Abstract
Introduction
Ospemifene (Osphena, Shionogi Inc, Florham, NJ, USA) is an estrogen agonist and antagonist approved by the U.S. Federal Drug Administration for the treatment of “moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause.” Although published, peer-reviewed, placebo-controlled studies have shown objective improvement in dyspareunia and in vaginal atrophy, there are no published data that have assessed changes in vulvar atrophy after the use of ospemifene.
Aim
To present two cases of women with severe vulvar atrophy that showed no improvement with the use of ospemifene.
Methods
A review of two recent cases of a clinic specializing in the treatment of vulvovaginal disorders was performed. Case 1 was a 53-year-old menopausal woman who presented with non-provoked vulvar and vaginal discomfort and introital dyspareunia. She had used ospemifene 60 mg/d for 1.5 years without improvement in her symptoms before presentation. Case 2 was a 57-year-old menopausal woman who also presented with non-provoked vulvar rawness, burning, irritation, vaginal dryness, and introital dyspareunia. She had started ospemifene 60 mg/d 1 year before presentation and reported mild improvement in her vaginal dryness but no improvement in her vulvar irritation or introital dyspareunia.
Main Outcome Measures
Change in vulvar atrophy and introital dyspareunia.
Conclusion
These cases highlight the need to perform additional clinical trials that specifically assess the efficacy of ospemifene for changes in vulvar atrophy.
Key Words: Ospemifene, Genitourinary Syndrome of Menopause, Vulvovaginal Atrophy, Menopause
Introduction
Ospemifene (Osphena; Shionogi Inc, Florham NJ, USA) is a selective estrogen-receptor modulator that has been approved by the Food and Drug Administration for the treatment of moderate to severe vulvar and vaginal atrophy and moderate to severe dyspareunia (a symptom of vulvovaginal atrophy) with a suggested dosage of 60 mg once daily.1 The prescribing guidelines for ospemifene are based on two 12-week, double-blinded, placebo-controlled, parallel-group efficacy trials and one double-blinded, placebo-controlled, parallel-group, 52-week long-term safety trial.
The first clinical trial was a 12-week, double-blinded, placebo-controlled, parallel-group efficacy trial that included 826 menopausal women in three randomized groups.1, 2 The first group received ospemifene 30 mg/d for 12 weeks (n = 282), the second group received ospemifene 60 mg/d for 12 weeks (n = 276), and the third group received a placebo (n = 268). The co-primary end points included the mean change from baseline in vaginal dryness or dyspareunia as indicated on a four-point scoring system, with participants stratified and assessed by the symptoms that were most bothersome at baseline (none = 0, mild = 1, moderate = 2, severe = 3) as taken at the screening appointment and again at weeks 4 and 12. Vaginal dryness was further assessed by the percentage of superficial cells and parabasal cells and vaginal pH as seen on a vaginal culture wet mount. The results of the trial indicated that women who received ospemifene in the two strata showed an improvement from baseline in vaginal dryness or dyspareunia by the four-point scale and an improvement in total Female Sexual Function Index score that was evident at week 4 and increased in magnitude until week 12.
The second clinical trial was a 12-week, double-blinded, placebo-controlled, parallel-group efficacy and safety trial that included 919 generally healthy postmenopausal women diagnosed with vulvovaginal atrophy who had greater than or equal to 5% superficial cells on vaginal smear and a vaginal pH higher than 5.0.1, 3 The participants were stratified into two groups based on the symptoms they designated as most bothersome at baseline; the first group included those who were most bothered by vaginal dryness (dryness cohort) and the second group included those who were most bothered by pain with intercourse (dyspareunia cohort). Treatment groups included those who received ospemifene 60 mg/d for 12 weeks (n = 463) and those who received a placebo (n = 456). The co-primary end points included the mean change from baseline in their most bothersome symptoms, which were vulvar and/or vaginal dryness (combined as one variable) and dyspareunia as indicated on a four-point scoring system. Secondary end points included change from baseline in the domains of the Female Sexual Function Index as taken at weeks 4 and 12 and change from baseline in serum sex hormone levels (follicle-stimulating hormone, luteinizing hormone, and SHBG) collected at the screening appointment and at week 12. The results of this trial showed that significantly more women who reported dyspareunia as their most bothersome symptom had improvement (68.3% vs 54.1%; P = .0255) or relief (57.5% vs 41.8%; P = .0205) in the severity of their dyspareunia from baseline compared with week 12 with ospemifene use vs placebo. Participants with vulvar and/or vaginal dryness also had improvement (74.6% vs 57.7%; P = .0101), substantial improvement (42.4% vs 26.9%; P = .0172), and even relief (66.1% vs 49.0%; P = .0140) of their vulvar and/or vaginal dryness from baseline to week 12 with ospemifene use compared with placebo.
The third clinical trial was a 52-week, double-blinded, placebo-controlled, parallel-group extension safety trial that was conducted in 301 women 40 to 80 years old without a uterus who were recruited from the 12-week efficacy study.4 The total elapsed time for patients was 68 weeks, including the initial 12-week study, the extension of 52 weeks, and a post-trial follow-up after 4 weeks. Women continued the 60-mg/d ospemifene dose or switched from the blinded placebo or 30-mg/d ospemifene dose to the open-label 60-mg/d ospemifene dose. Safety assessments included adverse events, laboratory studies, physical and gynecologic examination, vital signs, breast palpation, and mammography. Ospemifene was found to be clinically safe and generally well tolerated in postmenopausal patients with dyspareunia.
The two efficacy studies included an objective assessment of vaginal atrophy, However, somewhat surprisingly, ospemifene had been approved for (and is currently being marketed as) a treatment for vaginal and vulvar atrophy, although none of the published trials included any objective assessment of the vulvar tissue. Examples of tests that could have been included are quantitative sensory testing and changes in mucosal thickness as measured by biopsy examinations before and after treatment.
This report describes two cases of postmenopausal women who used ospemifene for at least 1 year and found no improvement in vulvar atrophy or introital allodynia.
Case 1
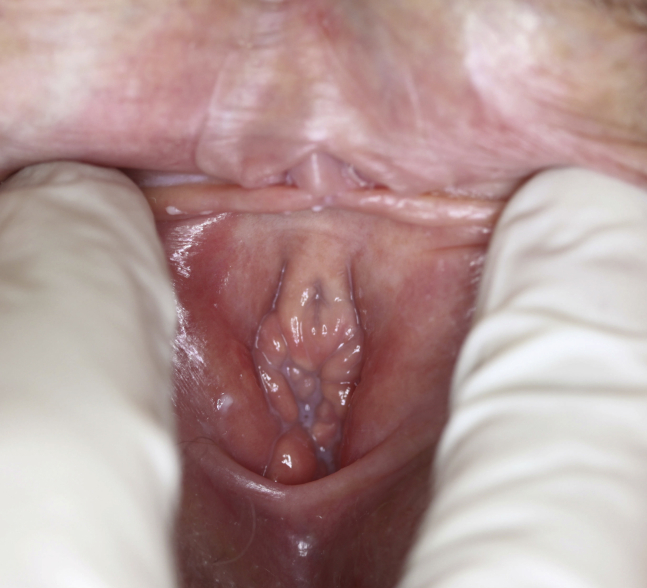
The patient was a 53-year-old gravida 1, para 2, aborta 0 menopausal woman who presented to a clinic specializing in the treatment of vulvovaginal disorders with complaints of non-provoked vulvar and vaginal rawness and pain with intercourse that she described as burning pain upon penetration. She reported that her symptoms had become progressively worse during the past 5 years. She had been menopausal for 10 years and had pain-free intercourse with normal lubrication and sexual arousal before the past 5 years. She had tried an estradiol vaginal ring and estradiol intravaginal tablets, without improvement in her symptoms. For the past 1.5 years, she had been taking ospemifene 60 md/d, without improvement in her symptoms. On physical examination, the patient had profound atrophy, erythema—especially of the major and minor vestibular gland ostia—and severe tenderness of the vulvar vestibule (Figure 1). There was no evidence of vulvar dermatoses. The vaginal mucosa was mildly atrophic and non-tender, without ulcerations or erosions. The bladder and urethra were non-tender to palpation. The levator ani muscles were not significantly tight or tender. The vaginal pH was 5.0, and a saline wet mount showed predominantly mature squamous cells with few leukocytes and few parabasal cells.
Figure 1.
Severe atrophy of vulvar vestibule of a patient taking ospemifene.
Case 2
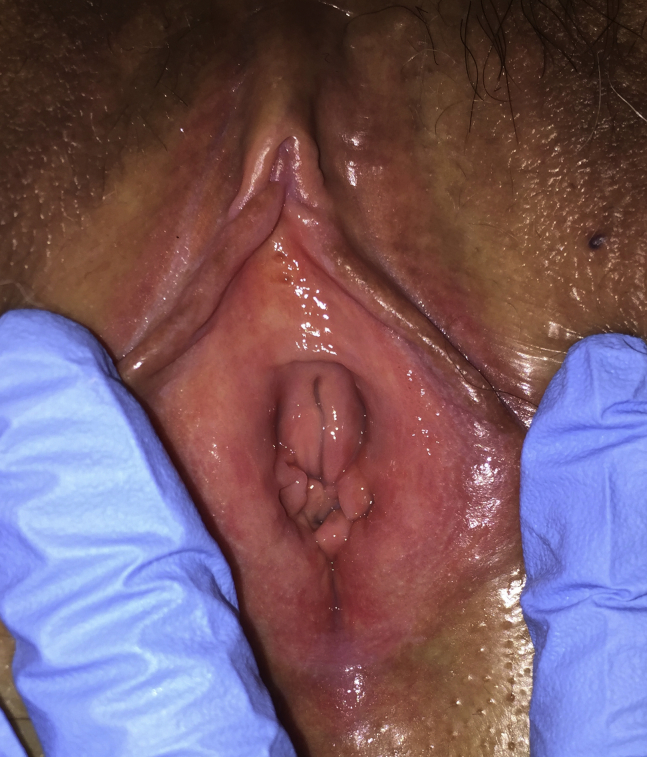
The patient was a 53-year-old gravida 5, para 3, aborta 2 menopausal woman who presented with the complaint of non-provoked vulvovaginal rawness, burning, dryness, and irritation. In addition, she complained of severe insertional dyspareunia of 2.5 years' duration. Her dyspareunia was so severe that she had not engaged in sexual intercourse for 16 months. The patient had been experiencing severe hot flashes and night sweats since she became menopausal 3 years previously. She had been treated with water- and silicon-based lubricants, topical lidocaine ointment, topical metronidazole, fluconazole, topical miconazole, and coconut oil, with minimal improvement in her provoked or non-provoked allodynia. In addition, at presentation, she had been taking ospemifene 60 mg/d for the past 13 months. She reported that her vaginal pain had been alleviated slightly within 3 months after starting the ospemifene, but she continued to have significant provoked, and non-provoked, vulvar pain. The patient's history was significant for menorrhagia, for which she had an endometrial ablation. In addition, she had a history of anxiety, depression, and bulimia that she attributed to a previous abusive relationship, and she was currently taking escitalopram 10 mg/d. Genitourinary examination showed normal labia majora, normal interlabial sulci, and a normal clitoral prepuce. The labia minora and glans clitoris were mildly atrophic. The vulvar vestibule was severely erythematous, severely atrophic, and severely tender throughout, without lesions, ulcerations, or erosions (Figure 2). There was no evidence of vulvar dermatoses. The vaginal mucosa was well estrogenized and non-tender, without ulcerations or erosions. The vaginal pH was 4.0. Saline wet mount showed all mature squamous without parabasal cells. There were few white blood cells and fewer than 5% clue cells. Lactobacilli were present and there were no fungal elements present.
Figure 2.
Severe atrophy of the vulvar vestibule with erythema of the gland ostia and clitoral atrophy.
Discussion
Ospemifene was recently approved by the Food and Drug Administration for the treatment of moderate to severe dyspareunia and moderate to severe vulvar and vaginal atrophy.1 However, the published trials on which the prescribing indications are based only reported changes from baseline in vaginal dryness and dyspareunia and changes in Female Sexual Function Index scores.2, 3, 4 These studies did not include any primary or secondary end points that exclusively examined vulvar symptoms: the assessment of the most bothersome symptom combined the symptoms of vulvar and vaginal atrophy. In addition, there were no objective measurements of vulvar atrophy such as quantitative sensory testing or post-treatment histologic evaluation.
These two case studies provide evidence that ospemifene does not treat vulvar atrophy or introital dyspareunia. Although no medication can be expected to be efficacious in all patients, there is a complete absence of published data that show that ospemifene does treat vulvar atrophy, and the present cases represent the only data currently available to prescribing providers. At the very least, these cases illustrate the need for additional studies that specifically examine vulvar atrophy and introital dyspareunia. For example, these studies could examine change in vulvar vestibular pain threshold before and after treatment with ospemifene as measured by a calibrated instrument such as a vulvogeisometer.5
It is also plausible that ospemifene, a selective estrogen receptor modulator, does not significantly reverse atrophy of the vulvar vestibule because the non-keratinized, squamous endothelium of the vulvar vestibule is highly androgen dependent.6, 7 In addition, the Bartholin glands, Skene glands, and minor vestibular glands are androgen-dependent mucin-secreting glands. To date, there are no published data that have examined the interaction between ospemifene and the androgen receptor (AR). In contrast, lasofoxifene, another selective estrogen receptor modulator, has shown agonist activity of the AR.8 Unless ospemifene acts as an AR agonist, it is unlikely that it will significantly reverse vulvar atrophy or introital dyspareunia.
It should be noted that in case 1, the patient’s vulvar atrophy and introital dyspareunia were not alleviated with the use of an estradiol-containing vaginal ring (Estring; Pfizer, New York, NY, USA) or an estradiol-containing vaginal tablet (Vagifem; Novo Nordisk, Plainsboro, NJ, USA). However, neither of these products has been approved for the indication of vulvar atrophy. If one considers the embryologic and anatomic differences between the vagina and the vulva, this is not surprising, primarily because the blood supply of the vulva is different from that of the vagina. Therefore, because neither product provides estradiol in a dose large enough for significant systemic absorption, it would be unlikely that either product would alleviate vulvar atrophy. This is in contrast to cream-based estradiol products that can be applied directly to the vulva or can leak out of the vaginal lumen and then come in contact with the vulvar endothelium. In addition, recent studies have shown that creams that contain testosterone (alone or in combination with estrogen) are superior in treating female sexual dysfunction in menopausal women with genitourinary syndrome of menopausal (previously known as vulvovaginal atrophy) than creams that contain only estrogen.9, 10 Therefore, the results of these studies clearly illustrate the need for studies on the activity of ospemifene on the AR.
Statement of authorship
Category 1
-
(a)Conception and Design
- Andrew Goldstein
-
(b)Acquisition of Data
- Andrew Goldstein
-
(c)Analysis and Interpretation of Data
- Andrew Goldstein; Michelle King
Category 2
-
(a)Drafting the Article
- Andrew Goldstein; Michelle King
-
(b)Revising It for Intellectual Content
- Andrew Goldstein
Category 3
-
(a)Final Approval of the Completed Article
- Andrew Goldstein; Michelle King
Footnotes
Conflict of Interest: Dr. Goldstein is on the advisory board of Emotional Brain and SST Research, and receives research funding from Palatin and Bayer. The other authors report no conflicts of interest.
Funding: None.
References
- 1.Ospemifene prescribing information. Shionogi Inc; Florham, NJ: 2013. [Google Scholar]
- 2.Constantine G., Graham S., Portman D.J. Female sexual function improved with ospemifene in post-menopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18:226–232. doi: 10.3109/13697137.2014.954996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nappi R.E., Bruyniks N., Castelo-Branco C. The clinical relevance of the effect of ospemifene on symptoms of vulvar and vaginal atrophy. Climacteric. 2015;18:233–240. doi: 10.3109/13697137.2014.975199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simon J., Portman D., Maby R.G., Jr. Long-term safety of ospemifene (52-week extension) in the treatment of vulvar and vaginal atrophy in hysterectomized postmenopausal women. Maturitas. 2014;77:274–281. doi: 10.1016/j.maturitas.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 5.Pukall C.F., Binik Y.M., Khalifé S. A new instrument for pain assessment in vulvar vestibulitis syndrome. J Sex Marital Ther. 2004;30:69–78. doi: 10.1080/00926230490275065. [DOI] [PubMed] [Google Scholar]
- 6.Burrows L.J., Goldstein A.T. The treatment of vestibulodynia with topical estradiol and testosterone. Sex Med. 2013;1:30–33. doi: 10.1002/sm2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johannesson U., Sahlin L., Masironi B. Steroid receptor expression in the vulvar vestibular mucosa—effects of oral contraceptives and menstrual cycle. Contraception. 2007;76:319–325. doi: 10.1016/j.contraception.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Wang X.N., Simmons H.A., Salatto C.T. Lasofoxifene enhances vaginal mucus formation without causing hypertrophy and increases estrogen receptor beta and androgen receptor in rats. Menopause. 2006;13:609–620. doi: 10.1097/01.gme.0000227337.73738.c9. [DOI] [PubMed] [Google Scholar]
- 9.Fernandes T., Costa-Paiva L.H., Pinto-Neto A.M. Efficacy of vaginally applied estrogen, testosterone, or polyacrylic acid on sexual function in postmenopausal women: a randomized controlled trial. J Sex Med. 2014;11:1262–1270. doi: 10.1111/jsm.12473. [DOI] [PubMed] [Google Scholar]
- 10.Raghunandan C., Agrawal S., Dubey P. A comparative study of the effects of local estrogen with or without local testosterone on vulvovaginal and sexual dysfunction in postmenopausal women. J Sex Med. 2010;7:1284–1290. doi: 10.1111/j.1743-6109.2009.01667.x. [DOI] [PubMed] [Google Scholar]