Abstract
The Hippo signaling pathway is an evolutionarily conserved kinase cascade, playing multiple roles in embryonic development that controls organ size, cell proliferation, and apoptosis. At the center of this network lie the Hippo kinase target and downstream pathway effector Yes-associated protein (YAP) and its paralog TAZ. In its phosphorylated form, cytoplasmic YAP is sequestered in an inactive state. When it is dephosphorylated, YAP, a potent oncogene, is activated and relocates to the nucleus to interact with a number of transcription factors and signaling regulators that promote cell growth, differentiation, and survival. The identification of YAP activation in human cancers has made it an attractive target for chemotherapeutic drug development. Little is known to date about the function of the Hippo pathway in the kidney, but that is rapidly changing. Recent studies have shed light on the role of Hippo-YAP signaling in glomerular and lower urinary tract embryonic development, maintenance of podocyte homeostasis, the integrity of the glomerular filtration barrier, regulation of renal tubular cyst growth, renal epithelial injury in diabetes, and renal fibrogenesis. This review summarizes the current knowledge of the Hippo-YAP signaling axis in the kidney under normal and disease conditions.
Keywords: Hippo, kidney, podocyte
like other well-known signal transduction pathways such as WNT, transforming growth factor (TGF)-β, and EGFR, the Hippo pathway transfers plasma membrane signals to the nucleus, resulting in altered gene expression profiles that control cell survival. The pathway was initially identified in Drosophila during a genetic screen for mutations that cause clonal overgrowth (27, 70). It is named after the Ste20-like protein kinase Hippo (Hpo), whose inactivation in flies results in a phenotype resembling a hippopotamus with enlarged folded eyes and excess head cuticle (Fig. 1, A and B) (18, 69). Conditional silencing of the mammalian ortholog of Hpo, Mst, results in hepatomegaly (Fig. 1, C and D) and hepatocellular carcinoma (Fig. 2, A and B) (12). Significant attention is being directed toward the development of pharmacological agents that inhibit YAP function (26, 61). These developments are highly relevant to kidney researchers and practicing nephrologists, where the role of YAP specifically and the Hippo pathway in general are only recently being deciphered. With free passage of molecules <40 kDa, kidney glomerular and tubular components are susceptible to injury from novel therapeutic agents that target ubiquitous Hippo components potentially essential for maintaining normal tissue homeostasis. Conversely, pharmacological manipulation of the Hippo pathway could provide therapeutic benefit for some renal disorders. Discussed in this review is a summary of what is currently known about the role of the pathway in kidney development, homeostasis, and disease.
Fig. 1.
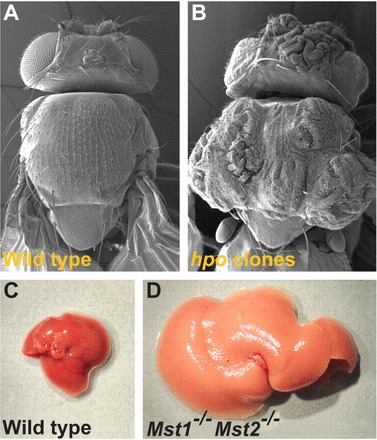
Hippo-mutant phenotypes in Drosophila and mice. Electron micrographs of wild-type (A) and homozygous mutant Hippo (Hpo) gene (B) are shown. C: mouse liver from a wild-type animal at 2 mo of age. D: mouse liver from a Mst1−/−Mst2−/− conditional knockout animal. This image was reproduced from Ref. 18 with permission (Company of Biologists, Ltd., 2011).
Fig. 2.
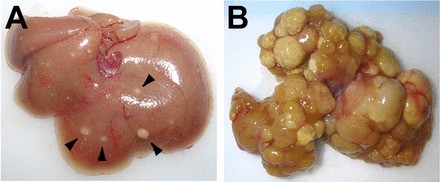
Dysregulation of the mammalian Hippo pathway leads to tumorigenesis in vivo. A: liver from an ApoE/rtTA-YAP mouse raised on Dox for 8 wk, starting at 3 wk after birth. Note the presence of discrete nodules scattered throughout the liver (arrowheads). B: liver from an ApoE/rtTA-YAP mouse raised on Dox for 3 mo, starting at birth. Note the widespread development of hepatocellular carcinoma throughout the liver. This image was reproduced from Ref. 12 with permission (Elsevier, 2012).
Overview of the Pathway
The core kinases in mammals are the Hpo ortholog Mst1/2 in a complex with its regulatory protein SAV1, acting upstream of Lats1/2 also in a complex with regulatory Mob1 (Fig. 3). MST is activated in a phosphorylation-dependent manner by upstream tumor suppressors NF2/Merlin and KIBRA (1, 16, 22, 40, 72, 76). MST phosphorylation in turn induces phosphorylation of LATS, which induces the phosphorylation and sequestration of the ultimate pathway target YAP or TAZ in the cytoplasm (19, 25, 46, 75). In this sense, an “active” Hippo pathway is one in which YAP/TAZ are phosphorylated in an inactive state in the cytoplasm. An “inactive” pathway is one where YAP/TAZ are dephosphorylated and expressed in the nucleus where they bind one of four TEAD transcription factors (TEAD1-4). The YAP/TAZ-TEAD transcription complex in turn mediates context-dependent transcriptional gene expression such as ctgf and others involved in cell survival, cell polarity, and cell fate determination (74, 77, 78). Several components of the pathway have been implicated in mammalian models of tumorigenesis. Nf2 is a tumor suppressor gene responsible for neurofibromatosis type II. Nf2 loss is associated with a wide range of tumors, including osteosarcoma and hepatocellular carcinoma (39). Lats1 deficiency results in soft tissue sarcomas and ovarian tumors (62). Mst-deficient mice develop hepatocellular carcinoma, cholangiocarcinoma, and colonic adenoma (37, 60, 80). Mob1 mutant mice develop skin, liver, and breast cancer as well as osteosarcomas and fibrosarcomas (44). Yap is an oncogene that is amplified in hepatocellular and squamous cell carcinomas, gastrointestinal dysplasia, and pancreatic duct metaplasia (4, 12, 55, 73).
Fig. 3.
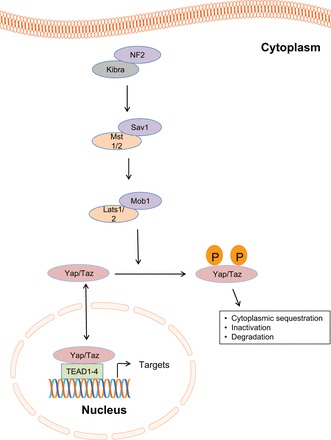
The core Hippo pathway. KIBRA/NF2 promote phosphorylation and activation of MST 1/2 and Sav1. This in turn phosphorylates and activates LATS1/2 and MOB1. LATS 1/2 phosphorylates YAP/TAZ, leading to its cytoplasmic sequestration, inactivation, and degradation. When YAP/TAZ are dephosphorylated, they enter the nucleus to induce gene transcription by interacting with TEAD1-4 transcription factors.
Kidney Development
Kidney development requires a complex, well-orchestrated signaling interplay between the epithelial ureteric bud (UB) and the surrounding metanephric mesenchyme (11, 13, 36, 52). The mesenchyme promotes UB branching, resulting in renal collecting duct formation. Cap mesenchyme (CM) cells are in turn induced by the UB to form a pretubular aggregate (PA), which are mesenchymal preepithelial cells that form the renal vesicle (RV). The RV, the most primitive stage of nephron development, results in subsequent elongation to a comma-shaped and then S-shaped body, making contact and fusing with the distal ureteric bud. The S-shaped body undergoes elongation and differentiation in association with invasion of endothelial cells to give rise to the glomerulus and various nephron segments. The signaling mediators critical in this intricate morphogenetic process remain poorly understood. The role of the Hippo pathway in nephrogenesis has been the subject of recent attention. Constitutive Yap deletion results in embryonic lethality at embryonic day 8.5 (E8.5) with a failure to develop an organized yolk sac vascular plexus (42). Conditional gene silencing approaches have therefore been utilized to define the role of YAP and TAZ at various stages in kidney development. Animals in which Yap is silenced at the CM do not survive beyond 48 h postbirth (49). The kidneys from these animals show a multitude of anatomical and histological anomalies: hypoplastic kidneys and an empty bladder, suggesting failure to produce urine; smaller papilla and a reduced nephrogenic zone; indistinguishable convoluted renal tubules and glomeruli in the inner cortex; a medulla mainly composed of collecting ducts; a dramatic reduction in the number of detectable glomeruli and proximal tubules; abnormal structures in the glomeruli; defects in Henle's loop and distal tubule formation; and abnormal morphogenesis of S-shaped bodies. The loss of Yap also significantly reduced the number of ureteric bud tips at P0, revealing a late-onset function of Yap in branching morphogenesis in the developing nephron. These data support the conclusion that Yap-depleted CM cells are unable to undergo normal nephrogenesis and morphogenesis during renal development. Interestingly, the loss of the Rho-GTPase Cdc42 from the CM results in reduced YAP activation and decreased Yap-dependent gene expression that phenocopies YapCM−/− cells (49).
In contrast to YapCM−/− kidneys, deletion of Taz in the CM does not result in dysplastic kidneys or defective nephrogenesis. TazCM−/− do, however, display highly cystic cortical tubules. These investigators also silenced Taz in YapCM−/− mice, generating double YapCM−/−; TazCM−/− mutants, which did not have an exacerbation of the YapCM−/− phenotype. Yap and Taz therefore appear to have divergent but critical roles in nephrogenesis (49).
Lower urinary tract.
A crucial step in urinary tract maturation is the movement of the ureter from its initial branch point on the nephric duct (ND) to its final insertion site in the cloaca (primitive bladder and urethra) (10, 45). Mutations in the tyrosine kinase receptor Ret result in delay or failure of the ND to insert into the cloaca (10). Approximately 5% of living patients with congenital abnormalities of the kidney and urinary tract (CAKUT) harbor mutations in the RET pathway (7). Astonishingly, Ret mutations are present in 30% of fetuses with bilateral or unilateral renal agenesis (59). Silencing of Yap in the ND in mice results in death within 24 h of birth due to severe anomalies of the kidney and urinary tract, including hydroureter and nephrosis (48). This phenotype mimics hyperactive Ret signaling accompanied by increased expression of Ret downstream target genes. Interestingly, the YapND−/− phenotype is largely reduced by decreasing Ret gene dosage (48). YAP could therefore be a potential modifier of Ret in human CAKUT.
YAP and TAZ appear to have some redundant functions in urinary tract development. TazND−/− kidneys develop normally, suggesting that YAP expression is sufficient in the absence of TAZ. However, silencing of Taz in YapND−/− mice severely worsens the phenotype, with kidneys being dysplastic, showing no ureters at E18.5, aberrant branching morphogenesis, blind-ending NDs, a defective ND-cloaca connection, and markedly increased apoptosis (48). These findings highlight the crucial role YAP and TAZ play in lower urinary tract development and lay the groundwork for further diagnostic, mechanistic, and therapeutic approaches to CAKUT. The synergistic roles of Yap and Taz in lower urinary tract development also contrast with their distinct ones in nephrogenesis and highlight the cell- and context-dependent nature of their signaling properties.
Glomerulus
To date, the role of the Hippo signaling pathway in regulating glomerular endothelial, mesangial, or parietal cell function has not been determined. Some studies have been published to date, however, on Hippo signaling in glomerular visceral epithelial cells or podocytes. Podocytes are terminally differentiated cells that form the final barrier to urinary protein loss, and podocyte injury is usually associated with proteinuria (17, 33, 34). Persistence of podocyte injury results in loss of podocytes, then leading to irreversible glomerulosclerosis and kidney failure (29, 32, 67). The roles of YAP and KIBRA have been explored in podocyte homeostasis. YAP is present in dual compartments in podocytes, with abundant baseline nuclear and notable cytoplasmic expression (5). YAP inhibits podocyte apoptosis by binding to and inhibiting the proinjury signaling molecule dendrin. Yap gene silencing makes cultured podocytes more susceptible to adriamycin- and staurosporine-induced injury (5). In vivo, podocyte-specific Yap deletion results in proteinuria between 5 and 6 wk of age and histological lesions characteristic of FSGS at 12 wk (Fig. 4) (57). Yap-deficient podocytes undergo apoptosis, resulting in progressive podocyte depletion (57). Taken together these findings highlight the role of YAP in maintaining an intact adult glomerular filter. The cell- and context-dependent consequence of YAP silencing is once again reinforced where Six2-mediated Yap deletion in the CM impairs nephrogenesis without evident apoptosis (49) while podocin-Cre-mediated Yap deletion results in normal development but a later onset podocytopathy associated with apoptosis and podocyte depletion (57).
Fig. 4.
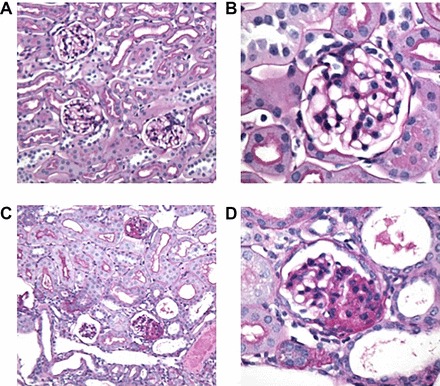
Periodic acid-Schiff (PAS) stainings of Yap-knockout (KO) and control (Ctrl) mice at 12 wk. A and B: Ctrl kidney sections at low and high power. C: Yap-KO mice with focal glomerulosclerosis and tubular dilatation and casts. D: Yap-KO mouse with a lesion of focal segmental glomerulosclerosis. This image was reproduced from Ref. 57 with permission (American Society of Nephrology, 2016).
KIBRA (“kidney brain”) protein, an upstream regulator of YAP via activation of the Hippo kinases, is important in podocyte homeostasis. KIBRA was initially characterized by a yeast two-hybrid screen as a putative binding partner for dendrin (31). KIBRA shares dendrin's role as a growth inhibitor and promoter of apoptosis as initially shown in Drosophila where KIBRA overexpression in imaginal disks results in decreased size of the adult Drosophila eye (72). Similar to other upstream members of the mammalian Hippo pathway, KIBRA antagonizes YAP function. In the MCF-10A human breast cancer cell line, KIBRA inhibits YAP-dependent growth and migration in these cells, suggesting that it may serve as a tumor suppressor (40). Two studies have investigated KIBRA signaling in podocytes. The first, by Duning et al. (14) in 2008, identified KIBRA's presence in podocytes of the glomerular tuft and in renal tubular cells. This study demonstrated that KIBRA interacts with the actin-bundling protein synaptopodin and the cell polarity protein PATJ. The functional impact of these interactions was highlighted by the finding that silencing the Wwc1 gene that encodes KIBRA in human podocytes resulted in impaired directed migration based on the reduced ability of knockdown podocytes to close a wound in a wound-healing assay (14). Despite increased velocity, the KIBRA-knockdown podocytes had impaired polarity that disrupted their capacity for directed cell migration. Further studies will be needed to clarify the role of KIBRA in the context of podocyte actin cytoskeleton integrity and its interaction with modulators of podocyte motility and polarity (15, 71).
Recently, it was demonstrated that overexpression of KIBRA in human podocytes activates LATS kinase, inducing the phosphorylation and cytoplasmic sequestration of YAP (66). Treatment of podocytes with latrunculin B, which disrupts the actin cytoskeleton, led to similar changes in podocytes as KIBRA overexpression, namely, an increase in the levels of phosphorylated LATS and the cytoplasmic redistribution and phosphorylation of YAP. KIBRA overexpression in podocytes was also associated with increased levels of apoptosis (66). This study further highlights the existence of canonical Hippo signaling in podocytes and its role in podocyte survival and homeostasis. KIBRA, like its binding partner dendrin, promotes podocyte injury through enhanced apoptosis.
Tubules and Interstitium
Hippo in cystic kidney disease.
Hippo signaling is also relevant in cystic kidney disease. A study by Hossain et al. (24) from 2006 demonstrated that constitutive Taz null adult mice presented with cystic kidney disease by 8 wk of age, without evidence of cyst formation in other organs (24). Interestingly, most of the renal cysts were of glomerular origin, with a minority derived from the collecting duct and proximal and distal tubules. These knockout mice developed progressive renal insufficiency by 4–6 mo of age. The renal cysts were characterized by abnormalities of the epithelial cilia, including shortened ciliary length and structural abnormalities, and some epithelial cells lining the cystic or dilated ducts lacked cilia completely. The importance of TAZ/Wwtr1 to ciliary integrity and cyst formation was further suggested by the corresponding decreased expression of PKD-associated genes that have also been linked to the renal ciliary system, including khd1, Tg737, Kif3a, and well as Tsc1 (tuberous sclerosis complex), which has been associated with glomerular cyst development (24).
The link between Taz deletion and the development of cystic renal disease was further demonstrated with a Taz-null/lacZ knockin mouse (38). These animals also developed cystic renal disease, with cysts mainly derived from glomeruli and proximal tubular epithelia, as well as dilated air spaces in the lung resembling human pulmonary emphysema. As observed in human PKD, these Taz-null/lacZ knockin mutants also had urinary concentration defects, polyuria, and hydronephrosis. Interestingly, here there were no changes in expression levels of genes involved in human polycystic kidney disease (PKD), including autosomal dominant PKD (ADPKD)-associated polycystin-1 (Pkd1) and polycystin-2 (Pkd2), and Pkhd1, which has been linked to ARPKD (autosomal recessive PKD).
Taz serves as a transcriptional coactivator of Glis3, a subfamily of Kruppel-like zinc finger proteins that contain a DNA binding domain and regulate gene transcription (2, 28). Similar to Taz knockout mice, Glis3-deleted mice develop renal cysts mostly from glomeruli but also from proximal and distal tubules and collecting ducts. In addition, Glis3 localized to primary cilia, and a significant number of epithelial cells in renal cysts either lacked primary cilia or had truncated primary cilia. Thus it is possible that TAZ interacts with Glis3 to suppress cyst formation.
Another interesting observation lies with the signaling properties of cysts in Taz null mice. Wnt/β-catenin signaling has been implicated in cyst formation in PKD. Activated mutant β-catenin gene expression results in a phenotype that mimics human ADPKD (51). Like cysts in humans with ADPKD (35), kidney cysts in Taz null mice display enhanced Wnt/β-catenin signaling (65). This provides evidence of cross talk between Wnt and Hippo signaling pathways that has been further validated in other model systems such as colorectal carcinoma and in osteogenesis (30, 58). It also provides a potential mechanistic explanation for cyst formation in Taz-null mice.
Epithelial-specific deletion of the Pkd1 gene in mice, which results in renal cystogenesis, is associated with the development of aberrations in Four-jointed 1 (Fjx1), a regulator of the Hippo pathway (20, 68). Fjx1 is also a member of the planar cell polarity (PCP) signaling pathway, which is normally active during the repair phase of epithelial injury but displays abnormalities in the setting of Pkd1 deletion (20). Specifically, Fjx1 expression is reduced in the kidneys of Pkd1-deleted mice in the precystic stages but later increased significantly in the cystic kidneys. The same investigators found that in adult conditional Pkd1-deleted mice (cKO), nuclear localization of YAP was present in the cystic epithelial cells that developed in the proximal tubules 10 wk after injury with the nephrotoxin 1,2-dichlorovinylcysteine (DCVC). Strong nuclear YAP expression was detected in cystic epithelial cells using an alternate model, the Pkd1 null mice, which develop renal cysts derived mostly from the distal tubules or collecting ducts by 3 wk of age. Analysis of gene expression levels of YAP target genes revealed significant upregulation of baculoviral IAP-repeat-containing-3 (Birc-3) and inhibin β-A (InhbA) in the cystic stages of the cKO mice and in cystic kidneys of the Pkd1 null mice. The findings from animal studies turned out to be relevant to human pathology, as evidenced by YAP staining of tissue from renal biopsies. Nuclear accumulation of YAP was found in cystic epithelial cells in human ADPKD and ARPKD, as well as in cysts associated with various renal tumors, including clear-cell renal cell carcinoma (ccRCC), papillary renal cell carcinoma, and renal mixed epithelial and stromal tumors. In contrast, no nuclear YAP was present in the proximal tubular epithelial cells from normal human biopsy renal tissue (21).
In summary, there is emerging evidence that the Hippo signaling pathway plays a role in the development of PKD. Cross talk between the Hippo and Wnt signaling pathways appears to regulate cyst growth. The expression of TAZ and the nuclear/cytoplasmic distribution of YAP are key regulatory determinants of cystogenesis.
Diabetic kidney disease.
Diabetics are at high risk for renal disease progressing to end-stage renal disease (3). The pathogenesis of disease and factors that distinguish progressors from nonprogressors remain unclear. Like the Hippo pathway, the epidermal growth factor receptor (EGFR) pathway was initially proposed as a target for cancer therapy (54). It has since been implicated in diabetic kidney disease. Prolonged EGFR activation in diabetic animals enhances TGF-β-mediated renal injury, while attenuation of EGFR signaling is protective (8, 47, 50, 53). A recently published study establishes a link between EGFR and Hippo signaling in the diabetic kidney (9). YAP expression and phosphorylation were found to be increased in animal models of type 1 (streptozotocin-induced) and type 2 (db/db) diabetes, as well as in proximal tubule-like epithelial cells exposed to high glucose for 24 h. These effects were attenuated by in vivo and in vitro silencing of EGFR expression as well as treatment of mice and cells with the EGFR inhibitor erlotinib. In mice with proximal tubule EGFR deletion, TAZ expression was decreased, indicating differential upstream regulation of the paralogs YAP and TAZ. Another interesting study finding with respect to the pathogenesis of diabetic kidney disease was the observation that expression of CTGF and another YAP transcriptional target gene, amphiregulin (AREG), was increased after high-glucose treatment. The high-glucose effects on CTGF and AREG were blunted after either Egfr or Yap was silenced by their specific small interfering (si) RNA and also after treatment with vertoporforin, which is an inhibitor of YAP-TEAD interaction (9). These findings suggest that the diabetic milieu promotes differential YAP expression, phosphorylation, and target gene expression in an EGFR-dependent manner.
Renal cell carcinoma.
Aberrant Hippo pathway signaling resulting in hyperactive YAP function has been detected in various human cancers, including those affecting the kidney. Nuclear overexpression of YAP has been detected in a subset of patients with ccRCC (56). Yap silencing also reduces proliferation, migration, and anchorage-independent growth of ccRCC cells (56). Yap mRNA and protein expression levels are also increased in ccRCC tissue and cell lines. Furthermore, Yap silencing in 786-0 ccRCC lines results in cell cycle arrest and increased apoptosis (6). Targeted deletion of the Nf2 gene that encodes the upstream Hippo pathway regulator Merlin in proximal convoluted epithelium results in intratubular neoplasia that progresses to invasive carcinoma (43). Interestingly, early lumen-filling lesions in this model exhibited hyperactive EGFR signaling with EGFR inhibition halting tumor cell proliferation (43). These findings highlight the potentially deleterious effects of unrestrained YAP signaling and provide further confirmation of cross talk between the Hippo and EGFR pathways.
Renal fibrogenesis.
Renal fibrosis, arising as a result of numerous divergent processes, culminates in the deposition of extracellular matrix that classically accompanies chronic kidney disease (23). Stiff extracellular matrix enhances TGF-β-induced profibrotic Smad signaling in a process mediated by YAP and TAZ (63). In the unilateral ureteral obstruction (UUO) model of renal fibrosis, YAP/TAZ display nuclear expression unlike sham-operated controls. Furthermore, verteporfin, a drug that inhibits YAP-TEAD interaction and YAP transcriptional activity, reduces interstitial YAP/TAZ staining, associated nuclear Smad 2/3 nuclear accumulation, and renal fibrosis (63). An antibody that recognized both YAP/TAZ was used here, so a distinction cannot be made regarding divergent functions of the two molecules. This study identified a potential therapeutic strategy to target both classic Smad 2/3 and mechanosensitive YAP/TAZ in renal fibrogenesis.
Table 1.
Hippo pathway modulators of kidney disease
| Hippo Mediator | In Vitro Data | Mouse Model Phenotype | Human Kidney Disease | Reference(s) |
|---|---|---|---|---|
| KIBRA | 1. KIBRA silencing impairs human podocyte polarity; | ? | ? | 14, 66 |
| 2. KIBRA overexpression promotes podocyte apoptosis. | ||||
| Mst | ? | ? | ? | |
| Lats | 1. LATS promotes YAP phosphorylation in podocytes. | ? | ? | 66 |
| 2. High glucose induces LATS phosphorylation in proximal tubular epithelial cells. | ||||
| YAP | 1. Promotes podocyte survival. | 1. Constitutive YAP KO → embryonic lethal. | 1. FSGS: reduced podocyte YAP. | 5, 9, 48, 49, 56, 57, 63 |
| 2. Proximal tubule epithelial cells exposed to high glucose → increased YAP expression. | 2. Cap mesenchyme YAP deletion → Death at 48 h postbirth, hypoplastic kidneys, empty bladder, dramatic reduction in detectable glomeruli and proximal tubules. | 2. ADPKD and ARPKD: nuclear YAP. | ||
| 3. Rat fibroblasts grown of stiff matrix → increased nuclear YAP/TAZ. | 3. Nephric duct YAP deletion → death within 24 h; hydronephrotic kidneys with blind-ending megaureters at birth. | 3. Renal carcinoma (clear cell, papillary, renal mixed epithelial and stromal): nuclear YAP. | ||
| 4. Podocyte-specific YAP deletion → FSGS. | ||||
| 5. Streptozotocin-induced (type 1) and db/db diabetic models: YAP expression and phosphorylation increased. | ||||
| TAZ | Rat fibroblasts grown of stiff matrix → increased nuclear YAP/TAZ. | 1. Cap mesenchyme TAZ deletion → cystic cortical tubules. | ? | 9, 48, 49, 63 |
| 2. Nephric duct deletion → normal development but double YAP/TAZ deletion in nephric duct → no ureters at E18.5. | ||||
| 3. Constitutive TAZ null mice → cystic kidney disease. | ||||
| 4. Streptozotocin-induced (type 1) and db/db diabetic models: TAZ expression decreased. |
KO, knockout; FSGS, focal segmented glomerulosclerosis; ADPKD, autosomal dominant polycystic kidney disease; ARPKD, autosomal recessive polycystic kidney disease.
Conclusion
Limited but compelling evidence exists that the Hippo signaling pathway has a significant role in kidney and urinary tract development, podocyte homeostasis, fibrotic, and cystic and diabetic kidney disease. In the kidney as in other model systems, the relationship between YAP and its paralog TAZ is cell and context dependent. The Hippo pathway communicates with EGFR in diabetic kidney disease and Wnt/β-catenin in cystic kidney disease. Further studies are needed to determine the relationship between Hippo signaling and other established interacting cascades such as TGF-β and Notch. The potential utility of pharmacological manipulation of the Hippo pathway in developmental and chronic kidney disorders is yet to be determined.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.W., K.M., J.R., and K.C. drafted manuscript; J.W., K.M., and K.C. edited and revised manuscript; K.C. prepared figures; K.C. approved final version of manuscript.
REFERENCES
- 1.Baumgartner R, Poernbacher I, Buser N, Hafen E, Stocker H. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev Cell 18: 309–316, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Beak JY, Kang HS, Kim YS, Jetten AM. Functional analysis of the zinc finger and activation domains of Glis3 and mutant Glis3(NDH1). Nucleic Acids Res 36: 1690–1702, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brancati FL, Whelton PK, Randall BL, Neaton JD, Stamler J, Klag MJ. Risk of end-stage renal disease in diabetes mellitus: a prospective cohort study of men screened for MRFIT. Multiple Risk Factor Intervention Trial. JAMA 278: 2069–2074, 1997. [PubMed] [Google Scholar]
- 4.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, Brummelkamp TR. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol 17: 2054–2060, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Campbell KN, Wong JS, Gupta R, Asanuma K, Sudol M, He JC, Mundel P. Yes-associated protein (YAP) promotes cell survival by inhibiting proapoptotic dendrin signaling. J Biol Chem 288: 17057–17062, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao JJ, Zhao XM, Wang DL, Chen KH, Sheng X, Li WB, Li MC, Liu WJ, He J. YAP is overexpressed in clear cell renal cell carcinoma and its knockdown reduces cell proliferation and induces cell cycle arrest and apoptosis. Oncol Rep 32: 1594–1600, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Chatterjee R, Ramos E, Hoffman M, VanWinkle J, Martin DR, Davis TK, Hoshi M, Hmiel SP, Beck A, Hruska K, Coplen D, Liapis H, Mitra R, Druley T, Austin P, Jain S. Traditional and targeted exome sequencing reveals common, rare and novel functional deleterious variants in RET-signaling complex in a cohort of living US patients with urinary tract malformations. Hum Genet 131: 1725–1738, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen J, Chen JK, Nagai K, Plieth D, Tan M, Lee TC, Threadgill DW, Neilson EG, Harris RC. EGFR signaling promotes TGFbeta-dependent renal fibrosis. J Am Soc Nephrol 23: 215–224, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen J, Harris RC. Interaction of the EGF receptor and the Hippo pathway in the diabetic kidney. J Am Soc Nephrol 27: 1689–1700, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chia I, Grote D, Marcotte M, Batourina E, Mendelsohn C, Bouchard M. Nephric duct insertion is a crucial step in urinary tract maturation that is regulated by a Gata3-Raldh2-Ret molecular network in mice. Development 138: 2089–2097, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev Cell 18: 698–712, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, Gayyed MF, Anders RA, Maitra A, Pan D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 130: 1120–1133, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Duning K, Schurek EM, Schluter M, Bayer M, Reinhardt HC, Schwab A, Schaefer L, Benzing T, Schermer B, Saleem MA, Huber TB, Bachmann S, Kremerskothen J, Weide T, Pavenstadt H. KIBRA modulates directional migration of podocytes. J Am Soc Nephrol 19: 1891–1903, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genevet A, Wehr MC, Brain R, Thompson BJ, Tapon N. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev Cell 18: 300–308, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greka A, Mundel P. Cell biology and pathology of podocytes. Annu Rev Physiol 74: 299–323, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development 138: 9–22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hao Y, Chun A, Cheung K, Rashidi B, Yang X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J Biol Chem 283: 5496–5509, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Happe H, Leonhard WN, van der Wal A, van de Water B, Lantinga-van Leeuwen IS, Breuning MH, de Heer E, Peters DJ. Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum Mol Genet 18: 2532–2542, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Happe H, van der Wal AM, Leonhard WN, Kunnen SJ, Breuning MH, de Heer E, Peters DJ. Altered Hippo signalling in polycystic kidney disease. J Pathol 224: 133–142, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114: 457–467, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Hewitson TD. Renal tubulointerstitial fibrosis: common but never simple. Am J Physiol Renal Physiol 296: F1239–F1244, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Hossain Z, Ali SM, Ko HL, Xu J, Ng CP, Guo K, Qi Z, Ponniah S, Hong W, Hunziker W. Glomerulocystic kidney disease in mice with a targeted inactivation of Wwtr1. Proc Natl Acad Sci USA 104: 1631–1636, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122: 421–434, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Johnson R, Halder G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat Rev Drug Discov 13: 63–79, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev 9: 534–546, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Kang HS, Beak JY, Kim YS, Herbert R, Jetten AM. Glis3 is associated with primary cilia and Wwtr1/TAZ and implicated in polycystic kidney disease. Mol Cell Biol 29: 2556–2569, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim YH, Goyal M, Kurnit D, Wharram B, Wiggins J, Holzman L, Kershaw D, Wiggins R. Podocyte depletion and glomerulosclerosis have a direct relationship in the PAN-treated rat. Kidney Int 60: 957–968, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Konsavage WM Jr, Kyler SL, Rennoll SA, Jin G, Yochum GS. Wnt/beta-catenin signaling regulates Yes-associated protein (YAP) gene expression in colorectal carcinoma cells. J Biol Chem 287: 11730–11739, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kremerskothen J, Plaas C, Buther K, Finger I, Veltel S, Matanis T, Liedtke T, Barnekow A. Characterization of KIBRA, a novel WW domain-containing protein. Biochem Biophys Res Commun 300: 862–867, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int 67: 404–419, 2005. [DOI] [PubMed] [Google Scholar]
- 33.Kriz W, Lemley KV. The role of the podocyte in glomerulosclerosis. Curr Opin Nephrol Hypertens 8: 489–497, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Kriz W, Shirato I, Nagata M, LeHir M, Lemley KV. The podocyte's response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol 304: F333–F347, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Lal M, Song X, Pluznick JL, Di Giovanni V, Merrick DM, Rosenblum ND, Chauvet V, Gottardi CJ, Pei Y, Caplan MJ. Polycystin-1 C-terminal tail associates with beta-catenin and inhibits canonical Wnt signaling. Hum Mol Genet 17: 3105–3117, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Little MH, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol 4: 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lu L, Li Y, Kim SM, Bossuyt W, Liu P, Qiu Q, Wang Y, Halder G, Finegold MJ, Lee JS, Johnson RL. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci USA 107: 1437–1442, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makita R, Uchijima Y, Nishiyama K, Amano T, Chen Q, Takeuchi T, Mitani A, Nagase T, Yatomi Y, Aburatani H, Nakagawa O, Small EV, Cobo-Stark P, Igarashi P, Murakami M, Tominaga J, Sato T, Asano T, Kurihara Y, Kurihara H. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol 294: F542–F553, 2008. [DOI] [PubMed] [Google Scholar]
- 39.McClatchey AI, Saotome I, Mercer K, Crowley D, Gusella JF, Bronson RT, Jacks T. Mice heterozygous for a mutation at the Nf2 tumor suppressor locus develop a range of highly metastatic tumors. Genes Dev 12: 1121–1133, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moleirinho S, Chang N, Sims AH, Tilston-Lunel AM, Angus L, Steele A, Boswell V, Barnett SC, Ormandy C, Faratian D, Gunn-Moore FJ, Reynolds PA. KIBRA exhibits MST-independent functional regulation of the Hippo signaling pathway in mammals. Oncogene 32: 1821–1830, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, Magnuson TR, O'Neal W, Milgram SL. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol 26: 77–87, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morris ZS, McClatchey AI. Aberrant epithelial morphology and persistent epidermal growth factor receptor signaling in a mouse model of renal carcinoma. Proc Natl Acad Sci USA 106: 9767–9772, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishio M, Hamada K, Kawahara K, Sasaki M, Noguchi F, Chiba S, Mizuno K, Suzuki SO, Dong Y, Tokuda M, Morikawa T, Hikasa H, Eggenschwiler J, Yabuta N, Nojima H, Nakagawa K, Hata Y, Nishina H, Mimori K, Mori M, Sasaki T, Mak TW, Nakano T, Itami S, Suzuki A. Cancer susceptibility and embryonic lethality in Mob1a/1b double-mutant mice. J Clin Invest 122: 4505–4518, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Obara-Ishihara T, Kuhlman J, Niswander L, Herzlinger D. The surface ectoderm is essential for nephric duct formation in intermediate mesoderm. Development 126: 1103–1108, 1999. [DOI] [PubMed] [Google Scholar]
- 46.Oka T, Mazack V, Sudol M. Mst2 and Lats kinases regulate apoptotic function of Yes kinase-associated protein (YAP). J Biol Chem 283: 27534–27546, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Reddy BV, Irvine KD. Regulation of Hippo signaling by EGFR-MAPK signaling through Ajuba family proteins. Dev Cell 24: 459–471, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reginensi A, Hoshi M, Boualia SK, Bouchard M, Jain S, McNeill H. Yap and Taz are required for Ret-dependent urinary tract morphogenesis. Development 142: 2696–2703, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reginensi A, Scott RP, Gregorieff A, Bagherie-Lachidan M, Chung C, Lim DS, Pawson T, Wrana J, McNeill H. Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet 9: e1003380, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saad S, Stevens VA, Wassef L, Poronnik P, Kelly DJ, Gilbert RE, Pollock CA. High glucose transactivates the EGF receptor and up-regulates serum glucocorticoid kinase in the proximal tubule. Kidney Int 68: 985–997, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene 20: 5972–5981, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Saxen L, Sariola H, Lehtonen E. Sequential cell and tissue interactions governing organogenesis of the kidney. Anat Embryol (Berl) 175: 1–6, 1986. [DOI] [PubMed] [Google Scholar]
- 53.Sayed-Ahmed N, Besbas N, Mundy J, Muchaneta-Kubara E, Cope G, Pearson C, el Nahas M. Upregulation of epidermal growth factor and its receptor in the kidneys of rats with streptozotocin-induced diabetes. Exp Nephrol 4: 330–339, 1996. [PubMed] [Google Scholar]
- 54.Scaltriti M, Baselga J. The epidermal growth factor receptor pathway: a model for targeted therapy. Clin Cancer Res 12: 5268–5272, 2006. [DOI] [PubMed] [Google Scholar]
- 55.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, Rodriguez JR, Zhou D, Kreger BT, Vasioukhin V, Avruch J, Brummelkamp TR, Camargo FD. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell 144: 782–795, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schutte U, Bisht S, Heukamp LC, Kebschull M, Florin A, Haarmann J, Hoffmann P, Bendas G, Buettner R, Brossart P, Feldmann G. Hippo signaling mediates proliferation, invasiveness, and metastatic potential of clear cell renal cell carcinoma. Transl Oncol 7: 309–321, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwartzman M, Reginensi A, Wong JS, Basgen JM, Meliambro K, Nicholas SB, D'Agati V, McNeill H, Campbell KN. Podocyte-specific deletion of Yes-associated protein causes FSGS and progressive renal failure. J Am Soc Nephrol 27: 216–226, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seo E, Basu-Roy U, Gunaratne PH, Coarfa C, Lim DS, Basilico C, Mansukhani A. SOX2 regulates YAP1 to maintain stemness and determine cell fate in the osteo-adipo lineage. Cell Rep 3: 2075–2087, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Skinner MA, Safford SD, Reeves JG, Jackson ME, Freemerman AJ. Renal aplasia in humans is associated with RET mutations. Am J Hum Genet 82: 344–351, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Song H, Mak KK, Topol L, Yun K, Hu J, Garrett L, Chen Y, Park O, Chang J, Simpson RM, Wang CY, Gao B, Jiang J, Yang Y. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci USA 107: 1431–1436, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stanger BZ. Quit your YAPing: a new target for cancer therapy. Genes Dev 26: 1263–1267, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.St. John MA, Tao W, Fei X, Fukumoto R, Carcangiu ML, Brownstein DG, Parlow AF, McGrath J, Xu T. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nat Genet 21: 182–186, 1999. [DOI] [PubMed] [Google Scholar]
- 63.Szeto SG, Narimatsu M, Lu M, He X, Sidiqi AM, Tolosa MF, Chan L, De Freitas K, Bialik JF, Majumder S, Boo S, Hinz B, Dan Q, Advani A, John R, Wrana JL, Kapus A, Yuen DA. YAP/TAZ are mechanoregulators of TGF-beta-Smad signaling and renal fibrogenesis. J Am Soc Nephrol [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol 5: 914–920, 2003. [DOI] [PubMed] [Google Scholar]
- 65.Varelas X, Miller BW, Sopko R, Song S, Gregorieff A, Fellouse FA, Sakuma R, Pawson T, Hunziker W, McNeill H, Wrana JL, Attisano L. The Hippo pathway regulates Wnt/beta-catenin signaling. Dev Cell 18: 579–591, 2010. [DOI] [PubMed] [Google Scholar]
- 66.Wennmann DO, Vollenbroker B, Eckart AK, Bonse J, Erdmann F, Wolters DA, Schenk LK, Schulze U, Kremerskothen J, Weide T, Pavenstadt H. The Hippo pathway is controlled by Angiotensin II signaling and its reactivation induces apoptosis in podocytes. Cell Death Dis 5: e1519, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005. [DOI] [PubMed] [Google Scholar]
- 68.Willecke M, Hamaratoglu F, Sansores-Garcia L, Tao C, Halder G. Boundaries of Dachsous cadherin activity modulate the Hippo signaling pathway to induce cell proliferation. Proc Natl Acad Sci USA 105: 14897–14902, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu S, Huang J, Dong J, Pan D. Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 114: 445–456, 2003. [DOI] [PubMed] [Google Scholar]
- 70.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development 121: 1053–1063, 1995. [DOI] [PubMed] [Google Scholar]
- 71.Yanagida-Asanuma E, Asanuma K, Kim K, Donnelly M, Young Choi H, Hyung Chang J, Suetsugu S, Tomino Y, Takenawa T, Faul C, Mundel P. Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. Am J Pathol 171: 415–427, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yu J, Zheng Y, Dong J, Klusza S, Deng WM, Pan D. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev Cell 18: 288–299, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zender L, Spector MS, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan ST, Luk JM, Wigler M, Hannon GJ, Mu D, Lucito R, Powers S, Lowe SW. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell 125: 1253–1267, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang H, Liu CY, Zha ZY, Zhao B, Yao J, Zhao S, Xiong Y, Lei QY, Guan KL. TEAD transcription factors mediate the function of TAZ in cell growth and epithelial-mesenchymal transition. J Biol Chem 284: 13355–13362, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang J, Smolen GA, Haber DA. Negative regulation of YAP by LATS1 underscores evolutionary conservation of the Drosophila Hippo pathway. Cancer Res 68: 2789–2794, 2008. [DOI] [PubMed] [Google Scholar]
- 76.Zhang N, Bai H, David KK, Dong J, Zheng Y, Cai J, Giovannini M, Liu P, Anders RA, Pan D. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell 19: 27–38, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao B, Kim J, Ye X, Lai ZC, Guan KL. Both TEAD-binding and WW domains are required for the growth stimulation and oncogenic transformation activity of yes-associated protein. Cancer Res 69: 1089–1098, 2009. [DOI] [PubMed] [Google Scholar]
- 78.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC, Guan KL. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22: 1962–1971, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, Lauwers GY, Thasler W, Lee JT, Avruch J, Bardeesy N. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell 16: 425–438, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


