [PubMed] [Google Scholar]
- Meredith FL, Benke TA, Rennie KJ. Hyperpolarization-activated current (Ih) in vestibular calyx terminals: characterization and role in shaping postsynaptic events. J Assoc Res Otolaryngol 13: 745–758, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith FL, Li GQ, Rennie KJ. Postnatal expression of an apamin-sensitive kca current in vestibular calyx terminals. J Membr Biol 244: 81–91, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouvian R, Beutner D, Parsons TD, Moser T. Structure and function of the hair cell ribbon synapse. J Membr Biol 209: 153–165, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perachio AA, Correia MJ. Responses of semicircular canal and otolith afferents to small angle static head tilts in the gerbil. Brain Res 280: 287–298, 1983. [DOI] [PubMed] [Google Scholar]
- Peretz A, Degani N, Nachman R, Uziyel Y, Gibor G, Shabat D, Attali B. Meclofenamic acid and diclofenac, novel templates of KCNQ2/Q3 potassium channel openers, depress cortical neuron activity and exhibit anticonvulsant properties. Mol Pharmacol 67: 1053–1066, 2005. [DOI] [PubMed] [Google Scholar]
- Perez C, Limon A, Vega R, Soto E. The muscarinic inhibition of the potassium M-current modulates the action-potential discharge in the vestibular primary-afferent neurons of the rat. Neuroscience 158: 1662–1674, 2009. [DOI] [PubMed] [Google Scholar]
- Purcell IM, Perachio AA. Three-dimensional analysis of vestibular efferent neurons innervating semicircular canals of the gerbil. J Neurophysiol 78: 3234–3248, 1997. [DOI] [PubMed] [Google Scholar]
- Rennie KJ, Streeter MA. Voltage-dependent currents in isolated vestibular afferent calyx terminals. J Neurophysiol 95: 26–32, 2006. [DOI] [PubMed] [Google Scholar]
- Risner JR, Holt JR. Heterogeneous potassium conductances contribute to the diverse firing properties of postnatal mouse vestibular ganglion neurons. J Neurophysiol 96: 2364–2376, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha-Sanchez SM, Morris KA, Kachar B, Nichols D, Fritzsch B, Beisel KW. Developmental expression of Kcnq4 in vestibular neurons and neurosensory epithelia. Brain Res 1139: 117–125, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüsch A, Lysakowski A, Eatock RA. Postnatal development of type I and type II hair cells in the mouse utricle: acquisition of voltage-gated conductances and differentiated morphology. J Neurosci 18: 7487–7501, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer FE, Savin D, Luu C, Sultemeier DR, Hoffman LF. Distribution of high-conductance calcium-activated potassium channels in rat vestibular epithelia. J Comp Neurol 517: 134–145, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songer JE, Eatock RA. Tuning and timing in mammalian type I hair cells and calyceal synapses. J Neurosci 33: 3706–3724, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto E, Vega R. Neuropharmacology of vestibular system disorders. Curr Neuropharmacol 8: 26–40, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa AD, Andrade LR, Salles FT, Pillai AM, Buttermore ED, Bhat MA, Kachar B. The septate junction protein caspr is required for structural support and retention of KCNQ4 at calyceal synapses of vestibular hair cells. J Neurosci 29: 3103–3108, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzmaul G, Tolosa L, Winkelman BH, Heidenreich M, Frens MA, Chabbert C, de Zeeuw CI, Jentsch TJ. Vestibular role of KCNQ4 and KCNQ5 K+ channels revealed by mouse models. J Biol Chem 288: 9334–9344, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung VW, Di Marco S, Lim R, Brichta AM, Camp AJ. An isolated semi-intact preparation of the mouse vestibular sensory epithelium for electrophysiology and high-resolution two-photon microscopy. J Vis Exp 76: e50471, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Kim HJ, Lv P, Tempel B, Yamoah EN. Association of the Kv1 family of K+ channels and their functional blueprint in the properties of auditory neurons as revealed by genetic and functional analyses. J Neurophysiol 110: 1751–1764, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng T, Correia MJ. Regional distribution of ionic currents and membrane voltage responses of type II hair cells in the vestibular neuroepithelium. J Neurophysiol 82: 2451–2461, 1999. [DOI] [PubMed] [Google Scholar]
- Wooltorton JR, Gaboyard S, Hurley KM, Price SD, Garcia JL, Zhong M, Lysakowski A, Eatock RA. Developmental changes in two voltage-dependent sodium currents in utricular hair cells. J Neurophysiol 97: 1684–1704, 2007. [DOI] [PubMed] [Google Scholar]


