Abstract
Introduction
Attitudes toward masturbation are extremely varied, and this practice is often perceived with a sense of guilt.
Aim
To evaluate the prevalence of ego-dystonic masturbation (EM), defined as masturbation activity followed by a sense of guilt, in a clinical setting of sexual medicine and the impact of EM on psychological and relational well-being.
Methods
A series of 4,211 men attending an andrology and sexual medicine outpatient clinic was studied retrospectively. The presence and severity of EM were defined according to ANDROTEST items related to masturbation, determined by the mathematical product of the frequency of masturbation and the sense of guilt after masturbation.
Main Outcome Measures
Clinical, biochemical, and psychological parameters were studied using the Structured Interview on Erectile Dysfunction, ANDROTEST, and modified Middlesex Hospital Questionnaire.
Results
Three hundred fifty-two subjects (8.4%) reported any sense of guilt after masturbation. Subjects with EM were younger than the remaining sample (mean age ± SD = 51.27 ± 13.43 vs 48.31 ± 12.04 years, P < .0001) and had more psychiatric comorbidities. EM severity was positively associated with higher free-floating (Wald = 35.94, P < .001) and depressive (Wald = 16.85, P < .001) symptoms, and subjects with a higher EM score reported less phobic anxiety (Wald = 4.02, P < .05) and obsessive-compulsive symptoms (Wald = 7.6, P < .01). A higher EM score was associated with a higher alcohol intake. Subjects with EM more often reported the partner's lower frequency of climax and more problems achieving an erection during sexual intercourse. EM severity was positively associated with worse relational and intrapsychic domain scores.
Conclusion
Clinicians should consider that some subjects seeking treatment in a sexual medicine setting might report compulsive sexual behaviors. EM represents a clinically relevant cause of disability, given the high level of psychological distress reported by subjects with this condition, and the severe impact on quality of life in interpersonal relationships.
Key Words: Ego-Dystonic Masturbation, Depression, Anxiety
Introduction
Masturbation is a common sexual practice and a part of normal sexuality. However, it has a long history of general condemnation.1, 2 Masturbation has been commonly perceived as a detrimental practice, and it has been banned by several religions such as Judaism, Islam, and Christianity.3 Attitudes toward masturbation are extremely varied in the general population.4 The main effect of masturbatory taboos is a pervasive sense of guilt without a relevant decrease in occurrence. The guilty actor accepts responsibility for a behavior that violates internalized standards or causes another's distress and desires to make amends or punish oneself.5, 6, 7
In different types of hypersexuality referral, Cantor et al8 described cases of sexual guilt, with clients reporting distress related to sexual behaviors often sufficient to have warranted previous diagnoses of depression. Greenberg and Archambault9 found that guilty feelings connected with masturbation occurred in 40% of a sample of university students. More recently, the masturbatory experience of young Korean men in military service was examined. Feelings of guilt were reported by approximately 10.9% of the sample (132 of 1,212).10 In a previous study,11 a feeling of guilt after masturbation was reported by 274 patients (15.4%). For this study, we defined a masturbation activity followed by a sense of guilt as ego-dystonic masturbation (EM). Distress after masturbation could be determined by the act of masturbating per se or by an excessive involvement of sexual cognitions and behaviors. The latter condition has been associated with several psychopathologic features.12 Excessive masturbation with a sense of guilt is one example of hypersexual behavior that is often reported as poorly controlled and leading to functional impairment.12, 13 The general consensus is that frequent EM is characterized by inappropriate or excessive sexual cognitions or behaviors that lead to subjective distress or impaired functioning in at least one important life domain.14 The subjective perception of masturbation is a multifactorial construct, which is neglected by the scientific literature. It probably encompasses psychological and cultural dimensions, rather than biological factors, and it is supposed to be associated with different psychiatric conditions. Accordingly, we attempted to evaluate the psychopathologic and clinical correlates of EM in a comprehensive analysis.
In light of these considerations and our clinical observations, the aims of the present study were to:
-
•
Evaluate the impact of EM on psychological and relational well-being and determine whether the frequency of episodes and the sense of guilt afterward are associated with general psychological distress as measured by a psychiatric symptom rating scale
-
•
Assess the prevalence of EM in a sexual medicine clinical setting
-
•
Exclude possible biological correlates of EM
Methods
Participants
A consecutive series of 4,211 men attending an andrology and sexual medicine outpatient clinic for sexual dysfunction for the first time were studied retrospectively provided they met the following inclusion criteria: (i) male biological sex and (ii) at least 18 years old. The exclusion criteria were (i) illiteracy and (ii) mental retardation. All data provided were collected as part of the routine clinical procedure. The study was approved by the institution's ethics committee. An informed consent for the study was obtained from all patients.
Assessment
Patients were interviewed using the Structured Interview on Erectile Dysfunction (SIEDY). The SIEDY is a 13-item structured interview composed of three scales that identify and quantify components concurrent with erectile dysfunction (ED).15 Scale 1 deals with organic disorders and its questions concern medical history, morning and nocturnal erections, and ejaculate volume. Scale 2 deals with disturbances in the relationship with the primary partner, and its questions concern the presence of disease in the primary partner, the primary partner's climax and desire, and menopausal symptoms. Scale 3 deals with psychological factors, and its questions concern the presence of life stressors, conflict in the primary relationship and within the family, extramarital affairs, and the patient's hypoactive sexual desire. Validation studies have confirmed that the SIEDY subscales have good sensitivity and specificity in detecting biological and psychological components of sexual dysfunction.15
In addition, patients were interviewed using the ANDROTEST structured interview, a previously validated tool for screening for hypogonadism in patients with ED.16
Frequency of masturbation was assessed using question 7 of the ANDROTEST (“How often have you practiced autoeroticism [masturbation] in the past 3 months?” 0 = none, 1 = one to two times, 2 = three to seven times, 3 = more than seven times per month). Feeling of discomfort or guilt after autoeroticism was investigated using question 8 of the ANDROTEST (“How do you feel after autoeroticism?” 0 = well, 1 = somewhat guilty, 2 = very guilty, 3 = very guilty as previously reported). The diagnosis of EM was established for those subjects reporting any feeling of guilt after masturbation with a frequency of masturbation rating higher than 0.
Subjects were categorized by degree of masturbation frequency and degree of guilt. Severity of EM was defined according to ANDROTEST items related to masturbation as determined by the mathematical product of the frequency of masturbation episodes by the sense of guilt after masturbation. Therefore, subjects were categorized according to the following product scores of the two variables: 0, 1, 2, 3, 4, 6, and 9. Given the small number of subjects reporting a 9 score, the last group was composed of subjects with a score equal to or higher than 6.
Patients also were asked to complete the modified Middlesex Hospital Questionnaire (MHQ),17 a brief self-report questionnaire for the screening of symptoms of mental disorders in a non-psychiatric setting, which provides scores for free-floating anxiety (MHQ-A), phobic anxiety (MHQ-P), obsessive-compulsive traits and symptoms (MHQ-O), somatization (MHQ-S), depressive symptoms (MHQ-D), and histrionic-hysterical symptoms (MHQ-H).
Previous diagnoses of mental disorders were assessed using criteria of the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition.18 Patients were asked to report the use of any drug. Among psychoactive medications, we included antipsychotics, mood stabilizers, antidepressants, and benzodiazepines. Cardiovascular risk was evaluated using the Progetto CUORE risk engine.19
Clinical and Biochemical Analyses
All patients underwent a complete physical examination, with measurement of blood pressure (mean of three measurements 5 minutes apart, in a sitting position, with a standard sphygmomanometer), height, weight, and body mass index. Pulse pressure was calculated as the difference between systolic and diastolic blood pressure, as previously reported.20 Blood samples were drawn in the morning, after an overnight fast, for determination of blood glucose (by a glucose oxidase method; Aeroset Abbott, Rome, Italy); total cholesterol, high-density lipoprotein cholesterol, and triglycerides (by an automated enzymatic colorimetric method; Aeroset Abbott); and luteinizing hormone, follicle-stimulating hormone, total testosterone, prolactin, and thyroid-stimulating hormone (by an electro-chemiluminescent method, Modular Roche, Milan, Italy).
All patients received an intracavernous alprostadil injection (10 mg); the response was assessed after 20 minutes. Responses were recorded on a four-point scale (1 = no response; 2 = rigidity insufficient for intercourse [<50%]; 3 = rigidity sufficient for intercourse [>50%]; 4 = full erection [>90%]) as previously described.15, 20
Statistical Analyses
Data were expressed as mean ± SD when normally distributed and as median (quartile) for parameters with non-normal distribution, unless otherwise specified. Subject with and without EM were compared for all considered clinical variables by χ2 test and unpaired two-sided Student t-test (or Mann-Whitney U-test for non-normally distributed variables) for categorical and continuous variables, respectively. One-way analysis of variance (or Kruskal-Wallis test) was adopted to compare groups of subjects with EM categorized according to different ANDROTEST scores on frequency and sense of guilt after masturbation and their product for MHQ scores.
Binary logistic regressions were applied to compare subjects with and without EM (categorized as a dummy variable) for all clinical variables being considered (Table 1); age was entered as a covariate; odds ratios with 95% CIs were calculated, expressing the association between EM and other clinical variables. Groups of subjects with EM categorized by the product of frequency of and sense of guilt after masturbation were compared for the MHQ and other clinical variables by ordinal logistic models; age was entered in these models as a covariate.
Table 1.
Characteristics of sample according to ego-dystonic masturbation∗
| Characteristics | No ego-dystonic masturbation (n = 3,859) | Ego-dystonic masturbation (n = 352) | P value adjusted for age and psychiatric comorbidities† |
|---|---|---|---|
| Age (y) | 51.27 ± 13.43 | 48.31 ± 12.04 | <.0001 |
| Marital status | |||
| Stable relationship | 486 (12.6) | 34 (9.7) | .007 |
| No stable relationship | 3,373 (87.4) | 318 (90.3) | |
| Education | |||
| None, primary school, or secondary school | 1,717 (44.5) | 114 (32.5) | .015 |
| Higher secondary or university | 2,142 (55.5) | 238 (67.5) | |
| Current smoker | 1,177 (30.5) | 104 (29.6) | .307 |
| Alcohol intake | |||
| >2 drinks daily | 3,157 (81.8) | 260 (73.8) | .101 |
| <4 drinks daily | 702 (18.2) | 92 (26.2) | |
| Psychiatric diseases | 274 (7.1) | 48 (13.7) | <.0001 |
| Reported psychoactive medications | 444 (11.5) | 71 (20.1) | <.0001 |
| Full erection during sexual intercourse | |||
| Sometimes (<25%) | 1,023 (26.7) | 56 (15.8) | <.0001 |
| Quite often (25%–50%) | 203 (5.3) | 18 (5.1) | |
| Often (50%–75%) | 269 (7) | 19 (5.4) | |
| Always (>75%) | 2,333 (61) | 259 (73.7) | |
| Clinical, laboratory, and instrumental parameters | |||
| BMI (kg/m2) | 26.53 ± 4.14 | 26.00 ± 3.79 | .114 |
| Waist circumference (cm) | 97.60 ± 10.69 | 96.37 ± 9.87 | .184 |
| SBP (mmHg) | 135.87 ± 17.13 | 133.78 ± 13.88 | .363 |
| DBP (mmHg) | 83.21 ± 9.68 | 82.71 ± 8.06 | .939 |
| Pulse pressure | 52.66 ± 12.82 | 51.07 ± 11.06 | .254 |
| Glycemia (mg/dL) | 95 (86–108) | 91 (84–101) | .004 |
| Total cholesterol (mg/dL) | 201.55 ± 41.33 | 203.11 ± 31.77 | .249 |
| Triglycerides (mg/dL) | 115 (82–163) | 111 (84–158) | .399 |
| HDL cholesterol (mg/dL) | 48.52 ± 12.54 | 46.53 ± 9.57 | .011 |
| LH (U/L) | 3.8 (2.63–5.50) | 3.6 (2.55–5.10) | .288 |
| FSH (U/L) | 4.6 (3.0–7.8) | 4.03 (2.7–6.3) | .104 |
| Total testosterone (nmol/L) | 15.64 ± 6.40 | 15.56 ± 6.69 | .376 |
| TSH (mU/L) | 1.44 (1.02–2.05) | 1.42 (1.03–1.94) | .037 |
| Prolactin (mU/L) | 156 (111.15–225) | 147 (105–210) | .117 |
| PGE1, test response (%) | |||
| Grade 1 | 53 (2.3) | 2 (0.6) | <.0001 |
| Grade 2 | 1,005 (43.8) | 109 (31) | |
| Grade 3 | 893 (38.9) | 128 (36.3) | |
| Grade 4 | 344 (15) | 113 (32.1) | |
| SIEDY scale score | |||
| Scale 1 (organic domain of ED) | 2.92 ± 2.53 | 2.64 ± 2.26 | .648 |
| Scale 2 (relational domain of ED) | 1.81 ± 1.98 | 2.16 ± 1.89 | <.0001 |
| Scale 3 (intrapsychic domain of ED) | 3.17 ± 2.14 | 3.99 ± 2.08 | <.0001 |
| Intrapsychic parameters as derived by MHQ | |||
| MHQ-A score (free-floating anxiety symptoms) | 5.05 ± 3.67 | 6.49 ± 3.39 | <.0001 |
| MHQ-P score (phobic anxiety symptoms) | 4.14 ± 2.76 | 3.78 ± 2.50 | .023 |
| MHQ-O score (obsessive-compulsive traits and symptoms) | 5.48 ± 3.88 | 4.80 ± 3.42 | .002 |
| MHQ-S score (somatization) | 3.31 ± 2.85 | 3.68 ± 2.64 | .042 |
| MHQ-D score (depressive symptoms) | 4.32 ± 3.32 | 5.17 ± 3.29 | <.0001 |
| MHQ-H score (hysterical symptoms and traits) | 4.58 ± 3.29 | 4.29 ± 3.15 | .068 |
BMI = body mass index; DBP = diastolic blood pressure; ED = erectile dysfunction; FSH = follicle-stimulating hormone; HDL = high-density lipoprotein; LH = luteinizing hormone; MHQ = Middlesex Hospital Questionnaire; PGE1 = prostaglandin E1; SIEDY = Structured Interview on Erectile Dysfunction; SBP = systolic blood pressure; TSH = thyroid-stimulating hormone.
Data are expressed as mean ± SD when normally distributed, median (quartiles) when not normally distributed, and percentage when categorical.
Adjusted P values were calculated by binary logistic regression analysis.
All statistical analysis was performed using SPSS 20.1 for Windows (SPSS, Inc, Chicago, IL, USA).
Results
Of the entire sample, 352 subjects (8.4%) reported any sense of guilt after masturbation (product > 1) and thus classified as having EM. Table 1 presents the general characteristics of the sample comparing subjects with EM with those without EM, which were computed as dummy variables. Subjects with EM were younger than the remaining sample and showed a higher frequency of psychiatric comorbidities. Therefore, the binary logistic models comparing subjects EM with subjects without EM were adjusted for age and psychiatric comorbidities.
Psychological Parameters
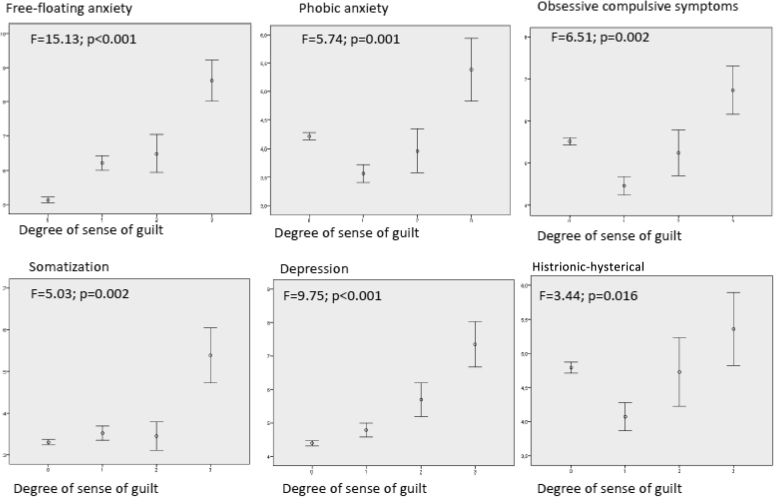
For the psychopathologic correlates of frequency of masturbation, only subjects in the group with highest masturbation frequency showed higher free-floating anxiety compared with the other groups (F = 6.98, P < .001); other comparisons in frequency were not significant. The degree of sense of guilt was associated with different psychological domains: in particular, the group with the highest sense of guilt showed higher free-floating anxiety, phobic anxiety, somatization, and depressive symptoms (Figure 1).
Figure 1.
Psychopathologic correlates of sense of guilt after masturbation. MSQE = Middlesex Hospital Questionnaire; x axis = sense of guilt after masturbation (scores on question 8 of the ANDROTEST); y axis = MHQ subscale scores.
The association between EM severity (defined as the mathematical product of the frequency of masturbation episodes by the sense of guilt after masturbation) and different clinical variables is presented in Table 2. When psychological dimensions (as assessed by MHQ scores) were considered, the ordinal logistic models showed positive associations with higher free-floating and somatized anxiety and with depressive symptoms (Wald = 16.85, P < .001). Subjects with greater EM severity reported less phobic anxiety and obsessive-compulsive traits and symptoms (Table 2).
Table 2.
Association of ego-dystonic masturbation severity (product of frequency by severity of sense of guilt during masturbation) and main clinical variables of the sample
| Characteristics | Wald | OR (95% CI) | P value adjusted for age and psychiatric comorbidities∗ |
|---|---|---|---|
| Intrapsychic parameters | |||
| MHQ-A score (free-floating anxiety symptoms) | 35.94 | 1.10 (1.07–1.14) | <.0001 |
| MHQ-P score (phobic anxiety symptoms) | 4.02 | 0.95 (0.91–0.99) | .040 |
| MHQ-O score (obsessive-compulsive traits and symptoms) | 7.66 | 0.95 (0.92–0.98) | .006 |
| MHQ-S score (somatization) | 4.02 | 1.04 (1.01–1.09) | .004 |
| MHQ-D score (depressive symptoms) | 16.86 | 1.07 (1.04–1.11) | <.0001 |
| Lifestyle parameters | |||
| Education level | 9.19 | 0.64 (0.44–0.91) | .002 |
| Stress at work | 20.87 | 1.28 (1.15–1.43) | <.0001 |
| Dissatisfaction at work | 32.72 | 1.46 (1.28–1.66) | <.0001 |
| Alcohol abuse | 12.66 | 1.39 (1.13–1.61) | <.0001 |
| Conflicts within family | 53.24 | 1.82 (1.53–2.13) | <.0001 |
| Conflicts within couple | 23.29 | 1.44 (1.24–1.68) | <.0001 |
| Clinical variables | |||
| Frequency of partner’s climax | 39.96 | 0.39 (0.27–0.52) | <.0001 |
| Intracavernous PGE1 | 28.32 | 1.73 (1.40–2.13) | <.0001 |
| Erection during sexual intercourse | 32.61 | 0.77 (0.68–0.82) | <.0001 |
| Prolactin | 5.28 | 0.58 (0.36–0.92) | .022 |
| Glycemia | 8.12 | 0.47 (0.29–0.77) | .004 |
| HDL cholesterol | 6.72 | 0.98 (0.97–0.99) | .010 |
| Progetto CUORE | 5.62 | 0.92 (0.86–0.98) | .017 |
| SIEDY scale parameters | |||
| Scale 2 (relational domain of ED) | 18.77 | 1.13 (1.07–1.21) | <.0001 |
| Scale 3 (intrapsychic domain of ED) | 21.57 | 1.15 (1.08–1.22) | <.0001 |
ED = erectile dysfunction; HDL = high-density lipoprotein; MHQ = Middlesex Hospital Questionnaire; OR = odds ratio; PGE1 = prostaglandin E1; SIEDY = Structured Interview on Erectile Dysfunction.
By linear regression analysis.
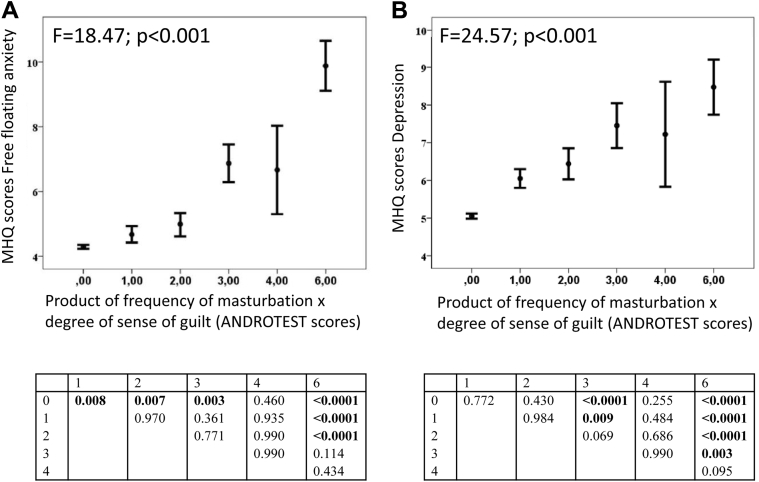
Subjects with EM categorized according to the product of frequency of and sense of guilt after masturbation were compared by MHQ parameters (analysis of variance). The Tukey-Kramer test indicated that subjects with the highest EM score (ie, ≥ 6) had higher levels of depression and anxiety compared with groups with scores 0, 1, 2, and 3, which did not differ among these groups (Figure 2A and B). Furthermore, EM severity was positively associated with current therapy with any psychiatric medication (data not shown).
Figure 2.
Psychopathologic correlates of ego-dystonic masturbation severity. Panel A shows MHQ scores for depression according to severity of ego-dystonic masturbation. Panel B shows MHQ scores for anxiety according to severity of ego-dystonic masturbation. Severity of ego-dystonic masturbation was defined according to Structured Interview on Erectile Dysfunction items related to masturbation determined as the mathematical product of the frequency of masturbation episodes and the sense of guilt after masturbation. Therefore, subjects were categorized according to the product scores of the two variables as 0, 1, 3, 4, 6, or 9. Because of the small number of subjects reporting a score of 9, this group was composed of subjects with a score equal to or higher than 6. MSQ = Middlesex Hospital Questionnaire.
Lifestyle Parameters
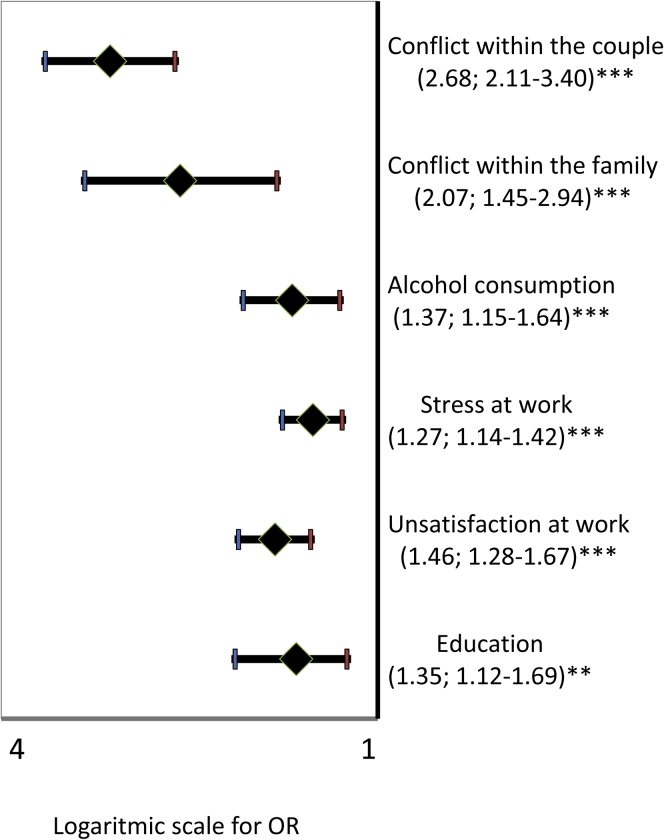
For previous analyses, ordinal logistic models were applied to test the association between the product of frequency by sense of guilt after masturbation and lifestyle parameters. In the same ordinal logistic model, EM severity was associated with a lower education level, higher stress and dissatisfaction at work, and alcohol abuse. Social relationships were found to be more conflictual within the family (and within the couple; P < .01 for all comparisons; Table 2). Similar results were obtained when considering EM as a dummy variable (Figure 3).
Figure 3.
Lifestyle variables and ego-dystonic masturbation. ORs with 95% CIs show the association between lifestyle variables and presence of ego-dystonic masturbation as a dummy variable (0 = no ego-dystonic masturbation, 1 = ego-dystonic masturbation). All data were adjusted for age. ∗∗P < .01; ∗∗∗P < .001 by logistic regression analysis. The abscissa shows log scale values. OR = odds ratio.
Clinical Variables
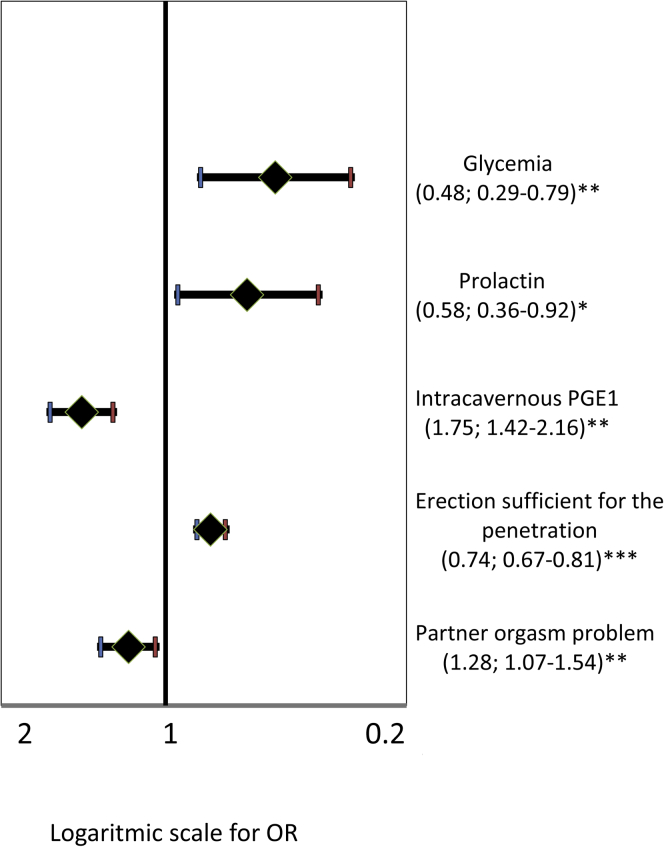
When sexual parameters were evaluated (binary logistic models for comparison between subjects with EM and those without EM), subjects with EM more often reported a lower frequency of the partner's climax. Furthermore, although subjects with EM were more responsive to intracavernous prostaglandin E1 injection, they reported more problems in obtaining an erection during sexual intercourse.
Ordinal logistic models were applied to test the association between product of frequency by sense of guilt after masturbation and biological parameters. No significant association was detected between EM score and hormonal levels, with the exception of prolactin, which was lower in subjects with EM (Table 1). Glycemia and high-density lipoprotein cholesterol levels were inversely related to EM score (Table 2). No association was found with the other metabolic parameters investigated (Table 1). Similar results were obtained when considering EM as a dummy variable (Figure 4). In line with these data, cardiovascular risk as predicted by the Progetto CUORE risk engine was inversely related to EM score (Table 2).
Figure 4.
Clinical variables and ego-dystonic masturbation. ORs with 95% CIs show the association between clinical variables and presence of ego-dystonic masturbation coded as a dummy variable (0 = no ego-dystonic masturbation, 1 = ego-dystonic masturbation). All data were adjusted for age. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001 by logistic regression analysis. The abscissa shows log scale values. OR = odds ratio; PGE1 = prostaglandin E1.
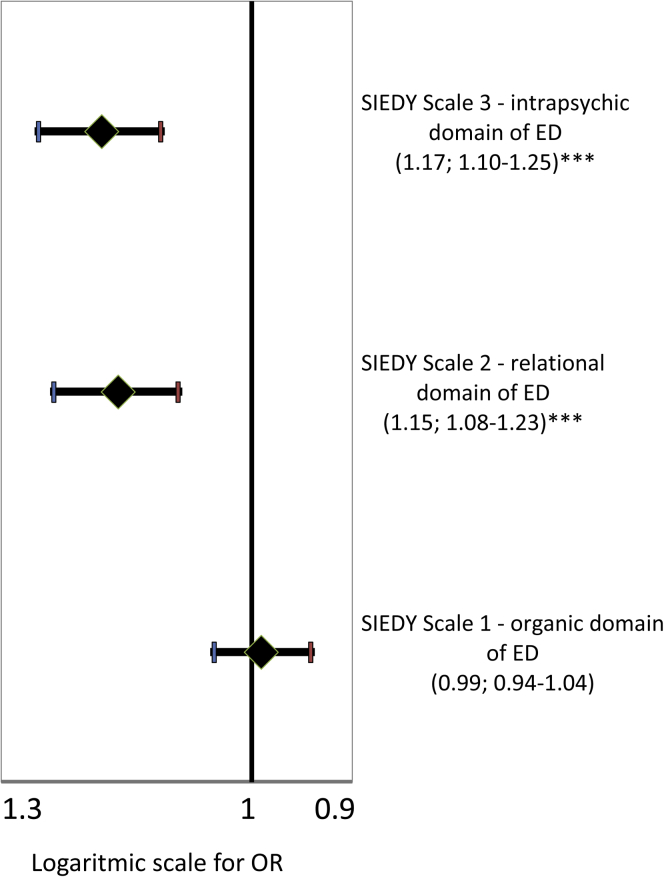
SIEDY Scale Parameters
Ordinal logistic models were applied to test the association between product of frequency by sense of guilt after masturbation and SIEDY scores. EM severity was positively associated with worse relational (SIEDY Scale 2) and intrapsychic (SIEDY Scale 3) domains, but no correlation was found with the organic domain (SIEDY Scale 1; not shown; Table 2). The odds ratios with 95% CIs of the SIEDY scales as predictors of any EM are presented in Figure 5.
Figure 5.
SIEDY scale parameters and ego-dystonic masturbation. ORs with 95% CIs show the association between SIEDY scores and ego-dystonic masturbation coded as a dummy variable (0 = no ego-dystonic masturbation, 1 = ego-dystonic masturbation). All data were adjusted for age and psychiatric comorbidities. ∗∗∗P < .001 by logistic regression analysis. The abscissa shows log scale values. ED = erectile dysfunction; OR = odds ratio; SIEDY = Structured Interview on Erectile Dysfunction.
Discussion
To our knowledge, this is one of the few recent studies to consider the psychopathologic and biological correlates of EM. According to our main results, EM seems to be a psychological problem because it shows (i) an almost 10% prevalence in clinical settings of sexual medicine; (ii) a clear association with psychiatric symptoms such as depression and anxiety and with psychological distress in general; (iii) worse sexuality, with an impairment of successful sexual intercourse for the couple, leading to significant relational problems; and (iv) a tripling of the risk of EM from conflict between the patient and his partner.
According to our results, 8.4% of subjects attending our clinic reported frequent masturbation associated with a sense of guilt, demonstrating that EM is a relevant health issue in a sexual medicine clinical setting. This statistic could underestimate the problem because of the private nature of sex and the continuing stigma of these behaviors likely leading to underreporting owing to embarrassment or shame.14 We did not compare the results with a healthy (non-clinical) comparison or control group, so we cannot be sure whether each difference represents an atypically low score in the EM group or an atypically high score in the non-EM group. However, subjects with EM represent a more dysfunctional subpopulation in this clinical sample that might show greater psychopathologic and relational problems compared with a non-clinical population. Furthermore, EM seemed to affect especially young men with higher education, suggesting that guilt might be more associated with initial sexual activities and cultural stereotypes. Our data suggest that EM should be considered a clinically relevant condition. Indeed, the sense of guilt after masturbation was associated with significant psychological distress. Our data confirm previous observations that psychiatric comorbidities, especially mood, anxiety, and personality disorders, are the rule rather the exception for people with compulsive sexual behaviors.21, 22, 23, 24 However, EM could be associated with a non-specific anxious activation.
As a further demonstration of the impact of EM on personal well-being, subjects with EM reported worse sexual functioning with their partners. Overall, EM was associated with a worse quality of life in the relational domain, because subjects with EM scored higher on the SIEDY Scale 2 (marital domain) and the risk of conflictual relationships with their partners was tripled. One of the main issues in the field of compulsive sexual behavior is the need to define qualitative and quantitative thresholds in eventually establishing a clinical entity. According to general agreement in psychiatry, a behavior should be considered pathologic when it leads to subjective distress or impaired functioning in at least one important life domain.14 Therefore, a possible strategy could be to evaluate the degree to which the product of frequency by sense of guilt after masturbation (EM severity) determines relevant psychological uneasiness. Kafka25 suggested that a high frequency of the behavior (eg, more than seven weekly orgasms over 6 consecutive months) should be considered the definition of hypersexuality. However, Kinsey et al1 criticized the notion of high-frequency sexual behavior as being inherently pathologic. Others have agreed and have focused on subjective distress and psychosocial dysfunction, not frequency of orgasm.23 According to a previous study,11 frequency of masturbation alone did not show any significant association with psychological distress; rather, the sense of guilt showed an increasing pattern of association with several domains of the MHQ questionnaire. Compared with this study, we considered a combined measurement of frequency with sense of guilt after masturbation: this variable can be considered a suitable measurement of the pervasiveness of a behavior, which is subjectively perceived as wrong.
It is important to note that the cross-sectional design of the study does not allow establishing a cause-and-effect relation between the variables considered. Indeed, it has been reported that subjects with anxiety disorders might adopt masturbation as a way to manage their negative emotions. In this regard, we found that subjects with EM showed more alcohol consumption than the other subjects, confirming the findings of Carnes and Delmonico26 who reported a high rate of alcohol or drug dependency, eating disorders, tobacco and caffeine addiction, compulsive working and spending, and compulsive gambling in relation to compulsive sexual behaviors. These results can be interpreted in different ways. EM could represent a dysfunctional coping strategy to manage emotions in people with deficient emotional regulation. The proposed model by Carnes27, 28 and later by Goodman29 hypothesizes that EM can be viewed as a particular form of addiction. Therefore, the repetitive misuse of sexual behavior allows specific people to manage dysphoric affects (ie, self-medication); this leads to an escalation or progression of sexual behaviors (tolerance and risk taking) and to “loss of control,” adverse psychosocial consequences, and a withdrawal state.
From a different perspective, other investigators have proposed that compulsive sexual behavior disorders are repetitive behaviors mediated by behavioral attempts to lessen anxiety and other dysphoric affects (eg, shame, depression) and are symptomatic of an “underlying obsessive compulsive disorder.”22, 30, 31, 32, 33 However, our data do not support this position, because we found that EM severity was associated less often with obsessive-compulsive symptoms, which represent the opposite pole of impulsivity in the obsessive-impulsive continuum.
In contrast, we found that fewer phobic anxiety symptoms were reported by the EM group compared with the non-EM group. The opposite psychological dimension of phobic anxiety is “sensation seeking,”34, 35, 36 which is typically associated with impulsive disorders.37 In the different editions of the Diagnostic and Statistical Manual of Mental Disorders,18, 38, 39, 40, 41 impulse control disorders have been characterized by the failure to resist an impulse, drive, or temptation to perform an act that is harmful to the person or others. A person might feel an increased sense of tension or arousal before committing the act and then experience pleasure, gratification, or relief at the time the act is committed. After the act, there might or might not be regret, self-reproach, or guilt.41 Considering our data, the degree of psychological distress was clearly correlated with the sense of guilt. Unfortunately, more data regarding impulsivity were not available in the present dataset, and this hypothesis requires a specific study for confirmation. As confirmation of the impulsivity-spectrum hypothesis of EM, disorders of impulse control were relatively common in the studies of Black et al22 and Raymond et al.23
The biological markers examined and scores of SIEDY Scale 1 (organic domain) did not show any significant association with EM. From an etiologic viewpoint, this lack of association appeared to contradict the involvement of testosterone-mediated mechanisms in the pathogenesis of EM.42 The association of EM with lower levels of prolactin is in line with previous findings, which showed an association between decreased prolactin levels and anxious43 or depressive44 symptomatology, most probably because it reflects a lower central serotoninergic tone.45 Animal models also have shown that prolactin levels in the brain contribute to the modulation of neuronal circuits involved in the regulation of stress responses.46, 47, 48 Therefore, prolactin could represent a biological marker of the psychological distress associated with the sense of guilt after EM.
The results of the present study should be considered in light of some limitations. First, we could not establish the personal reasons why subjects with EM perceived their sexual behavior with a sense of guilt. Investigators have observed that the complex cultural role of sex makes it difficult to consider EM a discrete psychiatric disorder rather than a deviation from the cultural norm.49 Others, although acknowledging the complicated nature of sex, have focused on the adverse consequences of compulsive sexual behaviors and considered impairment a key element of the disorder.24, 34 Second, some important information was obtained by self-reported measurements and could be subject to errors of recall. Third, because EM was not investigated by a specific instrument, it was not possible to establish the onset and duration of this behavior. Fourth, the data were collected in a clinical setting and should not be directly applied to the general population.
Conclusion
Clinicians should consider reports of ego-dystonic sexual behaviors from subjects seeking treatment in a sexual medicine setting. EM represents a clinically relevant cause of disability, given the high level of psychological distress reported by subjects with this condition and the severe impact on quality of life in interpersonal relationships. The psychological, and biological, underpinnings of this condition are still unknown. Specific treatments should be implemented, and further longitudinal studies could clarify the intricate aspects of this condition.
Statement of authorship
Category 1
-
(a)Conception and Design
- Giovanni Castellini; Ricca Valdo; Mario Maggi
-
(b)Acquisition of Data
- Egidia Fanni; Giovanni Corona; Elisa Maseroli
-
(c)Analysis and Interpretation of Data
- Giovanni Castellini; Egidia Fanni; Mario Maggi
Category 2
-
(a)Drafting the Article
- Giovanni Castellini; Egidia Fanni
-
(b)Revising It for Intellectual Content
- Giovanni Corona; Ricca Valdo; Mario Maggi
Category 3
-
(a)Final Approval of the Completed Article
- Ricca Valdo; Mario Maggi
Footnotes
Conflict of Interest: Authors report no conflicts of interest.
Funding: None.
References
- 1.Kinsey A.C., Pomeroy W.B., Martin C.E. Saunders; Philadelphia, PA: 1948. Sexual behavior in the human male. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dearborn L.W. Autoeroticism. In: Ellis A., Abarbanel A., editors. The encyclopedia of sexual behavior. Hawthorn Books; New York: 1967. [Google Scholar]
- 3.Gerressu M., Mercer C.H., Graham C.A. Prevalence of masturbation and associated factors in a British national probability survey. Arch Sex Behav. 2008;37:266–278. doi: 10.1007/s10508-006-9123-6. [DOI] [PubMed] [Google Scholar]
- 4.Ford C., Beach F.A. Harper and Row; New York: 1951. Patterns of sexual behavior; p. 194. [Google Scholar]
- 5.Ferguson T.J., Stegge H. Measuring guilt in children: a rose by any other name still has thorns. In: Bybee J., editor. Guilt and children. Academic Press; San Diego: 1998. pp. 19–74. [Google Scholar]
- 6.Hoffman M.L. Varieties of empathy based guilt. In: Bybee J., editor. Guilt and children. Academic Press; New York: 1998. pp. 91–112. [Google Scholar]
- 7.Tangney J.P. Moral affect: the good, the bad, and the ugly. J Pers Soc Psychol. 1991;61:598–607. doi: 10.1037//0022-3514.61.4.598. [DOI] [PubMed] [Google Scholar]
- 8.Cantor J.M., Klein C., Lykins A. A treatment-oriented typology of self-identified hypersexuality referrals. Arch Sex Behav. 2013;42:883–893. doi: 10.1007/s10508-013-0085-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg J.S., Archambault F.X. Masturbation, self-esteem and other variables. J Sex Res. 1973;9:41–51. [Google Scholar]
- 10.Choi Y.J., Lee W.H., Rha K.H. Masturbation and its relationship to sexual activities of young males in Korean military service. Yonsei Med J. 2000;41:205–208. doi: 10.3349/ymj.2000.41.2.205. [DOI] [PubMed] [Google Scholar]
- 11.Corona G., Ricca V., Boddi V. Autoeroticism, mental health, and organic disturbances in patients with erectile dysfunction. J Sex Med. 2010;7:182–191. doi: 10.1111/j.1743-6109.2009.01497.x. [DOI] [PubMed] [Google Scholar]
- 12.Kafka M.P. Hypersexual disorder: a proposed diagnosis for DSM-V. Arch Sex Behav. 2010;39:377–400. doi: 10.1007/s10508-009-9574-7. [DOI] [PubMed] [Google Scholar]
- 13.Bancroft J. Sexual behavior that is “out of control”: a theoretical conceptual approach. Psychiatr Clin North Am. 2008;31:593–601. doi: 10.1016/j.psc.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Black D.W. The epidemiology and phenomenology of compulsive sexual behavior. CNS Spectr. 2000;5:26–72. doi: 10.1017/s1092852900012645. [DOI] [PubMed] [Google Scholar]
- 15.Petrone L., Mannucci E.G., Bartolini M. Structured Interview on Erectile Dysfunction (SIEDY): a new, multidimensional instrument for quantification of pathogenetic issues on erectile dysfunction. Int J Impot Res. 2003;15:210–220. doi: 10.1038/sj.ijir.3901006. [DOI] [PubMed] [Google Scholar]
- 16.Corona G., Mannucci E., Petrone L. ANDROTEST: a structured interview for the screening of hypogonadism in patients with sexual dysfunction. J Sex Med. 2006;3:706–715. doi: 10.1111/j.1743-6109.2006.00262.x. [DOI] [PubMed] [Google Scholar]
- 17.Crown S., Crisp A.H. A short clinical diagnostic self-rating scale for psychoneurotic patients. The Middlesex Hospital Questionnaire (M.H.Q.) Br J Psychiatry. 1966;112:917–923. doi: 10.1192/bjp.112.490.917. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association . 5th ed. American Psychiatric Association; Washington, DC: 2013. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 19.Palmieri L., Panico S., Vanuzzo D., Gruppo di Ricerca del Progetto CUORE Evaluation of the global cardiovascular absolute risk: the Progetto CUORE individual score. Ann Ist Super Sanita. 2004;40:393–399. [PubMed] [Google Scholar]
- 20.Corona G., Mannucci E., Lotti F. Pulse pressure, an index of arterial stiffness, is associated with androgen deficiency and impaired penile blood flow in men with ED. J Sex Med. 2009;6:285–293. doi: 10.1111/j.1743-6109.2008.01059.x. [DOI] [PubMed] [Google Scholar]
- 21.Kafka M.P., Prentky R. A comparative study of nonparaphilic sexual addictions and paraphilias in men. J Clin Psychiatry. 1992;53:345–350. [PubMed] [Google Scholar]
- 22.Black D.W., Kehrberg L.L., Flumerfelt D.L. Characteristics of 36 subjects reporting compulsive sexual behavior. Am J Psychiatry. 1997;154:243–249. doi: 10.1176/ajp.154.2.243. [DOI] [PubMed] [Google Scholar]
- 23.Raymond N.C., Coleman E., Miner M.H. Psychiatric comorbidity and compulsive/impulsive traits in compulsive sexual behavior. Compr Psychiatry. 2003;44:370–380. doi: 10.1016/S0010-440X(03)00110-X. [DOI] [PubMed] [Google Scholar]
- 24.Kuzma J.M., Black D.W. Epidemiology, prevalence, and natural history of compulsive sexual behavior. Psychiatr Clin North Am. 2008;31:603–611. doi: 10.1016/j.psc.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 25.Kafka M.P. Hypersexual desire in males: an operational definition and clinical implications for males with paraphilias and paraphilia-related disorders. Arch Sex Behav. 1997;26:505–526. doi: 10.1023/a:1024507922470. [DOI] [PubMed] [Google Scholar]
- 26.Carnes P.J., Delmonico D.L. Childhood abuse and multiple addictions: research findings in a sample of self-identified sexual addicts. Sex Addict Compulsiv. 1996;3:258–268. [Google Scholar]
- 27.Carnes P.J. CompCare; Minneapolis: 1983. Out of the shadows: understanding sexual addiction. [Google Scholar]
- 28.Carnes P.J. Hazelden; Center City, MN: 1989. Contrary to love: helping the sexual addict. [Google Scholar]
- 29.Goodman A. Neurobiology of addiction. An integrative review. Biochem Pharmacol. 2008;75:266–322. doi: 10.1016/j.bcp.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 30.Coleman E. Sexual compulsivity: definition, etiology, and treatment considerations. J Chem Depend Treat. 1987;1:189–204. [Google Scholar]
- 31.Anthony D.T., Hollander E. Sexual compulsions. In: Hollander E., editor. Obsessive compulsive-related disorders. American Psychiatric Press; Washington, DC: 1993. [Google Scholar]
- 32.Hollander E. American Psychiatric Press; Washington, DC: 1993. Obsessive-compulsive related disorders. [Google Scholar]
- 33.Travin S. Compulsive sexual behaviors. Psychiatr Clin North Am. 1995;18:155–169. [PubMed] [Google Scholar]
- 34.Black D.W. Compulsive sexual behavior: a review. J Psychiatr Pract. 1998;4:217–229. [Google Scholar]
- 35.Zuckerman M., Neeb M. Sensation seeking and psychopathology. Psychiatry Res. 1979;1:255–264. doi: 10.1016/0165-1781(79)90007-6. [DOI] [PubMed] [Google Scholar]
- 36.Zuckerman M., Myers P.L. Sensation seeking in homosexual and heterosexual males. Arch Sex Behav. 1983;12:347–356. doi: 10.1007/BF01542195. [DOI] [PubMed] [Google Scholar]
- 37.Kalichman S.C., Rompa D. Sexual sensation seeking and Sexual Compulsivity Scales: reliability, validity, and predicting HIV risk behavior. J Pers Assess. 1995;65:586–601. doi: 10.1207/s15327752jpa6503_16. [DOI] [PubMed] [Google Scholar]
- 38.Hoyle R.H., Fejfar M.C., Miller J.D. Personality and sexual risk taking: a quantitative review. J Pers. 2000;68:1203–1231. doi: 10.1111/1467-6494.00132. [DOI] [PubMed] [Google Scholar]
- 39.American Psychiatric Association . 3rd ed. American Psychiatric Association; Washington, DC: 1980. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 40.American Psychiatric Association . 3rd ed, text revision. American Psychiatric Association; Washington, DC: 1987. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 41.American Psychiatric Association . 4th ed, text revision. American Psychiatric Association; Washington, DC: 2000. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 42.Levy F. The dopamine theory of attention deficit hyperactivity disorder (ADHD) Aust N Z J Psychiatry. 1991;25:277–283. doi: 10.3109/00048679109077746. [DOI] [PubMed] [Google Scholar]
- 43.Corona G., Mannucci E., Jannini E.A. Hypoprolactinemia: a new clinical syndrome in patients with sexual dysfunction. J Sex Med. 2009;6:1457–1466. doi: 10.1111/j.1743-6109.2008.01206.x. [DOI] [PubMed] [Google Scholar]
- 44.Corona G., Wu F.C., Rastrelli G., EMAS Study Group Low prolactin is associated with sexual dysfunction and psychological or metabolic disturbances in middle-aged and elderly men: the European Male Aging Study (EMAS) J Sex Med. 2014;11:240–253. doi: 10.1111/jsm.12327. [DOI] [PubMed] [Google Scholar]
- 45.Corona G., Jannini E.A., Vignozzi L. The hormonal control of ejaculation. Nat Rev Urol. 2012;9:508–519. doi: 10.1038/nrurol.2012.147. [DOI] [PubMed] [Google Scholar]
- 46.Torner L., Toschi N., Pohlinger A. Anxiolytic and anti-stress effects of brain prolactin: improved efficacy of antisense targeting of the prolactin receptor by molecular modeling. J Neurosci. 2001;21:3207–3214. doi: 10.1523/JNEUROSCI.21-09-03207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torner L., Karg S., Blume A. Prolactin prevents chronic stress-induced decrease of adult hippocampal neurogenesis and promotes neuronal fate. J Neurosci. 2009;29:1826–1833. doi: 10.1523/JNEUROSCI.3178-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker T.L., Vukovic J., Koudijs M.M. Prolactin stimulates precursor cells in the adult mouse hippocampus. PLoS One. 2012;7:e44371. doi: 10.1371/journal.pone.0044371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bancroft J., Vukadinovic Z. Sexual addiction, sexual compulsivity, sexual impulsivity, or what? Toward a theoretical model. J Sex Res. 2004;41:225–234. doi: 10.1080/00224490409552230. [DOI] [PubMed] [Google Scholar]