Abstract
Introduction
Hyaluronic acid has been shown to be efficacious in decreasing scar formation, inflammation, and oxidative stress.
Aim
To assess the efficacy of intralesional injection of hyaluronic acid in patients affected by Peyronie's disease.
Methods
In this prospective, single-arm, self-controlled, interventional, multicenter pilot study, 65 patients underwent a 10-week cycle of weekly intraplaque injections with hyaluronic acid (0.8% highly purified sodium salt hyaluronic acid 16 mg/2 mL; Sinovial, IBSA, Lodi, Italy). Patients were re-evaluated 2 months after the end of therapy.
Main Outcome Measures
Plaque size (millimeters), penile curvature (degrees), International Index of Erectile Function (IIEF-5) score, visual analog scale (VAS) score for sexual satisfaction, and Patient's Global Impressions of Improvement (PGI-I) score.
Results
Median age was 57 years (range = 23–70). At baseline, mean plaque size was 10 mm (range = 3–30 mm), mean penile curvature was 30° (range = 0°–50°), and mean IIEF-5 score was 20 (range = 0–25), with slight to moderate erectile dysfunction (IIEF score < 21) in 36 of 65 patients (55%). A median VAS score of 6 (range = 2–10) was found. Mean follow-up was 12 months (range = 6–24 months). Statistically significant post-treatment improvements were detected for plaque size (before treatment = 10 mm [3–30 mm], after treatment = 8 mm [1–30 mm], P < .0001), penile curvature (before treatment = 30° [0°–50°], after treatment = 20° [0°–40°], P < .0001), IIEF-5 score (before treatment = 20 [11–25], after treatment = 21 [15–25], P < .0001), and VAS score (before treatment = 6 [2–10], after treatment 8 [2–10], P < .0001). After treatment, the rate of patients with an IIEF score lower than 21 decreased from 55% (36 patients) to 40% (25 patients). Overall improvement on the PGI-I questionnaire was 69%.
Conclusion
Intralesional treatment with hyaluronic acid can improve plaque size, penile curvature, and overall sexual satisfaction and seems preferably indicated in the early (active) phase of the disease. Furthermore, it is easy to perform and well tolerated.
Key Words: Hyaluronic Acid, Intralesional Therapy, Penile Curvature, Peyronie's Disease
Introduction
Peyronie's disease (PD) is an acquired, localized fibrotic disorder characterized by the deposition of collagen and fibrin in the form of plaque on the tunica albuginea of the penis. Its pathophysiology is unknown and frequently results in penile deformity, penile pain, and erectile dysfunction,1 leading to a significant negative impact on patient quality of life, with associated rates of significant depression up to 48%.2 Recent prevalence estimates vary from 3.2% to 8.9%.3, 4
The etiology of PD is largely unknown. According to current popular theories, a single traumatic event or repeated microtraumas during sexual activity can lead to a low-level autoimmune response arising from a prolonged and complex inflammatory reaction of the tunica albuginea fibers,5 which leads to plaque formation.6, 7, 8, 9
From a historical point of view, it is worth highlighting that the first descriptions of a penile condition that could refer to PD probably date to the 13th century, and the first accurate description of PD was reported by the Italian anatomist Gabriele Falloppio in the 16th century. In 1743, Francois Gigot de la Peyronie's wrote a landmark article on PD, and his name was associated with the disease. Despite this long history, there are no medical or surgical therapies10 and there is a paucity of knowledge on the natural history of PD, although deformity stabilization and improvements in specific subsets of men with untreated PD have recently been reported.11
The European Association of Urology and the American Association of Urology have released clinical practice guidelines for the diagnosis, evaluation, treatment, and follow-up of patients with PD.12, 13 Conservative treatment of PD is focused primarily on patients in the early (acute inflammatory) stage, and surgical remediation is used to correct curvature, allow for satisfactory intercourse, and is reserved for patients who have stable disease for at least 12 months.12
Several options have been suggested for non-operative PD treatment, including oral pharmacotherapy, intralesional injection therapy, and other topical treatments. Of these, injection of pharmacologically active agents directly into penile plaques represents the most valid treatment option, because current evidence discourages the use of oral and topical agents. In particular, current American Association of Urology clinical practice guidelines suggest intralesional administration of Clostridium histolyticum collagenase (CHC) in combination with modeling or intralesional interferon alfa-2b or verapamil.14 However, these compounds have been found to produce the potential occurrence of mild to severe adverse events, including penile ecchymosis, swelling, pain, and corporal rupture with collagenase; sinusitis, flulike symptoms, and minor penile swelling with interferon alfa-2b; and penile bruising, dizziness, nausea, and pain at the injection site with verapamil.14 In addition, therapy with collagenase is limited by the high cost of the compound. Based on currently available scientific evidence, the intralesional agent presenting the optimal benefit-risk ratio is far from determined, and further research of novel intralesional agents is strongly needed.
Hyaluronic acid has been shown to be efficacious in decreasing scar formation and blocking the effects of substances that generate inflammation and oxidative stress. For this reason, it is used widely in numerous medical applications, including cosmetic surgery (for treatment of wrinkles and scar) and orthopedics (intra-articular therapy for osteoarthritis) because of its consolidated therapeutic efficacy and treatment-cycle feasibility.15, 16
The present study investigated whether intralesional use of hyaluronic acid could provide a positive effect on the pathogenesis of PD by its interference with inflammatory and pro-fibrotic processes. Therefore, the aim of this prospective, single-arm, self-controlled, interventional, multicenter pilot study was to assess the efficacy of intralesional injections of hyaluronic acid to decrease plaque size, decrease penile curvature, and increase sexual satisfaction in patients affected by PD.
Methods
This is a single-arm, self-controlled, interventional study. Patients were enrolled from 10 Italian andrology centers and pretreatment clinical evaluations were carried out by urologists experienced with PD management.
Sexually active men older than 18 years affected by PD were eligible for this study. Study criteria included (i) a palpable nodule or plaque in the tunica of the penis and (ii) pain in the flaccid state or during painful erections. Exclusion criteria were (i) calcified plaques or hourglass deformity as defined at duplex Doppler ultrasonography, (ii) previous PD therapy with oral agents or intralesional injections, and (iii) severe concomitant erectile dysfunction (International Index of Erectile Function [IIEF-5] score < 7).
After the preliminary evaluation for eligibility, including medical and sexual histories, physical examination, and self-administration of IIEF-5 questionnaires, enrolled patients were invited to undergo a 10-week cycle of weekly intraplaque injections with hyaluronic acid (0.8% highly purified sodium salt hyaluronic acid 16 mg/2 mL,; Sinovial, IBSA, Lodi, Italy).
All patients provided written informed consent and the study was approved by the institutional review board (ethics committee of Perugia University, Perugia, Italy).
Pretreatment Workup
All patients underwent Duplex Doppler ultrasonography in the basal condition and after the induction of penile erection with the assistance of an intracavernous injection of a tri-mix (papaverine 30 mg/mL, phentolamine 1 mg/mL, alprostadil 10 μg/mL). Repeat dosing was administered to a maximum of three doses until full erectile rigidity was achieved. All procedures were performed by the same experienced operator at each center. Plaque position and size (the longest diameter was considered when evaluating the results) were carefully assessed. Penile curvature was measured using a goniometer at maximum penile rigidity. Patients were stratified in six categories according to penile curvature: 0° to 10°, 10° to 20°, 20° to 30°, 30° to 40°, 40° to 50°, and greater than 50°. Self-assessment of sexual satisfaction was measured using a visual analog scale (VAS) from 0 to 10, where 0 represented “not satisfied” and 10 represented “satisfied.”
Follow-Up
Two months after the end of therapy, penile curvature and plaque size were re-evaluated with Duplex Doppler ultrasonography as described earlier and the results were compared with the pretreatment values. To assess sexual satisfaction, patients repeated the IIEF-5 questionnaire and VAS for sexual satisfaction. They also filled out the Patient's Global Impressions of Improvement (PGI-I) questionnaire, which is a one-item questionnaire designed to assess the patient's impression of changes in his condition.16 The PGI-I asks the patient to best describe his current condition compared with before treatment. Answers are given on a seven-point scale (1 = a lot better, 2 = much better, 3 = a little better, 4 = no change, 5 = a little worse, 6 = much worse, 7 = a lot worse).
Statistical Analysis
The Shapiro-Wilk test was used to assess the normal distribution of variables. The Wilcoxon signed rank test was used to compare paired data and Spearman ρ was used to examine correlations. Statistical analyses were performed using IBM-SPSS 22.0 (IBM Corp, Armonk, NY, USA). A two-sided P value less than 0.05 was considered significant.
Results
From December 2012 through December 2014, 118 patients with PD were screened. Of these, 65 patients met the inclusion criteria and received 10 weekly treatments with hyaluronic acid. All patients completed the treatment cycle and attended the first follow-up visit (2 months after the end of therapy).
Table 1 presents the demographics and clinical data of the study group. Median age was 57 years (range = 23–70 years). All patients described the onset of PD as pain, tension, or “tingling” during erection, curvature, and/or erectile dysfunction. Mean elapsed time from disease onset (symptoms and/or curvature) to diagnosis was 4.3 months (range = 1–18 months). All patients had at least one palpable plaque on the shaft at physical examination. At baseline, mean plaque size was 10 mm (range = 3–30 mm), mean curvature was 30° (range = 0°–50°), and mean baseline IIEF-5 score was 20 (range = 0–25), with slight to moderate erectile dysfunction (IIEF score < 21) present in 36 of 65 patients (55%). A median VAS score of 6 (range = 2–10) was detected. Mean follow-up was 12 months (range = 6–24 months).
Table 1.
Demographics and baseline clinical data
| Baseline parameter | Median | Range | SD |
|---|---|---|---|
| Age (y) | 57 | 23–70 | 10.88 |
| Plaque dimensions (mm) | 10 | 3–30 | 6.76 |
| Penile curvature (°) | 30 | 0–50 | 1.13 |
| IIEF-5 score | 20 | 11–25 | 4.72 |
| VAS score | 6 | 2–10 | 2.08 |
IIEF = International Index of Erectile Function; VAS = visual analog scale.
Table 2 lists the results after treatment for plaque size, penile curvature, and IIEF-5 and VAS scores. Statistically significant post-treatment improvements were detected for plaque size (before treatment = 10 mm [3–30 mm], after treatment = 8 mm [1–30 mm], P < .0001), curvature (before treatment = 30° [0°–50°], after treatment = 20° [0°–40°], P < .0001), IIEF-5 score (before treatment = 20 [11–25], after treatment = 21 [15–25], P < .0001), and VAS score (before treatment = 6 [2–10], after treatment = 8 [2–10], P < .0001). After treatment, the rate of patients with an IIEF score lower than 21 decreased from 55% (36 patients) to 40% (25 patients).
Table 2.
Clinical results after 10 intraplaque injections with hyaluronic acid∗
| Parameter | Before treatment | After treatment | P value |
|---|---|---|---|
| Plaque size (mm) | 10 (3–30) | 8 (1–30) | <.0001 |
| Penile curvature (°) | 30 (0–50) | 20 (0–40) | <.0001 |
| IIEF-5 (score) | 20 (11–25) | 21 (15–25) | <.0001 |
| Sexual satisfaction (VAS score) | 6 (2–10) | 8 (2–10) | <.0001 |
IIEF = International Index of Erectile Function; VAS = visual analog scale.
Data are expressed as median (range).
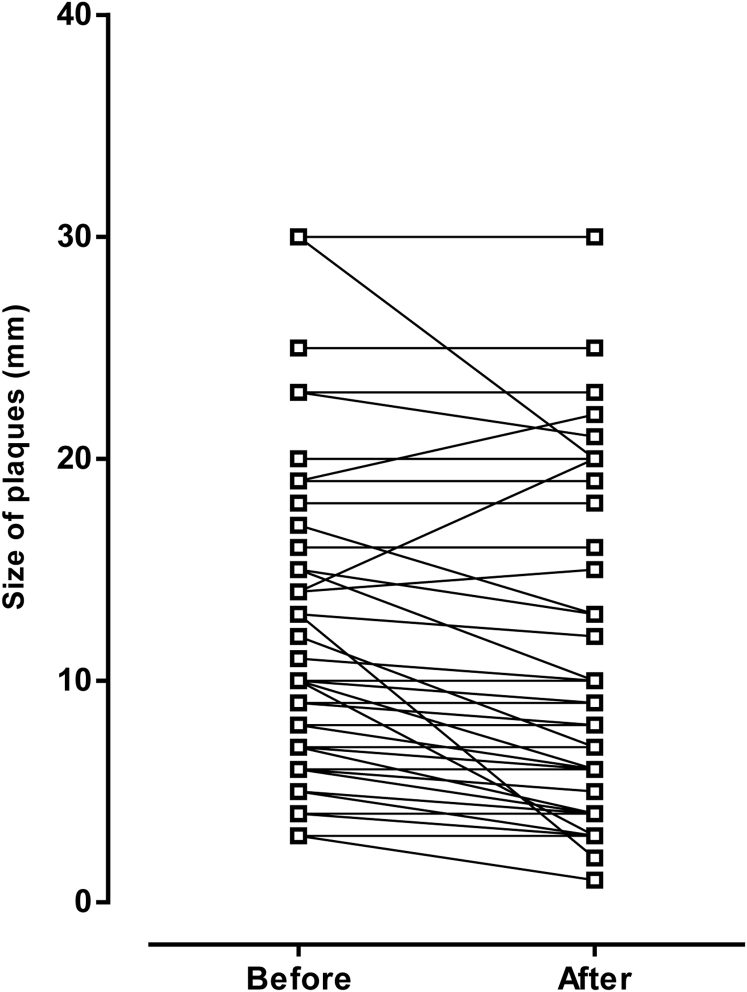
Figure 1 presents additional details of the treatment effect on plaque size. At the end of treatment, plaque size was smaller in 37 patients (57%), remained unchanged (stable) in 24 (37%), and was larger in 4 (6%).
Figure 1.
Plaque size before and after treatment course.
Overall improvement on the PGI-I questionnaire was 69%. Eight patients declared they felt “a lot better,” 16 felt “much better,” 21 felt “a little better,” 14 perceived “no change,” and 6 (9%) felt worse after treatment.
No injection-site ecchymosis or hematomas and no patient-reported pain during injection, local or systemic drug reactions, including adverse side effects or sensory changes at the tip of the penis, were noted.
Discussion
This prospective, single-arm, self-controlled, interventional, multicenter pilot study investigated the efficacy of hyaluronic acid as intralesional treatment for patients with PD and showed encouraging preliminary results for improvement in plaque size, penile curvature, and overall sexual satisfaction.
Hyaluronic acid is a glycosaminoglycan found in the extracellular matrix. At physiologic pH, the hyaluronate molecule is highly polarized and, hence, highly soluble in water. The marked uptake of water molecules by hyaluronic acid maintains hydration, turgor, plasticity, and viscosity in the amorphous connective tissue matrix. It also acts as an antishock molecule and a lubricant as, for example, in synovial fluid, thus protecting cells from physical stress.15, 16 It is most commonly used to treat osteoarthritis of the knee, where oxidative stress plays a major role in cartilage degeneration and disease onset. Oxygen-free radicals, such as hydrogen peroxide (H2O2), hypochlorite ions, (OCl−), the hydroxyl radical (·OH), and superoxide anions (O2−), are involved in intracellular signal transduction and degenerative cellular processes.15, 16 High levels of oxygen-free radicals cause chondrocyte apoptosis and cartilage degeneration, thus contributing to the pathogenesis of osteoarthritis.17, 18 A general consensus is that, in osteoarthritis, hyaluronic acid decreases oxygen-free radicals in the synovial fluid, inhibits apoptosis, promotes cell survival,19 and decreases concentrations of inflammatory proteins.20 As a result, hyaluronic acid acts as an antioxidant and as an anti-inflammatory agent.
The same type of cells involved in osteoarthritis play a fundamental role in the pathophysiology of PD. In the early stages of PD, oxidative stress induces overexpression of fibrogenic cytokines and increases collagen synthesis. Tumor growth factor-β1 increases levels of oxygen free radicals,21 and these are the main mediators of collagen synthesis because they decrease local collagenase activity and stimulate overproduction of extracellular matrix proteins. The inflammation associated with PD involves overexpression of inducible nitric oxide synthase and peroxynitrite production in the corpus cavernosum and fibroblasts. This results when nitric oxide levels increase and oxygen and nitrogen radicals react with superoxide dismutase to produce highly toxic and pro-fibrotic peroxynitrite (ONOO−). Adverse effects of ONOO− in the corpus cavernosum include lipid peroxidation, DNA fragmentation, protein damage, and nitration that cause cellular damage and lead to collagen accumulation and eventually organ dysfunction.22, 23
PD has been treated with some success by intralesional injection of different drugs, particularly when patients are in the early, painful progressive stages, do not have calcified plaques, and have a short case history. Currently, corticosteroids and verapamil are those most commonly used.12, 13, 14 However, only one single-blinded, placebo-controlled study has been performed using corticosteroids.24 In a recent non-randomized study, Bennett et al25 treated 94 patients with six intralesional verapamil injections administered on alternating weeks. Penile pain was alleviated in all patients and 18% showed decreased curvature. A randomized, single-blinded, placebo-controlled trial comparing intralesional verapamil with saline showed no significant difference or improvement in penile curvature, pain, plaque features, or sexual function,26 whereas administration of the calcium channel blocker nicardipine provided significant improvements in IIEF-5 score and plaque size.27
A single-blinded, placebo-controlled study investigated the efficacy of interferon in treating plaques in 103 patients with PD who received intralesional infiltrations of interferon alfa-2b for 12 weeks. Compared with placebo, interferon infiltration significantly decreased mean penile curvature (27% vs 8.9%), mean plaque size (54.6% vs 19.8%), and mean plaque density (33.3% vs 11.1%).28
In patients presenting with stable PD, CHC was proposed as effective in alleviating symptoms. In a double-blinded, placebo-controlled study of 49 patients, CHC decreased plaque number and size and penile curvature in 36% of patients compared with 4% in those who received placebo.29 It is worth noting that CHC is indicated in patients with stable plaque, whereas hyaluronic acid should be considered an alternative option in early-stage PD; thus, the two compounds should not be directly compared.
Table 3 presents a summary comparison of major series investigating intralesional therapies for every stage of PD.
Table 3.
Comparison among major series investigating intralesional therapies for Peyronie's disease
| Levine et al,14 2002 | Hellstrom et al,28 2006 | Bennett et al,25 2007 | Present study | |
|---|---|---|---|---|
| Patients, n | 156 | 117 | 94 | 65 |
| Drug | Verapamil | Interferon alfa2b | Verapamil | Hyaluronic acid |
| Dose | 10 mg every 2 wk | 2 × 106 U biweekly | 10 mg every 2 wk | 8 mg/wk |
| Study design | Prospective, non-randomized, single-arm study | Single-blinded, multicenter, placebo-controlled, parallel-group study | Prospective, non-randomized, single-arm study | Prospective, non-randomized, single-arm, multicenter, pilot study |
| Injections, n | 12 | 6 | 6 | 10 |
| Decrease in penile curvature, % | 60 | 27 | 18 | 37 |
| Increased sexual satisfaction, % | 71 | Not available | Monopoli | Monopoli |
| Decrease in plaque size, % | Not available | 54.6 | Not available | 57 |
Currently, most drugs used for intraplaque injection in PD are “off-label,” with a risk of systemic (verapamil, steroids) and local (collagenase) side effects. In contrast, hyaluronic acid showed a low risk of local or systemic adverse effects.
In the present study, hyaluronic acid was used because of its anti-inflammatory and antioxidant effects, which led to early resolution of plaque-related symptoms and its antifibrotic effects, which limited plaque growth. A decrease in plaque size was found in more than half the patients (57%), whereas an increase was observed in only 6%.
Although the plaque size measurement might be influenced by inter-operator variability, we interpreted the resulting decrease in plaque size as directly relevant to treatment and a sign of limited plaque evolution attributable to treatment efficacy.
Statistical analysis showed that curvature was decreased in 37% of patients and significant improvements were found in the IIEF-5 and VAS sexual satisfaction scores compared with pretreatment data. Although the net numeric increase of the IIEF-5 score was minimal, it is worth noting that, after the treatment course, most patients presented with restored erectile function (IIEF-5 score >21) and significant overall subjective improvement in sexual function. Furthermore, the reported improvement in plaque size and penile curvature, even if statistically meaningful, is numerically minimal. Regardless of statistical power, these results are clinically relevant because they trigger a positive behavior in the patient affected by PD, as demonstrated by the net increase in sexual satisfaction. Unfortunately, the lack of comparison with a placebo arm limits the relevancy of these data.
Hyaluronic acid was well tolerated in all patients in terms of local and systemic side effects, which can be considered a true advantage compared with other available intralesional drugs. Another point is that in our patient cohort the mean elapsed time from disease onset (symptoms and/or curvature) to diagnosis was 4.3 months, suggesting early-stage disease in most cases. Based on the mechanisms of action of hyaluronic acid, we speculate that its optimal efficacy rate can be achieved in this specific subset of patients with PD. Nevertheless, there are several limitations to the present study that must be considered when interpreting the results. First, the study design was not randomized and not controlled with placebo. Second, the study sample was limited and was recruited from 10 different centers with a limited number of cases in some centers; a sub-analysis of results according to single-center experience was not performed. We emphasize that if the multicenter enrollment is considered a bias for patients' baseline characteristics, then it should be considered representative of a “real-life” setting, thus supporting the strength of the study. Third, improvement in sexual function satisfaction was assessed using a VAS rather than other available validated tools.
Further prospective, randomized, placebo-controlled studies with larger patient cohorts stratified by disease stage are sorely needed to establish the real impact of intralesional hyaluronic acid as a therapeutic option for early-stage PD, other than confirming the high treatment tolerability.
Conclusions
This prospective, multicenter, pilot study assessing the efficacy of hyaluronic acid as intralesional treatment for patients with PD showed encouraging preliminary results for improvement of plaque size, penile curvature, and overall sexual satisfaction. This therapeutic approach seems to be preferably indicated in the early (active) phase of the disease. Intralesional treatment with hyaluronic acid is easy to perform and well tolerated, with null risk of local or systemic side effects.
Statement of Authorship
Category 1
-
(a)
Conception and Design
Alessandro Zucchi; Giorgio Franco; Alessandro Palmieri
-
(b)
Acquisition of Data
Alessandro Zucchi; Elisabetta Constantini; Tommaso Cai; Giorgio Cavallini; Giovanni Liguori; Vincenzo Favilla; Gaetano De Grande; Guiseppe D'Achille; Mauro Silvani; Giorgio Franco; Alessandro Palmieri; Paolo Verze; Vincenzo Mirone
-
(c)
Analysis and Interpretation of Data
Alessandro Zucchi; Paolo Verze
Category 2
-
(a)
Drafting the Article
Alessandro Zucchi; Paolo Verze
-
(b)
Revising It for Intellectual Content
Alessandro Zucchi; Elisabetta Constantini; Tommaso Cai; Giorgio Cavallini; Giovanni Liguori; Vincenzo Favilla; Gaetano De Grande; Guiseppe D'Achille; Mauro Silvani; Giorgio Franco; Alessandro Palmieri; Paolo Verze; Vincenzo Mirone
Category 3
-
(a)
Final Approval of the Completed Article
Alessandro Zucchi; Elisabetta Constantini; Tommaso Cai; Giorgio Cavallini; Giovanni Liguori; Vincenzo Favilla; Gaetano De Grande; Guiseppe D'Achille; Mauro Silvani; Giorgio Franco; Alessandro Palmieri; Paolo Verze; Vincenzo Mirone
Footnotes
Conflict of Interest: The authors report no conflict of interest.
Funding: None.
This study was conducted under the scientific supervision of the SERIA Group (Research in Andrology Working Group) of the Italian Society of Andrology (SIA).
References
- 1.Brock G., Hsu G.L., Nunes L. The anatomy of the tunica albuginea in the normal penis and Peyronie's disease. J Urol. 1997;157:276–281. [PubMed] [Google Scholar]
- 2.Nelson C.J., Diblasio C., Kendirci M. The chronology of depression and distress in men with Peyronie’s disease. J Sex Med. 2008;5:1985–1990. doi: 10.1111/j.1743-6109.2008.00895.x. [DOI] [PubMed] [Google Scholar]
- 3.Schwarzer U., Sommer F., Klotz T. The prevalence of Peyronie's disease: results of a large survey. BJU Int. 2001;88:727–730. doi: 10.1046/j.1464-4096.2001.02436.x. [DOI] [PubMed] [Google Scholar]
- 4.Mulhall J.P., Creech S.D., Boorjian S.A. Subjective and objective analysis of the prevalence of Peyronie's disease in a population of men presenting for prostate cancer screening. J Urol. 2004;171:2350–2353. doi: 10.1097/01.ju.0000127744.18878.f1. [DOI] [PubMed] [Google Scholar]
- 5.Jarow J.P., Lowe F.C. Penile trauma: an etiologic factor in Peyronie's disease and erectile dysfunction. J Urol. 1997;158:1388–1390. doi: 10.1016/s0022-5347(01)64222-8. [DOI] [PubMed] [Google Scholar]
- 6.El-Sakka A.I., Hassoba H.M., Chui R.M. An animal model of Peyronie's-like condition associated with an increase of transforming growth factor beta mRNA and protein expression. J Urol. 1997;158:2284–2290. doi: 10.1016/s0022-5347(01)68236-3. [DOI] [PubMed] [Google Scholar]
- 7.Somers K.D., Dawson D.M. Fibrin deposition in Peyronie's disease plaque. J Urol. 1997;157:311–315. [PubMed] [Google Scholar]
- 8.Van de Walter L. Mechanisms by which fibrin and fibronectin appear in healing wounds: implications for Peyronie's disease. J Urol. 1997;157:306–310. doi: 10.1097/00005392-199701000-00103. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez-Cadavid N.F., Rajfer J. Mechanisms of disease: new insights into the cellular and molecular pathology of Peyronie's disease. Nat Clin Pract Urol. 2005;2:291–297. doi: 10.1038/ncpuro0201. [DOI] [PubMed] [Google Scholar]
- 10.Musitelli S., Bossi M., Jallous H. A brief historical survey of ‘Peyronie's disease’. J Sex Med. 2008;5:1737–1746. doi: 10.1111/j.1743-6109.2007.00692.x. [DOI] [PubMed] [Google Scholar]
- 11.Berookhim B.M., Choi J., Alex B. Deformity stabilization and improvement in men with untreated Peyronie's disease. BJU Int. 2014;113:133–136. doi: 10.1111/bju.12346. [DOI] [PubMed] [Google Scholar]
- 12.Hatzimouratidis K., Eardley I., Giuliano F., European Association of Urology EAU guidelines on penile curvature. Eur Urol. 2012;62:543–552. doi: 10.1016/j.eururo.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 13.American Urological Association. Peyronie's disease: AUA guideline. Available at: https://www.auanet.org/education/guidelines/Peyronie'ss-disease.cfm. Accessed August 1, 2015.
- 14.Levine L.A., Goldman K.E., Greenfield J.M. Experience with intraplaque injection of verapamil for Peyronie's disease. J Urol. 2002;168:621–625. doi: 10.1016/s0022-5347(05)64691-5. [DOI] [PubMed] [Google Scholar]
- 15.Henrotin Y., Kurz B., Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthritis Cartilage. 2005;13:643–654. doi: 10.1016/j.joca.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Henrotin Y.E., Bruckner P., Pujol J.P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11:747–755. doi: 10.1016/s1063-4584(03)00150-x. [DOI] [PubMed] [Google Scholar]
- 17.Viktrup L., Hayes R.P., Wang P. Construct validation of patient global impression of severity (PGI-S) and improvement (PGI-I) questionnaires in the treatment of men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. BMC Urol. 2012;12:30. doi: 10.1186/1471-2490-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asada S., Fukuda K., Oh M. Effect of hydrogen peroxide on the metabolism of articular chondrocytes. Inflamm Res. 1999;48:399–403. doi: 10.1007/s000110050478. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K., Hashimoto S., Kubo T. Effect of hyaluronan on chondrocyte apoptosis and nitric oxide production in experimentally induced osteoarthritis. J Rheumatol. 2000;27:1713–1720. [PubMed] [Google Scholar]
- 20.Yu C.J., Ko C.J., Hsieh C.H. Proteomic analysis of osteoarthritic chondrocyte reveals the hyaluronic acid–regulated proteins involved in chondroprotective effect under oxidative stress. J Proteomics. 2014;99:40–53. doi: 10.1016/j.jprot.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Chen C.P., Hsu C.C., Pei Y.C. Changes of synovial fluid protein concentrations in supra-patellar bursitis patients after the injection of different molecular weights of hyaluronic acid. Exp Gerontol. 2014;52:30–35. doi: 10.1016/j.exger.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Vernet D., Nolazco G., Cantini L. Evidence that osteogenic progenitor cells in the human tunica albuginea may originate from stem cells: implications for Peyronie's disease. Biol Reprod. 2005;73:1199–1210. doi: 10.1095/biolreprod.105.041038. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Cadavid N.F., Magee T.R., Ferrini M. Gene expression in Peyronie's disease. Int J Impot Res. 2002;14:361–374. doi: 10.1038/sj.ijir.3900873. [DOI] [PubMed] [Google Scholar]
- 24.Cipollone G., Nicolai M., Mastroprimiano G. Betamethasone versus placebo in Peyronie's disease. Arch Ital Urol Androl. 1998;70:165–168. [PubMed] [Google Scholar]
- 25.Bennett N.E., Guhring P., Mulhall J.P. Intralesional verapamil prevents the progression of Peyronie's disease. Urology. 2007;69:1181–1184. doi: 10.1016/j.urology.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 26.Shirazi M., Haghpanah A.R., Badiee M. Effect of intralesional verapamil for treatment of Peyronie's disease: a randomized single-blind, placebo-controlled study. Int Urol Nephrol. 2009;4:467–471. doi: 10.1007/s11255-009-9522-4. [DOI] [PubMed] [Google Scholar]
- 27.Soh J., Kawauchi A., Kanemitsu N. Nicardipine vs saline injection as treatment for Peyronie's disease: a prospective, randomized, single-blind trial. J Sex Med. 2010;7:3743–3749. doi: 10.1111/j.1743-6109.2010.01924.x. [DOI] [PubMed] [Google Scholar]
- 28.Hellstrom W.J., Kendirci M., Matern R. Single-blind, multicenter, placebo controlled, parallel study to assess the safety and efficacy of intralesional interferon alpha-2B for minimally invasive treatment for Peyronie's disease. J Urol. 2006;176:394–398. doi: 10.1016/S0022-5347(06)00517-9. [DOI] [PubMed] [Google Scholar]
- 29.Gelbard M., Goldstein I., Hellstrom W.J. Clinical efficacy, safety and tolerability of collagenase clostridium histolyticum for the treatment of Peyronie's disease in 2 large double-blind, randomized, placebo controlled phase 3 studies. J Urol. 2013;190:199–207. doi: 10.1016/j.juro.2013.01.087. [DOI] [PubMed] [Google Scholar]