Abstract
Background
Among the various ginseng strains, Shizhu ginseng is endemic to China, mainly distributed in Kuandian Manchu Autonomous County (Liaoning Province, China); however, not much is known about the compounds (especially saponins) in Shizhu ginseng.
Methods
A rapid, sensitive, and reliable ultra-high performance liquid chromatography coupled with MS/MS (UHPLC–MS/MS) method was developed to separate and identify saponins in Shizhu ginseng.
Results
The separation was carried out on a Waters ACQUITY UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 μm) with acetonitrile and 0.1% formic acid aqueous solution as the mobile phase under a gradient elution at 40°C. The detection was performed on a Micromass Quattro Micro API mass spectrometer equipped with electrospray ionization source in both positive and negative modes. Under the optimized conditions, a total of 31 saponins were identified or tentatively characterized by comparing retention time and MS data with related literatures and reference substances.
Conclusion
The developed UHPLC–MS/MS method was suitable for identifying and characterizing the chemical constituents in Shizhu ginseng, which provided a helpful chemical basis for further research on Shizhu ginseng.
Keywords: identification, saponins, separation, Shizhu ginseng, ultra-high performance liquid chromatography coupled with MS/MS
1. Introduction
Panax ginseng Meyer is an important Chinese herbal medicine that has served as a rich source of natural nourishment for a long time and has become increasingly popular in China and Korea in recent years [1], [2], [3]. Shizhu ginseng as one of the excellent artificial cultivation herbs for more than 400 years of cultivation history [4], [5], derived from the dry root and rhizome of P. ginseng Meyer and is mainly cultivated in the northeastern region of China [6]. Shizhu ginseng has the same morphological characteristics and medicinal value as wild ginseng; additionally, it also contains many different constituents such as amino acids, vitamins, ginseng polysaccharides, and dozens of saponins. Studies have shown that saponins in Shizhu ginseng play important roles in the treatment and prevention of diabetes, aging, and arteriosclerosis, etc. [7], [8], [9]. In particular, saponins such as Rg1, Re, Rf, Rg2, Rb1 are active ingredients used in the treatment of various tumors and cancers. In this paper, we attempt to separate and characterize a variety of saponins in Shizhu ginseng.
In recent years, various methods for determination of saponins in P. ginseng, including micellar electrokinetic capillary chromatography, HPLC, high-performance thin-layer chromatography, liquid chromatography coupled with MS (LC–MS), and LC–MS/MS (LC–MS/MS), have been well described in the literature [10], [11], [12]. Among these methods, LC–MS had been increasingly applied in studies on natural products. Compared with the common methods, application of ultra-high performance LC coupled with MS/MS (UHPLC–MS/MS) can effectively resist the interference caused by multicomponent samples, improve accuracy on the quantitative detection, and have the advantage of higher sensitivity and selectivity, which is suitable not only for the structural confirmation of known ingredients but also for the rapid identification of unknown ingredients in traditional Chinese medicine [13]. Because of these advantages, UHPLC–MS/MS has become one of the effective technologies used for determination of saponins in ginseng. In is also widely used method for pharmaceutical analysis [14], [15]. Therefore, in this paper, a UHPLC–MS/MS system equipped with an electrospray ionization source in both positive and negative modes was chosen to separate and identify saponins in Shizhu ginseng.
The aim of our study was to develop a direct and rapid UHPLC–MS/MS method for simultaneously identifying 31 saponins in Shizhu ginseng. In the full-scan mode, the structure of mother nucleuses and the molecular weight of 31 major saponins were established by analyzing the mass-to-charge ratios from [M+Na]+ ions produced in the electron spray ionization-positive (ESI+) mode and from [M-H]– ions produced in the ESI– spectra. The structures of 29 saponins in Shizhu ginseng were determined by comparing them with reference substances, analyzing the MS/MS spectra data, and comparing the fragmentation pattern with related literatures. Our study results show that the analytic method adopted here can be used as a valuable tool for further research on Shizhu ginseng.
2. Materials and methods
2.1. Chemicals and materials
Shizhu ginseng specimens were collected from Kuandian, Liaoning. The plant was identified by Professor Jincai Lu of Shenyang Pharmaceutical University (Shenyang, Liaoning, China). The reference standards of ginseng saponins Rg1, Re, and Rb1 were purchased from the National Institute for Food and Drug Control (Beijing, China). Ginseng saponins Rb2, Rb3, Rc, Rd, Rf, Rg2, Rh1, and notoginsenoside R1 were obtained from College of Pharmacy, Jilin University (Changchun, Jilin Province, China). Acetonitrile and formic acid of HPLC grade were purchased from Fisher Scientific (Fair Lawn, NJ, USA), and HPLC-grade ammonium acetate was purchased from Sigma (Saint Louis, MO, USA). Other reagents were of analytical grade. Deionized water was purified using a Milli-Q system (Millipore, USA).
2.2. Instrumentation and analytical conditions
Chromatographic analysis was carried out on an ACQUITY ultra-high performance liquid chromatography column (Waters, USA) equipped with a quaternary pump, a vacuum degasser, an autosampler, and a diode array detector. Separation was achieved on an ACQUITY UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 μm; Waters, USA) with the column temperature maintained at 40°C. The mobile phase consisted of acetonitrile (A) and 0.1% formic acid in water (B), with a flow rate of 0.25 mL/min. The gradient elution program was as follows: 0–5.0 min, 20–20% A; 5–10 min, from 20% to 30% A; 10–30 min, from 30% to 35% A. The Photo-Diode Array (PDA) spectrum was recorded from 200 nm to 400 nm.
Mass spectrometric detection was performed on a Micromass Quattro Micro API mass spectrometer equipped with an ESI source operating in both positive- and negative-ion modes. The optimal MS parameters were as follows: capillary voltage, 2.9 kV; cone voltage, 50 V; cone gas flow, 300 L/h; ion-source temperature, 105°C; desolvation gas temperature, 350°C; desolvation gas flow rate, 600 L/h; collision chamber pressure, helium 2.50 × 10−3 mbar. Full-scan MS data were collected from 100 Da to 1,200 Da in positive- and negative-ion modes. MassLynx 4.0 software (Waters, USA) was used to control the equipment and for data acquisition and analysis.
2.3. Sample preparation
Approximately 3 g of Shizhu ginseng powder was weighed and extracted with 25 mL of 70% ethanol by ultrasonic treatment for 30 min. The solution was filtered and evaporated to dryness under reduced pressure. The residue was dissolved with 10 mL of 20% acetonitrile. The solution obtained was then filtered through a 0.22-μm filter membrane and 10 μL of the solution was injected onto the UHPLC–MS/MS system for analysis.
3. Results and discussion
3.1. Total ion current of Shizhu ginseng
Test solutions were analyzed according to the aforementioned conditions. The total ion current (TIC) chromatograms were acquired in the positive and negative ionization modes and UV chromatograms at different wavelengths were recorded. The TIC chromatograms are shown in Fig. 1.
Fig. 1.
Total ion current chromatogram of Shizhu Ginseng at (A) positive ionization and (B) negative ionization modes.
3.2. Optimum conditions for Shizhu ginseng
According to previous reports [16], [17], [18], [19], saponins in Shizhu ginseng were particularly soluble in water and dilute alcohol, and therefore, different concentrations of alcohol adopting reflux, cold soak, and other extraction methods were commonly used to treat the samples. In addition, the solubilities of saponins are very high in water-saturated ethanol, n-butyl alcohol, and pentanol, which are frequently used as extraction solvent. In consideration of the environmentally friendly factor, ethanol was better than other extraction solvents. Thus, different concentrations of ethanol (range 20–90%) were used in this study. The extraction method is as follows: approximately 3 g of Shizhu ginseng powder was weighed and extracted with 50 mL of different concentrations of ethanol, and then 20-mL extracts were taken, respectively, on evaporating dishes and evaporated to dryness in a water bath at 100°C. Then the residues were placed in an oven and dried to constant weight at 105°C. Finally, the weights of total solids were obtained. The results (data not shown) indicated that 70% ethanol extracted most of the saponin contents (i.e., it had best extraction efficiency), and thus, 70% ethanol was chosen as extraction solvent.
In this study, two extraction methods [20], [21], namely, reflux and ultrasonic, were used. Because the samples contained malonyl ginsenosides, which are thermally unstable, ultrasonic extraction at room temperature was chosen for sample extraction. Under this condition, nine malonyl ginsenosides compounds were identified.
Optimization of chromatographic conditions was also investigated systematically. Because the components of saponins are very complex and isocratic elution was hard to separate, gradient elution was carried out for the following research. It is well-known that the mobile phase composition plays an important role in the separation and identification of compounds, and therefore, several trials were carried out in this study to identify the best mobile phase composition. There are many hydroxyl groups in the structure of monomer saponin and because different kinds of additives in the mobile phase could inhibit the ionization of hydroxyl groups, improve the effect of separation, and affect the peak symmetry, we chose a solvent system consisting of acetonitrile and 0.1% aqueous formic acid for further analysis. We also investigated the effect of temperature on the column. From the results, it was found that column temperature had no influence on the retention time; however, with the increase of temperature, the column efficiency improved and the resolution also increased. Taking account of the tolerance of column, the analysis temperature was determined at 40°C. To obtain a detailed structural information of saponins, both positive and negative modes were used for detection.
3.3. Analysis of Shizhu ginseng saponins by UHPLC–MS/MS
In the ESI+ spectra, saponins generated large amounts of [M+Na]+ ions, but very low amount of [M+H]+ ions. Saponins produced a group of characteristic fragment ions at m/z 350–500 during endogenous cracking, which contributed to identifying the structure of the nucleus. The spectra showed that saponins with protopanaxatriol as the mother nucleus produced characteristic fragment ions at m/z 405, m/z 423, and m/z 441 (Fig. 2A), whereas those with protopanaxadiol produced characteristic fragment ions at m/z 407 and m/z 425 (Fig. 2B). A previous study [22] had also reported that those with oleanolic acid as the mother nucleus produced characteristic fragment ions at m/z 439. Saponins with different mother nucleuses generated different characteristic fragment ions, and thus, by analyzing the characteristic fragment ions, the structures of mother nucleus could be determined.
Fig. 2.
Ultra-high performance liquid chromatography–electron spray ionization–MS positive mass spectra of (A) Re and (B) Rd and negative mass spectra of (C) Re, (D) Rf, and (E) Rh1.
In the ESI− spectra, saponins generated large amounts of deprotonated molecular ions ([M-H]–) and a few adductive ions ([M+Cl]− and [M+HCOO]−; Fig. 2C and D). However, some saponins with small molecular weight such as ginseng saponin Rh1 showed stronger peaks of the [M+HCOO–]– adductive ions and weaker peaks of the [M-H]– deprotonated molecular ions (Fig. 2E). Thus, it could be concluded that the deprotonated molecular ions, adductive ions, and characteristic fragment ions played an important role in determination of the molecular weight of compounds.
The structure of mother nucleuses and molecular weight of 31 saponins were determined by studying the ESI-negative and ESI-positive mass spectra of saponins in Shizhu ginseng (Table 1). In ESI−, the MS spectra of saponins containing a malonyl group showed a stronger peak of the fragment ions of [M-H-44]– and a weaker peak of the fragment ions of [M-H]–. This can attributed to the following reasons: thermal stability of malonyl ginsenosides was destroyed and the compounds endogenously released a carboxylic group and produced a large number of [M-H-44]– and a few [M-H]– (Fig. 3A); however, in ESI+, the adductive ion of [M+Na]+ (Fig. 3B) showed a strong peak, which was a characteristic feature that helped us to determine its relative molecular weight. The ESI mass spectra of Peak 15 are shown in Fig. 3. Besides, a new kind of saponin (molecular weight 1,342 Da) was identified. The fragment ions at m/z 427 and m/z 457 produced by endogenous cracking can be observed in the ESI+ mass spectra of this new compound (Fig. 3C). However, the obtained ions were not in accordance with the characteristic fragment ions of saponins having either of the three certain mother nucleuses referred earlier, and therefore, its mother nucleus could not be identified. The ESI-positive and ESI-negative ions mass spectra are shown in Fig. 3C and D.
Table 1.
Major negative and positive ions observed in the ultra-high performance liquid chromatography–electron spray ionization–MS spectra of extract of Shizhu ginseng
| No. | tR (min) | [M-H]– | [M+Na]+ | Positive major ions |
|---|---|---|---|---|
| 1 | 5.6 | 961 | 985 | 405, 423, 441 |
| 2 | 6.7 | 931 | 955 | 405, 423, 441 |
| 3 | 8.1 | 799 | 823 | 405, 423, 441 |
| 4 | 8.3 | 945 | 969 | 405, 423, 441 |
| 5 | 9.2 | 885 | 909 | 405, 423, 441 |
| 6 | 10.0 | 1,031 | 1,055 | 405, 423, 441 |
| 7 | 13.4 | 799 | 823 | 405, 423, 441 |
| 8 | 13.8 | 1,239 | 1,263 | 407, 425, 443 |
| 9 | 14.1 | 1,341 | 1,365 | 427, 457 |
| 10 | 14.5 | 769 | 793 | 405, 423, 441 |
| 11 | 14.6 | 783 | 807 | 405, 423, 441 |
| 12 | 15.9 | 1,209 | 1,233 | 407, 425, 443 |
| 13 | 16.6 | 1,209 | 1,233 | 407, 425, 443 |
| 14 | 17.5 | 1,107 | 1,131 | 407, 425, 443 |
| 15 | 18.4 | 1,193 | 1,217 | 407, 425, 443 |
| 16 | 18.8 | 955 | 979 | 439 |
| 17 | 19.1 | 1,077 | 1,101 | 407, 425, 443 |
| 18 | 19.2 | 637 | 661 | 405, 423, 441 |
| 19 | 19.5 | 1,209 | 1,233 | 407, 425, 443 |
| 20 | 19.8 | 1,163 | 1,187 | 407, 425, 443 |
| 21 | 20.2 | 1,295 | 1,319 | 407, 425, 443 |
| 22 | 21.1 | 1,077 | 1,101 | 407, 425, 443 |
| 23 | 21.6 | 1,077 | 1,101 | 407, 425, 443 |
| 24 | 21.9 | 1,163 | 1,187 | 407, 425, 443 |
| 25 | 22.8 | 1,295 | 1,319 | 407, 425, 443 |
| 26 | 23.2 | 1,149 | 1,173 | 407, 425, 443 |
| 27 | 24.8 | 1,163 | 1,187 | 407, 425, 443 |
| 28 | 25.1 | 945 | 969 | 407, 425, 443 |
| 29 | 25.4 | 1,119 | 1,143 | 407, 425, 443 |
| 30 | 26.3 | 1,031 | 1,055 | 407, 425, 443 |
| 31 | 27.6 | 1,119 | 1,143 | 407, 425, 443 |
Fig. 3.
Ultra-high performance liquid chromatography–electron spray ionization–MS positive mass spectra (B, C, and F) and negative mass spectra (A, D, and E) of Peak 15, the compound with molecular weight of 1,342 Da, and Peak 10.
3.4. Analysis of Shizhu ginseng saponin by UHPLC–MS/MS
In ESI–, to identify the structures, the deprotonated molecular ion peaks of each saponin were selected to obtain the MS/MS spectra with high collision energy using UHPLC–MS/MS. By analyzing the data from the MS/MS spectra, the structures of mother nucleuses and the numbers and types of glycones attached to saponins could be further inferred. The MS/MS spectra of selected ginseng saponins are illustrated in Fig. 4. Fig. 4A shows that the ginsenoside Re with protopanaxatriol as the mother nucleus produced the quasi-molecular ion (QMI) at m/z 475, which was caused by the complete deglycosylation of the ginsenoside Re. In addition, in the structure of ginsenoside Re, one carbohydrate chain with rhamnosyl and glucosyl groups was connected at the C-6 position and one glucosyl group was connected at the C-20 position, and therefore, the diagnostic fragment ions of [M-H-162]– (m/z 784.1), [M-H-146-162]– (m/z 637.4), and [M-H-162-146-162]– (m/z 475.3) were observed, which can be attributed to the sequential loss of one glucosyl group, one rhamnosyl group, and one glucosyl group from the [M-H]– ion (m/z 945.4). Fig. 4B demonstrates that the ginsenoside Rb1 with panaxadiol as the mother nucleus generated the QMI at m/z 459, which was formed following the loss of all glycones from ginsenoside Rb1. Besides, the structures of ginsenoside Rb1 contain four glucosyl groups, and therefore, the fragment ions of [M-H-162-x × n]– (n = 1, 2, 3, 4) (m/z 944.4, m/z 784.0, m/z 621.3, and m/z 459.7) were produced, which can be attributed to the consecutive loss of four glucosyl groups from the [M-H]– ion (m/z 1,107.5). Fig. 4C illustrates that the notoginsenoside R1 produced the fragment ions of [M-H-132]– (m/z 799), [M-H-132-162]– (m/z 637), and [M-H-132-162 × 2]– (m/z 476), which can be attributed to the sequential loss of one xylosyl group, one glucosyl group, and another glucosyl group from the [M-H]– ion (m/z 931). In Fig. 4D, the ginsenoside Rc produced the characteristic fragments ions of [M-H-132]– and [M-H-132-162 × n]– (n = 1, 2, 3) (m/z 635.7 and m/z 474.9), which respectively resulted from the sequential loss of one arabinosyl group and three glucosyl groups from the [M-H]– ion (m/z 768.9).
Fig. 4.
Collision-induced dissociation mass spectra of ginsenosides (A) Re and (B) Rb1, and (C) notoginsenoside R1, (D) Peak 10, (E) Peak 15, and (F) the compound with molecular weight of 1,342 Da. (A) The charge-to-mass ratio of [M-H-162]–, [M-H-146-162]–, and [M-H-162-146-162]– are m/z 784.1, m/z 637.4, and m/z 475.3, respectively. (B) The charge-to-mass ratio of [M-H-162-x × n]– (n = 1, 2, 3, 4) are m/z 944.4, m/z 784.0, m/z 621.3, and m/z 459.7, respectively. (C) The charge-to-mass ratio of [M-H-132]–, [M-H-132-162]–, and [M-H-132-162 × 2]– are m/z 799, m/z 637, and m/z 476. (D) The charge-to-mass ratio of [M-H-132]– and [M-H-132-162]– are m/z 635.7 and m/z 474.9. (E) The charge-to-mass ratio of [M-H-44]–, [M-H-86]–, [M-H-104]–, and [M-H-86-162-162]– are m/z 1,149.2, m/z 1,106.8, m/z 1,088.8, and m/z 783.2. (F) The charge-to-mass ratio of [M-H-132]–, [M-H-132 × 2]–, [M-H-132 × 3]–, [M-H-132 × 3-162]–, and [M-H-132 × 3-162 × 2]– are m/z 1,209.3, m/z 1,077.0, m/z 945.1, m/z 783.5, and m/z 620.3.
The structures of 29 saponins in Shizhu ginseng were established by analyzing the data of MS/MS spectra and comparing the fragmentation pattern with related literatures [23], [24], [25], [26]. By comparing the data on retention time of reference substances, Peaks 2, 3, 4, 7, 11, 14, 17, 21, 22, and 27 were identified as notoginsenoside R1, ginsenosides Rg1, Re, Rf, Rg2, Rb1, Rc, Rb2, Rb3, and Rd, respectively. We also determined some other peaks: Peaks 1, 8, 10, 16, 18, and 25 were identified as 20-Glu-Rf; ginsenosides Ra3, Ro, and F1; notoginsenoside R2; and acetyl ginsenoside Rb1, respectively. Peaks 12 and 13 were identified as belonging to the ginsenoside Ra1/Ra2 or its isomer; Peaks 28 and 30 were identified as belonging to the ginsenoside Rs1/Rs2, in accordance with related literatures and retention times. As described in the MS spectra of Peak 10 (Fig. 3E and F), the [M-H]– ion at m/z 769 and the [M+Na]+ ion at m/z 793 were observed, and thus, the relative molecular weight was estimated to be 770 Da; moreover, the characteristic fragment ions at m/z 405, m/z 423, and m/z 441 were also observed, which may be produced by the cleavage of the mother nucleus. This confirms the mother nucleus of this compound is protopanaxatriol. The fragment ions of [M-H-132]– at m/z 637 and [M-H-132-162]– at m/z 475 are shown in Fig. 3E, which indicated that the structure of Peak 10 contained one glucosyl group and one pentose group. From these results, we further identified that Peak 10 was notoginsenoside R2.
Compared with ginsenoside, malonyl ginsenoside possesses an extra malonyl group, and therefore, its chromatographic behavior is characterized by longer retention time. By analyzing the MS and MS/MS spectra of malonyl ginsenoside according to related literatures [27], [28], [29], Peaks 5, 6, 15, 19, 20, 23, 24, 26, and 29 were found to belong to malonyl ginsenosides Rg1, Re, Rb1, Rc, Ra1/Ra2, Rb2, Ra1/Ra2, Rb3, Rd, respectively. As described in the MS spectra of Peak 15 (Fig. 3A and B), the characteristic fragment ions [M-H]– at m/z 1,193; [M-H-CO2]– at m/z 1,149; [M-H-C2H2O2]– at m/z 1,107; and [M+Na]+at m/z 1,217 were seen. Thus, we could confirm that the relative molecular weight of this saponin is 1,194 Da. Furthermore, in the ESI-positive MS spectra (Fig. 3B), the characteristic fragment ions at m/z 407, m/z 425, and m/z 443 were seen due to the cracking of the molecule, which proved that its mother nucleus was protopanaxadiol. In addition, in its ESI negative MS/MS spectra (Fig. 4E), the fragment ions of [M-H-44]–, [M-H-86]–, [M-H-104]–, and [M-H-86-162-162]– (m/z 1,149.2, m/z 1,106.8, m/z 1,088.8, m/z 783.2) were produced, based on which we speculated the existence of a malonyl group and a few glucosyl groups in its structure. By comparing our data with a previous study [28], we identified Peak 15 as belonging to the malonyl ginsenoside Rb1. The structures of each compound proposed are presented in Table 2.
Table 2.
Chemical structures of constituents identified in extracts of Shizhu ginseng
| No. | R1 | R2 | Compound name |
|---|---|---|---|
| 1 | Glc2-glc | Glc | 20-Glc-Rf |
| 3 | Glc | Glc | Rg1 |
| 4 | Glc2-Rha | Glc | Re |
| 11 | Glc2-Rha | H | Rg2 |
| 18 | H | Glc | F1 |
| 10 | Glc-Xyl | H | Notoginsenoside-R2 |
| 7 | Glc2-glc | H | Rf |
| 2 | Glc2-Xyl | Glc | Notoginsenoside-R1 |
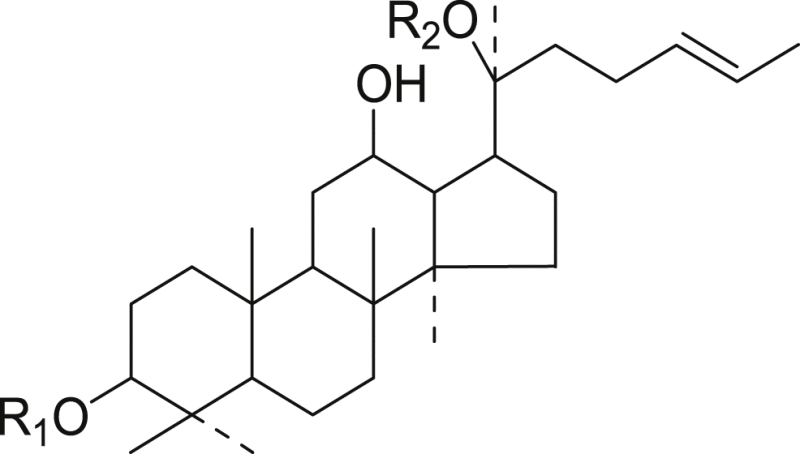 | |||
| 12 | Glc2-Glc | Glc6-Ara(p)4-Xyl | Ra1 |
| 13 | Glc2-Glc | Glc6-Ara(f)4-Xyl | Ra2 |
| 8 | Glc2-Glc | Glc6-Glc3-Xyl | Ra3 |
| 14 | Glc2-Glc | Glc6-Glc | Rb1 |
| 22 | Glc2-Glc | Glc6-Ara(p) | Rb2 |
| 23 | Glc2-Glc | Glc6-Xyl | Rb3 |
| 16 | Glc2-Glc | Glc6-Ara(f) | Rc |
| 28 | Glc2-Glc | Glc | Rd |
| 29 | Glc2-Glc6-AC | Glc6-Ara(p) | Rs1 |
| 31 | Glc2-Glc6-AC | Glc6-Ara(f) | Rs2 |
| 26 | Glc2-Glc6-AC | Glc6-Glc | Acetyl-Rb1 |
| 15 | Glc2-Glc6-Ma | Glc6-Glc | Malonyl-Rb1 |
| 24 | Glc2-Glc6-Ma | Glc6-Ara(p) | Malonyl-Rb2 |
| 27 | Glc2-Glc6-Ma | Glc6-Xyl | Malonyl-Rb3 |
| 20 | Glc2-Glc6-Ma | Glc6-Ara(f) | Malonyl-Rc |
| 30 | Glc2-Glc6-Ma | Glc | Malonyl-Rd |
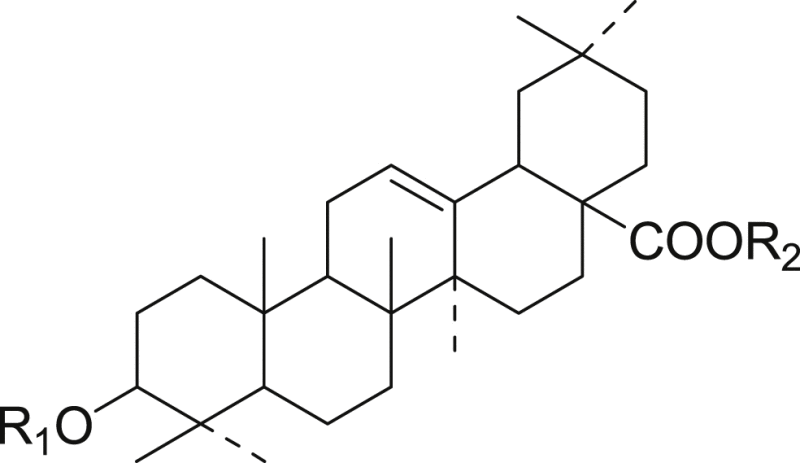 | |||
| 16 | GlcUA-Glc | Glc | Ro |

Peak 9 belonged to the compound with the molecular weight of 1,342 Da. In ESI+, the characteristic fragment ions at m/z 427 and m/z 457 in its MS spectra (Fig. 3C) and the fragment ion at m/z 455 in its MS/MS spectra (Fig. 4F) could be observed, which shared the same mass-to-charge ratio with fragment ions of oleanolic acid ginsenoside reported in the literature. Because the characteristic fragment ion at m/z 439 was not clearly seen in the ESI-positive MS spectra, its mother nucleus could not be confirmed. The fragment ions of [M-H-132]–, [M-H-132 × 2]–, [M-H-132 × 3]–, [M-H-132 × 3-162]–, and [M-H-132 × 3-162 × 2]– (m/z 1,209.3, m/z 1,077.0, m/z 945.1, m/z 783.5, and m/z 620.3) were produced, based on which it can be assumed that the compound contained at least two hexose sugars and three pentose sugars; however, we failed to determine the fragment ion at m/z 455, and thus, further structural identification was achieved with a combination of other analysis methods. The results of different kinds of saponins from ESI-negative MS/MS spectra are presented in Table 3.
Table 3.
Fragment ions observed in collision-induced dissociation mass spectra of extract of Shizhu ginseng using [M-H]– as the precursor ions
| No. | Precursor ions [M-H]– (m/z) | Product ions (m/z) | Identification |
|---|---|---|---|
| 1 | 961 | 799[M-H-(Glc-H2O)]–; 637[M-H-2(Glc-H2O)]–; 475[M-H-3(Glc-H2O)]–; 161[Glc-H2O]-H]–; 961[M-H]– | 20-Glu-Rf |
| 2 | 931 | 769[M-H-(Glc-H2O)]–; 637[M-H-(Glc-H2O)-(Xyl-H2O)]–; 475[M-H-2(Glc-H2O)-(Xyl-H2O)]–; 931[M-H]–; | Notoginsenoside-R1 |
| 3 | 799 | 637[M-H-(Glc-H2O)]–; 475[M-H-2(Glc-H2O)]–; 161[Glc-H2O]-H]–; 799[M-H]– | Rg1 |
| 4 | 945 | 765[M-H-Glc]–; 637[M-H-(Glc-H2O)-(Rha-H2O)]–; 619[M-H-Glc-(Rha-H2O)]–; 475[M-H-2(Glc-H2O)-(Rha-H2O)]–; 945[M-H]– | Re |
| 5 | 885 | 841[M-H-CO2]–; 799[M-H-Ma]–; 781[M-H-Ma-H2O]– | Malonyl-Rg1 |
| 6 | 1,031 | 987[M-H-CO2]–; 945[M-H-Ma]–; 927[M-H-Ma-H2O]–; 799[M-H-(Rha-H2O)]–; 783[M-H-(Glc-H2O)-Ma]–; 637[M-H-(Rha-H2O)-(Glc-H2O)-Ma]–; 475[M-H-(Rha-H2O)-2(Glc-H2O)-Ma]– | Malonyl-Re |
| 7 | 799 | 637[M-H-(Glc-H2O)]–; 475[M-H-2(Glc-H2O)]–; 619[M-H-(Glc-H2O)-H2O]–; 161[(Glc-H2O)-H]–; 799[M-H]–; | Rf |
| 8 | 1,239 | 1,107[M-H-(Xyl-H2O)]–; 1,077[M-H-(Glc-H2O)]–; 945[M-H-(Xyl-H2O)-(Glc-H2O)]–; 783[M-H-2(Glc-H2O)-(Xyl-H2O)]–; 621[M-H-3(Glc-H2O)-(Xyl-H2O)]–; 459[M-H-4(Glc-H2O)-(Xyl-H2O)]– 1,239[M-H]–; |
Ra3 |
| 9 | 1,341 | 1,341[M-H]–; 1,209[M-H-(Ara/Xyl-H2O)]–; 1,179[M-H-(Glc-H2O)]–; 1,077[M-H-2(Ara/Xyl-H2O)]–; 945[M-H-3(Ara/Xyl-H2O)]–; 783[M-H-3(Ara/Xyl-H2O)-(Glc-H2O)]–; 621[M-H-3(Ara/Xyl-H2O)-2(Glc-H2O)]–; 455 |
Not reported |
| 10 | 769 | 637[M-H-(Ara/Xyl-H2O)]; 619[M-H-Ara/Xyl]–; 475[M-H-(Ara/Xyl-H2O)-(Glc-H2O)]–; 161[(Glc-H2O)-H]– ; 769[M-H]–; | Notoginsenoside-R2 |
| 11 | 783 | 783[M-H]–; 637[M-H-(Rha-H2O)]–; 619[M-H-Rha]–; 475[M-H-(Rha-H2O)-(Glc-H2O)]; 161[(Glc-H2O)-H]– | Rg2 |
| 12 | 1,209 | 1,077[M-H-(Xyl-H2O)]–; 1,047[M-H-(Glc-H2O)]–; 945[M-H-(Xyl-H2O)-(Ara-H2O)]–; 783[M-H-(Xyl-H2O)-(Ara-H2O)-(Glc-H2O)]–; 621[M-H-(Xyl-H2O)-(Ara-H2O)-2(Glc-H2O)]–; 459[M-H-(Xyl-H2O)-(Ara-H2O)-3(Glc-H2O)]–; 323[2(Glc-H2O)-H]–; 1,209[M-H]– | Ra1/Ra2/isomer |
| 13 | 1,209 | 1,077[M-H-(Xyl-H2O)]–; 1,047[M-H-(Glc-H2O)]–; 945[M-H-(Xyl-H2O)-(Ara-H2O)]–; 783[M-H-(Xyl-H2O)-(Ara-H2O)-(Glc-H2O)]–; 621[M-H-(Xyl-H2O)-(Ara-H2O)-2(Glc-H2O)]–; 459[M-H-(Xyl-H2O)-(Ara-H2O)-3(Glc-H2O)]–; 323[2(Glc-H2O)-H]–; 1,209[M-H]– | Ra1/Ra2/isomer |
| 14 | 1,107 | 1,107[M-H]–; 945[M-H-(Glc-H2O)]–; 783[M-H-2(Glc-H2O)]–;459[M-H-4(Glc-H2O)]–; 323[2(Glc-H2O)-H]–; 179[Glc-H]– |
Rb1 |
| 15 | 1,193 | 1,193[M-H]–; 1,149[M-H-CO2]–; 1,107[M-H-Ma]–; 1,089[M-H-Ma-H2O]; 945[M-H-(Glc-H2O)-Ma]–; 927[M-H-(Glc-H2O)-Ma-H2O]–; 783[M-H-2(Glc-H2O)-Ma]–; |
Malonyl-Rb1 |
| 16 | 955 | 955[M-H]–; 793[M-H-(Glc-H2O)]–; 731[M-H-Glc-CO2]–; 613[M-H-2(Glc-H2O)-H2O]–; 588[M-H-2(Glc-H2O)-CO2]–; 570[M-H-2(Glc-H2O)-H2O-CO2]–; 523[M-H-formylglucose-Glc-CO2]– |
Ro |
| 17 | 1,077 | 1,077[M-H]–; 945[M-H-(Ara-H2O)]–; 783[M-H-(Glc-H2O)-(Ara-H2O)]–; 621[M-H-2(Glc-H2O)-(Ara-H2O)]–; 459[M-H-3(Glc-H2O)- (Ara-H2O)]– | Rc |
| 18 | 637 | 475[M-(Glc-H2O)]–;457[M-(Glc-H2O)-H2O]–; 161[(Glc-H2O)-H]– | F1 |
| 19 | 1,163 | 1,120[M-H-CO2]–; 1,059[M-H-Ma-H2O]; 945[M-H-(Ara-H2O)-Ma]–; 783[M-H-(Glc-H2O)-(Ara-H2O)-Ma]–; 621[M-H-2(Glc-H2O)-(Ara-H2O)-Ma]; 459[M-H-3(Glc-H2O)-(Ara-H2O)-Ma]–; 233[Glc+C2OH+H2O]; 1,077[M-H-Ma]–; | Malonyl-Rc |
| 20 | 1,295 | 1,251[M-H-CO2]–; 149[Ara-H]–; 1,209[M-H-Ma]–; 1,191[M-H-Ma-H2O]–;1,077[M-H-(Xyl-H2O)-Ma]–; 1,059[M-H-(Xyl-H2O)-Ma-H2O]–; 945[M-H-(Xyl-H2O)-(Ara-H2O)-Ma]–; 765[M-H-(Xyl-H2O)-(Ara-H2O)-(Glc-H2O)-Ma-H2O]–783[M-H-(Xyl-H2O)-(Ara-H2O)-(Glc-H2O)-Ma]–; 621[M-H-(Xyl-H2O)-(Ara-H2O)-2(Glc-H2O)-Ma]–; 603[M-H-(Xyl-H2O)-(Ara-H2O)-(Glc-H2O)-Ma-H2O]– 323[2(Glc-H2O)-H]–; 131[(Ara/Xyl-H2O)-H]– | Malonyl-Ra1/ Ra2 |
| 21 | 1,077 | 1,077[M-H]–; 945[M-H-(Ara-H2O)]–; 783[M-H-(Glc-H2O)-(Ara-H2O)]–; 621[M-H-2(Glc-H2O)-(Ara-H2O)]–; 459[M-H-3(Glc-H2O)- (Ara-H2O)]– | Rb2 |
| 22 | 1,077 | 1,077[M-H]–; 945[M-H-(Xyl-H2O)]–; 783[M-H-(Glc-H2O)-(Xyl-H2O)]–; 621[M-H-2(Glc-H2O)-(Xyl-H2O)]–; 459[M-H-3(Glc-H2O)- (Xyl-H2O)]– | Rb3 |
| 23 | 1,163 | 1,120[M-H-CO2]–; 1,059[M-H-Ma-H2O]; 945[M-H-(Ara-H2O)-Ma]–; 783[M-H-(Glc-H2O)-(Ara-H2O)-Ma]–; 621[M-H-2(Glc-H2O)-(Ara-H2O)-Ma]; 459[M-H-3(Glc-H2O)-(Ara-H2O)-Ma]–; 233[Glc+C2OH+H2O]; 1,077[M-H-Ma]–; | Malonyl-Rb2 |
| 24 | 1,295 | 1,251[M-H-CO2]–; 149[Ara-H]–; 1,209[M-H-Ma]–; 1,191[M-H-Ma-H2O]–; 1,077[M-H-(Xyl-H2O)-Ma]–; 1,059[M-H-(Xyl-H2O)-Ma-H2O]–; 945[M-H-(Xyl-H2O)-(Ara-H2O)-Ma]–; 765[M-H-(Xyl-H2O)-(Ara-H2O)-(Glc-H2O)-Ma-H2O]–783[M-H-(Xyl-H2O)-(Ara-H2O)-(Glc-H2O)-Ma]–; 621[M-H-(Xyl-H2O)-(Ara-H2O)-2(Glc-H2O)-Ma]–; 603[M-H-(Xyl-H2O)-(Ara-H2O)-(Glc-H2O)-Ma-H2O]– 323[2(Glc-H2O)-H]–; 131[(Ara/Xyl-H2O)-H]– | Malonyl-Ra1/ Ra2 |
| 25 | 1,149 | 1,149[M-H]–; 1,107[M-AC-H2O]–; 963[M-(Glc-H2O)-AC]–; 945[M-(Glc-H2O)-AC-H2O]–; 783[M-2(Glc-H2O)-AC-H2O]–;621[M-3(Glc-H2O)-AC-H2O]–459[M-4(Glc-H2O)-AC-H2O]– | AC-Rb1 |
| 26 | 1,163 | 1,120[M-H-CO2]–;1,077[M-H-Ma]–; 1,059[M-H-Ma-H2O]–; 945[M-H-(Xyl-H2O)-Ma]–; 783[M-H-(Glc-H2O)-(Xyl-H2O)-Ma]–; 621[M-H-2(Glc-H2O)-(Xyl-H2O)-Ma]–; 459[M-H-3(Glc-H2O)-(Xyl-H2O)-Ma]–; 233[Glc+C2OH+H2O] | Malonyl-Rb3 |
| 27 | 945 | 783[M-H-(Glc-H2O)]–;621[M-H-2(Glc-H2O)]–; 945[M-H]– 459[M-H-3(Glc-H2O)]–; 161[(Glc-H2O)-H]–; 179[Glc-H]–; |
Rd |
| 28 | 1,119 | 1,077[M-AC]–; 1,059[M-AC-H2O]–; 945[M-(Ara-H2O)-AC]–; 783[M-(Ara-H2O)-(Glc-H2O)-AC]–; 621[M-(Ara-H2O)-2(Glc-H2O)-AC]–; 459[M-(Ara-H2O)-3(Glc-H2O)-AC]– |
Rs1/Rs2 |
| 29 | 1,031 | 987[M-H-CO2]–; 945[M-H-Ma]–; 927[M-H-Ma-H2O]–; 783[M-H-(Glc-H2O)-Ma]–;621[M-H-2(Glc-H2O)-Ma]–459[M-H-3(Glc-H2O)-Ma]– | Malonyl-Rd |
| 30 | 1,119 | 1,077[M-AC]–; 1,059[M-AC-H2O]–; 945[M-(Ara-H2O)-AC]–; 783[M-(Ara-H2O)-(Glc-H2O)-AC]–; 621[M-(Ara-H2O)-2(Glc-H2O)-AC]–; 459[M-(Ara-H2O)-3(Glc-H2O)-AC]– |
Rs1/Rs2 |
Glc, β-D-glucose; Ara(p), α-L-arabinose (pyranose); Ara(f), α-L-arabinose (furanose); Xyl, β-D-xylose; Rha, α-L-rhamnose; AC, 6-O-acetyl; GlcUA, β-D-glucuronide.
3.5. Others
The molecular weight of saponins can be determined by analyzing the mass-to-charge ratios from [M+Na]+ ions produced in ESI+ spectra and from [M-H]– ions produced in ESI– spectra. In addition, saponins with different mother nucleuses were fragmented into specific ions with characteristic mass-to-charge ratios, and thus, it seemed a good approach to deduce the types of mother nucleuses by analyzing the fragment ions shown in the ESI mass spectra. The MS/MS spectra of fragment ions of the targeted compounds were obtained with high collision energy. By identifying the D value of mass-to-charge ratio of fragmented ions shown in the MS/MS spectra, the kinds of glucoses attached to the saponins can be identified. Similarly, by calculating the mass-to-charge ratio of fragmented ions formed through deglycosylation in the MS/MS spectra, the types of mother nucleus can be further established.
Through the research of literatures, it has been proved that all 29 compounds speculated in this study existed in ginseng research reports, some of which, such as malonyl ginsenosides Rg1, Re, Ra1, Ra2, and Rb3, were only reported in LC–MS analysis; however, no reports on malonyl ginsenosides were found, which were isolated as reference substance. Compound 9, a novel saponin from Shizhu ginseng, was extracted and analyzed for the first time. Its relative molecular weight was determined and the MS/MS data were also analyzed, which provided a basis for its further studies.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
The UHPLC–ESI–MS/MS method was successfully carried out by many researchers to identify saponins in Shizhu ginseng because of their passion for their work. We are grateful to Dr CS Sun, Dr F Qin, and Dr N Li for carrying out UHPLC–ESI–MS/MS.
References
- 1.Coleman C.I., Hebert J.H., Reddy P. The effects of Panax ginseng on quality of life. J Clin Pharm Ther. 2003;28:5–15. doi: 10.1046/j.1365-2710.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim J.H. Cardiovascular diseases and Panax ginseng: a review on molecular mechanisms and medical applications. J Ginseng Res. 2012;36:16–26. doi: 10.5142/jgr.2012.36.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin B.-K., Kwon S.W., Park J.H. Chemical diversity of ginseng saponins from Panax ginseng. J Ginseng Res. 2015;39:287–298. doi: 10.1016/j.jgr.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao C.P., Yang L.M., Zhang L.X., Liu C.J., Han M. Effects of cultivation ages and modes on microbial diversity in the rhizosphere soil of Panax ginseng. J Ginseng Res. 2016;40:28–37. doi: 10.1016/j.jgr.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim C., Choo G.C., Cho H.S., Lim J.T. Soil properties of cultivation sites for mountain-cultivated ginseng at local level. J Ginseng Res. 2015;39:76–80. doi: 10.1016/j.jgr.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peng X.L., Fu Y.H., Xiong Z.L., Sun C.S., Li F.M. Quantitative determination of five ginsenosides in Shizhu ginseng and its tablets by HPLC. Shenyang Yao Ke Da Xue Xue Bao. 2010;55:48–51. [Google Scholar]
- 7.Vogler B.K., Pittler M.H., Ernst E. The efficacy of ginseng. A systematic review of randomised clinical trials. Eur J Clin Pharmacol. 1999;55:567–575. doi: 10.1007/s002280050674. [DOI] [PubMed] [Google Scholar]
- 8.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 9.Xiang Y.Z., Shang H.C., Gao X.M., Zhang B.L. A comparison of the ancient use of ginseng in traditional Chinese medicine with modern pharmacological experiments and clinical trials. Phytother Res. 2008;22:851–858. doi: 10.1002/ptr.2384. [DOI] [PubMed] [Google Scholar]
- 10.Avula B., Wang Y.H., Rumalla C.S., Ali Z., Smillie T.J., Khan I.A. Analytical methods for determination of magnoflorine and saponins from roots of Caulophyllum thalictroides (L.) Michx. using UPLC, HPLC and HPTLC. J Pharm Biomed Anal. 2011;56:895–903. doi: 10.1016/j.jpba.2011.07.028. [DOI] [PubMed] [Google Scholar]
- 11.Wang X.K., He Y.Z., Qian L.L. Determination of polyphenol components in herbal medicines by micellar electrokinetic capillary chromatography with Tween 20. Talanta. 2007;74:1–6. doi: 10.1016/j.talanta.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Ying H., Liu J., Du Q. Analysis and determination of oestrogen-active compounds in fructus amomi by the combination of high-speed counter-current chromatography and high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;958:36–42. doi: 10.1016/j.jchromb.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Wang X., Sakuma T., Asafu-Adjaye E., Shiu G.K. Determination of ginsenosides in plant extracts from Panax ginseng and Panax quinquefolius L. by LC/MS/MS. Anal Chem. 1999;71:1579–1584. doi: 10.1021/ac980890p. [DOI] [PubMed] [Google Scholar]
- 14.Zhu J., Guo X., Fu S., Zhang X., Liang X. Characterization of steroidal saponins in crude extracts from Dioscorea zingiberensis C. H. Wright by ultra-performance liquid chromatography/electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J Pharm Biomed Anal. 2010;53:462–474. doi: 10.1016/j.jpba.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Li X., Xiong Z., Ying X., Cui L., Zhu W., Li F. A rapid ultra-performance liquid chromatography-electrospray ionization tandem mass spectrometric method for the qualitative and quantitative analysis of the constituents of the flower of Trollius ledibouri Reichb. Anal Chim Acta. 2006;580:170–180. doi: 10.1016/j.aca.2006.07.069. [DOI] [PubMed] [Google Scholar]
- 16.Prokudina E.A., Havlíček L., Al-Maharik N., Lapčíka O., Strnad M., Gruz J. Rapid UPLC–ESI–MS/MS method for the analysis of isoflavonoids and other phenylpropanoids. J Food Compost Anal. 2012;26:36–42. [Google Scholar]
- 17.Jung C.-H., Seog H.M., Choi I.-W., Park M.-W., Cho H.-Y. Antioxidant properties of various solvent extracts from wild ginseng leaves. LWT – Food Sci Technol. 2006;39:266–274. [Google Scholar]
- 18.Cheok C.Y., Abdel H., Salman K., Sulaiman R. Extraction and quantification of saponins: a review. Food Res Int. 2014;59:16–40. [Google Scholar]
- 19.De Souza L.R., Jenkins A.L., Jovanovski E., Rahelić D., Vuksan V. Ethanol extraction preparation of American ginseng (Panax quinquefolius L) and Korean Red Ginseng (Panax ginseng C.A. Meyer): differential effects on postprandial insulinemia in healthy individuals. J Ethnopharmacol. 2015;159:55–61. doi: 10.1016/j.jep.2014.10.057. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y.C., Liu C.M., Qi Y.J., Li S.N., Wang J. Application of accelerated solvent extraction coupled with counter-current chromatography to extraction and online isolation of saponins with a broad range of polarity from Panax notoginseng. Sep Sci Technol. 2013;106:82–89. [Google Scholar]
- 21.Lin H., Zhang Y., Han M., Yang L. Aqueous ionic liquid based ultrasonic assisted extraction of eight ginsenosides from ginseng root. Ultrason Sonochem. 2013;20:680–684. doi: 10.1016/j.ultsonch.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Chen M., Zhao Y., Yu S. Optimisation of ultrasonic-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses. Food Chem. 2015;172:543–550. doi: 10.1016/j.foodchem.2014.09.110. [DOI] [PubMed] [Google Scholar]
- 23.Sakai S., Katsumata M., Satoh Y., Nagasao M., Miyakoshi M., Ida Y., Shoji J. Oleanolic acid saponins from root bark of Aralia elata. Phytochemistry. 1994;35:1319–1324. doi: 10.1016/s0031-9422(00)94846-5. [DOI] [PubMed] [Google Scholar]
- 24.Fuzzati N., Gabetta B., Jayakar K., Pace R., Peterlongo F. Liquid chromatography-electrospray mass spectrometric identification of ginsenosides in Panax ginseng roots. J Chromatogr A. 1999;854:69–79. doi: 10.1016/s0021-9673(99)00463-x. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., You J., Yu Y., Qu C., Zhang H., Ding L., Zhang H., Li X. Analysis of ginsenosides in Panax ginseng in high pressure microwave-assisted extraction. Food Chem. 2008;110:161–167. doi: 10.1016/j.foodchem.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 26.Chan T.W., But P.P., Cheng S.W., Kwok I.M., Lau F.W., Xu H.X. Differentiation and authentication of Panax ginseng, Panax quinquefolius, and ginseng products by using HPLC/MS. Anal Chem. 2000;72:1281–1287. doi: 10.1021/ac990819z. [DOI] [PubMed] [Google Scholar]
- 27.Ma X., Xiao H., Liang X. Identification of ginsenosides in Panax quinquefolium by LC-MS. Chromatographia. 2006;64:31–36. [Google Scholar]
- 28.Liu S., Cui M., Liu Z., Song F., Mo W. Structural analysis of saponins from medicinal herbs using electrospray ionization tandem mass spectrometry. J Am Soc Mass Spectrom. 2004;15:133–141. doi: 10.1016/j.jasms.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Song F.R., Liu Z.Q., Liu S.Y., Cai Z.W. Differentiation and identification of ginsenoside isomers by electrospray ionization tandem mass spectrometry. Anal Chim Acta. 2005;531:69–77. [Google Scholar]






