Abstract
Background
Panax ginseng root is used in traditional oriental medicine for human health. Its main active components such as saponins and polysaccharides have been widely evaluated for treating diseases, but secondary active components such as oligosaccharides have been rarely studied. This study aimed to assess the impact of water-soluble ginseng oligosaccharides (WGOS), which were isolated from the warm-water extract of Panax ginseng root, on scopolamine-induced cognitive impairment in mice and its antineuroinflammatory mechanisms.
Methods
We investigated the impact of WGOS on scopolamine-induced cognitive impairment in mice by using Morris water maze and novel object recognition task. We also analyzed the impact of WGOS on scopolamine-induced inflammatory response (e.g., the hyperexpression of proinflammatory cytokines IL-1β and IL-6 and astrocyte activation) by quantitative real-time polymerase chain reaction and glial fibrillary acid protein (GFAP) immunohistochemical staining.
Results
WGOS pretreatment protected against scopolamine-induced learning and memory deficits in the Morris water maze and in the novel object recognition task. Furthermore, WGOS pretreatment downregulated scopolamine-induced hyperexpression of proinflammatory cytokines interleukin (IL)-1β and IL-6 mRNA and astrocyte activation in the hippocampus. These results indicate that WGOS can protect against scopolamine-induced alterations in learning and memory and inflammatory response.
Conclusion
Our data suggest that WGOS may be beneficial as a medicine or functional food supplement to treat disorders with cognitive deficits and increased inflammation.
Keywords: antineuroinflammation, astrocyte activation, cognitive impairment, proinflammatory cytokine, water-soluble ginseng oligosaccharide
1. Introduction
Alzheimer's disease (AD) is characterized by the progressive loss of memory and deterioration of cognitive function. The pathological hallmark of AD is the presence of β-amyloid (Aβ) plaques. The accumulation of Aβ may trigger the degeneration of the cholinergic system in the basal forebrain and result in dementia [1]. Acetylcholinesterase inhibitors, which elevate acetylcholine levels in the brain, are accordingly clinically utilized for treating AD [2]; however, this treatment only improves symptoms of AD temporarily without preventing the progression of the disease [3].
Chronic inflammation is an invariant component in the pathogenesis of most neurodegenerative diseases such as AD. After a local injection of Aβ, the inflammatory response occurs in the brain, which is characterized by the upregulation of proinflammatory cytokines and the activation of glial cells [4], [5]. Activated glial cells, along with the overexpression of proinflammatory cytokines such as interleukin (IL)-1β) and IL-6 have been associated with the lesions of AD as neurotoxic agents [6], [7], [8]. Therefore, traditional nonsteroidal anti-inflammatory drugs were considered a potential treatment to prevent or slow the onset of AD in epidemiologic and clinical studies [9], [10]; however, significant adverse effects of the drugs at high therapeutic doses were anticipated [11]. Thus, researchers have shifted toward discovering natural compounds that may decrease inflammation in AD [12], [13].
Panax ginseng root has been used in traditional oriental medicine for human health, and its neuroprotective effect on different neurologic diseases has been studied [14], [15], [16]. The main active components of Panax ginseng such as saponins and polysaccharides have been widely evaluated, but secondary active components such as ginseng oligosaccharides have not been widely studied. Water-soluble ginseng oligosaccharides (WGOS), which comprise polymers of 2–14 d-glucose molecules, were recently purified from an aqueous extract of ginseng roots. Studies have been demonstrated that WGOS exert an immunoregulatory effect in vitro and in vivo [17], [18] and display antitumor activity [19]. Thus, we hypothesize WGOS may possess an antineuroinflammatory effect, which may delay the development of neurodegenerative diseases such as AD. To investigate this possibility, we assessed the potential protective effects of WGOS on learning and memory defects and on the hyperexpression of proinflammatory cytokines and astrocyte activation in a scopolamine-induced dementia model.
To date, the only study examining the effect of ginseng oligosaccharides on cognition demonstrated that a mixture of oligosaccharides and peptides from ginseng root can enhance memory in scopolamine-induced dementia in rats; however, the mechanism of this protection was not elucidated [20]. In the current study, we assessed the protective effects of purified ginseng oligosaccharides (i.e., WGOS) against the development of a cognition defect induced by scopolamine. We found that WGOS significantly attenuated scopolamine-induced impairment in the Morris water maze and in the novel object recognition task. In addition, WGOS pretreatment attenuated scopolamine-induced hyperexpression of the inflammatory markers IL-1β and IL-6, and attenuated astrocyte activation in all hippocampal subregions examined. The data from this study shed light on a new potential prophylactic approach toward treating disorders with cognitive deficits and increased inflammation.
2. Materials and methods
2.1. Animals
Male ICR mice (weight, 25–30 g; age, 6–7 weeks old) were obtained from the Animal Center of the College of Basic Medical Sciences in Jilin University (Jilin, China) with approval from the Animal Research Ethics Committee. They were housed 3–4 mice per cage at room temperature (22 ± 2°C) with a 12-h alternating light-dark cycle and free access to food and water. Drug treatment was initiated 5 d after the animals arrived. All behavioral experiments were performed between 8:00 a.m. and 11:00 a.m.
2.2. Drugs and experimental protocol
Water-soluble ginseng oligosaccharides obtained from the water extract of Panax ginseng roots [17], [18] was provided by Jilin Ginseng Academy at the Changchun University of Chinese Medicine (Changchun, China). Scopolamine hydrobromide was purchased from Shanghai Hefeng Pharmaceutical Co., Ltd. (Shanghai, China). Before the experiments, WGOS was dissolved and scopolamine was diluted with sterile 0.9% saline. All of drugs were administered intraperitoneally (IP).
The mice were assigned to one of six groups, as indicated in Table 1. Three different pretreatments were administered beginning on Day 1 and continued throughout the course of the experiment, as indicated in Fig. 1. The three pretreatments were (1) saline, (2) 40 mg/kg WGOS, or (3) 80 mg/kg WGOS. These dosages for the WGOS were chosen because they produce a robust protection in scopolamine-treated mice, as demonstrated by their performance in the Morris water maze task in our pilot studies. On Day 4 and on all subsequent days, 15 min after the first injection, the mice were administered a second injection with either saline or 3 mg/kg scopolamine. The six groups are the following (presented as pretreatment/treatment): (1) saline/saline (SAL); (2) 40 mg/kg WGOS/saline (WGOS40); (3) 80 mg/kg WGOS/saline (WGOS80); (4) saline/scopolamine (SCO); (5) 40 mg/kg WGOS/scopolamine (WGOS40+SCO); and (6) 80 mg/kg WGOS/scopolamine (WGOS80+SCO). Thirty minutes after the second injection, the mice underwent the behavior tests. One subset of mice in each treatment group was tested with the Morris water maze. One day after the conclusion of this test, these mice were administered the same drug treatments before they were sacrificed for quantitative polymerase chain reaction (qPCR) analysis. Another subset of each treatment group was tested with the novel object recognition task. One day after the conclusion of this test, these mice were sacrificed for immunohistochemistry analysis. The timeline of experimental protocols is shown in Fig. 1.
Table 1.
The experimental groups and drug treatments
| Group | Pretreatment | Experiment treatment1) |
|
|---|---|---|---|
| 1st injection | 2nd injection | ||
| SAL | Saline, IP | Saline, IP | Saline, IP |
| SCO | Saline, IP | Saline, IP | Scopolamine (3 mg/kg), IP |
| WGOS40 | WGOS (40 mg/kg), IP | WGOS (40 mg/kg), IP | Saline, IP |
| WGOS40+SCO | WGOS (40 mg/kg), IP | WGOS (40 mg/kg), IP | Scopolamine (3 mg/kg), IP |
| WGOS80 | WGOS (80 mg/kg), IP | WGOS (80 mg/kg), IP | Saline, IP |
| WGOS80+SCO | WGOS (80 mg/kg), IP | WGOS (80 mg/kg), IP | Scopolamine (3 mg/kg), IP |
IP, intraperitoneal, SAL, saline; SCO, scopolamine; WGOS, water-soluble ginseng oligosaccharides.
Three different pretreatments were administered once daily for 3 d. On Day 4 and all subsequent days, the mice were administered two injections. The first injection was the same drug as the pretreatment drug and the second injection, administered 15 min after the first injection, was saline or scopolamine. Thirty minutes after the second injection, the behavior tests were administered to the mice.
Fig. 1.
The timeline of the experimental protocols. Experiment 1 (Exp 1) is designed to investigate the protective effect of water-soluble ginseng oligosaccharides (WGOS) on scopolamine-induced spatial memory impairment by using the Morris water maze. These animals were subsequently used to examine mRNA levels in the hippocampus using real-time polymerase chain reaction (PCR). Experiment 2 (Exp 2) is designed to investigate the protective effect of WGOS on scopolamine-induced in recognition memory impairment by using the novel object recognition task. These animals were subsequently sacrificed to examine glial fibrillary acidic protein immunohistochemistry in the hippocampus. IHC, immunohistochemistry; qPCR, quantitative polymerase chain reaction; Scop, scopolamine; WGOS, water-soluble ginseng oligosaccharide.
2.3. Behavioral tests
2.3.1. Morris water maze task
The Morris water maze is a circular pool that is 80 cm in diameter and 40 cm high, and filled to a depth of 19 cm with water containing milk and maintained at 25 ± 2°C. The pool was conceptually divided into four equal quadrants. An escape platform, 11 cm in diameter and 18 cm high, was located at a fixed position in the one quadrant and submerged 1 cm below the water surface.
The mice were pretreated with saline or WGOS for three days. On Day 4, the acquisition phase began, which consisted of five training days (Days 4–8 of the timeline are coincident with training Days 1–5) with three consecutive trials per day to find the platform. When a mouse reached the platform within 80 s, it was allowed to stay on the platform for 20 s until the start of the next trial. If a mouse did not reach the platform within 80 s, it was guided to the platform by handlers. The time to reach the platform for all three trials (i.e., the escape latency) was averaged.
On the day after finishing the acquisition trial—Day 9 on the timeline—a probe trial was performed in which the platform was removed and replaced by a virtual platform in the exact same position. The mice were allowed to swim freely for 80 s. The time spent in the platform quadrant and the number of times the mouse crossed the virtual platform were recorded. Swimming velocity was computed using the video-tracking software, Ethovision (Nodulus, Wageningen, The Netherlands).
2.3.2. Novel object recognition task
The novel object recognition task was performed in an open-field arena (50 cm × 50 cm × 40 cm) in a dimly lit, quiet room. After 3 d of pretreatment with saline or WGOS (i.e., Days 1–3), the task began with a habituation session on Day 4. During this session, each mouse was exposed to the arena for 10 min to habituate them to the apparatus. On Day 5 (i.e., the training session), the mouse was allowed 10 min to explore two identical objects placed in opposite corners of the arena at 12 cm from the walls. On Day 6 (i.e., the retention session), the mouse was again allowed 10 min to explore two different objects, one familiar object and one novel object. To prevent position preference, the locations of the familiar and novel object were varied. After each trial, the arena and objects were cleaned with 75% ethanol.
The mice's behavior was recorded using the video-tracking software, Ethovision (Nodulus). “Exploring” was defined as having the head oriented toward and within 2 cm of the objects. The total exploring time for both objects and the time spent exploring the novel object were quantified. The discrimination index, defined as the percent of time spent exploring the novel object was calculated using Eq. (1):
| discrimination index = Time with novel object/total time × 100% [21]. | (1) |
2.4. Quantitative real-time PCR
The mice were anesthetized with an overdose of barbiturate (50 mg/kg, IP). The hippocampus was rapidly dissected and stored at −80°C. The samples were sonicated in Trizol reagent (Invitrogen Co., Carlsbad, CA, USA) and the total RNA was extracted. The RNA concentration and quality were determined using a microplate reader (BioTek Epoch; Winooski, VT, USA). The cDNA was synthesized using the GoScriptTM Reverse Transcription System (Promega Co., Madison, WI, USA) and amplification reactions were performed using SYBR Premix Ex Taq TM (Takara Biological Co., Dalian, China). The qPCR test was run by using ABI Prism 7900 HT (Applied Biosystems, Foster City, CA, USA). Fold changes were calculated by the 2−ΔΔCT method using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as the internal control. Amplification of cDNA was performed employing the following primers: GAPDH (forward): 5′-TGT GTC CGT CGT GGA TCT GA-3′, GAPDH (reverse): 5′-TTG CTG TTG AAG TCG CAG GAG-3′; IL-1β (forward): 5′-ACG GAC CCC AAA AGA TGA AG-3′, IL-1β (reverse): 5′-TTC TCC ACA GCC ACA ATG AG-3′; IL-6 (forward): 5′-GAT AAG CTG GAG TCA CAG AAG G-3′, IL-6 (reverse): 5′-GGA ATG TCC ACA AAC TGA TAT GC-3′; and GFAP (forward): 5′-GAA AAC CGC ATC ACC ATT CC-3′, GFAP (reverse): 5′-CTT AAT GAC CTC ACC ATC CCG-3′.
2.5. Immunohistochemistry
The mice were anesthetized with an overdose of the barbiturate (50 mg/kg, IP), and then perfused with 10 mL normal saline through the left ventricle, followed by 20 mL of 4% paraformaldehyde in 0.1M phosphate buffered saline (PBS). The brain was removed and postfixed overnight in a perfusing fixative. Relevant brain tissues were dissected, embedded in paraffin wax, and cut into 1-μm thick coronal sections with a microtome (RM 2245; Leica Microsystems, Wetzlar, Germany).
Based on the atlas of the mouse brain [22], each 20th serial section throughout the dorsal hippocampus were collected from approximately −1.6 mm to −2.3 mm posterior of the bregma; the sections were used for immunohistochemistry analysis in accordance with previous protocols [23]. Rabbit anti-GFAP polyclonal antibody (1:200, Boster, Wuhan, China), biotin-conjugated secondary antibody, and 3,3′-diaminobenzidine (Zhongshan Golden Bridge Inc., Beijing, China) were used for immunostaining. The sections were counterstained with hematoxylin and coverslipped with neutral balsam. Images were captured using a digital camera (DP72; Olympus, Tokyo, Japan) connected to a light microscope (Nikon Corp., Tokyo, Japan).
For quantification of the immunostained cells, five sections from the same plane within the dorsal hippocampus were examined from each mouse. Cells that were stained brown were considered GFAP-positive, and blue cells were considered not immunoreactive. Using a light microscope, the GFAP-positive cells in five visual fields of each hippocampal subregions [CA1, CA3, and the dentate gyrus (DG)] were counted in each of the five sections at 200× magnification. The results were averaged within each hippocampal subregion to provide a mean number of GFAP-positive cells for each animal group.
2.6. Statistical analysis
The data were analyzed using GraphPad Prism version 5 software (GraphPad Software Inc., San Diego, CA, USA). All data are presented as the mean ± the standard deviation (SD) and statistical significance was determined by using a series of analysis of variance (ANOVA), which included F-values. For the Morris water maze, the escape latency was analyzed using two-way repeated measures ANOVA. The data from the probe trial were analyzed by one-way ANOVA, followed by Bonferroni's test. For all other experiments, the data were analyzed using a one-way ANOVA, followed by Bonferroni's post hoc test for multiple comparisons. Statistical significance was p < 0.05. The number of mice in each experiment is indicated in the figure legends.
3. Results
3.1. Water-soluble ginseng oligosaccharides protect against scopolamine-induced spatial learning and memory impairment
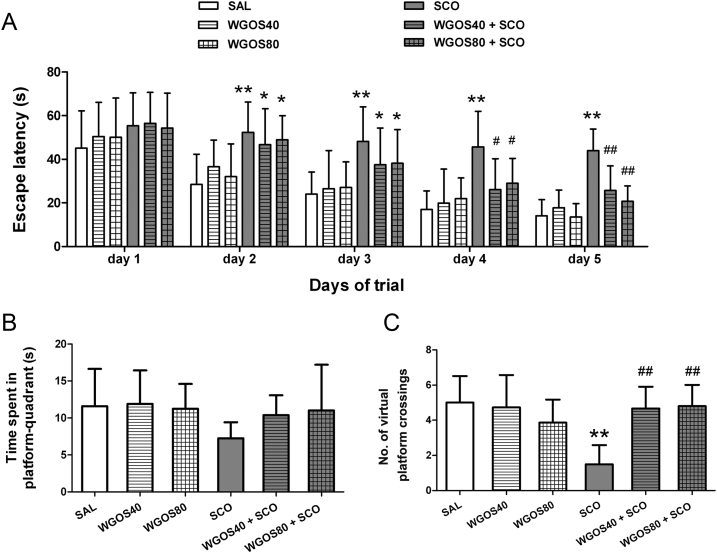
Spatial learning and memory, as measured by escape latency during the training sessions of the Morris water maze, were significantly different between the treatment groups [F (5, 415) = 29.4, p < 0.0001]. The escape latency decreased progressively over the course of the training sessions [F (4, 415) = 67.47, p < 0.0001]. However, scopolamine administration induced a more prolonged escape latency, compared to the saline treatment for training Days 2–5 (p < 0.01, respectively). The mice with WGOS (40 mg/kg or 80 mg/kg) pretreatment before scopolamine had a significantly shorter escape latency in comparison to mice treated with scopolamine on training Day 4 (p < 0.05 for both doses) and Day 5 (p < 0.01 for both doses), but not on the other days (p > 0.05). The results are presented in Fig. 2A. This finding suggests that WGOS can not immediately attenuate the effect of scopolamine on memory. Pretreatment/treatment with WGOS alone did not affect the escape latency, compared to the escape latency of the saline-treated group (p > 0.05).
Fig. 2.
Water-soluble ginseng oligosaccharides protect against scopolamine-induced spatial learning and memory impairment in the Morris water maze task. (A) The mean escape latency during the training trials was analyzed by using two-way repeated measures of ANOVA. It significantly differs between treatment groups and days. (B) The time spent in the platform quadrant and (C) the number of the virtual platform crossings during the probe trials were analyzed by one-way ANOVA, followed by Bonferroni's post hoc test. There is a significant difference between the groups for the number of virtual platform crossings. Eighty-nine mice were tested with 14–15 animals per group. Data are presented as mean ± SD. *p < 0.05 and **p < 0.01, compared to the saline-treated mice on the same day. #p < 0.05 and ##p < 0.01, compared with scopolamine-treated mice on the same day. ANOVA, analysis of variance; SAL, saline; SCO, scopolamine; WGOS40/WGOS80, 40 mg/kg dose/80 mg/kg dose of water-soluble ginseng oligosaccharides.
The time spent in the target quadrant during the probe trial session of the Morris water maze was not statistically different among all groups [F (5, 83) = 2.29, p = 0.05, Fig. 2B]. However, WGOS treatment at either dosage (i.e., WGOS40 + SCO or WGOS80 + SCO) attenuated the slight decrease caused by scopolamine, but did not achieve significance. However, a significant difference was detected among the groups for the number of virtual platform crossings [F (5, 83) = 13.05, p < 0.0001, Fig. 2C]. Therefore, we used this measure to evaluate the ability of WGOS to attenuate learning and memory deficits caused by scopolamine. Compared to the saline-treated mice, the mice treated with scopolamine had a fewer number of crossings (p < 0.01), which indicated that scopolamine could induce spatial learning and memory impairment. Pretreatment by WGOS (40 mg/kg or 80 mg/kg) attenuated the decreased number of crossings in mice treated with scopolamine alone (p < 0.01, respectively), which indicated that WGOS pretreatment markedly ameliorated scopolamine-induced spatial learning and memory impairment. However, WGOS alone at either dose did not positively or negatively affect memory because there was no significant difference between these animals and those treated with saline alone (p > 0.05).
3.2. Water-soluble ginseng oligosaccharides protect against scopolamine-induced recognition memory impairment
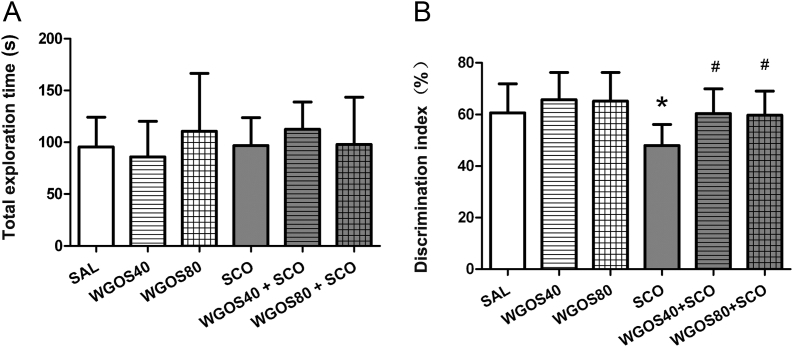
To further confirm the protective effect of WGOS on learning and memory impairment, we used the novel object recognition task. During the retention session of the novel object recognition task, all groups exhibited similar behavior because the total time spent exploring both objects did not differ among the groups [F (5, 80) = 0.99, p = 0.43, Fig. 3A]. However, the time spent exploring the novel object, expressed as the discrimination index, was significantly different between the groups [F (5, 80) = 5.87, p < 0.0001, Fig. 3B]. The discrimination index in animals treated with scopolamine alone was approximately 50%, which represents a chance level [21]; this level was markedly decreased in comparison to that of the saline-treated group (p < 0.05, Fig. 3B), which indicated that scopolamine can induce recognition memory impairment. Pretreatment with 40 mg/kg or 80 mg/kg WGOS before administering scopolamine increased the time spent exploring the novel object and revealed a significantly higher discrimination index than scopolamine alone (p < 0.05, respectively, Fig. 3B). These results indicate that WGOS pretreatment (40 mg/kg or 80 mg/kg) markedly ameliorated scopolamine-induced recognition memory impairment. However, WGOS alone at either dose did not positively or negatively affect recognition memory because these animals explored the novel object for similar amounts of time, compared to the saline-treated group (p > 0.05, respectively, Fig. 3B).
Fig. 3.
Water-soluble ginseng oligosaccharides (WGOS) protect against scopolamine-induced recognition memory impairment in the novel object recognition task. (A) One-way analysis of variance shows that the total time of exploring both objects was not different. (B) By contrast, the discrimination index is significantly different between the groups. The Bonferroni's post hoc test reveals that the discrimination index is lower in scopolamine-treated mice and that this effect is attenuated by WGOS pretreatment during the retention session. Eighty-six mice were tested with 13–15 animals per group. Data are presented as mean ± SD. *p < 0.05, compared to the saline-treated mice. #p < 0.05, compared to the scopolamine group. SAL, saline; SCO, scopolamine; WGOS40/WGOS80, 40 mg/kg dose/80 mg/kg dose of water-soluble ginseng oligosaccharides.
3.3. Water-soluble ginseng oligosaccharides protect against scopolamine-induced hyperexpression of proinflammatory cytokines mRNA in the hippocampus
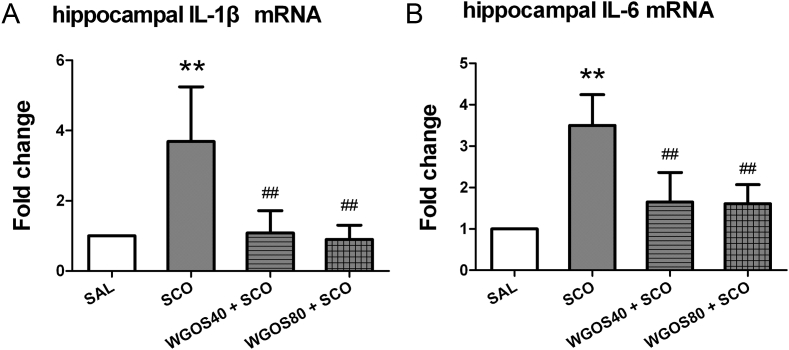
To determine whether the attenuation of scopolamine-induced behavior by WGOS was associated with antineuroinflammatory effects, we examined the mRNA expression levels of proinflammatory cytokines IL-1β and IL-6 in the hippocampus by using qPCR. The results are quantitated in Figs. 4A, 4B. The treatment significantly affected the expression of IL-1β mRNA [F (3, 14) = 11.10, p = 0.0005, Fig. 4A] and IL-6 mRNA [F (3, 14) = 14.56, p = 0.0001, Fig. 4B] with scopolamine markedly increasing the expression of IL-1β mRNA and IL-6 mRNA in the hippocampus, compared to the saline-treated mice (p < 0.01 for both factors). Pretreatment with WGOS (40 mg/kg or 80 mg/kg) prevented the scopolamine-induced hyperexpression of IL-1β mRNA (p < 0.01 for both doses) and IL-6 mRNA (p < 0.01 for both doses), and were similar to the levels in the saline-treated group (p > 0.05). These data indicate that WGOS pretreatment at either dose could attenuate the scopolamine-induced hyperexpression of proinflammatory cytokines IL-1β and IL-6 mRNA in the hippocampus.
Fig. 4.
Water-soluble ginseng oligosaccharides (WGOS) protect against scopolamine-induced hyperexpression of proinflammatory cytokines in the hippocampus. The levels of (A) IL-1β mRNA and (B) IL-6 mRNA in the hippocampus of each treatment group are quantitated and presented as the fold change. One-way analysis of variance shows that the treatment significantly affects mRNA expression of proinflammatory cytokines. Bonferroni's post hoc test reveals that WGOS pretreatment prevents the scopolamine-induced hyperexpression of IL-1β and IL-6 mRNA. Eighteen mice were used for mRNA analysis with 4–5 animals per group. Data are presented as mean ± SD. **p < 0.01, compared to the saline group. ##p < 0.01, compared to the scopolamine group. SAL, saline; SCO, scopolamine; WGOS40/WGOS80, 40 mg/kg dose/80 mg/kg dose of water-soluble ginseng oligosaccharides.
3.4. Water-soluble ginseng oligosaccharides suppress scopolamine-induced astrocyte activation in the hippocampus
Based on earlier results, we concluded that WGOS pretreatment at either dose (40 mg/kg or 80 mg/kg) had similar effects on behavior and proinflammatory cytokines levels. Therefore, rather than examining both doses, we only examined the effect of 40 mg/kg WGOS pretreatment on the expressions of GFAP, a marker for astrocyte activation. We examined the mRNA levels of GFAP using qPCR, the morphology, and number of GFAP-positive cells by immunohistochemistry in the hippocampus for three treatment groups: (1) the saline-treated controls, (2) the scopolamine-treated mice, and (3) the mice pretreated with 40 mg/kg of WGOS before scopolamine treatment.
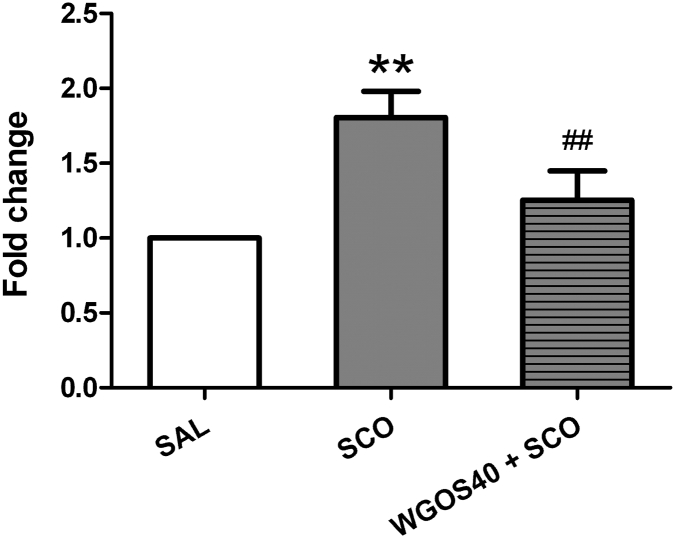
The mRNA levels of GFAP are quantitated in Fig. 5. The treatment significantly affected the expression of GFAP mRNA [F (2, 12) = 37.32, p < 0.0001] with scopolamine markedly increasing the expression of GFAP mRNA in the hippocampus, compared to the expression in the saline-treated mice (p < 0.01). Pretreatment with 40 mg/kg WGOS prevented the hyperexpression of GFAP mRNA induced by scopolamine (p < 0.01).
Fig. 5.
Water-soluble ginseng oligosaccharides (WGOS) prevent scopolamine-induced hyperexpression of glial fibrillary acid protein (GFAP) mRNA in the hippocampus. The levels of GFAP mRNA in the hippocampus of each treatment group were quantitated and are presented as the fold change. One-way analysis of variance shows that treatment significantly affected the expression of GFAP mRNA. The Bonferroni's post hoc test reveal that WGOS pretreatment prevents the hyperexpression of GFAP mRNA by scopolamine. The data are presented as mean ± SD. Fifteen mice were used for mRNA analysis with five animals per group. **p < 0.01, compared to the saline group. ##p < 0.01, compared to the scopolamine group. SAL, saline; SCO, scopolamine; WGOS40, 40 mg/kg dose of water-soluble ginseng oligosaccharides.
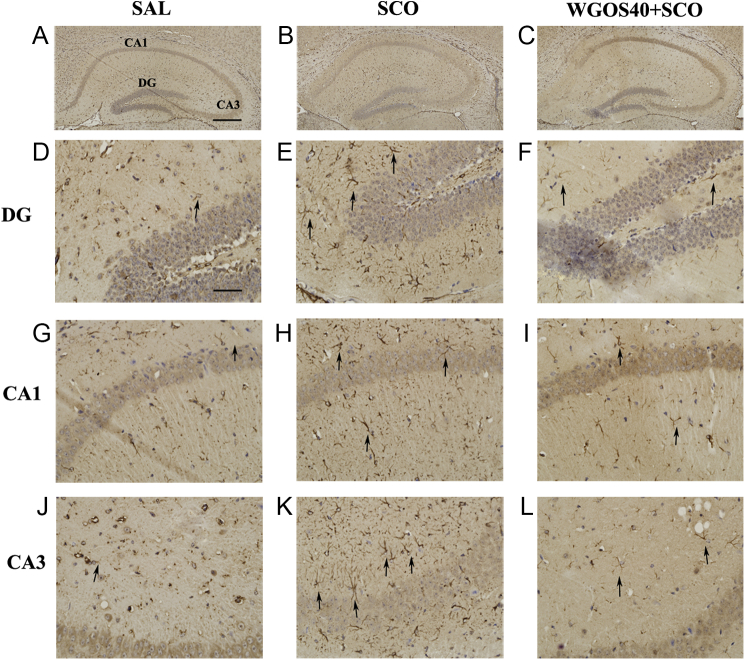
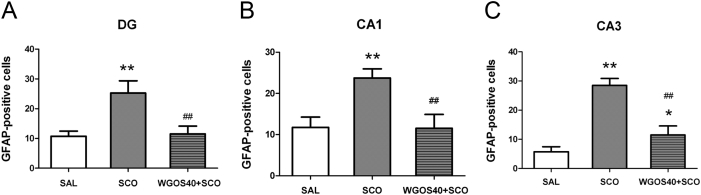
Fig. 6 shows representative images of GFAP-immunostained cells. In the saline-treated mice, GFAP-positive cells were rarely observed in any hippocampal subregion; however, when GFAP-positive cells were present, they were small and dispersed (Fig. 6D, 6G, 6J). For the scopolamine-treated mice, a large number of GFAP-positive cells were evident in all hippocampal subregions, and the cells were hypertrophied and condensed (Fig. 6E, 6H, 6K), which is indicative of astrocyte activation. Pretreatment with 40 mg/kg WGOS attenuated scopolamine-induced astrocyte activation in all hippocampal subregions (Fig. 6F, 6I, 6L). The number of activated astrocytes was significantly different in all three subregions: DG [F (2, 9) = 29.8, p = 0.0001], CA1 [F (2, 9) = 25.76, p = 0.0002], and CA3 [F (2, 9) = 92.01, p < 0.0001] (Fig. 7). In particular, the number of GFAP-positive cells in all three regions (i.e., DG, CA1, CA3) were significantly increased in the scopolamine-treated mice, compared to the saline-treated controls (p < 0.01, Fig.7). When the mice were pretreated with 40 mg/kg WGOS before scopolamine, the increase in the number of GFAP-positive cells triggered by scopolamine was significantly attenuated in all hippocampal subregions (p < 0.01 for all three subregions; Fig. 7), compared to scopolamine. In the DG and CA1, the levels were similar to those in the saline-treated group (p > 0.05 for both; Fig. 7A, 7B), but slightly elevated in the CA3 subregion (p < 0.05; Fig. 7C).
Fig. 6.
Water-soluble ginseng oligosaccharides (WGOS) prevent the scopolamine-induced activation of astrocytes in each hippocampal subregion. Representative images of glial fibrillary acid protein (GFAP)-immunopositive cells show the dorsal hippocampus in mice that received treatment with (A) saline, (B) scopolamine (3 mg/kg), or (C) WGOS (40 mg/kg) before scopolamine (3 mg/kg). The images are at 100× magnification. Higher resolution images of the morphology of astrocytes in the three hippocampal subregions (DG, CA1, and CA3) are below the original image. Scale bar is 200 μm in A–C and 50 μm in D–L. DG, dentate gyrus; SAL, saline; SCO, scopolamine; WGOS40, 40 mg/kg dose of water-soluble ginseng oligosaccharides.
Fig. 7.
Statistical analysis of glial fibrillary acid protein (GFAP)-positive cells in each hippocampal region. The mean number of GFAP-positive cells per section is counted in (A) the dentate gyrus (DG), (B) CA1, and (C) CA3 in mice that received treatment with saline, scopolamine (3 mg/kg), or WGOS (40 mg/kg) before scopolamine (3 mg/kg), respectively. One-way analysis of variance shows that treatment significantly affects the number of GFAP-positive cells. Bonferroni's post hoc test reveals that WGOS pretreatment significantly attenuates the scopolamine-induced increase in the number of GFAP-positive cells. Twelve mice were used with four animals per group. Data are presented as mean ± SD. *p < 0.05, **p < 0.01, compared to the saline-treated mice. #p < 0.05, ##p < 0.01, compared to the scopolamine-treated mice. SAL, saline; SCO, scopolamine; WGOS40, 40 mg/kg dose of water-soluble ginseng oligosaccharides.
4. Discussion
In the present study, we demonstrated for the first time the ability of WGOS (i.e., purified oligosaccharides from aqueous extract of the ginseng root) to protect against scopolamine-induced learning and memory deficits in the Morris water maze and novel object recognition task in mice.
The escape latency, the time spent in the platform quadrant, and the number of virtual platform crossings in the Morris water maze have been frequently used to evaluate the ability of spatial learning and memory. In our experiments, WGOS pretreatment attenuated the effects of scopolamine on spatial learning and memory, prevented the time delay in escape latency, and decreased the number of virtual platform crossings in the probe trial in scopolamine-treated mice. The time spent in the platform quadrant was not statistically different among the groups; however, this time was slightly increased in the WGOS +SCO group, compared to the scopolamine group. Because the number of virtual platform crossings can most precisely reflect the platform's location, we believe that WGOS can ameliorate the spatial learning and memory defect induced by scopolamine in mice.
To further confirm the effects of WGOS, we also examined the ability of WGOS to protect against scopolamine-induced recognition memory deficits by using the novel object recognition task. The results indicated that WGOS pretreatment also protected against recognition memory impairment caused by scopolamine, as indicated by a significantly higher discrimination index, which reflects good recognition memory.
Several studies have shown that oligosaccharides from various sources can attenuate learning and memory deficits in various animal models of AD by different mechanisms: (1) an acidic oligosaccharide from brown algae attenuated scopolamine-induced memory impairment in rats by exerting antioxidant activity [24]; (2) a heparin-derived oligosaccharide, C3, exerts a neuroprotective effect in cholinergic lesioned rats through reducing cholinergic damage [25]; and (3) cyclodextrins, which are cyclic oligosaccharides, improved spatial learning and memory deficits in Tg19959 mice and upregulated the expression of genes involved in cholesterol transport and Aβ clearance in cell and mouse models of AD [26]. To date, the only study using ginseng oligosaccharides is by Wang et al [20], who found that a mixture of oligosaccharides and peptides from Panax ginseng root administered to rats intraperitoneally could enhance memory activity in scopolamine-induced memory deficit, and enhance memory better than piracetam; however, they did not lucubrate the mechanisms. In the current study, we used pure ginseng oligosaccharides (i.e., WGOS) and examined the mechanism that protects against scopolamine-induced memory deficit in mice.
Neuroinflammation is an important mechanism that leads to neurodegenerative changes in the AD brain and activated glial cells (e.g., astrocytes and microglia). Proinflammatory cytokines are indicators of an inflammatory event in the brain. In the brains of AD patients and animal models, the Aβ-elicited expression of inflammatory genes such as IL-1β and IL-6 and inflammatory stimuli induced the signaling pathway of oxidative stress and cell death [27]. Chronic inflammation resulting from the recruitment of activated glial cells to Aβ plaques are an invariant component responsible for the induction of hippocampal neuronal damage [5], [28]. Therefore, controlling Aβ-stimulated neuroinflammation may be an important target for AD drugs, and active components from a variety of plants have been discussed as possible anti-inflammatory drug candidates for the treatment of AD [29].
Previous studies demonstrated that WGOS could exert antitumor activity via its immunomodulatory effects [19], which suggests that WGOS could function as an antineuroinflammatory agent and may be a potential treatment for AD. In this study, we used scopolamine to induce learning and memory deficits. The drug also induced a strong inflammatory response such as the hyperexpression of the proinflammatory cytokines IL-1β and IL-6 and astrocyte activation in the hippocampus. These results were consistent with previous reports in which scopolamine-induced dementia via damage to the cholinergic system was complicated by inflammation in the brain [13], [30]. The increased expression of IL-1β and IL-6 and astrocyte activation in the hippocampus that was evident in mice treated with scopolamine only was attenuated by pretreatment with WGOS. These results indicated that WGOS may protect against scopolamine-induced learning and memory deficits by regulating the inflammatory response in the brain, and suggests that this drug could have therapeutic value in the treatment of learning and memory deficits and various brain disease associated with neuroinflammation.
Studies have shown that some oligosaccharides that have a low molecular weight (MW) of 1,300–2,200 Da can enter the central nervous system [31], [32], although oligosaccharides were traditionally viewed as being difficult to pass through the blood–brain barrier (BBB). One study indicated that oligosaccharides cross the BBB, depending on their MW, and the process of transport is associated with glucose transporter 1 [33]. WGOS are composed of polymers of 2–14 glucose molecules with a lower MW ranging from 365 Da to 2,310 Da [17], [18], [19], which suggests that WGOS has the ability to cross the BBB and mediate their effects. By contrast, WGOS exerts an immunoregulatory effect in vivo [19]. Therefore, WGOS possibly prevent neuroinflammation through the immune system. Further studies are necessary to identify the mechanism by which WGOS exert their effects.
In summary, our experimental results demonstrated that WGOS pretreatment significantly protects against scopolamine-induced learning and memory deficits in the Morris water maze and in the novel object recognition task. Furthermore, these results indicate that WGOS may protect against learning and memory deficits by regulating the inflammatory response in the brain. This drug could also have a therapeutic value in the treatment of learning and memory deficits and various brain diseases associated with neuroinflammation.
Conflicts of interest
All authors have no conflicts of interests to declare regarding the publication of this paper.
Acknowledgements
This work was supported in part by the Natural Science Youth Foundation (Grant No. 20130522004JH) and by the Postdoctoral Foundation (Grant No. RB201332) of Jilin Province, China.
Contributor Information
Weihong Lin, Email: linweihong321@126.com.
Chunxiao Zhang, Email: chunxiao@jlu.edu.cn.
References
- 1.Kar S., Slowikowski S.P., Westaway D., Mount H.T. Interactions between beta-amyloid and central cholinergic neurons: implications for Alzheimer's disease. J Psychiatry Neurosci. 2004;29:427–441. [PMC free article] [PubMed] [Google Scholar]
- 2.Ibach B., Haen E. Acetylcholinesterase inhibition in Alzheimer's Disease. Curr Pharm Des. 2004;10:231–251. doi: 10.2174/1381612043386509. [DOI] [PubMed] [Google Scholar]
- 3.Golde T.E. Disease modifying therapy for AD? J Neurochem. 2006;99:689–707. doi: 10.1111/j.1471-4159.2006.04211.x. [DOI] [PubMed] [Google Scholar]
- 4.Perini G., Della-Bianca V., Politi V., Della Valle G., Dal-Pra I., Rossi F., Armato U. Role of p75 neurotrophin receptor in the neurotoxicity by beta-amyloid peptides and synergistic effect of inflammatory cytokines. J Exp Med. 2002;195:907–918. doi: 10.1084/jem.20011797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giovannini M.G., Scali C., Prosperi C., Bellucci A., Vannucchi M.G., Rosi S., Pepeu G., Casamenti F. Beta-amyloid-induced inflammation and cholinergic hypofunction in the rat brain in vivo: involvement of the p38MAPK pathway. Neurobiol Dis. 2002;11:257–274. doi: 10.1006/nbdi.2002.0538. [DOI] [PubMed] [Google Scholar]
- 6.Toro V.C., Tehranian R., Zetterström M., Eriksson G., Langel U., Bartfai T., Iverfeld K. Increased gene expression of interleukin-1alpha and interleukin-6 in rat primary glial cells induced by beta-amyloid fragment. J Mol Neurosci. 2001;17:341–350. doi: 10.1385/jmn:17:3:341. [DOI] [PubMed] [Google Scholar]
- 7.Mrak R.E., Griffin W.S. Interleukin-1, neuroinflammation, and Alzheimer's disease. Neurobiol Aging. 2001;22:903–908. doi: 10.1016/s0197-4580(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 8.Rubio-Perez J.M., Morillas-Ruiz J.M. A review: inflammatory process in Alzheimer's disease, role of cytokines. Scientific World Journal. 2012;2012:756357. doi: 10.1100/2012/756357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Craen A.J., Gussekloo J., Vrijsen B., Westendorp R.G. Meta-analysis of nonsteroidal antiinflammatory drug use and risk of dementia. Am J Epidemiol. 2005;161:114–120. doi: 10.1093/aje/kwi029. [DOI] [PubMed] [Google Scholar]
- 10.Vlad S.C., Miller D.R., Kowall N.W., Felson D.T. Protective effects of NSAIDs on the development of Alzheimer disease. Neurology. 2008;70:1672–1677. doi: 10.1212/01.wnl.0000311269.57716.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGeer P.L., McGeer E.G. NSAIDs and Alzheimer disease: epidemiological, animal model and clinical studies. Neurobiol Aging. 2007;28:639–647. doi: 10.1016/j.neurobiolaging.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Moon M., Kim H.G., Choi J.G., Oh H., Lee P.K., Ha S.K., Kim S.Y., Park Y., Huh Y., Oh M.S. 6-Shogaol, an active constituent of ginger, attenuates neuroinflammation and cognitive deficits in animal models of dementia. Biochem Biophys Res Commun. 2014;449:8–13. doi: 10.1016/j.bbrc.2014.04.121. [DOI] [PubMed] [Google Scholar]
- 13.Lee B., Jung K., Kim D.H. Timosaponin AIII, a saponin isolated from Anemarrhena asphodeloides, ameliorates learning and memory deficits in mice. Pharmacol Biochem Behav. 2009;93:121–127. doi: 10.1016/j.pbb.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Kim J., Kim S.H., Lee D.S., Lee D.J., Kim S.H., Chung S., Yang H.O. Effects of fermented ginseng on memory impairment and β-amyloid reduction in Alzheimer's disease experimental models. J Ginseng Res. 2013;37:100–107. doi: 10.5142/jgr.2013.37.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho I.H. Effects of Panax ginseng in neurodegenerative diseases. J Ginseng Res. 2012;36:342–353. doi: 10.5142/jgr.2012.36.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao Z., Kim Y.W., Wu Y., Zhang J., Lee J.H., Li X., Cho I.J., Park S.M., Jung D.H., Yang C.H. Korean Red Ginseng attenuates anxiety-like behavior during ethanol withdrawal in rats. J Ginseng Res. 2014;15:256–263. doi: 10.1016/j.jgr.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wan D.B., Jiao L.L., Yang H.M., Liu S.Y. Structural characterization and immunological activities of the water-soluble oligosaccharides isolated from the Panax ginseng roots. Planta. 2012;235:1289–1297. doi: 10.1007/s00425-011-1574-x. [DOI] [PubMed] [Google Scholar]
- 18.Jiao L., Wan D., Zhang X., Li B., Zhao H., Liu S. Characterization and immunostimulating effects on murine peritoneal macrophages of oligosaccharide isolated from Panax ginseng C.A. Meyer. J Ethnopharmacol. 2012;144:490–496. doi: 10.1016/j.jep.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Jiao L., Zhang X., Li B., Liu Z., Wang M., Liu S. Anti-tumour and immunomodulatory activities of oligosaccharides isolated from Panax ginseng C.A. Meyer. Int J Biol Macromol. 2014;65:229–233. doi: 10.1016/j.ijbiomac.2014.01.039. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y., Jiang R.Z., Li G.R., Chen Y.H., Luo H.M., Gao Y., Gao Q.P. Structural and enhanced memory activity studies of extracts from Panax ginseng root. Food Chem. 2010;119:969–973. [Google Scholar]
- 21.Han R.W., Zhang R.S., Chang M., Peng Y.L., Wang P., Hu S.Q., Choi C.L., Yin M., Wang R., Han Y.F. Reversal of scopolamine-induced spatial and recognition memory deficits in mice by novel multifunctional dimers bis-cognitins. Brain Res. 2012;1470:59–68. doi: 10.1016/j.brainres.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G., Franklin K.B.J. Academic Press; Waltham, MA: 2001. The mouse brain in stereotaxic coordinates. [Google Scholar]
- 23.Zhang C.X., Zhang H., Xu H.Y., Li M.X., Wang S. The lateral habenula is a common target of cocaine and dexamethasone. Neurosci Lett. 2013;555:12–17. doi: 10.1016/j.neulet.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Fan Y., Hu J., Li J., Yang Z., Xin X., Wang J., Ding J., Geng M. Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci Lett. 2005;374:222–226. doi: 10.1016/j.neulet.2004.10.063. [DOI] [PubMed] [Google Scholar]
- 25.Rose M., Dudas B., Cornelli U., Hanin I. Protective effect of the heparin-derived oligosaccharide C3, on AF64A-induced cholinergic lesion in rats. Neurobiol Aging. 2003;24:481–490. doi: 10.1016/s0197-4580(02)00093-3. [DOI] [PubMed] [Google Scholar]
- 26.Yao J., Ho D., Calingasan N.Y., Pipalia N.H., Lin M.T., Beal M.F. Neuroprotection by cyclodextrin in cell and mouse models of Alzheimer disease. J Exp Med. 2012;209:2501–2513. doi: 10.1084/jem.20121239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vukic V., Callaghan D., Walker D., Lue L.F., Liu Q.Y., Couraud P.O., Romero I.A., Weksler B., Stanimirovic D.B., Zhang W. Expression of inflammatory genes induced by beta-amyloid peptides in human brain endothelial cells and in Alzheimer's brain is mediated by the JNK-AP1 signaling pathway. Neurobiol Dis. 2009;34:95–106. doi: 10.1016/j.nbd.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwab C., McGeer P.L. Inflammatory aspects of Alzheimer disease and other neurodegenerative disorders. J Alzheimer's Dis. 2008;13:359–369. doi: 10.3233/jad-2008-13402. [DOI] [PubMed] [Google Scholar]
- 29.Millington C., Sonego S., Karunaweera N., Rangel A., Aldrich-Wright J.R., Campbell I.L., Gyengesi E., Münch G. Chronic neuroinflammation in Alzheimer's disease: new perspectives on animal models and promising candidate drugs. Biomed Res Int. 2014;2014:309129. doi: 10.1155/2014/309129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee B., Shim I., Lee H., Hahm D.H. Rehmannia glutinosa ameliorates scopolamine-induced learning and memory impairment in rats. J Microbiol Biotechnol. 2011;21:874–883. doi: 10.4014/jmb.1104.04012. [DOI] [PubMed] [Google Scholar]
- 31.Ma Q., Dudas B., Daud A., Iqbal O., Hoppensteadt D., Jeske W., Cornelli U., Lee J., Lorens S., Mervis R. Molecular and biochemical profiling of a heparin-derived oligosaccharide, C3. Thromb Res. 2002;105:303–309. doi: 10.1016/s0049-3848(01)00413-3. [DOI] [PubMed] [Google Scholar]
- 32.Guo X., Xin X., Gan L., Nie Q., Geng M. Determination of the accessibility of acidic oligosaccharide sugar chain to blood-brain barrier using surface plasmon resonance. Biol Pharm Bull. 2006;29:60–63. doi: 10.1248/bpb.29.60. [DOI] [PubMed] [Google Scholar]
- 33.Guo X., Geng M., Du G. Glucose transporter 1, distribution in the brain and in neural disorders: its relationship with transport of neuroactive drugs through the blood-brain barrier. Biochem Genet. 2005;43:175–187. doi: 10.1007/s10528-005-1510-5. [DOI] [PubMed] [Google Scholar]