Abstract
Introduction
Female sexual arousal disorder is a pathophysiologic state characterized clinically by persistent or recurrent inability to attain or maintain an adequate lubrication-swelling response of sexual excitement until completion of sexual activity. Prior clinical experience with alprostadil products for men with erectile dysfunction supports its use in women with female sexual arousal disorder.
Aim
To compare the effect of topical alprostadil with over-the-counter (OTC) lubricant on female genital arousal in the absence of visual sexual stimuli.
Methods
Healthy premenopausal women without sexual dysfunction were recruited from the community to participate in the study. Of 17 women who consented, 10 were enrolled and completed the trial. The mean age of subjects was 32 years (range = 27–43). Study drug or placebo was applied topically to the genitals. Continuous temperature monitoring was performed. Participants completed questionnaires assessing genital sensation, effect, intensity, and duration.
Main Outcome Measures
Change in temperature from baseline in vestibule, clitoris and vulva.
Results
In all 10 subjects, topical alprostadil induced a statistically significant increase in temperature of the vestibule, clitoris, and vulva compared with the OTC lubricant. The most rapid difference in genital temperature between placebo and alprostadil was seen on the vulva, which demonstrated a significant difference at approximately 9 minutes. There was a significant difference in temperature seen for the vestibule and clitoris at 11 and 19 minutes, respectively. Sixty percent of women reported being aware or conscious of genital sensations with topical alprostadil, but not with OTC lubricant. Discordance was noted in 30% of subjects who reported being aware or conscious of genital sensations with the two treatments and 10% who reported not being aware or conscious of genital sensations with either treatment.
Conclusion
Topical alprostadil administered to healthy premenopausal women induced statistically significant, sustained increases in genital temperatures of the vestibule, clitoris, and vulva within 20 minutes compared with OTC lubricant.
Key Words: Female Sexual Arousal Disorder, Alprostadil, Thermography, Female Sexual Dysfunction, Genitals
Introduction
Female sexual dysfunction (FSD) is a multidisciplinary, biopsychosocial concern that can present with many distressing symptoms such as low sexual desire, decreased peripheral and/or central sexual arousal, orgasm dysfunction, and/or pain or discomfort during sexual activity.1, 2 The prevalence of overall FSD, independent of associated distress, has been estimated to be 43% of women.3 The prevalence of FSD surpasses that of male sexual dysfunction.4, 5 Symptoms of FSD, when associated with distress, have been shown to negatively affect quality of life.3
In women, physiologic sexual stimulation results in pelvic nerve-mediated genital smooth muscle relaxation and subsequent increases in hypogastric-pudendal arterial blood inflow. Initiation and maintenance of female sexual arousal consists, in part, of clitoral, vulvar, vestibular, and vaginal vasodilation, genital engorgement, and enhanced genital lubrication.6, 7 Female sexual arousal disorder (FSAD) is a pathophysiologic state characterized clinically by persistent or recurrent inability to attain or maintain an adequate lubrication-swelling response of sexual excitement until completion of sexual activity.8 The prevalence of women with arousal complaints accompanied by distress is estimated to be 3% to 8% across all age groups.1, 9 Shifren et al10 reported that the prevalence of distressing FSAD was 5.4%. Epidemiologic studies have shown that comorbidities of metabolic syndrome, such as obesity, diabetes, cardiovascular disease, and hypertension, can contribute to female genital arousal disorder.11, 12, 13, 14, 15, 16, 17 Such comorbidities are associated, in part, with atherosclerotic lesions in the hypogastric-pudendal arterial bed that negatively restrict blood inflow increases to the peripheral genitalia during sexual arousal. Although the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition has suggested that FSAD should be linked to hypoactive sexual desire disorder (HSDD) in the entity “female sexual interest-arousal disorder,” this remains controversial. The International Society for the Study of Women's Sexual Health and other societies have determined that the use of FSAD and HSDD as separate conditions should continue in clinical and research settings. The present research was performed in healthy women without sexual dysfunctions.
Prostaglandin E1 (PGE1) naturally occurs in humans. PGE1 is a vasodilator that produces an increase in intracellular cyclic adenosine monophosphate and activation of protein kinase A. PGE1, unlike phosphodiesterase type 5 inhibitors (PDE5Is), increases blood flow without the need for sexual arousal. Increased protein kinase A activity has been shown to induce genital smooth muscle relaxation that in women can result in vulvovaginal vasodilation and enhanced genital lubrication, which are components of genital arousal.6, 7 Alprostadil is a vasoactive, synthetic form of PGE1 that has been approved worldwide for several intracavernosal and intraurethral products to treat erectile dysfunction.18, 19, 20, 21 Prior clinical experience with alprostadil products for men with erectile dysfunction supports its use in women with FSAD.22, 23, 24, 25 Several studies have examined the use of alprostadil in the treatment of FSAD, but effects on genital arousal from these previous investigations have largely been measured in a sexual context.22, 23, 24, 25 Becher et al26 studied topical alprostadil on the clitoris alone using duplex Doppler sonography, but heretofore no one has studied the genital changes from application of alprostadil to the external genitalia compared with placebo. It is unknown whether topical alprostadil increases genital temperature (blood flow) and arousal without visual stimulation.
The objective of this proof-of-principle study was to evaluate the effect of a topical alprostadil cream (Femprox, Apricus Biosciences, Inc, San Diego, CA, USA) 1,000 μg on the external genitalia of sexually healthy women in the absence of sexual stimulus compared with an over-the-counter (OTC) marketed lubricant using forward-looking infrared (FLIR) thermography as a measurement of genital blood flow. The use of thermography as a method of measuring female sexual arousal has been well validated.27, 28, 29, 30, 31, 32 This was an investigator-initiated pilot study to determine whether a larger study in women with FSAD might be feasible. This novel study design was more rigorous than previous studies in its ability to assess genital blood flow changes in women using alprostadil. This single-blinded, placebo-controlled clinical trial used a non-invasive tool for measuring blood flow changes and timing of those changes without the confounding influence of sexual arousal in an office setting.
Methods
Study Design
A prospective, randomized, single-center, single-blinded, crossover, proof-of-principle study was performed under independent review board approval. The study involved two counterbalanced crossover visits, each consisting of thermographic assessment of vestibular, clitoral region, and vulvar responses to non-sexual stimuli (ie, travel film) and completion of several self-report questionnaires. Alprostadil and placebo were randomized at the start of the trial and applied by an unblinded clinician who had no other involvement in the study, so that all other study procedures were performed and results were assessed in blinded fashion. The two visits of study drug application were scheduled with a washout (minimum = 1 day, maximum = 7 days) between visits.
Subjects
Healthy premenopausal women without sexual dysfunction were recruited from the community to participate in the study. The intention of the study was to enroll up to 15 subjects to obtain 10. Of 17 who consented, 10 were enrolled and completed the trial. The mean age of the subjects was 32 years (range = 27–43). Six subjects were Caucasian (five of whom identified as Hispanic), two were Pacific Islanders, one was Black, and 1 was East Asian. Only heterosexual women were included in the study.
Subjects had to be in good general health as determined by medical history, physical examination, and laboratory testing, have a normal body mass index (range = 20–30 kg/m2), exhibit no clinically significant electrocardiographic abnormalities, have regular menstrual cycles (with not more than one missed menstrual period in the past 6 months), and be sexually active. All subjects were screened for pregnancy and were required to use a consistent form of contraception during the course of the study. Breastfeeding subjects were not allowed to participate.
Subjects were excluded if they did not meet all the inclusion criteria or had any musculoskeletal condition that would not allow them to remain in a supine position with their legs spread or in stirrups for a prolonged time; had a history of alcohol or drug abuse in the previous year; or had sensitivity to any drugs similar to alprostadil. Women who previously underwent genital or major pelvic surgery or trauma, had a vestibule that appeared not to be healthy in any way, or scored lower than 26.55 on the Female Sexual Function Index also were excluded.33 Subjects were not allowed to use any vaginal cream or lubricant within 24 hours of the thermographic procedure or any prescription medication or OTC medication that might interfere with study drug evaluation within 14 or 7 days, respectively, before study drug administration (Table 1 lists specifically excluded medications).
Table 1.
Excluded prescription medications
| Antidepressant medications |
| Selective serotonin reuptake inhibitors |
| Serotonin-norepinephrine reuptake inhibitor antidepressants |
| Tricyclic antidepressants and monoamine oxidase inhibitors |
| All anticonvulsant and antiepileptic medications |
| All antipsychotic medications |
| Lithium for 6 mo before enrollment |
| All β-blockers and α-blockers |
| Gonadotropin-releasing hormone agonists |
| Antiandrogens |
| Narcotics |
| Including cannabis and tetrahydrocannabinol, barbiturates, heroin, morphine, codeine, oxycodone, hydrocodone |
| Amphetamines including phentermine |
| Aromatase inhibitors and tamoxifen |
| Regular or frequent benzodiazepine use if commenced within past 6 mo |
Subjective Measurements
Each participant was provided with two questionnaires at each visit: one before dose administration and one after study drug application (Appendix 1). The predose questionnaire asked women to rate current genital sensation (eg, warmth in genitals, genital wetness or lubrication, tingling, or fullness) on a scale from 1 to 5, with 1 representing barely noticeable, 3 representing neutral, and 5 representing intense. The postdose questionnaire asked women to report a change, if any, in genital sensation. Women reporting a noticeable change were asked to rate its intensity on a scale from 1 to 5, with 1 representing barely noticeable, 3 representing neutral, and 5 representing intense (question 1a). Subjects were asked to describe the sensations on a positive-negative scale from 1 to 5, with 1 representing negative, 3 representing neutral, and 5 representing positive (question 1b). These women also were asked to indicate when the sensation change was most intense, with the following options: almost immediately after applying medication; within 5 to 10 minutes after applying medication; more than 30 minutes after applying medication; or unable to tell (question 1c). These women were asked to quantify how long the sensation change lasted: less than 5 minutes; 5 to 10 minutes; longer than 30 minutes; or unable to tell (question 1d).
Adverse Events
All adverse events, whether volunteered by the subject, discovered during general questioning, or detected through physical examination or other testing, were recorded. Adverse events were reported as serious or non-serious and expected or unexpected.
Data Analysis
The thermographic imaging camera (FLIR system ThermosVision A-Series; FLIR Systems, Inc, N. Billerica, MA, USA) and validated software from FLIR examinIR and QuickPlot were used to generate data in the form of genital temperature readings from thermographic imaging. After positioning the subject (Figure 1), thermography was started to capture baseline recordings and then allowed to run continuously through 1 hour after dosing. The subjects were asked to remain still; however, if they shifted slightly, then the area of interest could be moved when measuring the temperature on the recording afterward. Values were recorded continuously, but temperatures were measured in the three areas of interest every other minute from 5 minutes before dosing until 60 minutes after dosing.
Figure 1.
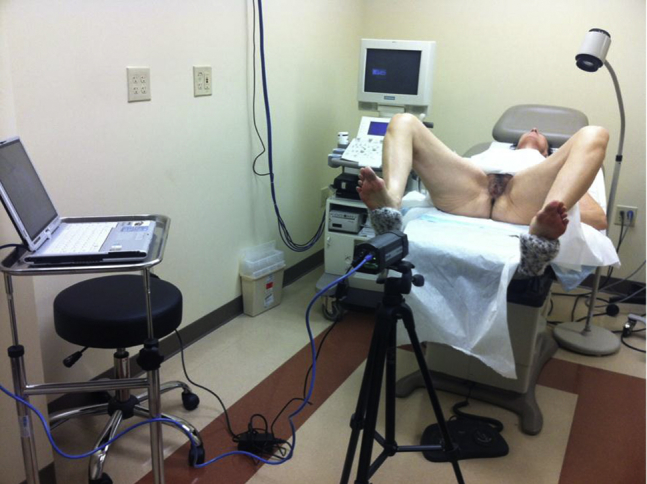
Arrangement of subject and thermographic camera.
Time to peak response, peak temperature increase from baseline after study drug application, and area under the curve for temperature measurements were recorded during this same interval. Comparisons of the time to peak response, peak temperature increase, and area under the curve between OTC lubricant and alprostadil topical cream were examined using paired t-test.
Subjective questionnaires were completed at baseline and at approximately 60 minutes after the dose for each treatment period. The frequency distributions for responses to postdosing question 1a and differences between treatments were assessed by the Mann-Whitney U-test. Responses to other Likert scale-type items were characterized by frequency distributions only.
Results
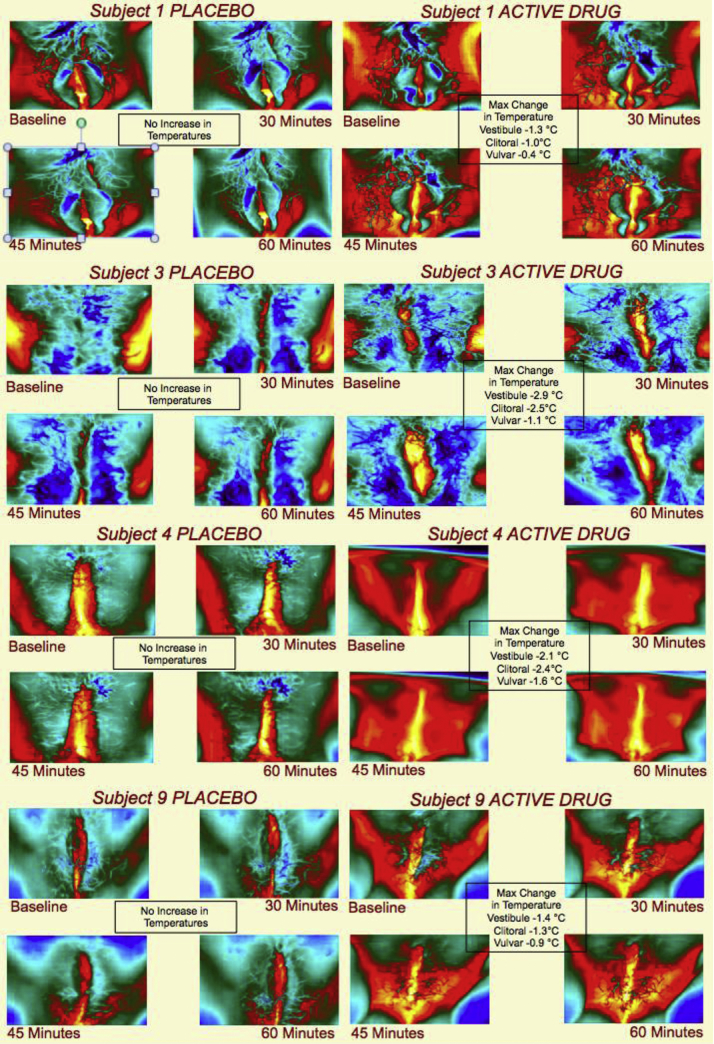
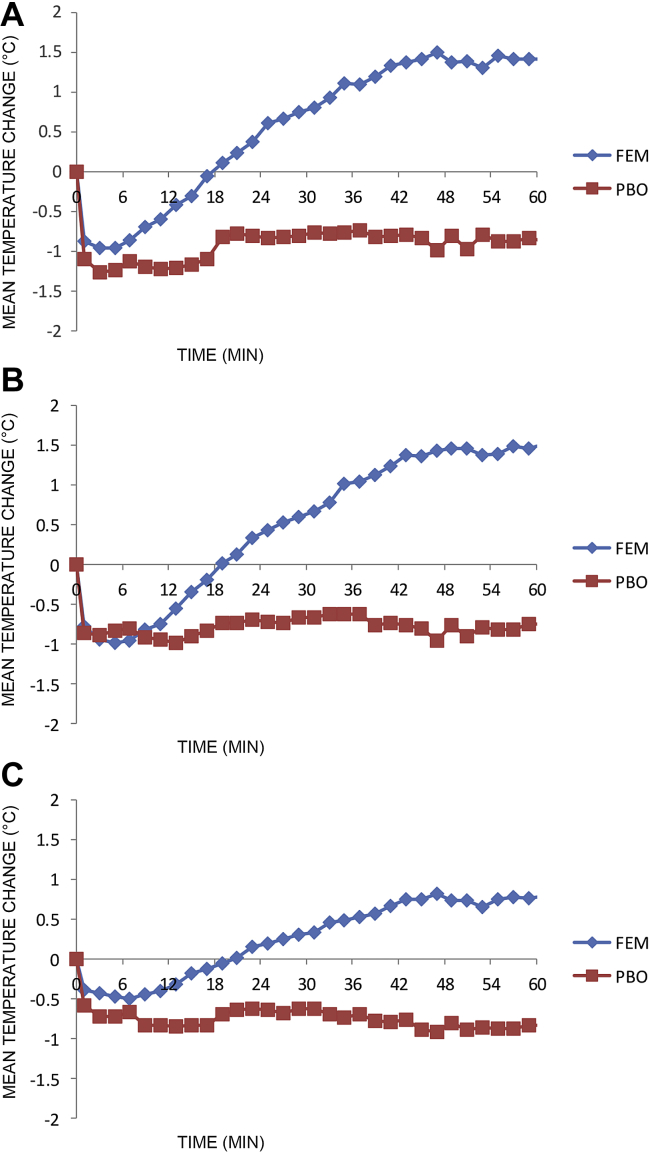
In all 10 subjects, topical alprostadil cream (Femprox) induced a statistically significant increase in temperature of the vestibule, clitoris, and vulva compared with the OTC lubricant (Table 2). Temperature changes were captured with thermographic imaging (Figure 2). Significant differences in genital temperature occurred at different time points depending on the specific region (Figure 3). The most rapid difference in genital temperature between placebo and alprostadil was seen on the vulva, which demonstrated a significant difference at approximately 9 minutes. There was a significant difference in temperature seen for the vestibule and clitoris shortly thereafter, at 11 and 19 minutes, respectively.
Table 2.
Change in genital temperature (°C) from baseline in over-the-counter lubricant vs alprostadil/DDAIP-HCl cream
| Variable | Least squares mean |
P value | |
|---|---|---|---|
| Placebo | Alprostadil | ||
| Vestibule | |||
| Baseline | 34.52 | 34.70 | 0.6672 |
| Change to postdose average | −1.04 | 0.56 | <0.0001 |
| Maximum postdose temperature | 34.00 | 36.32 | <0.0001 |
| Clitoris | |||
| Baseline | 34.24 | 34.52 | 0.4447 |
| Change to postdose average | −0.85 | 0.51 | <0.0001 |
| Maximum postdose temperature | 33.85 | 36.16 | <0.0001 |
| Vulva | |||
| Baseline | 33.98 | 34.22 | 0.5413 |
| Change to postdose average | −0.84 | 0.26 | <0.0001 |
| Maximum postdose temperature | 33.57 | 35.17 | 0.0014 |
DDAIP-HCl = dodecyl 2-(N,N-dimethyl amino) propionate plus dodecyl-2-(N,N-dimethyl amino) propionate hydrochloride.
Figure 2.
Thermography of subjects using over-the-counter lubricant vs alprostadil (dodecyl 2-[N,N-dimethyl amino] propionate plus dodecyl-2-[N,N-dimethyl amino] propionate hydrochloride) cream.
Figure 3.
Mean temperature change from baseline for the (A) vestibule, (B) clitoris, and (C) vulva using PBO vs FEM. FEM = alprostadil (dodecyl 2-[N,N-dimethyl amino] propionate plus dodecyl-2-[N,N-dimethyl amino] propionate hydrochloride) cream; PBO = placebo.
Sixty percent of women reported being aware or conscious of genital sensations with the topical alprostadil cream, but not with the OTC lubricant, demonstrating concordance between physiologic and subjective assessments (Table 3). Discordance was noted in 30% who reported being aware or conscious of genital sensations with the two treatments and 10% who reported not being aware or conscious of genital sensations with either treatment. No adverse events were reported.
Table 3.
Subjective evaluation of change in temperature (°C)
| Variable—visit | Femprox: subjective change |
Placebo: subjective change |
||
|---|---|---|---|---|
| No (n = 1) | Yes (n = 9) | No (n = 7) | Yes (n = 3) | |
| Predose genital sensation | 3.0 | 2.6 | 1.9 | 2.3 |
| Vestibule | ||||
| Baseline temperature | 35.4 | 34.6 | 34.4 | 34.9 |
| Mean change over 61 min | 0.44 | 0.58 | −1.05 | −1.00 |
| Maximum temperature over 61 min | 36.5 | 36.3 | 33.9 | 34.3 |
| Time (min) of maximum temperature | 43 | 48 | 33 | 34 |
| Clitoris | ||||
| Baseline temperature | 35.1 | 34.5 | 34.1 | 34.5 |
| Mean change over 61 min | 0.30 | 0.53 | −0.86 | −0.83 |
| Maximum temperature over 61 min | 36.2 | 36.2 | 33.8 | 34.0 |
| Time (min) of maximum temperature | 43 | 49 | 32 | 32 |
| Vulva | ||||
| Baseline temperature | 34.6 | 34.2 | 33.9 | 34.2 |
| Mean change over 61 min | 0.31 | 0.26 | −0.91 | −0.71 |
| Maximum temperature over 61 min | 35.6 | 35.1 | 33.5 | 33.7 |
| Time (min) of maximum temperature | 61 | 48 | 14 | 22 |
Discussion
FSAD is a common problem, affecting millions of women of all ages. The Prevalence of Female Sexual Problems Associated with Distress and Determinants of Treatment Seeking study found that nearly one-fourth of women surveyed endorsed arousal difficulty, with 5% reporting associated distress.10 Prior studies have demonstrated that women with sexual distress are more likely to have lower overall well-being, higher negative mood, lower positive mood, more negative feelings toward their partner, more reports of partners having problems with sexual performance, and higher depression scores.34, 35 The comorbidity between FSAD and HSDD also has been recognized.33
Despite its prevalence, there have been only a few clinical trials that have examined pharmaceutical therapies for FSAD in large, double-blinded, placebo-controlled trials.36, 37 There are no current government-approved topically applied arousal drugs for FSAD. Use of peripherally acting oral PDE5Is has yielded inconsistent results. Berman et al38 found a significant improvement in arousal sensation, lubrication, and orgasm in women who received sildenafil vs placebo, whereas a large randomized double-blinded study of subjects with FSAD found no significant physical response seen in patients given the active drug PDE5I.39 Daily tadalafil studied in a small group of premenopausal women with type 1 diabetes with arousal difficulty showed subjective sexual improvement vs placebo.40 Vardenafil and systemic testosterone caused an improvement in genital response, as measured by vaginal photoplethysmography, in women with arousal dysfunction and/or HSDD.41
PDE5Is are taken orally and are associated with bothersome and unwanted systemic side effects that act to discourage treatment continuation. The most commonly reported adverse events include headache, flushing, nausea, rhinitis, and visual disturbances.42 On-demand subcutaneously delivered bremelanotide, a centrally acting melanocortin receptor agonist, was used in the treatment of women with FSAD and HSDD and demonstrated significantly greater improvement in intercourse satisfaction than placebo.43 This agent has reported side effects of nausea, flushing, somnolence, and increases in blood pressure.
The use of topical vasodilator products for FSAD, such as alprostadil, are not systemically delivered and thus might be not be associated with the bothersome, unwanted systemic side effects of systemically administered vasodilator treatment. In prior clinical trials examining the safety of alprostadil in women with FSAD, adverse events were primarily classified as mild and were usually transient.23, 25 These included local, topical reactions, such as vaginal itching and burning, and might have been related to the delivery base compound and not the active drug. Another advantage of topical products is quick absorption within the genital tissues, yielding fast onset of effect.
The present study examined the local genital physiologic effect of topically applied alprostadil to the clitoris and periurethral tissue in healthy women without visual stimulation. A statistically significant increase in temperature, a marker of increased blood flow, was recorded in genital tissues. We measured objective changes in temperature, using FLIR thermography, as a marker of blood flow in addition to recording subjective change in arousal. To our knowledge, this is the first study to measure local peripheral genital arousal objectively by measuring temperature change in women not subjected to visual sexual stimulation. In three different genital regions—the vulva, vestibule, and clitoris—there was a significant difference in temperature between alprostadil and placebo. These differences occurred at different time points, with the most rapid difference occurring in the vulva. Furthermore, increases in genital temperature and arousal occurred with no reported systemic or local adverse events.
In previous studies using topical alprostadil, Becher et al26 used duplex Doppler ultrasound as the objective measurement of arousal. They showed that topical application of alprostadil to the clitoris resulted in a doubling of the peak systolic velocity of the clitoral artery. These physiologic engorgement changes were associated with a reported pleasurable sensation of warmth. In contrast to duplex Doppler ultrasonography, thermography measurements do not require a human operator in the room performing “genital arousal” assessments. Thus, thermography has the ability to record “peripheral genital arousal” in on-going fashion throughout the study. The use of duplex Doppler ultrasound does require placement of the measuring device on the genitals and interruption of the overall arousal condition of the subject. Moreover, genital arousal measurements with duplex Doppler ultrasound are taken only at the time of intervention. The strong benefits of thermography are that it is non-invasive and non-obtrusive to study subjects. Privacy of the subject is maintained and genital arousal is obtained continuously throughout a study.
There are some limitations to this study. First, unrecognized psychological and other non-physiologic factors might have influenced results in the treatment groups. We did try to minimize this effect by including only healthy subjects without sexual dysfunction and excluding those currently using antidepressants or other psychotropic drugs and β-blockers and other medications known to influence blood flow and/or arousal. Second, manual application of the active drug and OTC lubricant might have inadvertently contributed to genital arousal and biased our results. However, one would expect to see a similar significant increase in genital temperature in the placebo group, if this were the case, which was not identified in the present study. Third, the sample was small because this was merely a proof-of-principle study.
There remain several strengths to our work. This is the first study to use an objective, easily reproducible method such as thermography to measure changes in peripheral genital arousal without visual sexual stimulation. Previous studies investigating the efficacy of alprostadil in treating arousal dysfunction have relied only on questionnaires and/or diary logs, which are subject to response bias and error, or technically difficult measuring tools such as photoplethysmography.23, 24, 25 Furthermore, our data highlight the potential discordance that can exist between objective arousal and subjective experience and demonstrate that harmony between the two is likely necessary for a satisfactory sexual event to occur.
Previous work has highlighted the importance of cerebral activation in sexual arousal.44 Liao et al24 found that alprostadil affects patterns of cerebral activation at functional magnetic resonance imaging and postulated that, when applied to the genitals, the drug might act not only on local vascular dilation but also on local chemoreceptors, which could facilitate sexually related nerve reflexes. They offered this theory as a possible explanation for why topical alprostadil successfully increased subjective reports of sexual satisfaction, whereas other vasoactive drugs such as sildenafil did not. Future research on women with FSAD should investigate the association between central and subjective arousal and peripheral arousal so that more, successful therapeutic options, pharmaceutical and non-pharmaceutical, can be developed to treat this condition.
Conclusions
In summary, topical alprostadil cream (Femprox) administered to healthy premenopausal women without visual sexual stimulation induced statistically significant, sustained increases in genital temperatures of the vestibule, clitoris, and vulva within 20 minutes compared with the OTC lubricant. Local arousal pharmacologic therapies for FSAD approved by the U.S. Food and Drug Administration are currently lacking and necessitate further inquiry, but this proof-of-principle study highlights the safety and efficacy of topical alprostadil, just one of many potential therapeutic options. Treatment of arousal dysfunction can be challenging and multifactorial. Successful management of FSAD, and FSD in general, needs to be approached from a biopsychosocial perspective to address organic and psychological components of sexual dysfunction. It remains unclear whether improving peripheral arousal will result in satisfactory sexual experiences if central arousal is in disagreement. Future studies are needed to investigate this relation and to collect objective data on women with diagnosed, distressing arousal dysfunction.
Statement of authorship
Category 1
-
(a)Conception and Design
- Sue W. Goldstein; Catherine Gagnon; Irwin Goldstein
-
(b)Acquisition of Data
- Sue W. Goldstein; Catherine Gagnon
-
(c)Analysis and Interpretation of Data
- Sue W. Goldstein; Joshua R. Gonzalez; Catherine Gagnon; Irwin Goldstein
Category 2
-
(a)Drafting the Article
- Joshua R. Gonzalez; Catherine Gagnon
-
(b)Revising It for Intellectual Content
- Sue W. Goldstein; Joshua R. Gonzalez; Catherine Gagnon; Irwin Goldstein
Category 3
-
(a)Final Approval of the Completed Article
- Sue W. Goldstein; Joshua R. Gonzalez; Catherine Gagnon; Irwin Goldstein
Footnotes
Conflict of Interest: Irwin Goldstein serves on the advisory board of Apricus Biosciences, Inc.
Funding: Investigator-initiated grant from Apricus Biosciences, Inc.
Appendix
Appendix 1.
Subjective questionnaires
| Predose | ||||
| Initials: | Number: | Date: | ||
| Using the scale below, please rate your current genital sensations (eg, warmth in genitals, genital wetness or lubrication, tingling, or fullness). | ||||
| 1 | 2 | 3 | 4 | 5 |
| Barely noticeable | Neutral | Intense | ||
| Postdose | ||||
| Initials: | Number: | Date: | ||
| 1. Did you notice any change in genital sensations (eg, warmth in genital, genital tingling or fullness) after applying the study medication? | ||||
| ☐ Yes | ☐ No | |||
| a. If yes, how intense were those sensations (please circle the number)? | ||||
| 1 | 2 | 3 | 4 | 5 |
| Barely noticeable | Neutral | Intense | ||
| b. If yes, how would you describe those sensations (please circle the number)? | ||||
| 1 | 2 | 3 | 4 | 5 |
| Negative | Neutral | Positive | ||
| c. If yes, when were the sensations the most intense (please circle the number)? | ||||
| 1. Almost immediately after applying the medication | ||||
| 2. Within 5–10 minutes after applying the medication | ||||
| 3. More than 30 minutes after applying the medication | ||||
| 4. I couldn't tell | ||||
| d. If yes, how long did the sensations last (please circle the number)? | ||||
| 1. Less than 5 minutes | ||||
| 2. Within 5–10 minutes | ||||
| 3. Longer than 30 minutes | ||||
| 4. I couldn't tell | ||||
References
- 1.Giraldi A., Rellini A.H., Pfaus J. Female sexual arousal disorders. J Sex Med. 2013;10:58–73. doi: 10.1111/j.1743-6109.2012.02820.x. [DOI] [PubMed] [Google Scholar]
- 2.Jordan R., Hallam T.J., Molinoff P. Developing treatments for female sexual dysfunction. Clin Pharmacol Ther. 2011;89:137–141. doi: 10.1038/clpt.2010.262. [DOI] [PubMed] [Google Scholar]
- 3.Laumann E.O., Paik A., Rosen R.C. Sexual dysfunction in the United States: prevalence and predictors. JAMA. 1999;281:537–544. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 4.Heiman J.R. Sexual dysfunction: overview of prevalence, etiological factors, and treatments. J Sex Res. 2002;39:73–78. doi: 10.1080/00224490209552124. [DOI] [PubMed] [Google Scholar]
- 5.Lewis R.W., Fugl-Meyer K.S., Corona G. Definitions/epidemiology/risk factors for sexual dysfunction. J Sex Med. 2010;7:1598–1607. doi: 10.1111/j.1743-6109.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 6.Allahdadi K.J., Tostes R.C., Webb R.C. Female sexual dysfunction: therapeutic options and experimental challenges. Cardiovasc Hematol Agents Med Chem. 2009;7:260–269. doi: 10.2174/187152509789541882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berman J.R. Physiology of female sexual function and dysfunction. Int J Impot Res. 2005;17:S44–S51. doi: 10.1038/sj.ijir.3901428. [DOI] [PubMed] [Google Scholar]
- 8.Diagnostic and statistical manual of mental disorders. 4th ed., text rev. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- 9.Brotto L.A. The DSM diagnostic criteria for hypoactive sexual desire disorder in women. Arch Sex Behav. 2010;39:221–239. doi: 10.1007/s10508-009-9543-1. [DOI] [PubMed] [Google Scholar]
- 10.Shifren J.L., Monz B.U., Russo P.A. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970–978. doi: 10.1097/AOG.0b013e3181898cdb. [DOI] [PubMed] [Google Scholar]
- 11.Sarwer D.B., Lavery M., Spitzer J.C. A review of the relationships between extreme obesity, quality of life, and sexual function. Obes Surg. 2012;22:668–676. doi: 10.1007/s11695-012-0588-1. [DOI] [PubMed] [Google Scholar]
- 12.Moore R.H., Sarwer D.B., Lavenberg J.A. Relationship between sexual function and quality of life in obese persons seeking weight reduction. Obesity (Silver Spring) 2013;21:1966–1974. doi: 10.1002/oby.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhasin S., Enzlin P., Coviello A. Sexual dysfunction in men and women with endocrine disorders. Lancet. 2007;369:597–611. doi: 10.1016/S0140-6736(07)60280-3. [DOI] [PubMed] [Google Scholar]
- 14.Enzlin P., Mathieu C., Van Den Bruel A. Prevalence and predictors of sexual dysfunction in patients with type 1 diabetes. Diabetes Care. 2003;26:409–414. doi: 10.2337/diacare.26.2.409. [DOI] [PubMed] [Google Scholar]
- 15.Kazemi-Saleh D., Pishgou B., Farrokhi F. Gender impact on the correlation between sexuality and marital relation quality in patients with coronary artery disease. J Sex Med. 2008;5:2100–2106. doi: 10.1111/j.1743-6109.2007.00724.x. [DOI] [PubMed] [Google Scholar]
- 16.De Franciscis P., Mainini G., Messalli E.M. Arterial hypertension and female sexual dysfunction in postmenopausal women. Clin Exp Obstet Gynecol. 2013;40:58–60. [PubMed] [Google Scholar]
- 17.Martelli V., Valisella S., Moscatiello S. Prevalence of sexual dysfunction among postmenopausal women with and without metabolic syndrome. J Sex Med. 2012;9:434–441. doi: 10.1111/j.1743-6109.2011.02517.x. [DOI] [PubMed] [Google Scholar]
- 18.Porst H. The rationale for prostaglandin E1 in erectile failure: a survey of worldwide experience. J Urol. 1996;155:802–815. [PubMed] [Google Scholar]
- 19.Lee L.M., Stevenson R.W., Szasz G. Prostaglandin E1 versus phentolamine/papaverine for the treatment of erectile impotence: a double-blind comparison. J Urol. 1989;141:549–550. doi: 10.1016/s0022-5347(17)40889-5. [DOI] [PubMed] [Google Scholar]
- 20.Padma-Nathan H., Hellstrom W.J., Kaiser F.E. Treatment of men with erectile dysfunction with transurethral alprostadil. Medicated Urethral System for Erection (MUSE) Study Group. N Engl J Med. 1997;336:1–7. doi: 10.1056/NEJM199701023360101. [DOI] [PubMed] [Google Scholar]
- 21.Guay A.T., Perez J.B., Velásquez E. Clinical experience with intraurethral alprostadil (MUSE) in the treatment of men with erectile dysfunction. A retrospective study. Medicated Urethral System for Erection. Eur Urol. 2000;38:671–676. doi: 10.1159/000020360. [DOI] [PubMed] [Google Scholar]
- 22.Heiman J.R., Gittelman M., Costabile R. Topical alprostadil (PGE1) for the treatment of female sexual arousal disorder: in-clinic evaluation of safety and efficacy. J Psychosom Obstet Gynaecol. 2006;27:31–41. doi: 10.1080/01674820500237973. [DOI] [PubMed] [Google Scholar]
- 23.Islam A., Mitchel J., Rosen R. Topical alprostadil in the treatment of female sexual arousal disorder: a pilot study. J Sex Marital Ther. 2001;27:531–540. doi: 10.1080/713846804. [DOI] [PubMed] [Google Scholar]
- 24.Liao Q., Zhang M., Geng L. Efficacy and safety of alprostadil cream for the treatment of female sexual arousal disorder: a double-blind, placebo-controlled study in Chinese population. J Sex Med. 2008;5:1923–1931. doi: 10.1111/j.1743-6109.2008.00876.x. [DOI] [PubMed] [Google Scholar]
- 25.Padma-Nathan H., Brown C., Fendl J. Efficacy and safety of topical alprostadil cream for the treatment of female sexual arousal disorder (FSAD): a double-blind, multicenter, randomized, and placebo-controlled clinical trial. J Sex Marital Ther. 2003;29:329–344. doi: 10.1080/00926230390224710. [DOI] [PubMed] [Google Scholar]
- 26.Becher E.F., Bechara A., Casabe A. Clitoral hemodynamic changes after a topical application of alprostadil. J Sex Marital Ther. 2001;27:405–410. doi: 10.1080/713846798. [DOI] [PubMed] [Google Scholar]
- 27.Cherner R.A., Reissing E.D. A psychophysiological investigation of sexual arousal in women with lifelong vaginismus. J Sex Med. 2013;10:1291–1303. doi: 10.1111/jsm.12102. [DOI] [PubMed] [Google Scholar]
- 28.Kukkonen T.M., Binik Y.M., Amsel R. An evaluation of the validity of thermography as a physiological measure of sexual arousal in a non-university adult sample. Arch Sex Behav. 2010;39:861–873. doi: 10.1007/s10508-009-9496-4. [DOI] [PubMed] [Google Scholar]
- 29.Kukkonen T.M., Binik Y.M., Amsel R. Thermography as a physiological measure of sexual arousal in both men and women. J Sex Med. 2007;4:93–105. doi: 10.1111/j.1743-6109.2006.00399.x. [DOI] [PubMed] [Google Scholar]
- 30.Hodgson B, Kukkonen TM, Binik YM, et al. Using the dual control model to examine the relationship between mood, physiological and self-reported sexual arousal in men and women. J Sex Reshttp://dx.doi.org/10.1080/00224499.2015.1110107. E-pub ahead of print. [DOI] [PubMed]
- 31.Huberman J.S., Chivers M.L. Examining gender specificity of sexual response with concurrent thermography and plethysmography. Psychophysiology. 2015;52:1382–1395. doi: 10.1111/psyp.12466. [DOI] [PubMed] [Google Scholar]
- 32.Paterson L.Q.P., Shuo Jin E., Amsel R. Gender similarities and differences in sexual arousal, desire and orgasmic pleasure in the laboratory. J Sex Res. 2014;51:801–813. doi: 10.1080/00224499.2013.867922. [DOI] [PubMed] [Google Scholar]
- 33.Rosen R., Brown C., Heiman J. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 34.Dennerstein L., Guthrie J.R., Hayes R.D. Sexual function, dysfunction, and sexual distress in a prospective, population-based sample of mid-aged, Australian-born women. J Sex Med. 2008;5:2291–2299. doi: 10.1111/j.1743-6109.2008.00936.x. [DOI] [PubMed] [Google Scholar]
- 35.Hayes R.D., Dennerstein L., Bennett C.M. Risk factors for female sexual dysfunction in the general population: exploring factors associated with low sexual function and sexual distress. J Sex Med. 2008;5:1681–1693. doi: 10.1111/j.1743-6109.2008.00838.x. [DOI] [PubMed] [Google Scholar]
- 36.Berman L.A., Berman J.R., Chhabra S. Novel approaches to female sexual dysfunction. Expert Opin Investig Drugs. 2001;10:85–95. doi: 10.1517/13543784.10.1.85. [DOI] [PubMed] [Google Scholar]
- 37.Rosen R.C. Sexual pharmacology in the 21st century. J Gend Specif Med. 2000;3:45–52. [PubMed] [Google Scholar]
- 38.Berman J.R., Berman L.A., Toler S.M. Safety and efficacy of sildenafil citrate for the treatment of female sexual arousal disorder: a double-blind, placebo controlled study. J Urol. 2003;170:2333–2338. doi: 10.1097/01.ju.0000090966.74607.34. [DOI] [PubMed] [Google Scholar]
- 39.Basson R., McInnes R., Smith M.D. Efficacy and safety of sildenafil citrate in women with sexual dysfunction associated with female sexual arousal disorder. J Womens Health Gend Based Med. 2002;11:367–377. doi: 10.1089/152460902317586001. [DOI] [PubMed] [Google Scholar]
- 40.Caruso S., Cicero C., Romano M. Tadalafil 5 mg daily treatment for type 1 diabetic premenopausal women affected by sexual genital arousal disorder. J Sex Med. 2012;9:2057–2065. doi: 10.1111/j.1743-6109.2012.02777.x. [DOI] [PubMed] [Google Scholar]
- 41.van der Made F., Bloemers J., Yassem W.E. The influence of testosterone combined with a PDE5-inhibitor on cognitive, affective, and physiological sexual functioning in women suffering from sexual dysfunction. J Sex Med. 2009;6:777–790. doi: 10.1111/j.1743-6109.2008.01142.x. [DOI] [PubMed] [Google Scholar]
- 42.Nurnberg H.G., Hensley P.L., Heiman J.R. Sildenafil treatment of women with antidepressant-associated sexual dysfunction: a randomized controlled trial. JAMA. 2008;300:395–404. doi: 10.1001/jama.300.4.395. [DOI] [PubMed] [Google Scholar]
- 43.Buvat J. [2013 annual meeting of the International Society for the Study of Women’s Sexual Health (ISSWSH), 28 February–3 March 2013, New Orleans, Louisiana, USA. State of pharmacological research in Women's Sexual Medicine: from testosterone to bremelatonide] Gynecol Obstet Fertil. 2013;41:330–333. doi: 10.1016/j.gyobfe.2013.04.003. (in French) [DOI] [PubMed] [Google Scholar]
- 44.Park K., Kang H.K., Seo J.J. Blood-oxygenation-level–dependent functional magnetic resonance imaging for evaluating cerebral regions of female sexual arousal response. Urology. 2001;57:1189–1194. doi: 10.1016/s0090-4295(01)00992-x. [DOI] [PubMed] [Google Scholar]