Abstract
Introduction
Diabetes-induced sexual dysfunction is associated with an increase in oxidative stress. Scavengers of reactive oxygen species (ROS) have been shown to reduce oxidative stress and aid in the management of sexual dysfunction in diabetes.
Aim
The aim of the study was to test the hypothesis that antioxidant, which scavenge ROS and reduce formation of advanced glycation end products (AGEs), can potentiate efficacy of phosphodiesterase type 5 inhibitors in diabetes-induced sexual dysfunction that is associated with oxidative stress.
Materials and Methods
Effect of phloroglucinol and sildenafil on serum glucose level, sexual function, penile smooth muscle : collagen ratio, and phenylephrine precontracted corpus cavernosum smooth muscle (CCSM) was studied. The ability of phloroglucinol to reduce the formation of AGEs and its ability to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) and nitric oxide (NO) was also evaluated.
Main Outcome Measures
Antioxidant potential of phloroglucinol was studied in addition to its effect on diabetes-induced sexual dysfunction in presence and absence of sildenafil.
Results
Phloroglucinol (50 mg/kg, p.o.) significantly decreased serum glucose level and increased sexual function in streptozotocin-induced diabetic rats when compared with diabetic control rats. Sildenafil (5 mg/kg, p.o.) had no effect on glycemia but significantly increased sexual function of diabetic rats. Coadministration of phloroglucinol increased the efficacy of sildenafil by improving sexual function. Treatment of diabetic rats with phloroglucinol + sildenafil maintained smooth muscle : collagen levels similar to that of normal rat penile tissue. Phloroglucinol decreased formation of AGEs and significantly scavenged DPPH radical activity in vitro. Sildenafil relaxed isolated CCSM of normal rat and diabetic rat significantly, but phloroglucinol did not show any significant effect. Phloroglucinol also inhibited human CYP3A4 enzyme activity in vitro.
Conclusion
Phloroglucinol coadministration increases efficacy of sildenafil in diabetes-induced sexual dysfunction. However, further studies are required to ascertain the benefits of phloroglucinol owing to its undesirable CYP3A4 inhibition activity.
Key Words: Phloroglucinol, Diabetes-Induced Erectile Dysfunction, Sildenafil, CYP3A4, AGEs, Antioxidant
Introduction
Diabetes mellitus is a metabolic disorder that is characterized by increased blood sugar levels (hyperglycemia). India is home to largest number of patients with diabetes in the world, and the number is expected to reach 87 million by 2030.1 Type 1 diabetes is characterized by lack of insulin secretion from pancreas.2 Diabetic complications include nephropathy, neuropathy, retinopathy, cardiovascular disease, and sexual dysfunction.3, 4 Sexual dysfunction is an indirect indicator of cardiovascular disorder5 and can lead to loss of self-esteem.6
Oxidative stress associated with hyperglycemia damages the penile smooth muscle and nerves that leads to sexual dysfunction.7, 8 Though, phosphodiesterase inhibitors such as sildenafil is preferred medicine for managing erectile dysfunction (ED), a male sexual dysfunction in diabetics, but it is reported not to be 100% efficacious.9, 10 Co-treatment of an antioxidant and sildenafil has been reported to increase efficacy of sildenafil.7, 11
We evaluated the effect of co-treatment of phloroglucinol, an antioxidant and sildenafil on sexual function of streptozotocin-induced type 1 diabetic rats and compared it with the effect of phloroglucinol and sildenafil treatment under same physiological condition.7, 11, 12 Effect of sildenafil and phloroglucinol on phenylephrine precontracted isolated rat corpus cavernosum smooth muscles (CCSMs) was studied to evaluate their erectogenic potential in normal rats.11 Phloroglucinol (1,3,5-trihydroxybenzene), a polyphenol, is the monomeric building unit of phlorotannins, phenolic compounds known only from brown algae (Phaeophyceae).13 Polyphenols are known to reduce oxidative stress by scavenging reactive oxygen species, chelating iron, and modulating various enzymes.14 Increase in formation and accumulation of AGEs in corpus cavernosum in diabetes was linked to ED.15 Therefore, we studied the effect of phloroglucinol on formation of AGEs in vitro.16 Antioxidant potential of phloroglucinol was evaluated by studying its effect on scavenging of DPPH and nitric oxide (NO).17 Effect of phloroglucinol was studied on activity of cytochrome P450 3A4 (CYP3A4), which metabolizes sildenafil to find out possible pholoroglucinol–sildenafil interaction.18
Materials and Methods
Materials
Phloroglucinol and streptozotocin (STZ) were purchased from Sigma-Aldrich (St Louis, MO, USA), whereas glucose estimation kit was procured from Autospan (Surat, India). Human CYP3A4 substrate Benzyloxy-4-(trifluoromethyl) coumarin (BFC) was collected from ChemBridge Corporation (San Diego, CA, USA), whereas enzyme and BFC metabolite: 7-Hydroxy-4-(trifluoromethyl) coumarin (HFC) was from Corning Inc (Fremont, CA, USA). Other reagent and chemicals procured were of analytical grade.
Animals
Three-month-old adult male and female Wistar rats weighing about 175–225 g were used in the study. Thirty male rats and equal number of female rats were used for the study. The use of animals in these experiments was approved by Institutional Animal Ethical Committee of Al-Ameen College of Pharmacy. Experiments on rats were conducted in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals guidelines. Rats were maintained under controlled temperature at 25 ± 2°C and relative humidity of 45–55% with an alternating 12 h light/dark cycle (light ON 6:00–18:00 h). The animals were reared and maintained with free access to food and water throughout the day.
Induction of Type 1 Diabetes and Drug Treatment
Streptozotocin was dissolved in ice-cold 0.1 M sodium citrate buffer just prior to use and injected intraperitoneally to male rats at a dose of 55 mg/kg.7, 11 Three days after administration of STZ, fasting serum glucose level of overnight-fasted rats was analyzed as per procedure mentioned in glucose estimation kit (Autospan, India) to confirm induction of diabetes.11 This day was considered as day 0, following which drug treatment was started.
Phloroglucinol was dissolved in distilled water and administered daily to rats orally using per oral tube. The test solutions were freshly prepared every day before dosing the animals. Diabetic rats were administered with phloroglucinol for 4 weeks post-induction of diabetes. Male rats were divided into five groups as follows, each group containing six animals.
Group 1: Negative control, normal rats (water 4 mL/kg, p.o.)
Group 2: Positive control, diabetic rats (vehicle 4 mL/kg, p.o. for 4 weeks)
Group 3: Diabetic rats administered with phloroglucinol (50 mg/kg, p.o. for 4 weeks)
Group 4: Diabetic rats administered with sildenafil (5 mg/kg, p.o. for 4 weeks)
Group 5: Diabetic rats administered with phloroglucinol (50 mg/kg, p.o.) + sildenafil (5 mg/kg, p.o.) for 4 weeks
Though study started with six animals in each group, few animals were excluded from statistical calculation due to development of sexual dysfunction and inability of individual treatment to elevate sexual function in all animals.
Sexual Behavior Study of Rats
Before induction of diabetes, all the male rats were trained thrice for sexual activity in presence of ovariectomized estrous female rats in an observation chamber on different days. The male rats that did not perform intromission (vaginal penetration) within 10 minutes of introduction of female rats were removed from the study and those rats were replaced by sexually active male rats. Female rats were treated with diethylstilbestrol (1 mg/kg, p.o., administered 48 hours prior to sexual behavior study) and progesterone (5 mg/kg, s.c., administered 4 hours prior to the sexual behavior study) to induce artificial estrous phase. Female rats that did not exhibit lordosis posture were not paired with male rats for sexual behavior study.19, 20
Sexual behavior study was performed in evening and night on day 0 and 28. The study was performed as per published literature. Briefly, after male rat accustoms to observation chamber (5–10 minutes), a female rat was introduced and following sexual behavior parameters were observed.19, 20
Mount latency (ML): time from the introduction of female into the cage of the male up to the first mount
Intromission latency (IL): time from the introduction of the female up to the first intromission by the male
Ejaculation latency (EL): time from the first intromission to the ejaculation
Mount frequency (MF): number of mount in a sexual cycle before ejaculation
intromission frequency (IF) : number of intromission in a sexual cycle before ejaculation
Post-ejaculatory interval (PEI) : time from end of first ejaculation to start of next intromission
Determination of Smooth Muscle : Collagen Level
At the end of sexual behavior study, male rats were anesthetized (Ketamine 60 mg/kg, i.p.; Xylazine 10 mg/kg, i.p.), penectomy was performed, and rats were sacrificed by cervical dislocation. The shaft of penile tissue was dipped in 7% formalin saline for 24 hours and then transferred to 70% ethanol until the tissues were further processed. Masson's trichrome staining was performed to evaluate effect of treatment on smooth muscle:collagen ratio of penile tissue as per published literature.19
Isolated Rat Corpus Cavernosum Study
Effect of phloroglucinol and sildenafil was studied on isolated corpus cavernosum collected from anesthetized normal and diabetic rat (32 days after induction of diabetes) penile tissues.21 Briefly, CCSMs of dimension 3 × 3 × 15 mm were mounted in a four-channel organ bath containing modified Krebs–Henseleit salt solution (composition [mM]: 118 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 1.5 CaCl2, 25 NaHCO3, 11 glucose) maintained at 37°C. CCSMs were stretched up to 500 mg tension and washed four times within 1 hour of stabilzation period. The tissues were contracted with 3 μM of phenylephrine and then different concentrations of phrologlucinol and sildenafil were added separately to the organ bath in the interval of 5 minutes each.
Effect of Phloroglucinol and Sildenafil on Formation of AGEs In Vitro
Effect of phloroglucinol and sildenafil on advanced glycation end product (AGE) was estimated using bovine serum albumin (BSA)-glucose assay.16 Briefly, different concentrations of phloroglucinol, sildenafil, and aminoguanidine (reference standard) solution containing preservative sodium azide (0.02%) were incubated with final concentrations of BSA (2 mg/mL), glucose (40 mM) at 37°C. Positive control reaction without inhibitor was also set up. All the solutions were stored in capped bottles in dark for 15 days. Fluorescence intensity of each solution was measured at excitation wavelength of 370 nm and emission wavelength of 440 nm.
DPPH Free Radical and NO Scavenging Assay
DPPH free radical and NO scavenging assay are commonly employed to evaluate antioxidant potential of natural and synthetic products. Assays were performed as described elsewhere. Methanol was used as blank, and ascorbic acid was used as standard antioxidant. All the readings were taken in triplicate, that is, n = 3 and absorbance was measured using Shimadzu (UV-1601, Kyoto, Japan) UV-visible spectrophotometer. The IC50 value for each test compound as well as standard preparation was calculated using the following formula.17
For DPPH scavenging assay, to 150 μL DPPH solution (4.3 mg in 3.3 mL), 3 mL of methanol was added and absorbance was measured at 517 nm (control reading) after 15 minutes. Similarly, for determining DPPH scavenging capacity of phloroglucinol, to 3 mL solution of phloroglucinol in methanol, 150 μL of DPPH solution was added, and absorbance was measured after 15 minutes at 517 nm.
For NO scavenging assay, different concentrations of phloroglucinol were taken in separate test tubes, and the volume was made up to 150 μL with methanol. Two milliliters of 10 mM sodium nitroprusside in phosphate buffer saline was added to each tube, and solutions were incubated for 2.5 h at 27°C followed by addition of 5 mL of Griess reagent. Absorbance was measured at 546 nm.
Cytochrome P450 3A4 Study
Enzyme activity was studied as per available literature with slight modification.22, 23 Briefly, human CYP3A4 enzyme (5 pmol/mL) suspended in 100 mM potassium phosphate buffer (pH 7.4) containing magnesium chloride (35 μM) and ethylenediamine tetracacetic acid (10 μM), was incubated with BFC (10 μM). Reaction was started with addition of nicotinamide adenine dinucleotide phosphatase (NADPH) regenerating system containing 5 mM glucose-6-phosphate, 1 mM NADP+, and 1 unit/mL of glucose-6-phosphate dehydrogenase. Formation of HFC was recorded in kinetic mode (excitation 410 nm/emission 510 nm) for 30 minutes.
Statistics
One-way analysis of variance followed by Tukey's multiple comparison test was employed to evaluate statistical significance. Values are presented as mean ± standard deviation or standard error of mean. Statistical calculation was performed using GraphPad Prism version 5 (GraphPad software company, La Jolla, CA, USA). A P value < .05 was considered as statistically significant.
Results
Effect of Treatment on Diabetes
Serum glucose levels in STZ diabetic rats increased significantly in comparison with normal rats (P < .001). A slight but significant decrease in serum glucose levels was observed in diabetic rats treated with phloroglucinol in comparison with diabetic rats treated with vehicle (P < .05). Sildenafil had no effect on serum glucose level of diabetic rats (Figure 1).
Figure 1.
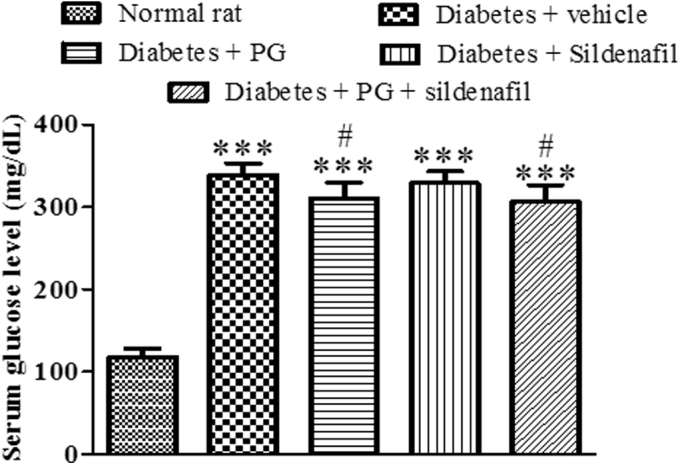
Effect of treatment on serum glucose level of diabetic rats. Administration of streptozotocin increased serum glucose level in rats significantly in comparison with normal rats not exposed to streptozotocin (∗∗∗P < .001). Phloroglucinol (PG) decreased serum glucose level significantly (#P < .05 vs diabetic rats treated with vehicle) while sildenafil had no effect on glycemia. One–way analysis of variance followed by Tukey multiple comparison test was used for calculating statistical significance. Values are represented as mean ± standard deviation of 6 observations.
Effect of Treatment on Sexual Function
Sexual function of all rats in different groups on day 0 was similar, but significant changes were observed 28 days after treatment. Sexual function of type 1 diabetic rats decreased significantly (P < .05) in comparison with normal rats. Phloroglucinol and sildenafil increased sexual function in diabetic rats, but effect was statistically significant only for sildenafil. Co-treatment of phloroglucinol and sildenafil preserved the sexual function in diabetic rats, and the effect was better when sildenafil was used alone (Table 1).
Table 1.
Phloroglucinol and sildenafil increases sexual function of diabetic rats
| Sexual behavior parameters (unit) | Normal rat (n = 6) | Diabetic rats |
|||
|---|---|---|---|---|---|
| Water (n = 5) | Phloroglucinol (n = 5) | Sildenafil (n = 5) | Phloroglucinol + Sildenafil (n = 6) | ||
| ML (second) | 28.7 ± 3.8 | 56.8 ± 4.4$ | 42.0 ± 4.0∗ | 33.8 ± 5.0∗∗ | 27.8 ± 3.5∗∗ |
| IL (second) | 41.8 ± 3.9 | 80.8 ± 6.9$ | 56.0 ± 5.2∗ | 48.4 ± 4.7∗∗ | 40.5 ± 4.5∗∗ |
| EL (second) | 400.8 ± 26.3 | 270.4 ± 14.1$ | 321.4 ± 27.6∗ | 383.0 ± 16.1∗∗ | 410.5 ± 15.8∗∗ |
| MF (number) | 14.0 ± 2.1 | 5.4 ± 0.7$ | 9.4 ± 1.2∗ | 12.6 ± 1.1∗∗ | 14.2 ± 1.4∗∗ |
| IF (number) | 9.8 ± 0.6 | 3.4 ± 0.6$ | 6.4 ± 0.9∗ | 8.6 ± 0.8∗∗ | 10.0 ± 1.0∗∗ |
| PEI (second) | 345.3 ± 16.9 | 718.6 ± 23.2$ | 553.2 ± 24.6∗ | 499.8 ± 33.1∗∗ | 402.7 ± 12.5∗∗ |
Diabetes decreased sexual function of rats significantly ($P < .05). ML, IL, and PEI of rats with diabetes increased significantly ($P < .05), whereas MF, IF, and EL of diabetic rats decreased significantly ($P < .05) in comparison with normal healthy rats inferring the development of sexual dysfunction in diabetic rats.11, 20 Phloroglucinol, sildenafil, and Phloroglucinol + sildenafil increased sexual function of diabetic rats significantly (∗P < .05, ∗∗P < .01). One rat each from water, phloroglucinol and sildenafil–treated diabetic group did not respond to treatment and were excluded from statistical data calculation. Data are presented as mean ± standard error of mean. One-way ANOVA followed by Tukey's multiple comparison test was used for statistical analysis.
Effect of Treatment on Smooth Muscle : Collagen Level
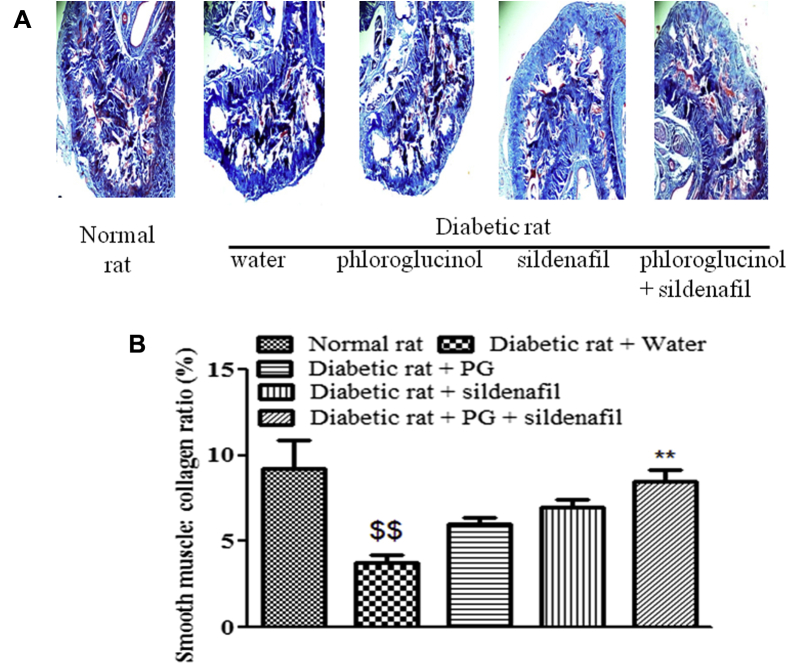
Smooth muscle:collagen level decreased in diabetic rat penile tissue in comparison with normal rats. Effect of treatment on smooth muscle:collagen ratio was in order: sildenafil + phloroglucinol > phloroglucinol > sildenafil. Co-treatment improved smooth muscle:collagen level in diabetic penile tissue compared with water (Figure 2).
Figure 2.
Effect of treatment on smooth muscle : collagen level in rat penile tissue (A) CCSM stained with Masson's trichrome staining and imaged through light microscopy (40×). Smooth muscle is stained pink, whereas collagen is stained as blue. (B) Images were analyzed using software ImageJ of NIH (Bethesda, MD, USA). Smooth muscle : collagen level had decreased ($$P < .01) in diabetic rat penile tissue in comparison with normal rats. Phloroglucinol (PG) + sildenafil treatment was effective (∗∗P < .01) in improving smooth muscle : collagen level in penile tissue of diabetic rats in comparison with diabetic rat treated with only water. Value represents mean ± standard error of mean of four observations. One way ANOVA followed by Tukeys multiple comparison test was used for statistical analysis.
Effect of Phloroglucinol and Sildenafil on Isolated Rat Corpus Cavernosum
Sildenafil relaxed isolated rat CCSMs significantly. Relaxation effect of sildenafil on isolated diabetic rat penile tissue was lesser than the effect that was seen with normal rat isolated CCSMs. Phloroglucinol did not significantly relax isolated CCSM from normal and diabetic rat (Table 2).
Table 2.
Sildenafil Relaxes Corpus Cavernosum of Normal and Diabetic Rats
| Concentration of phloroglucinol and sildenafil (μg/mL) | Normal rat (% relaxation) |
Diabetic rat (% relaxation) |
||||
|---|---|---|---|---|---|---|
| Water | Phloroglucinol | Sildenafil | Water | Phloroglucinol | Sildenafil | |
| 0.01 | 0.6 ± 0.2 | 0.8 ± 0.3 | 9.5 ± 1.7∗ | 0.5 ± 0.1$ | 0.4 ± 0.1 | 3.5 ± 0.9# |
| 0.1 | 1.2 ± 0.3 | 2.0 ± 0.4 | 19.3 ± 3.2∗ | 1.0 ± 0.2$ | 1.0 ± 0.2 | 11.3 ± 2.6# |
| 1 | 2.7 ± 0.5 | 6.2 ± 1.4 | 39.5 ± 5.8∗ | 1.6 ± 0.2$ | 2.0 ± 0.3 | 27.8 ± 5.2# |
| 10 | 4.7 ± 0.5 | 13.6 ± 1.9 | 85.7 ± 3.5∗ | 2.8 ± 1.1$ | 5.3 ± 1.0 | 65.8 ± 9.8# |
Table 2 illustrates effect of phloroglucinol and sildenafil on percent relaxation of phenylephrine precontracted isolated CCSMs of normal and diabetic rat in comparison with water. Sildenafil significantly (∗P < .05 vs water) relaxed CCSM of normal rat while phloroglucinol could not relax CCSM significantly. Relaxation effect of CCSMs of diabetic rat decreased significantly ($P < .05) in comparison with normal rats and sildenafil could significantly relax (#P < .05 vs water) CCSM of diabetic rat.11, 19, 21 n = 6. Values are presented as mean ± standard error of mean.
Effect of Phloroglucinol on Formation of AGEs
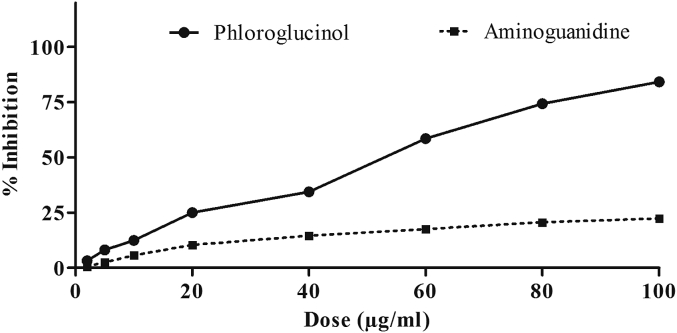
In vitro, AGE inhibition potential of phloroglucinol was better than aminoguanidine with IC50 48.38 ± 1.02 and 322.4 ± 0.98 μg/mL, respectively (Figure 3).
Figure 3.
Phloroglucinol inhibits formation of advanced glycation end products (AGEs) in vitro. Aminoguanidine known to inhibit AGEs is less effective than phloroglucinol. Data represent mean ± standard error of mean of three observations.
DPPH Free Radical and NO Scavenging Assay
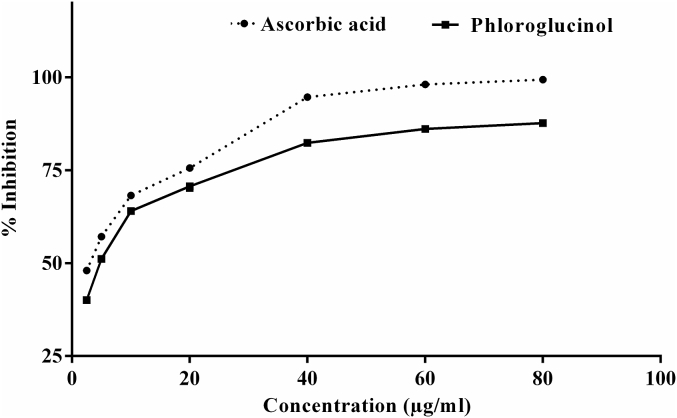
Phloroglucinol was very effective in scavenging DPPH free radicals with an IC50 of 4.48 ± 0.54 μg/mL. Ascorbic acid was better antioxidant than phloroglucinol in term of scavenging DPPH with IC50 3.07 ± 0.76 μg/mL (Figure 4).
Figure 4.
Percent reduction of DPPH by phloroglucinol and standard antioxidant ascorbic acid. Value represents mean ± standard error of mean of three observations.
Ascorbic acid was efficient in scavenging NO free radical with IC50 13.92 ± 1.62 μg/mL, but phloroglucinol was not effective in scavenging NO free radicals with an IC50 of 377.51 ± 1.31 μg/mL. (Figure 5).
Figure 5.
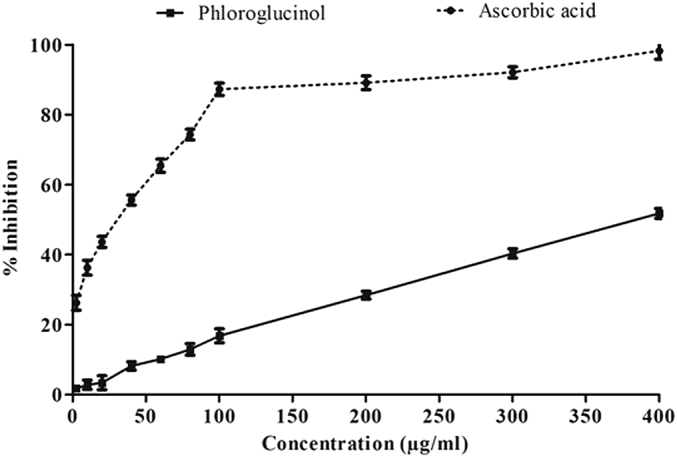
Percent reduction of NO by phloroglucinol and standard antioxidant ascorbic acid in vitro. Value presents mean ± standard error of mean. Assay was performed in triplicate.
CYP3A4 Inhibition Assay
Ketoconazole, used as positive control, inhibited 90% of CYP3A4 activity at a concentration of 10 nM, whereas IC50 of phloroglucinol was found to be 108 ± 10 nM. IC50 of ketoconazole, a standard human CYP3A4 inhibitor, was 1.8 ± 0.1 nM.
Discussion
This study proved usefulness of antioxidant therapy along with standard erectogenic agent in the management of diabetes-induced sexual dysfunction. Phloroglucinol decreased serum glucose level significantly (Table 1). Administration of both phloroglucinol and sildenafil prevented decrease in smooth muscle : collagen level thereby preserving sexual function (Figure 2, Table 1). Phloroglucinol inhibited AGEs formation in vitro suggesting its importance in minimizing AGEs-mediated sexual dysfunction (Figure 3, Table 1). Antioxidant property of phloroglucinol and its ability to inhibit CYP3A4 might be at least in part be responsible for increasing efficacy of sildenafil (Figures 4 and 5, Table 1). Phloroglucinol as such may not be useful in increasing sexual function in normal animals because it did not relax isolated CCSM (Table 2). However, the inhibition of CYP3A4 represents a bottleneck in the clinical use of phloroglucinol and further studies are required.
Diabetes is associated with increased risk of sexual dysfunction.4, 7, 8, 11, 24, 25 Increased AGEs, elevated levels of free radicals, impaired NO synthesis, decrease in smooth muscle : collagen ratio, impaired NO synthesis and availability, increased endothelin B receptor binding sites, and upregulated RhoA/Rho-kinase pathway have been proposed to facilitate diabetes-induced ED.24, 25, 26 Controlling serum glucose level is a primary factor in the management of diabetes-induced erectile dysfunction (DIED).24 Phloroglucinol increased efficacy of sildenafil by decreasing glucose level though it did not show any effect on CCSM.
Smooth muscles of corpus cavernosum play important role in penile erection. Relaxation of CCSM along with penile artery allows accumulation of blood in penis and stoppage of venous outflow ensures erection.26 Arginine/NO/cyclic guanosine monophosphate (cGMP) pathway relaxes CCSM, whereas RhoA/ROCK pathway contracts the smooth muscles in the penile tissue. A balance between relaxation and contraction of CCSM regulates penile erection.24, 25, 26 Oxidative stress-induced decrease in smooth muscle:collagen ratio was reported in CCSM of streptozotocin-induced diabetic rat.27 In our study, smooth muscle:collagen ratio decreased significantly in penile tissue of diabetic rats when compared with normal healthy rats. Smooth muscle:collagen ratio level in the penile tissue of diabetic rats treated with phloroglucinol + sildenafil was better in comparison with control diabetic rats (Figure 2). In addition to this, effect of phloroglucinol in decreasing formation of AGE and antioxidant property might have improved efficacy of sildenafil.
Mechanism of AGEs-induced sexual dysfunction in diabetes is extensively discussed elsewhere.15 Briefly, AGEs are generated by glycation of proteins. In diabetes, levels of AGEs are elevated due to increased blood glucose levels. AGEs-induced ED results from vascular wall thickening and decreased elasticity arising due to formation of covalent bond with collagen. Increase in levels of AGEs in serum is reported to increase the expression and activity of NADPH oxidase, which in turn increases oxidative stress in CCSM by generation of reactive free radicals. AGEs decreases expression of antioxidant enzyme Mn-superoxide dismutase, thereby decreasing antioxidant defense system. In addition, AGEs decrease endothelial nitric oxide synthase (eNOS) mRNA expression and uncouples eNOS, which decrease production of NO that relaxes CCSM by elevating production of cGMP. AGEs regulate smooth muscle contraction through Rho as Y27632, a Rho kinase inhibitor inhibits AGE-BSA-induced cell contraction.28 Antibody to AGE receptor inhibits AGE–BSA-induced cell contraction, and breakdown of preformed AGEs reverses ED in STZ-induced diabetic rats.29 We observed that in our study, phloroglucinol was more effective than aminoguanidine in decreasing AGE formation in vitro. Diabetes-induced oxidative stress and decrease in levels of antioxidant in penile tissue promote collagen deposition and fibrosis of penile tissue resulting in ED. Phloroglucinol exhibited potent antioxidant activity in vitro and efficacy of phloroglucinol to decrease DIED could be partly attributed to this antioxidant activity. Advantage of phloroglucinol over other antioxidants such as ellagic acid11 and vitamin E7 in DIED is due to its effect on reducing glycemia. Though phloroglucinol increases efficiency of sildenafil, it is least effective than sildenafil in management of DIED.
Apart from PDE5 inhibition, efficacy of sildenafil in DIED might be through different mechanisms due to its effect on testosterone, inflammation, oxidative stress, RhoA/ROCK signaling etc. DIED is associated with hypogonadism, decrease in the mRNA level of neural nitric oxide synthase (nNOS) and PDE5, and upregulation of RhoA/ROCK pathway in penile tissue. Testosterone supplementation increases the erectile response in diabetic rats along with increase in level of nNOS and PDE5 in comparison with diabetic control rats in addition to normalization of RhoA/ROCK pathway upregulation.30, 31, 32 Sildenafil also increases erectile function and serum testosterone level significantly in men with ED associated with low testosterone level.33 Limitation of this study is lack of data on level of testosterone after sildenafil treatment. PDE5 inhibitors such as tadalafil and vardenafil exert anti-inflammatory effect in human benign prostrate hyperplasia.34 Sildenafil decreases inflammation and oxidative stress in pelvic ganglia neurons associated with bilateral cavernosal nerve damage in rats. Sildenafil also restores endothelial NOS and PDE5 in addition to its ability to control oxidative/nitrosative stress in mouse penis.35, 36 Therefore, anti-inflammatory and antioxidant effect of PDE5 inhibitors such as sildenafil might help in mitigating DIED in addition to their effect on PDE5. Administration of tadalafil, a PDE5 reduces visceral fat weight, RhoA/ROCK signaling and smooth muscle over activity in a rabbit model of high fat diet-induced metabolic syndrome. Though sildenafil could not decrease glycemia in this study, it might have improved sexual function in diabetic animals by suppressing RhoA/ROCK signaling and smooth muscle over activity.37
Phloroglucinol inhibited CYP3A4, the enzyme that metabolizes sildenafil, therefore, co-treatment with sildenafil may increase the efficacy of sildenafil by increasing its concentration in the blood. Potential of phloroglucinol to inhibit CYP3A4 might increase level of sildenafil in treated individuals when coadministered thereby increasing its bioavailability, efficacy, and perhaps toxicity. A similar inhibition potential of phloroglucinol on rat CYP3A1, ortholog to human CYP3A4 is expected. This study also shed a light on potential herb–drug interaction that could come in picture when an herbal product containing phloroglucinol is co-administered with sildenafil. Though PDE5 inhibitor such as sildenafil is first line of drug for management of DIED, adverse events are reported. Phloroglucinol may increase adverse effect of sildenafil such as headache, flushing, abnormal vision, nasal congestion, myalgia, back pain, cardiovascular disorder, etc.38 Further studies are required to shed more light on the potential drug interactions of phloroglucinol with other drugs metabolized by CYP3A4.
Conclusion
Preventative treatment of phloroglucinol could be helpful to manage diabetes-induced ED. The efficacy of phloroglucinol could be due to its ability to decrease serum glucose level and formation of AGEs and its antioxidant property. Phloroglucinol coadministration with sildenafil increases efficacy of sildenafil in diabetic rats. Inhibition of CYP3A4 by phloroglucinol indicates a potential drug interaction with drugs that are substrates for the enzyme. We also propose that further studies are required to ascertain the usefulness of phloroglucinol in the management of diabetes-induced sexual dysfunction.
Acknowledgments
Authors are grateful to Dr. Bruce D. Hammock, University of California, Davis for providing facility and reagents to conduct CYP3A4 assay.
Footnotes
Conflict of Interest: The authors report no conflict of interest.
Funding: None.
References
- 1.Ramachandran A. Epidemiology of diabetes in India—Three decades of research. J Assoc Physicians India. 2005;53:34. [PubMed] [Google Scholar]
- 2.Daneman D. Type 1 diabetes. Lancet. 2006;367:847. doi: 10.1016/S0140-6736(06)68341-4. [DOI] [PubMed] [Google Scholar]
- 3.Clark C.M., Jr., Lee D.A. Prevention and treatment of the complications of diabetes mellitus. N Engl J Med. 1995;332:1210. doi: 10.1056/NEJM199505043321807. [DOI] [PubMed] [Google Scholar]
- 4.Enzlin P., Mathieu C., Van Den Bruel A., Vanderschueren D., Demyttenaere K. Prevalence and predictors of sexual dysfunction in patients with type 1 diabetes. Diabetes Care. 2003;26:409. doi: 10.2337/diacare.26.2.409. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez J.J., Al Dashti R., Schwarz E.R. Linking erectile dysfunction and coronary artery disease. Int J Impot Res. 2005;17:S12–S18. doi: 10.1038/sj.ijir.3901424. [DOI] [PubMed] [Google Scholar]
- 6.Yakubu M.T., Akanji M.A., Oladiji A.T. Male sexual dysfunction and methods used in assessing medicinal plants with aphrodisiac potentials. Pharmacogn Rev. 2007;1:49. [Google Scholar]
- 7.De Young L., Yu D., Bateman R.M., Brock G.B. Oxidative stress and antioxidant therapy: Their impact in diabetes-associated erectile dysfunction. J Androl. 2004;25:830. doi: 10.1002/j.1939-4640.2004.tb02862.x. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal A., Nandipati K.C., Sharma R.K., Zippe C.D., Raina R. Role of oxidative stress in the pathophysiological mechanism of erectile dysfunction. J Androl. 2006;27:335. doi: 10.2164/jandrol.05136. [DOI] [PubMed] [Google Scholar]
- 9.Lepore G., Nosari I. Efficacy of oral sildenafil in the treatment of erectile dysfunction in diabetic men with positive response to intracavernosal injection of alprostadil. Diabetes Care. 2001;24:409. doi: 10.2337/diacare.24.2.409. [DOI] [PubMed] [Google Scholar]
- 10.Rendell M.S., Rajfer J., Wicker P.A., Smith M.D. Sildenafil for treatment of erectile dysfunction in men with diabetes: A randomized controlled trial. Sildenafil Diabetes Study Group. JAMA. 1999;281:421. doi: 10.1001/jama.281.5.421. [DOI] [PubMed] [Google Scholar]
- 11.Goswami S.K., Vishwanath M., Gangadarappa S.K., Razdan R., Inamdar M.N. Efficacy of ellagic acid and sildenafil in diabetes-induced sexual dysfunction. Pharmacogn Mag. 2014;10:581. doi: 10.4103/0973-1296.139790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50:537. [PubMed] [Google Scholar]
- 13.Bae J.S. Antithrombotic and profibrinolytic activities of phloroglucinol. Food Chem Toxicol. 2011;49:1572. doi: 10.1016/j.fct.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Rah D.K., Han D.W., Baek H.S., Hyon S.H., Park J.C. Prevention of reactive oxygen species-induced oxidative stress in human microvascular endothelial cells by green tea polyphenol. Toxicol Lett. 2005;155:269. doi: 10.1016/j.toxlet.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 15.Neves D. Advanced glycation end-products: A common pathway in diabetes and age-related erectile dysfunction. Free Radic Res. 2013;47:49. doi: 10.3109/10715762.2013.821701. [DOI] [PubMed] [Google Scholar]
- 16.Perez Gutierrez R.M. Inhibition of advanced glycation end-product formation by Origanum majorana L. in vitro and in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med. 2012;2012:598638. doi: 10.1155/2012/598638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel R.M., Patel N.J. In vitro antioxidant activity of coumarin compounds by DPPH, super oxide and nitric oxide free radical scavenging methods. J Adv Pharm Educ Res. 2011;1:52. [Google Scholar]
- 18.Warrington J.S., Shader R.I., Von Moltke L.L., Greenblatt D.J. In vitro biotransformation of sildenafil (Viagra): Identification of human cytochromes and potential drug interactions. Drug Metab Dispos. 2000;28:392. [PubMed] [Google Scholar]
- 19.Goswami S.K., Inamdar M.N., Jamwal R., Dethe S. Effect of Cinnamomum cassia methanol extract and sildenafil on arginase and sexual function of young male Wistar rats. J Sex Med. 2014;11:1475. doi: 10.1111/jsm.12535. [DOI] [PubMed] [Google Scholar]
- 20.Agmo A. Male rat sexual behavior. Brain Res Brain Res Protoc. 1997;1:203. doi: 10.1016/s1385-299x(96)00036-0. [DOI] [PubMed] [Google Scholar]
- 21.Italiano G., Calabrò A., Pagano F. A simplified in vitro preparation of the corpus cavernosum as a tool for investigating erectile pharmacology in the rat. Pharmacol Res. 1994;30:325. doi: 10.1016/1043-6618(94)80012-x. [DOI] [PubMed] [Google Scholar]
- 22.Donato M.T., Jiménez N., Castell J.V., Gómez-Lechón M.J. Fluorescence-based assays for screening nine cytochrome P450 (P450) activities in intact cells expressing individual human P450 enzymes. Drug Metab Dispos. 2004;32:699. doi: 10.1124/dmd.32.7.699. [DOI] [PubMed] [Google Scholar]
- 23.Reed J.R., Cawley G.F., Ardoin T.G., Dellinger B., Lomnicki S.M., Hasan F., Kiruri L.W., Backes W.L. Environmentally persistent free radicals inhibit cytochrome P450 activity in rat liver microsomes. Toxicol Appl Pharmacol. 2014;277:200. doi: 10.1016/j.taap.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thorve V.S., Kshirsagar A.D., Vyawahare N.S., Joshi V.S., Ingale K.G., Mohite R.J. Diabetes-induced erectile dysfunction: Epidemiology, pathophysiology and management. J Diabetes Complications. 2011;25:129. doi: 10.1016/j.jdiacomp.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Musicki B., Burnett A.L. Endothelial dysfunction in diabetic erectile dysfunction. Int J Impot Res. 2007;19:129. doi: 10.1038/sj.ijir.3901494. [DOI] [PubMed] [Google Scholar]
- 26.Andersson K.E., Wagner G. Physiology of penile erection. Physiol Rev. 1995;75:191. doi: 10.1152/physrev.1995.75.1.191. [DOI] [PubMed] [Google Scholar]
- 27.Suresh S., Prakash S. Effect of Mucuna pruriens (Linn.) on oxidative stress-induced structural alteration of corpus cavernosum in streptozotocin-induced diabetic rat. J Sex Med. 2011;8:1943. doi: 10.1111/j.1743-6109.2011.02221.x. [DOI] [PubMed] [Google Scholar]
- 28.Hirose A., Tanikawa T., Mori H., Okada Y., Tanaka Y. Advanced glycation end products increase endothelial permeability through the RAGE/Rho signalling pathway. FEBS Lett. 2010;584:61. doi: 10.1016/j.febslet.2009.11.082. [DOI] [PubMed] [Google Scholar]
- 29.Usta M.F., Kendirci M., Gur S., Foxwell N.A., Bivalacqua T.J., Cellek S., Hellstrom W.J. The breakdown of preformed advanced glycation end products reverses erectile dysfunction in streptozotocin-induced diabetic rats: Preventive versus curative treatment. J Sex Med. 2006;3:242. doi: 10.1111/j.1743-6109.2006.00217.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X.H., Filippi S., Morelli A., Vignozzi L., Luconi M., Donati S., Forti G., Maggi M. Testosterone restores diabetes-induced erectile dysfunction and sildenafil responsiveness in two distinct animal models of chemical diabetes. J Sex Med. 2006;3:253. doi: 10.1111/j.1743-6109.2006.00207.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X.H., Filippi S., Morelli A., Vignozzi L., Luconi M., Donati S., Forti G., Maggi M. Testosterone restores diabetes-induced erectile dysfunction and sildenafil responsiveness in two distinct animal models of chemical diabetes [errata] J Sex Med. 2006;3:573. doi: 10.1111/j.1743-6109.2006.00207.x. [DOI] [PubMed] [Google Scholar]
- 32.Vignozzi L., Morelli A., Filippi S., Ambrosini S., Mancina R., Luconi M., Mungai S., Vannelli G.B., Zhang X.H., Forti G., Maggi M. Testosterone regulates RhoA/Rho-kinase signaling in two distinct animal models of chemical diabetes. J Sex Med. 2007;4:620. doi: 10.1111/j.1743-6109.2007.00440.x. [DOI] [PubMed] [Google Scholar]
- 33.Spitzer M., Bhasin S., Travison T.G., Davda M.N., Stroh H., Basaria S. Sildenafil increases serum testosterone levels by a direct action on the testes. Andrology. 2013;1:913. doi: 10.1111/j.2047-2927.2013.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vignozzi L., Gacci M., Cellai I., Morelli A., Maneschi E., Comeglio P., Santi R., Filippi S., Sebastianelli A., Nesi G., Serni S., Carini M., Maggi M. PDE5 inhibitors blunt inflammation in human BPH: A potential mechanism of action for PDE5 inhibitors in LUTS. Prostate. 2013;73:1391. doi: 10.1002/pros.22686. [DOI] [PubMed] [Google Scholar]
- 35.Garcia L.A., Hlaing S.M., Gutierrez R.A., Sanchez M.D., Kovanecz I., Artaza J.N., Ferrini M.G. Sildenafil attenuates inflammation and oxidative stress in pelvic ganglia neurons after bilateral cavernosal nerve damage. Int J Mol Sci. 2014;15:17204. doi: 10.3390/ijms151017204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bivalacqua T.J., Musicki B., Hsu L.L., Berkowitz D.E., Champion H.C., Burnett A.L. Sildenafil citrate-restored eNOS and PDE5 regulation in sickle cell mouse penis prevents priapism via control of oxidative/nitrosative stress. PLoS One. 2013;8:e68028. doi: 10.1371/journal.pone.0068028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vignozzi L., Filippi S., Comeglio P., Cellai I., Morelli A., Maneschi E., Sarchielli E., Gacci M., Carini M., Vannelli G.B., Maggi M. Tadalafil effect on metabolic syndrome-associated bladder alterations: An experimental study in a rabbit model. J Sex Med. 2014;11:1159. doi: 10.1111/jsm.12478. [DOI] [PubMed] [Google Scholar]
- 38.Balhara Y.P., Sarkar S., Gupta R. Phosphodiesterase-5 inhibitors for erectile dysfunction in patients with diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Indian J Endocrinol Metab. 2015;19:451. doi: 10.4103/2230-8210.159023. [DOI] [PMC free article] [PubMed] [Google Scholar]