Abstract
Placental insufficiency programs an increase in blood pressure associated with a twofold increase in serum testosterone in male growth-restricted offspring at 4 mo of age. Population studies indicate that the inverse relationship between birth weight and blood pressure is amplified with age. Thus, we tested the hypothesis that intrauterine growth restriction programs an age-related increase in blood pressure in male offspring. Growth-restricted offspring retained a significantly higher blood pressure at 12 but not at 18 mo of age compared with age-matched controls. Blood pressure was significantly increased in control offspring at 18 mo of age relative to control counterparts at 12 mo; however, blood pressure was not increased in growth-restricted at 18 mo relative to growth-restricted counterparts at 12 mo. Serum testosterone levels were not elevated in growth-restricted offspring relative to control at 12 mo of age. Thus, male growth-restricted offspring no longer exhibited a positive association between blood pressure and testosterone at 12 mo of age. Unlike hypertension in male growth-restricted offspring at 4 mo of age, inhibition of the renin-angiotensin system with enalapril (250 mg/l for 2 wk) did not abolish the difference in blood pressure in growth-restricted offspring relative to control counterparts at 12 mo of age. Therefore, these data suggest that intrauterine growth restriction programs an accelerated age-related increase in blood pressure in growth-restricted offspring. Furthermore, this study suggests that the etiology of increased blood pressure in male growth-restricted offspring at 12 mo of age differs from that at 4 mo of age.
Keywords: low birth weight, accelerated aging, cardiovascular risk, blood pressure, renin-angiotensin system
hypertension is a major risk factor for cardiovascular (CV) mortality worldwide (2). Age is also a risk factor for CV disease since blood pressure (BP) increases with age (31). Aging is a progressive process that results in decreased function in all organ systems (14), and increased susceptibility to diseases such as hypertension (41). In a recent study from the National Health and Nutrition Examination Survey, 50% of adults over the age of 55 develop hypertension (30). Of these, resistant hypertension is estimated to affect ∼55% (46). Despite the high rates of hypertension in the older population, the proper treatment for BP control in these patients is not well-studied due to a lack of clinical trials investigating age-related hypertension (17). Thus, studies addressing the etiology of essential hypertension in the elderly and its long-term effect on CV risk are warranted.
The developmental origins of adult health and disease (DOHaD) hypothesis suggests that an adverse environment during early life programs an increased risk for CV disease in later life (4). Birth weight is inversely related to BP in childhood (16, 26) and in a manner that is amplified with age (8, 24). BP is also elevated in individuals born preterm (9), in particular those born with intrauterine growth restriction (IUGR) (23). Experimental models of IUGR demonstrate that BP is increased in offspring exposed to a developmental insult (1, 34, 43, 44). Yet, few studies investigate the effect of aging on increased CV risk that has its origins in fetal life. In the experimental model of developmental programming induced by administration of an angiotensin II (ANG II) type 1 receptor antagonist (ARA) during postnatal days 1–14 in the rat, BP is elevated in ARA male offspring at 11 mo of age relative to vehicle-treated controls (37). BP remains elevated in ARA-treated animals at 17 mo of age compared with age-matched controls (37). Yet, BP is increased in ARA-treated male offspring at 17 mo of age relative to ARA-treated males at 11 mo of age (37), suggesting that BP in offspring exposed to this developmental insult is augmented with age. However, the mechanisms involved remain unclear.
To elucidate the long-term effects of IUGR on CV risk, our laboratory utilizes a rodent model of IUGR induced by placental insufficiency at day 14 of gestation in the Sprague-Dawley (SD) rat (1). Male growth-restricted offspring exhibit a significant increase in BP at 4 mo of age that is testosterone-dependent (32), whereas female growth-restricted offspring remain normotensive in young adulthood (1). While the female growth-restricted offspring are protected against an increase in BP at 4 mo of age (1), they develop an age-related increase in BP by 12 mo of age (21). Since female growth-restricted offspring exhibit an age-related increase in BP, the first aim of this study tested the hypothesis that IUGR programs an age-related increase in BP in male growth-restricted offspring.
Increased risk of CV disease in individuals born preterm or IUGR may involve programming of the renin-angiotensin system (RAS). Expressions of circulating ANG II and angiotensin-converting enzyme (ACE) are elevated in small for gestational age boys that exhibit a significant increase in BP relative to appropriate for gestational age boys (15). We previously reported that male growth-restricted offspring exhibit a significant increase in renal renin, angiotensinogen, and ACE mRNA expression at 4 mo of age (18) when male growth-restricted rats exhibit a significant increase in BP (32). In addition, we previously demonstrated that ACE inhibition administered for 2 wk before measure of BP abolishes hypertension in male growth-restricted offspring at 4 mo of age (32). RAS blockade also abolishes hypertension at 4 mo of age in rat offspring exposed to maternal undernutrition (6, 28), indicating a similar etiology of increased BP despite a different experimental model of fetal insult. Thus, these studies demonstrate a role for the RAS in the etiology of increased BP that has its origins in fetal life.
Our previous studies also indicate that male growth-restricted rats exhibit an enhanced BP response to acute ANG II at 4 mo of age that is also testosterone-dependent (33). Aging results in an increase in activity and responsiveness of the RAS in the human population (11). Whether enhanced BP sensitivity to acute ANG II persists with age and whether the RAS contributes to age-related increases in BP in male growth-restricted offspring is unknown. Therefore, we also tested the hypothesis that age increases enhanced BP sensitivity to acute ANG II in male growth-restricted offspring and that inappropriate activation of the RAS contributes to an age-related increase in BP in male growth-restricted offspring relative to control counterparts at 12 and 18 mo of age.
METHODS
All experimental protocols were approved by the Animal Care and Use Committee at the University of Mississippi Medical Center and procedures were conducted in accordance with National Institutes of Health guidelines. Rats were housed in a temperature-controlled room (23°C) with a 12:12-h light-dark cycle. Food and water were made available ad libitum. Timed pregnant SD rats were purchased from Harlan (Indianapolis, IN). At day 14 of gestation, rats intended for reduced uterine perfusion were clipped as described below. A sham procedure was conducted on dams at day 14 of gestation to generate control offspring. All pregnant rats were allowed to deliver at term with birth weights recorded within 12 h of delivery. Pups in control and reduced uterine perfusion litters were culled to four female and four male pups per dam to allow equal nutrition access for all offspring; however, only male offspring were utilized in this study and only one male offspring per litter was used per study variable. Male offspring from 21 control (sham) pregnant and 23 reduced uterine perfusion pregnant rats were randomly assigned to groups studied at 12 or 18 mo of age.
Reduced uterine perfusion in the pregnant rat.
Reduced uteroplacental perfusion or a sham procedure was performed on day 14 of gestation in timed pregnant SD rats as previously described to induce IUGR (1).
Measurement of mean arterial pressure.
Mean arterial pressure (MAP) was measured as previously described in control and growth-restricted male offspring at 12 mo of age in one study group and at 18 mo of age in a second study group (32). In brief, rats were surgically implemented with a catheter in the right carotid artery. MAP was measured 24 h after recovery in the conscious state using a data-acquisition kit (DATAQ Instruments, Akron, OH) followed by collection of blood and tissue samples for further analysis.
Chronic blockade of the RAS.
Male offspring were treated with enalapril, an ACE inhibitor, for 2 wk (250 mg/l) in drinking water (32) to block the endogenous RAS; vehicle-treated animals were maintained on drinking water. Both groups were provided water ad libitum. BP was measured at the end of the 2-wk administration of vehicle or enalapril as described above in one group of animals studied at 12 and another at 18 mo of age.
Measurement of the MAP response to acute ANG II in male control and growth-restricted offspring.
To determine the BP response to acute ANG II, male control and growth-restricted rats were pretreated with enalapril for 2 wk in one group of animals at 12 mo of age, and in a second group at 18 mo of age. In brief, animals were instrumented with a carotid arterial catheter and after a 24-h recovery, baseline MAP was measured in the conscious state for 30 min before and after an acute infusion of ANG II (100 ng·kg−1·min−1 in 0.9% saline solution; Sigma, St. Louis, MO) as previously described (33).
Isolation and quantitation of mRNA using real-time PCR (qRT-PCR).
Kidneys were separated into cortical and medullary sections and then were flash-frozen in liquid nitrogen and stored at −80°C. Total RNA was isolated from the renal cortex and medulla using NucleoSpin RNA (Machery-Nagel, Bethelhem, PA). cDNA was synthesized from 1 μg of RNA using the iScript cDNA Synthesis Kit (BioRad, Hercules, CA). qRT-PCR was performed using iQ SYBR Green Supermix (BioRad) and the CFX96 Touch Real-Time PCR Detection System (BioRad). The following primer sequences (Integrated DNA Technologies) were used for quantitation of renin (forward: tgatcctggtcatgtctactcc, reverse: cctcttgtagcttcagtctcc), angiotensinogen (forward: caggtcaggatgcagaagatg, reverse: ggatagctgtgcttgtctgg), ACE (forward: caccaattcctccatgttcac, reverse: tgttagagaagccaaccgatg), angiotensin type 1a receptor (forward: cactattcgaaatccacttgacc, reverse: ctctcagctctgccacattc), and angiotensin type 1b receptor (forward: tgctctctgacactatttaaaatgc, reverse: gacacacacagcctttcca). Levels of mRNA expression were calculated using the mathematical formula for delta/delta CT recommended by Applied Biosystems (Applied Biosystems User Bulletin, No. 2, 1997, Foster City, CA).
Serum testosterone levels were measured using a commercially available kit (Coat-A-Count Total Testosterone Assay kit; Diagnostics Products, Los Angeles, CA).
Body composition including total fat mass and total lean mass was determined in conscious animals using an Echo-MRI-700 (Echo Medical Systems, Houston, TX) at 12 and 18 mo of age as previously described (21).
Statistics.
Data are presented as means ± SE, with n representing the number of male offspring per group. Baseline MAP, systemic responses to ANG II, and plasma testosterone levels in each group were compared using two-way ANOVA (Prism 5.0, GraphPad, San Diego, CA). The two different factors studied via two-way ANOVA included 1) the effect of IUGR on each variable and 2) the effect of age on each variable. Post hoc testing was performed using Bonferroni's multiple-comparisons test where appropriate. Differences were reported as significant when P < 0.05.
RESULTS
Birth weight, body weight, and total fat mass; effect of age.
Birth weight was significantly reduced in growth-restricted compared with control (P < 0.05; Table 1). However, body weight did not differ between growth-restricted and control at 12 mo and remained similar at 18 mo of age (Table 1). Total fat mass was similar in growth-restricted offspring compared with their age-matched control counterparts at 12 and 18 mo of age (Table 1).
Table 1.
Body composition in male offspring at 12 and 18 mo of age
| 12 mo of Age |
18 mo of Age |
|||
|---|---|---|---|---|
| Control | IUGR | Control | IUGR | |
| Birth wt, g | 6.35 ± .1 | 4.74 ± .2* | 6.33 ± .13 | 5.28 ± .09* |
| Body wt, g | 572 ± 21 | 524 ± 27 | 576 ± 23 | 539 ± 25 |
| Fat mass, g | 80 ± 12 | 80 ± 11 | 79 ± 19 | 84 ± 16 |
| Lean mass, g | 370 ± 23 | 318 ± 24 | 398 ± 23 | 368 ± 18 |
Data values represent means ± SE. Birth weight, body weight, and fat mass in male control and male intrauterine growth-restricted (IUGR) offspring.
P < 0.05 vs. control offspring.
MAP in control and growth-restricted offspring; effect of age.
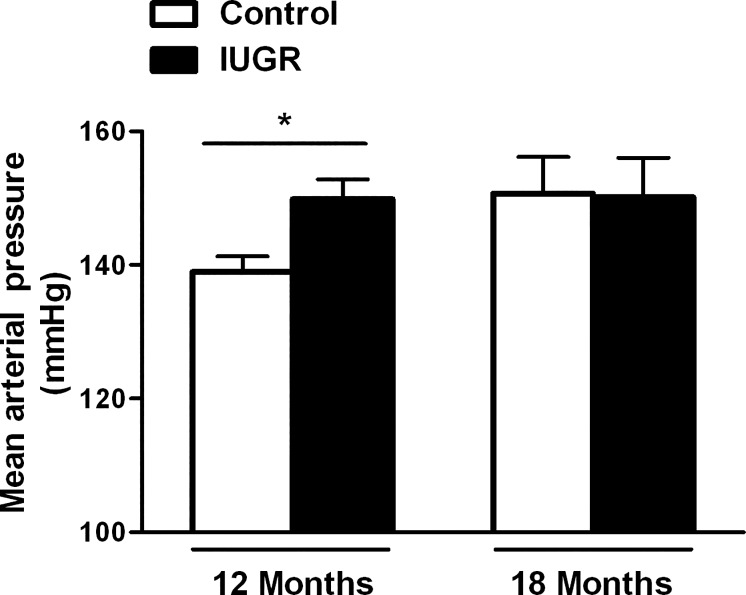
MAP was significantly higher in growth-restricted offspring compared with control offspring at 12 mo of age (P < 0.05, IUGR vs. control; Fig. 1). Yet, by 18 mo, MAP did not differ upon comparison of growth-restricted relative to age-matched control (Fig. 1).
Fig. 1.
Effect of age on mean arterial pressure (MAP). Male control and intrauterine growth-restricted (IUGR) offspring measured in conscious, chronically instrumented animals at 12 mo of age (control n = 14 and IUGR n = 9) and at 18 mo of age (control n = 5 and IUGR n = 6). *P < 0.05 vs. age-matched control. Data values represent means ± SE.
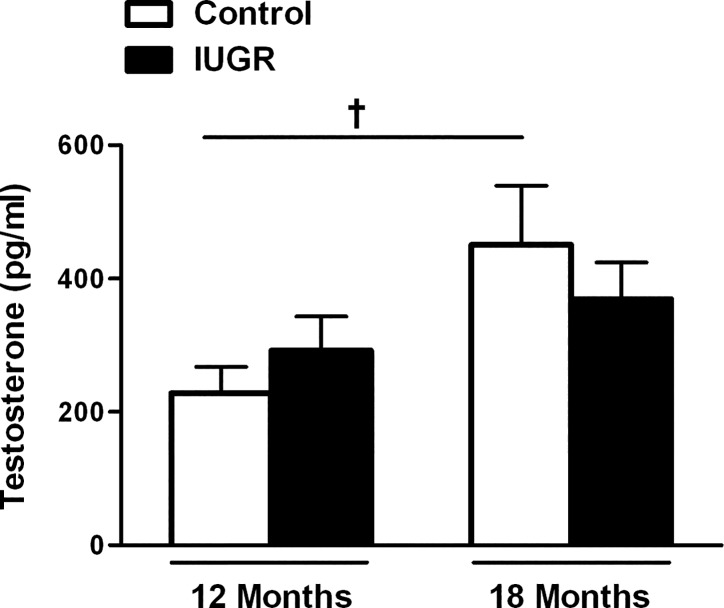
Serum testosterone levels in male control and growth-restricted offspring; effect of age.
Serum testosterone levels did not differ upon comparison of growth-restricted offspring relative to control counterparts at 12 or 18 mo of age (P > 0.05, IUGR vs. control; Fig. 2).
Fig. 2.
Serum testosterone in male control and male IUGR offspring at 12 mo of age (control n = 12 and IUGR n = 10) and at 18 mo of age (control n = 10 and IUGR n = 10). †P < 0.05 vs. 12 mo of age control. Data values represent means ± SE.
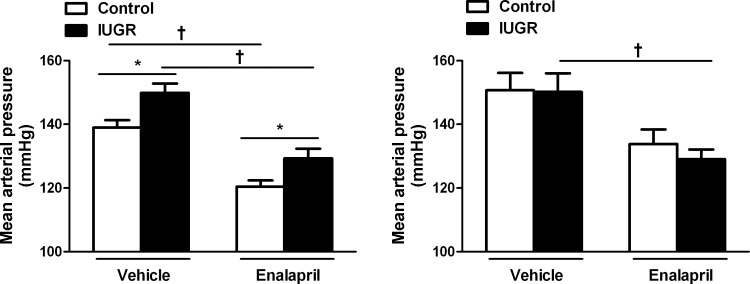
MAP in male control and growth-restricted offspring; effect of ACE inhibition.
MAP remained significantly elevated in enalapril-treated growth-restricted offspring relative to enalapril-treated control at 12 mo of age (P < 0.05, IUGR vs. control; Fig. 3A). MAP did not differ in enalapril-treated growth-restricted relative to enalapril-treated controls at 18 mo of age (Fig. 3B).
Fig. 3.
Effect of renin-angiotensin system (RAS) blockade with the angiotensin-converting enzyme (ACE) inhibitor enalapril (250 mg/l for 2 wk) on MAP in male control and IUGR rats. MAP was measured at 12 mo of age (control n = 11 and IUGR n = 12) and at 18 mo of age (control n = 5 and IUGR n = 6) following the 2-wk exposure to enalapril or vehicle. *P < 0.05 vs. age-matched control. †P < 0.05 vs. 12 mo IUGR. Data values represent means ± SE.
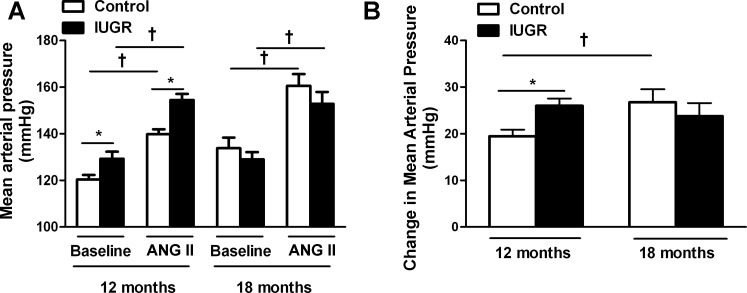
BP response to acute ANG II in male control and growth-restricted offspring; effect of age.
In response to acute ANG II, growth-restricted offspring exhibited an elevated MAP at 12 mo of age compared with control counterparts (Fig. 4A; P < 0.05, IUGR vs. control). By 18 mo of age, MAP following acute ANG II was similar in growth-restricted relative to control (Fig. 4A). The change in MAP between baseline BP vs. acute ANG II BP (ΔBP) was significantly greater in control offspring at 18 mo of age relative to the ΔBP in control at 12 mo of age (P < 0.05, 12-mo ΔBP vs. 18-mo ΔBP; Fig. 4B). However, the ΔBP in growth-restricted offspring at 12 mo of age did not differ relative to the ΔBP in growth-restricted offspring at 18 mo of age (P > 0.05, 12-mo ΔBP vs. 18-mo ΔBP; Fig. 4B).
Fig. 4.
Blood pressure response to acute ANG II infusion following inhibition of the endogenous RAS at 12 and 18 mo of age. A: change in blood pressure in response to ANG II. B: MAP in male control and IUGR offspring at baseline and in response to acute infusion of ANG II (100 ng·kg−1·min−1) in conscious, chronically instrumented animals at 12 and 18 mo of age that were pretreated with the ACE inhibitor enalapril. 12-mo group: control n = 11, IUGR n = 12; 18-mo group: control n = 5, IUGR n = 6. *P < 0.05 vs. controls at baseline. †P < 0.05 vs. IUGR at baseline. All data are means ± SE.
Intrarenal mRNA expression of the RAS in aged male control and growth-restricted offspring.
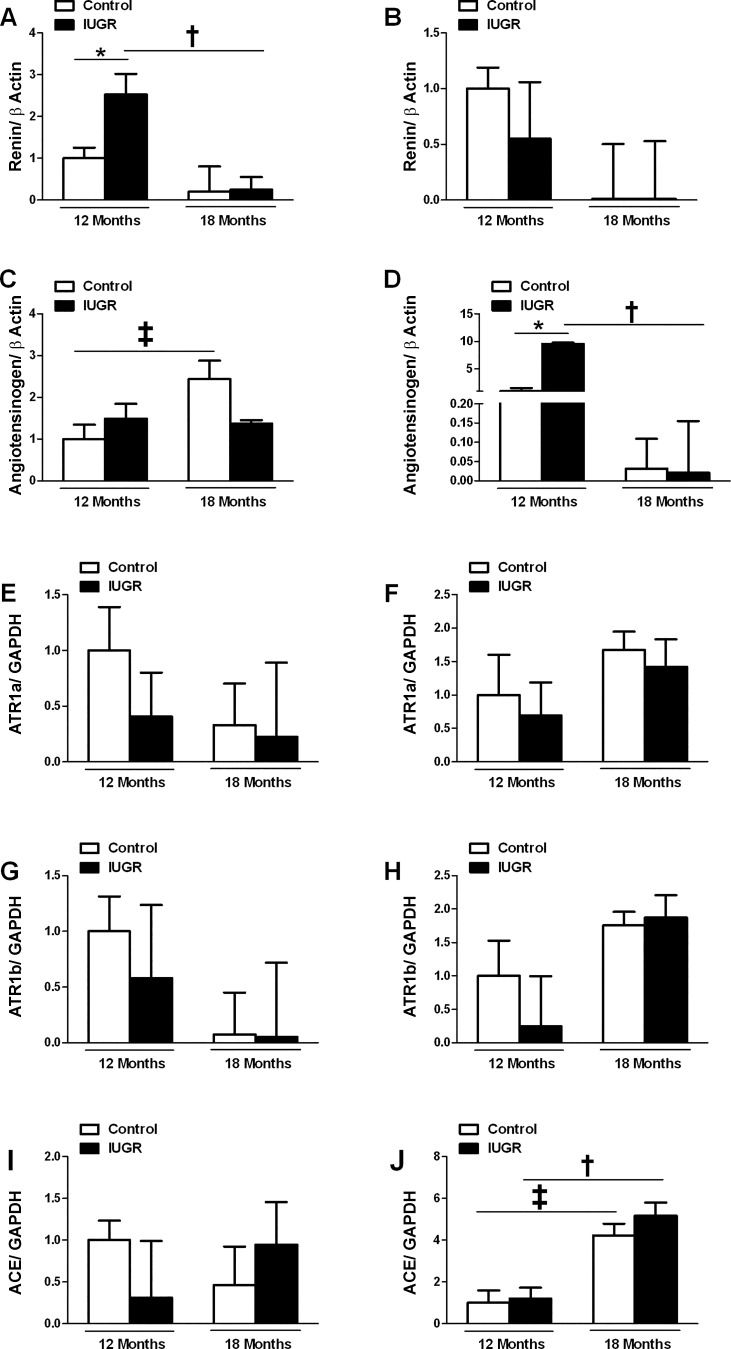
Intrarenal renin mRNA expression within the cortex was significantly increased in growth-restricted offspring at 12 mo of age compared with age-matched controls (Fig. 5A). Yet, the difference in renal cortical renin mRNA expression between control and growth-restricted offspring was abolished by 18 mo of age (Fig. 5A). Cortical renin mRNA expression did not differ in growth-restricted offspring at 12 mo of age relative to 18-mo-old counterparts (Fig. 5A). Medullary renin mRNA expression did not differ at 12 mo of age between control and growth-restricted (Fig. 5B). Age did not alter expression in either group. Cortical angiotensinogen mRNA expression also did not differ upon comparison of control and growth-restricted offspring at 12 or 18 mo of age; however, there was a significant increase in cortical angiotensinogen mRNA expression in control offspring at 18 mo of age relative to 12-mo counterparts (Fig. 5C). Medullary angiotensinogen mRNA expression was significantly increased in growth-restricted offspring relative to age-matched controls at 12 mo of age (Fig. 5D), but medullary angiotensinogen mRNA expression was lower at 18 mo of age in growth-restricted relative to 12-mo counterparts. Cortical or medullary expression of the angiotensin type 1a and 1b receptor (ATR1a and ATR1b) did not differ in control relative to growth-restricted offspring at 12 mo of age (Fig. 5, E–H); age did not alter expression (Fig. 5, E–H). Cortical ACE mRNA expression did not differ at either 12 or 18 mo of age upon comparison of control to growth-restricted offspring (Fig. 5I). Medullary ACE mRNA expression also did not differ between control and growth-restricted offspring at 12 or 18 mo of age; however, medullary ACE mRNA expression was significantly higher at 18 mo of age in control and growth-restricted offspring compared with their 12 mo of age counterparts (Fig. 5J).
Fig. 5.
Temporal changes in mRNA levels of the renal RAS in male control and IUGR offspring by real-time PCR. Cortical renin mRNA expression (A), medullary renin mRNA expression (B), cortical angiotensinogen mRNA expression (C), medullary angiotensinogen mRNA expression (D), cortical angiotensin receptor type 1a mRNA expression (E), medullary angiotensin receptor type 1a mRNA expression (F), cortical angiotensin receptor type 1b mRNA expression (G), medullary angiotensin receptor type 1b mRNA expression (H), cortical ACE mRNA expression (I), and medullary ACE mRNA expression (J) in male control (n = 8) and male IUGR offspring (n = 7) at 12 mo of age and male control (n = 5) and male IUGR offspring (n = 6) at 18 mo of age. Data are expressed as fold changes relative to the mean expression level of the vehicle-treated control rats that were arbitrarily defined as 1. *P < 0.05 vs. age-matched counterpart. †P < 0.05 vs. IUGR at 12 mo of age. ‡P < 0.05 vs. control at 12 mo of age.
DISCUSSION
The main findings from this study indicated that 1) growth-restricted offspring retained a significantly higher MAP at 12 but not 18 mo of age compared with age-matched controls. MAP was significantly elevated in control offspring at 18 mo of age relative to control counterparts at 12 mo of age; but MAP did not differ upon comparison of growth-restricted offspring at 18 mo of age relative to growth-restricted counterparts at 12 mo of age. Thus, these findings suggested that IUGR programmed an accelerated age-related increase in BP in male growth-restricted offspring. 2) Additional data from this study demonstrated that despite the retained increase in MAP at 12 mo of age, serum testosterone levels were not elevated in growth-restricted offspring relative to control. Thus, unlike their counterparts at 4 mo of age, male growth-restricted offspring no longer exhibited a positive association between BP and testosterone at 12 mo of age. 3) Age also altered the BP response to chronic blockade of the RAS. Unlike growth-restricted counterparts at 4 mo of age (32), MAP in enalapril-treated growth-restricted offspring at 12 mo of age remained increased relative to enalapril-treated control, suggesting that factors other than the RAS contribute to the significant increase in BP at 12 mo of age in male growth-restricted offspring. 4) Lastly, the ΔBP between baseline BP vs. acute ANG II BP was higher in control offspring at 18 mo of age relative to the ΔBP in control at 12 mo. Although growth-restricted offspring exhibited a higher ΔBP at 12 mo of age relative to age-matched control, the ΔBP was similar at 18 mo of age in growth-restricted relative to age-matched controls. Importantly, the ΔBP was similar in growth-restricted at 12 mo compared with the ΔBP in growth-restricted offspring at 18 mo of age, suggesting that IUGR programmed accelerated CV risk in the male growth-restricted rat.
The first aim of this study tested the hypothesis that IUGR programs an age-related increase in BP. Findings from this study demonstrated that growth-restricted offspring retained a significantly higher MAP at 12 mo of age relative to age-matched controls; yet, the significant difference in MAP between growth-restricted and age-matched controls was abolished at 18 mo of age. We previously reported that male growth-restricted offspring exhibit an enhanced BP response to acute ANG II infusion at 4 mo of age (33). Prenatal exposure to nicotine programs an enhanced sensitivity to acute ANG II that is heightened by 22 mo of age relative to 5 mo in male rats implicating that age augments sensitivity to acute ANG II following a developmental insult (40). Thus, the second aim of this proposal tested the hypothesis that age increases enhanced BP sensitivity to acute ANG II in male growth-restricted offspring relative to age-matched controls. The ΔBP between baseline and acute ANG II was significantly higher in control offspring at 18 mo of age relative to the ΔBP in controls at 12 mo. Tank et al. (39) previously reported that the BP response to acute ANG II (100 ng·kg−1·min−1) in male SD rats is similar at 3 and 15 mo of age. However, our studies indicated that in the presence of blockade of the endogenous RAS, the acute BP response to ANG II (100 ng·kg−1·min−1) was higher at 18 mo of age (ΔBP 27 mmHg) relative to that observed at 12 mo (ΔBP 19 mmHg) in control or normal birth weight SD offspring. Although growth-restricted offspring at 12 mo of age exhibited a higher MAP in response to acute ANG II relative to age-matched control counterparts, the ΔBP between baseline and acute ANG II was less in growth-restricted offspring at 12 mo of age (ΔBP 25 mmHg) relative to that previously reported for growth-restricted offspring at 4 mo of age (ΔBP 48 mmHg) (33). Furthermore, BP following acute ANG II did not differ upon comparison of growth-restricted offspring at 18 mo of age relative to age-matched control counterparts, and the ΔBP was similar in growth-restricted offspring at 12 mo of age relative to 18-mo growth-restricted rats (ΔBP 24 mmHg). Thus, these data indicate that an age-related increase in the BP response to acute ANG II was present in male control SD rats between 12 and 18 mo of age but not in male growth-restricted, suggesting that IUGR programs accelerated CV aging in male rats that was abolished relative to age-matched male controls by 18 mo of age.
Despite the significantly higher MAP at 12 mo of age in male growth-restricted offspring, testosterone levels were similar in the growth-restricted offspring relative to age-matched controls. Longitudinal studies in normal aging men demonstrate that testosterone levels decline with age (12, 20). Moreover, clinical studies report an inverse relationship between testosterone levels and BP in men (13, 38). Together, these studies suggest that a decrease in circulating testosterone levels contributes to the increased risk for hypertension in aging men. Studies addressing testosterone and BP in low birth weight men are limited. However, our laboratory previously reported that BP is positively associated with testosterone in male growth-restricted offspring at 4 mo of age (32). Furthermore, castration abolishes the significant increase in BP (32) and the enhanced sensitivity to acute ANG II (33) in male growth-restricted offspring at 4 mo of age, suggesting that increased CV risk following IUGR in the male rat is testosterone dependent. A role for elevated testosterone in the developmental programming of increased CV risk is also reported by others. In the model of IUGR induced via bilateral uterine ligation, serum testosterone levels are positively associated with BP in male offspring at 4 mo of age (5). Early life stress (ELS) programs exacerbated ANG II-hypertension associated with a significant increase in testosterone in male ELS offspring at 3 mo of age (25). Thus, these studies also suggest a permissive role for elevated testosterone in the etiology of increased CV risk in male offspring exposed to a developmental insult. Rodriguez-Gonzalez et al. (35) report that fetal exposure to maternal protein restriction programs an accelerated decline in circulating testosterone levels in male rats. Circulating testosterone levels in male growth-restricted offspring at 12 mo of age were similar to that previously reported by our laboratory for male control offspring at 4 mo of age (32, 33). A direct test of the importance of testosterone as a contributor to increased BP and enhanced sensitivity to acute ANG II at 12 mo of age in male growth-restricted offspring was not conducted. Yet, collectively, results from studies in male growth-restricted offspring at 4 (32, 33) and 12 mo of age in the current study suggest that increased circulating testosterone levels in male growth-restricted offspring contribute to accelerated CV. Yet, factors other than an increase in testosterone per se are involved at 12 mo of age, before catch-up to control counterparts by 18 mo of age, in the pathogenesis of elevated CV risk induced by IUGR in the male rat.
Testosterone upregulates renal renin and angiotensinogen expression in experimental models of hypertension (7, 45). We previously reported that hypertension is abolished by blockade of the RAS in male growth-restricted offspring at 4 mo of age (32). Although the significantly higher BP in male growth-restricted offspring at 12 mo of age was not associated with a significant elevation in testosterone, we also determined whether the RAS remained a contributor to increased BP in male growth-restricted offspring at 12 mo of age. The RAS plays a critical role in regulation of salt and water balance (19), and numerous experimental studies indicate that expression of the RAS is altered in response to a developmental insult (29). Male children born small for gestational age have increased circulating levels of ANG II and ACE activity that correlate to elevated BP compared with children born appropriate for gestational age (15). Whether increases in expression of the RAS persist into adulthood following low birth weight within the human population is not yet known. We previously reported that renal renin and angiotensinogen mRNA expressions are suppressed in newborn male growth-restricted offspring relative to male control at birth, are not altered in hypertensive male growth-restricted relative to male controls before puberty, but are significantly elevated in conjunction with increased ACE mRNA expression and activity in male growth-restricted offspring that remain hypertensive at 4 mo of age (18). Suppression of the intrarenal expression of the RAS at birth is reported in male growth-restricted offspring exposed to maternal protein restriction during fetal life (42, 44); however, mRNA expression for the renal AT1aR but not renal ANG II is increased at 1 mo of age in protein-restricted offspring (42). Plasma renin activity is decreased with age in normotensive populations (3) and intrarenal expressions of renin and ACE mRNA are decreased in male SD rats from 3 to 12 mo (22). However, the temporal expression and the relative importance of the RAS as it relates to age-matched changes in BP across the lifespan following a developmental insult are not yet reported. In the present study, cortical mRNA expression of angiotensinogen was higher in control offspring at 18 relative to 12 mo of age. Medullary mRNA expression of ACE was also higher in control offspring at 18 mo of age relative to 12-mo control when BP was elevated at 18 mo of age relative to BP in control at 12 mo. Thus, these results suggest that the age-related increase in BP in male control at 18 mo of age was associated with an age-related increase in expression of medullary angiotensinogen and ACE. Previously, using dot blot technology Jung et al. (22) reported no change in renal ACE at 3 vs. 12 mo of age in the male SD rat; whether ACE was elevated at 18 mo relative to 12 mo of age was not determined. Male growth-restricted offspring demonstrated a significant increase in cortical renin mRNA expression in association with the significant increase in BP at 12 mo of age relative to age-matched controls. However, cortical renin mRNA expression was lower at 18 mo of age in growth-restricted offspring relative to 12-mo counterparts. Medullary mRNA expression of angiotensinogen was also elevated in hypertensive growth-restricted offspring relative to age-matched normotensive control at 12 mo of age but this increase was not observed at 18 mo of age. Although blockade of the RAS decreased BP in growth-restricted rats at 12 mo of age, it did not abolish the significant increase in BP in growth-restricted rats relative to enalapril-treated controls at 12 mo of age. Thus, these data indicate that the RAS contributes to the increase in BP in male growth-restricted rats at 12 mo of age, but other mechanisms also play a contributory role. Additionally, data from this study indicate that increased expression of the RAS was present in the absence of increased testosterone at 12 mo of age and that temporal changes in expression of the RAS continue across the lifespan following a developmental insult.
Through the natural aging process, glomerular filtration rate (GFR) declines with age (10) in association with a decrease in nephron number (36). Nephron number is reduced in many experimental models of developmental programming (34, 44). We previously reported that GFR is not reduced in male growth-restricted offspring at 4 mo of age (1), an observation also noted in young adult male offspring programmed by in utero exposure to other developmental insults (34, 44). Glomerular hypertrophy sustains GFR in the presence of reduced nephron number in male rats exposed to maternal protein restriction (44), suggesting that male offspring exposed to a developmental insult are at an increased risk for an accelerated age-related decline in renal function that may also contribute to age-dependent increases in BP. Renal function was not determined in the present study. Whether nephron number is reduced in our model at 4 mo of age or with aging up to 12 or 18 mo of age is not yet known, but an age-related decline in renal function that is accelerated in male growth-restricted offspring may be another potential mechanism that contributes to accelerated CV aging following a developmental insult.
The prevalence of hypertension in the elderly approaches 60 to 80%. Yet, appropriate therapeutic regiments for treatment of age-associated increases in BP are difficult to recommend due to the lack of investigation into BP control in the elderly. The etiology of increased BP and CV risk in elderly patients exposed to a developmental insult is less understood. A recent study by Luu et al. (27) suggests that physicians consider gestational age and birth weight in the prevention and management of chronic health. Thus, further studies are needed to explore the etiology of accelerated CV aging and increased CV risk that has its origins in early life to properly manage hypertension in the elderly that were born low birth weight or preterm.
GRANTS
B. T. Alexander is supported by the American Heart Grant GRNT19900004 and National Institutes of Health (NIH) Grants HL074927 and HL51971. S. Intapad is supported by funding from the American Heart Association (AHA), 12POST1198002, and NIH Grant P20GM104357. J. H. Dasinger is supported by funding from the AHA, 15PRE24700010, and NIH Grant T32HL105324.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
J.H.D. and B.T.A. conception and design of research; J.H.D., S.I., M.A.B., and A.J.C. performed experiments; J.H.D., S.I., and B.T.A. analyzed data; J.H.D., S.I., and B.T.A. interpreted results of experiments; J.H.D. prepared figures; J.H.D. and B.T.A. drafted manuscript; J.H.D. and B.T.A. edited and revised manuscript; J.H.D., S.I., M.A.B., A.J.C., and B.T.A. approved final version of manuscript.
REFERENCES
- 1.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension 41: 457–462, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Allen N, Berry JD, Ning H, Van Horn L, Dyer A, Lloyd-Jones DM. Impact of blood pressure and blood pressure change during middle age on the remaining lifetime risk for cardiovascular disease: the cardiovascular lifetime risk pooling project. Circulation 125: 37–44, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson S. Ageing and the renin-angiotensin system. Nephrol Dial Transplant 12: 1093–1094, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ, Osmond C, Kajantie E, Eriksson JG. Growth and chronic disease: findings in the Helsinki birth cohort. Ann Hum Biol 36: 445–458, 2009. [DOI] [PubMed] [Google Scholar]
- 5.Baserga M, Bares AL, Hale MA, Callaway CW, McKnight RA, Lane PH, Lane RH. Uteroplacental insufficiency affects kidney VEGF expression in a model of IUGR with compensatory glomerular hypertrophy and hypertension. Early Hum Dev 85: 361–367, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceravolo GS, Franco MC, Carneiro-Ramos MS, Barreto-Chaves ML, Totes RC, Nigro D, Fortes ZB, Carvalho MH. Enalapril and losartan restored blood pressure and vascular reactivity in intrauterine undernourished rats. Life Sci 80: 782–787, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Chen YF, Naftilan AJ, Oparil S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension 19: 456–463, 1992. [DOI] [PubMed] [Google Scholar]
- 8.Davies AA, Smith GD, May MT, Ben-Shlomo Y. Association between birth weight and blood pressure is robust, amplifies with age, and may be underestimated. Hypertension 48: 431–436, 2006. [DOI] [PubMed] [Google Scholar]
- 9.de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta-analysis of preterm birth and later systolic blood pressure. Hypertension 59: 226–234, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis 2: 19–28, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duggan J, Nussberger J, Kilfeather S, O'Malley K. Aging and human hormonal and pressor responsiveness to angiotensin II infusion with simultaneous measurement of exogenous and endogenous angiotensin II. Am J Hypertens 6: 641–647, 1993. [DOI] [PubMed] [Google Scholar]
- 12.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 87: 578–598, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Fogari R, Preti P, Zoppi A, Fogari E, Rinadli A, Corradi L, Mugellini A. Serum testosterone levels and arterial blood pressure in the elderly. Hypertens Res 28: 625–630, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Franceschi C, Motta L, Motta M, Malaguarnera M, Capri M, Vasto S, Candore G, Caruso C, IMUSCE. The extreme longevity: the state of the art in Italy. Exp Gerontol 43: 46–52, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Franco MCP, Casarini DE, Carneiro-Rams MS, Sawaya AL, Barreto-Chaves MLM, Sesso R. Circulating renin-angiotensin system and catecholamines in childhood: is there a role for birthweight? Clin Sci (Lond) 114: 375–380, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Gamborg M, Byberg L, Rasmussen F, Andersen PK, Baker JL, Bengtsson C, Canoy D, Drøyvold W, Eriksson JG, Forsén T, Gunnarsdottir I, Järvelin MR, Koupil I, Lapidus L, Nilsen TI, Olsen SF, Schack-Nielsen L, Thorsdottir I, Tuomainen TP, Sørensen TI, NordNet Study Group. Birth weight and systolic blood pressure in adolescence and adulthood: meta-regression analysis of sex- and age-specific results from 20 Nordic studies. Am J Epidemiol 166: 634–645, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Goeres LM, Williams CD, Eckstrom E, Lee DS. Pharmacotheraphy for hypertension in older adults: a systemic review. Drugs Aging 31: 897–910, 2014. [DOI] [PubMed] [Google Scholar]
- 18.Grigore D, Ojeda NB, Robertson EB, Dawson AS, Huffman CA, Bourassa EA, Speth RC, Brosnihan KB, Alexander BT. Placental insufficiency results in temporal alterations in the renin angiotensin system in male hypertensive growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 293: R804–R811, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyton AC, Coleman TG, Cowley AV Jr, Scheel KW, Manning RD Jr, Norman RA Jr.. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 52: 584–594, 1972. [DOI] [PubMed] [Google Scholar]
- 20.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR, Baltimore Longitudinal Study of Aging. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86: 724–731, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Intapad S, Dasinger JH, Brown AD, Fahling JM, Esters J, Alexander BT. Glucose intolerance develops prior to increased adiposity and accelerated cessation of estrous cyclicity in female growth-restricted rats. Pediatr Res 79: 962–970, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung FF, Kennefick TM, Ingelfinger JR, Vora JP, Anderson F. Downregulation of the intrarenal renin-angiotensin system in the aging rat. J Am Soc Nephrol 5: 1573–1580, 1995. [DOI] [PubMed] [Google Scholar]
- 23.Juonala M, Cheung MM, Sabin MA, Burgner D, Skilton MR, Kahonen M, Hutri-Kahonen N, Lehtimaki T, Jula A, Laitinen T, Jokinen E, Taittonen L, Tossavainen P, Viikari JS, Magnussen CG, Raitakari OT. Effect of birth weight on life-course blood pressure levels among children born premature: the Cardiovascular Risk in Young Finns Study. J Hypertens 33: 1542–1548, 2015. [DOI] [PubMed] [Google Scholar]
- 24.Law CM, de Swiet M, Osmond C, Fayers PM, Barker DJ, Cruddas AM, Fall CH. Initiation of hypertension in utero and its amplification throughout life. BMJ 306: 24–27, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loria AS, Yamamoto T, Pollock DM, Pollock JS. Early life stress induces renal dysfunction in adult male rats but not female rats. Am J Physiol Regul Integr Comp Physiol 304: R121–R129, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lurbe E, Torro MI, Carvajal E, Alvarez V, Redon J. Birth weight impacts on wave reflections in children and adolescents. Hypertension 41: 646–650, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Luu TM, Katz SL, Leeson P, Thebaud B, Nuyt AM. Preterm birth: risk factor for early-onset chronic diseases. CMAJ pii: cmaj. 150450, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manning J, Vehaskari VM. Postnatal modulation of prenatally programmed hypertension by dietary Na and ACE inhibition. Am J Physiol Regul Integr Comp Physiol 288: R80–R84, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Moritz KM, Cuffe JS, Wilson LB, Dickinson H, Wlodek ME, Simmons DG, Denton KM. Sex specific programming: a critical role for the renal renin-angiotensin system. Placenta 31: S40–S46, 2010. [DOI] [PubMed] [Google Scholar]
- 30.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wiley JZ, Woo D, Yeh RW, Turner MB, . American Heart Association Statistics Committee, Stroke Statistics Committee. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation 131: e29–e322, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Odden MC, Shlipak MG, Whitson HE, Katz R, Kearney PM, defilippi C, Shastri S, Sarnak MJ, Siscovick DS, Cushman M, Psaty BM, Newman AB. Risk factors for cardiovascular disease across the spectrum of older age: The Cardiovascular Health Study. Atherosclerosis 237: 336–342, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ojeda NB, Grigore D, Yanes LL, Iliescu R, Robertson EB, Zhang H, Alexander BT. Testosterone contributes to marked elevations in mean arterial pressure in adult male intrauterine growth restricted offspring. Am J Physiol Regul Integr Comp Physiol 292: R758–R776, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Ojeda NB, Royals TP, Black JT, Dasinger JH, Johnson JM, Alexander BT. Enhanced sensitivity to acute angiotensin II is testosterone dependent in adult male growth-restricted offspring. Am J Physiol Regul Integr Comp Physiol 298: R1421–R1427, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oritz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension 41: 460–467, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rodriguez-Gonzalez GL, Reyes-Castro LA, Vega CC, Boeck L, Ibanez C, Nathanielsz PW, Larrea F, Zambrano E. Accelerated aging of reproductive capacity in male rat offspring of protein-restricted of mothers is associated with increased testicular and sperm oxidative stress. Age (Dordr) 36: 9721, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rule AD, Semret MH, Amer H, Cornell LD, Taler SJ, Lieske JC, Melton LF 3rd, Stegall MD, Textor SC, Kremers WK, Lerman LO. . Association of kidney function and metabolic risk factors with density of glomeruli on renal biopsy samples from living donors. Mayo Clin Proc 86: 282–290, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saez F, Reverte V, Paliege A, Moreno JM, Llinas MT, Bachmann S, Salazar FJ. Sex-dependent hypertension and renal changes in aged rats with altered renal development. Am J Physiol Renal Physiol 307: F461–F470, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Svartberg J, von Muhlen D, Schirmer H, Barrett-Connor E, Sundfjord J, Jorde R. Association of endogenous testosterone with blood pressure and left ventricular mass in men. The Tromso Study. Eur J Endocrinol 150: 65–71, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Tank JE, Vora JP, Houghton DC, Anderson S. Altered renal vascular responses in the aging rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 266: F942–F948, 1994. [DOI] [PubMed] [Google Scholar]
- 40.Tao H, Rui C, Zheng J, Tang J, Wu L, Shi A, Chen N, He R, Wu C, Li J, Yin X, Zhang P, Zhu Z, Tao J, Xiao J, Mao C, Xu Z. Angiotensin II-mediated vascular changes in aged offspring rats exposed to perinatal nicotine. Peptides 44: 111–119, 2013. [DOI] [PubMed] [Google Scholar]
- 41.Troen BR. The biology of aging. Mt Sinai J Med 70: 3–22, 2003. [PubMed] [Google Scholar]
- 42.Vehaskari VM, Stewart T, Lafont D, Soyez C, Seth D, Manning J. Kidney angiotensin and angiotensin receptor expression in prenatally programmed hypertension. Am J Physiol Renal Physiol 287: F262–F267, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Wlodek ME, Mibus A, Tan A, Siebel AL, Owens JA, Moritz KM. Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J Am Soc Nephrol 18: 1688–1696, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adulthypertension in rats. Pediatr Res 49: 460–467, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Yanes LL, Sartori-Valinotti JC, Iliescu R, Romero DG, Racusen LC, Zhang H, Reckelhoff JF. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am J Physiol Renal Physiol 296: F771–F779, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yoon SS, Carroll MD, Fryer CD. Hypertension prevalence and control among adults: United States, 2011–2014. NCHS Data Brief 220: 1–8, 2015. [PubMed] [Google Scholar]