Abstract
Background
Korean Red Ginseng (KRG) is a well-known natural product with anticarcinogenic and antioxidant effects. We evaluated the antifatigue effect of KRG in patients with nonalcoholic fatty liver disease (NAFLD).
Methods
Eighty patients with NAFLD were prospectively randomized to receive 3 wk of KRG or placebo in addition to counseling on healthy eating and regular exercise. Liver function test, proinflammatory cytokines, adiponectin, antioxidant activity, and fatigue score were measured and compared according to the body mass index between the KRG and placebo groups.
Results
The liver function tests were significantly improved after 3 wk of treatment in both groups. The mean levels (at baseline and after treatment) of tumor necrosis factor-α were 108.0 pg/mL ± 54.8 pg/mL and 92.7 pg/mL ± 39.0 pg/mL (p = 0.018) in the KRG group and 123.1 pg/mL ± 42.1 pg/mL and 127.5 pg/mL ± 62.2 pg/mL (p = 0.694) in the placebo group, respectively. There was a significant difference in change of adiponectin levels between the KRG (7,751.2 pg/mL ± 3,108.1 pg/mL and 8,197.3 pg/mL ± 2,714.5 pg/mL) and placebo groups (7,711.6 pg/mL ± 3,041.3 pg/mL and 7,286.1 pg/mL ± 5,188.7 pg/mL, p = 0.027). In patients with overweight, the fatigue score was significantly decreased in the KRG group (35.0 ± 13.2 and 24.5 ± 8.9, p = 0.019).
Conclusion
Our results show that KRG might be effective in reducing proinflammatory cytokine and fatigue in overweight patients with NAFLD, in addition to improvements in adiponectin levels.
Keywords: adiponectin, chronic hepatitis, fatty liver, Panax ginseng
1. Introduction
Fatty liver is defined as a condition in which triglycerides accumulate in hepatocytes to the extent that they comprise over 5% of the mass of the liver. It is divided into alcoholic fatty liver disease and nonalcoholic fatty liver disease (NAFLD) [1], [2]. NAFLD and nonalcoholic steatohepatitis are major global public health problems, because they are related to insulin resistance, obesity, and metabolic syndrome [3]. Most NAFLD patients show chronic fatigue with peripheral inflammation and immune activation, which causes serious social, economic, or medical problems and impairs physical function [4], [5].
The World Health Organization defines being overweight as having a body mass index (BMI) of 25 kg/m2 or more, whereas a BMI of 30 kg/m2 or more is considered to indicate obesity. Being overweight or obese significantly increases the risk of developing metabolic syndrome and liver disease [6]. In Western countries, 20–30% of NAFLD patients develop hepatocellular carcinoma [7]. Many overweight patients have been found to suffer from excessive daytime sleepiness [8]. Ginseng (Panax ginseng Meyer) root has been widely used as an herbal treatment in East Asia for more than 2,000 yr. Several studies have demonstrated that ginseng has anticarcinogenic, anti-inflammatory, and antioxidant activities, which were associated with fatigue syndrome [9], [10].
Ginsenoside Rb1, the most abundant ginsenoside in the herb, activates 5′-adenosine monophosphate-activated protein kinase, which suppresses the expression of genes that encode lipogenesis-inducing enzymes in rats with fatty liver disease [11]. Moreover, ginseng has been used to treat cancer-related fatigue without significant side effects [12]. Based on these findings, it is conceivable that ginseng might contribute to reduce fatigue by modulating inflammation and signal pathway in NAFLD patients. Some studies have demonstrated the antifatigue effect of ginseng [13], [14]. However, few studies have evaluated whether ginseng has a direct effect on fatigue resulting from liver disease or on the biomarker of liver disease. Therefore, in this study, we evaluated the anti-inflammatory antioxidant, and antifatigue activities of Korean Red Ginseng (KRG) in patients with NAFLD.
2. Materials and methods
2.1. Patients
Between April 2011 and August 2012, we conducted a single-blind, randomized, controlled clinical trial evaluating the efficacy of KRG (trial registration number: NCT02331589; http://clinicaltrials.gov/ct2/show/NCT02331589). Patients aged over 20 yr and with aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels of 50 IU/L or more were enrolled. Patients who had viral hepatitis, alcoholic hepatitis, autoimmune hepatitis, pancreatitis, hemochromatosis, Wilson's disease, drug-induced liver injury, or cancers were excluded. The study protocol conformed to the ethical guidelines of the 1975 Helsinki Declaration as reflected by a priori approval of the Institutional Review Board for Human Research in all participating hospitals. Informed consent for study participation was obtained from each patient.
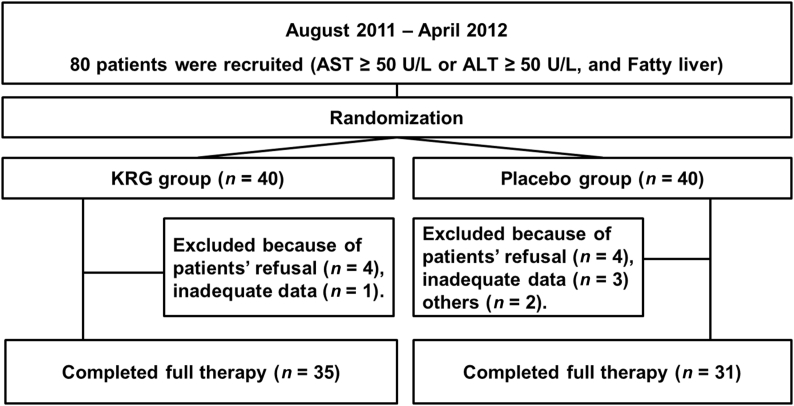
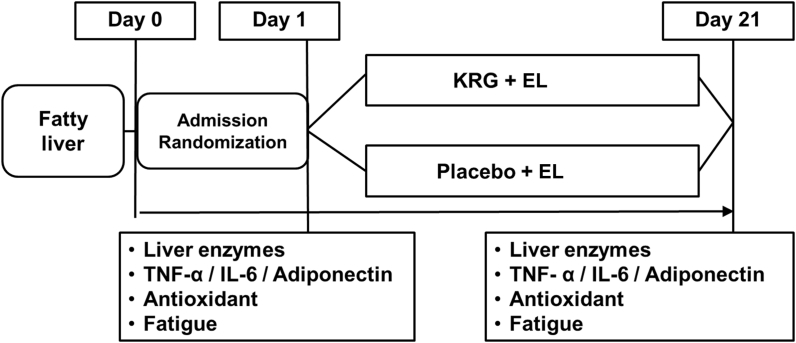
Randomization was performed using a computerized procedure.A total of 80 patients were enrolled. Among them, 14 patients (5 patients in the KRG group and 9 patients in the placebo group) were excluded (8 patients refused to participate, 4 patients had inadequate records, and 2 patients were consuming alcohol; Fig. 1). The levels of liver enzymes, proinflammatory cytokines [tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6)], adiponectin, antioxidant activity, and fatigue severity score were measured at the beginning of the study (baseline) and after 3 wk of medication (Fig. 2). We performed subgroup analysis by dividing the overweight and normal groups by BMI, because a previous report suggested that obesity was closely associated with fatigue [15].
Fig. 1.
Flow chart for enrollment. ALT, alanine aminotransferase; AST, aspartate aminotransferase; KRG, Korean Red Ginseng.
Fig. 2.
Study design. IL-6, interleukin-6; KRG, Korean Red Ginseng; TNF-α, tumor necrosis factor-α.
We conducted a baseline evaluation, which included obtaining data for the following: family history, BMI, abdominal ultrasound results, a complete blood count, a liver function test, and viral markers. Serum biochemical parameters included total bilirubin (TB), ALT, AST, gamma-glutamyl transpeptidase (γ-GT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), albumin, blood urea nitrogen, α1-antitrypsin, creatinine, α-fetoprotein, prothrombin time, blood glucose, triglycerides, total protein (TP), and total cholesterol. All patients were tested for several hepatitis viruses and human immunodeficiency virus. Hepatitis A virus was detected using antihepatitis A IgG and IgM antibodies, and hepatitis B virus was detected using the IgM antibody against the hepatitis B core antigen, the hepatitis B surface antigen, and the hepatitis B surface antibody. Hepatitis E virus was detected using antihepatitis E IgG and IgM antibodies, and hepatitis C virus was detected using antihepatitis C antibodies with or without the presence of hepatitis C RNA.
2.2. Medical treatment
All patients were treated with Silybum marianum (Legalon, Bukwang Pharmaceutical, Co., Ltd., Seoul, South Korea) capsule (450 mg/d) and all patients were advised by the same clinician (K.T.S.) to perform regular aerobic exercise for more than 30 min/d. In addition, all patients also received counseling on healthy eating. Patients who met the inclusion criteria were randomly assigned to receive a KRG capsule (ginsenosides Rg1 + Rb1 6.0 mg/g; 3,000 mg/d) or a placebo three times a day for 3 wk (Fig. 2). No other medication was prescribed. The placebos were manufactured at the Korea Ginseng Corporation (Seoul, South Korea), and resembled the KRG capsule powder in both shape and size.
2.3. Cytokines and adiponectin
For the measurements of cytokines and adiponectin, serum was processed using the TNF alpha human ELISA kit (ab100654; Abcam, Inc., Cambridge, United Kingdom), the IL-6 human ELISA kit (ab100572; Abcam, Inc.), and the adiponectin human ELISA kit (ab99968; Abcam, Inc.). All procedures were performed according to the manufacturer's instructions.
2.4. Antioxidant activity
A potentiometric method was used for the calculation of serum antioxidant activity. The value of antioxidant activity was measured as the redox potential difference of the K3[Fe(CN6)]/K4[Fe(CN6)] mediator system using an antioxidant activity measuring device (IVA Co. Ltd., Yekaterinburg, Russia). Concentrations of antioxidant activity were calculated using the following equation: antioxidant activity (ng/mL) = ±(Cox – αCred)/(1 + α), where Cox is the concentration of the oxidized form of the mediator (mol/L), Cred is the concentration of the reduced form of the mediator (mol/L); and , R is the gas constant, T is the temperature (K), n is the electron number (n = 1), F is the Faraday constant, and E and E1 are the potentials (V) of the mediator system before and after the addition of the serum, respectively.
In this study, the standard solution consisted of 0.01M K3[Fe(CN6)] and 0.1mM K4[Fe(CN6)] in a phosphate buffer solution (pH 7.2). To measure the redox potential within the solution, a platinum electrode and an Ag/AgCl electrode were used as the working electrode and the reference electrode, respectively. First, the value of E was measured for the 2 mL standard solution. Next, 0.4 mL of serum was added to the standard solution and the new redox potential E1 was measured. Based on the difference between E and E1, the value of antioxidant activity was quantitatively estimated using the aforementioned equation [16].
2.5. Fatigue Severity Scale
We used the Krupp Fatigue Severity Scale [17]. The survey had the following nine questions: (1) my motivation is lower when I am fatigued; (2) exercise brings on my fatigue; (3) I am easily fatigued; (4) fatigue interferes with my physical functioning; (5) fatigue causes frequent problems for me; (6) my fatigue prevents sustained physical functioning; (7) fatigue interferes with carrying out certain duties and responsibilities; (8) fatigue is among my three most disabling symptoms; and (9) fatigue interferes with my work, family, or social life. All patients scored each question on a 7-point scale (1 = strongly disagree, 4 = neither disagree nor agree, and 7 = strongly agree). All answers were collected and calculated by one research nurse in the Department of Internal Medicine.
2.6. Statistical analysis
This study is a clinical trial that evaluated fatigue and other outcomes in patients with nonalcoholic steatohepatitis. Therefore, we assumed the sample size by calculating with the difference of 0.7 and standard deviation (SD) of 1 in the fatigue score. A total of 60 patients were needed for this study. The primary outcome was change in liver enzyme levels and fatigue scale score at 3 wk. The secondary outcomes were improvement of cytokines, adiponectin, and antioxidant activities.
All data were expressed as mean ± SD unless otherwise stated. Significance was evaluated with a paired t test, independent samples t test, and analysis of covariance. Statistical significance was observed when p < 0.05. Data obtained from routine blood tests were analyzed with statistical software (SPSS, version 19.0, SPSS, Inc., Chicago, IL, USA) and GraphPad Prism version 6.0 for Windows (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Patient characteristics
Sixty-six patients (35 patients in the KRG group and 31 in the placebo group) completed the study. The mean age was 49.4 yr ± 12.2 yr and 50 patients (75.8%) were men. The patients' baseline characteristics are outlined in Table 1. Fifty-six patients (84.9%) had been diagnosed with fatty liver and acute hepatitis. The mean serum levels of AST, ALT, LDH, and cholesterol were significantly decreased after 3 wk (Table 1).
Table 1.
Clinical characteristics of patients
| Variables | All patients (n = 66) |
|---|---|
| Male | 56 (74.7) |
| Age (yr) | 47.8 ± 14.9 |
| BMI (kg/m2) | 27.9 ± 4.9 |
| Day 1 | Day 21 | p | |
|---|---|---|---|
| AST (IU/L) | 55.3 ± 33.7 | 41.7 ± 24.5 | < 0.001 |
| ALT (IU/L) | 81.3 ± 38.5 | 60.7 ± 36.8 | < 0.001 |
| γ-GT (IU/L) | 122.5 ± 145.7 | 107.8 ± 208.2 | 0.243 |
| LDH (IU/L) | 223.0 ± 76.0 | 201.3 ± 39.1 | 0.035 |
| TB (mg/dL) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.292 |
| Cholesterol (mg/dL) | 195.6 ± 66.8 | 182.8 ± 46.7 | 0.010 |
| ALP (IU/L) | 90.3 ± 42.2 | 87.2 ± 38.0 | 0.273 |
| Albumin (g/dL) | 4.5 ± 0.4 | 4.6 ± 0.5 | 0.695 |
| Total protein (g/dL) | 7.1 ± 0.5 | 7.1 ± 0.5 | 0.842 |
| Glucose (mg/dL) | 131.1 ± 67.0 | 125.3 ± 42.5 | 0.391 |
Data are presented as n (%) or mean ± SD.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; LDH, lactate dehydrogenase; TB, total bilirubin; γ-GT, gamma-glutamyl transferase.
3.2. Blood test
In the KRG group, the mean (from baseline to after 3 wk) levels of AST (from 52.1 IU/L ± 30.6 IU/L to 37.3 IU/L ± 14.5 IU/L), ALT (from 81.0 IU/L ± 43.7 IU/L to 56.9 IU/L ± 36.5 IU/L), and γ-GT (from 106.9 IU/L ± 68.6 IU/L to 81.3 IU/L ± 56.7 IU/L) improved after KRG therapy (p < 0.05). However, the levels of LDH, TB, cholesterol, ALP, albumin, TP, and glucose were not significantly different from the baseline to after 3 wk of KRG therapy (Table 2). Patients with a BMI of 25 kg/m2 or more who underwent KRG therapy showed particularly improved mean levels of ALT (from 83.7 IU/L ± 47.9 IU/L to 55.8 IU/L ± 24.6 IU/L) and γ-GT (from 112.6 IU/L ± 71.2 IU/L to 79.1 IU/L ± 63.9 IU/L; p < 0.05). Although patients in the KRG group with a BMI less than 25 kg/m2 did show improved mean levels of ALT (from 69.5 IU/L ± 23.3 IU/L to 57.3 IU/L ± 55.0 IU/L) and γ-GT (from 97.4 IU/L ± 73.4 IU/L to 83.5 IU/L ± 47.2 IU/L), the differences were not statistically significant (p > 0.05; Table 3).
Table 2.
Changes in liver function tests
| KRG (n = 35) |
Placebo (n = 31) |
p∗ | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post | p | Pre | Post | p | ||
| BMI (kg/m2) | 26.8 ± 3.8 | 27.4 ± 4.7 | 0.610 | ||||
| AST (IU/L) | 52.1 ± 30.6 | 37.3 ± 14.5 | 0.024 | 59.1 ± 37.2 | 47.1 ± 32.2 | 0.008 | 0.171 |
| ALT (IU/L) | 81.0 ± 43.7 | 56.9 ± 36.5 | 0.013 | 81.6 ± 32.2 | 65.1 ± 37.2 | 0.026 | 0.374 |
| γ-GT (IU/L) | 106.9 ± 68.6 | 81.3 ± 56.7 | 0.012 | 139.3 ± 198.2 | 136.4 ± 294.3 | 0.902 | 0.574 |
| LDH (IU/L) | 226.7 ± 99.0 | 200.3 ± 35.4 | 0.135 | 218.6 ± 34.1 | 202.5 ± 43.8 | 0.063 | 0.748 |
| TB (mg/dL) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.939 | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.045 | 0.081 |
| Cholesterol (mg/dL) | 197.4 ± 39.1 | 186.1 ± 31.4 | 0.110 | 193.6 ± 88.2 | 179.4 ± 59.4 | 0.043 | 0.493 |
| ALP (IU/L) | 92.2 ± 54.0 | 86.7 ± 45.2 | 0.209 | 88.1 ± 22.2 | 87.8 ± 28.0 | 0.925 | 0.414 |
| Albumin (g/dL) | 4.6 ± 0.3 | 4.7 ± 0.6 | 0.305 | 4.5 ± 0.4 | 4.4 ± 0.4 | 0.190 | 0.108 |
| TP (g/dL) | 7.1 ± 0.4 | 7.2 ± 0.5 | 0.507 | 7.1 ± 0.6 | 7.0 ± 0.6 | 0.240 | 0.125 |
| Glucose (mg/dL) | 125.9 ± 64.5 | 119.8 ± 39.5 | 0.470 | 137.1 ± 70.4 | 131.6 ± 45.7 | 0.621 | 0.399 |
Data are presented as mean ± SD.
∗ comparison between KRG and placebo.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; KRG, Korean Red Ginseng; LDH, lactate dehydrogenase; TB, total bilirubin; TP, total protein; γ-GT, gamma-glutamyl transferase.
Table 3.
Changes in liver function tests in difference of BMI
| BMI ≥ 25 kg/m2 | |||||||
|---|---|---|---|---|---|---|---|
| KRG (n = 24) |
Placebo (n = 18) |
p∗ | |||||
| Pre | Post | p | Pre | Post | p | ||
| BMI (kg/m2) | 28.3 ± 3.1 | 29.8 ± 4.0 | 0.214 | ||||
| AST (IU/L) | 55.5 ± 35.4 | 37.9 ± 13.9 | 0.053 | 51.4 ± 26.1 | 48.3 ± 30.7 | 0.438 | 0.119 |
| ALT (IU/L) | 83.7 ± 47.9 | 55.8 ± 24.6 | 0.031 | 76.6 ± 24.4 | 70.4 ± 37.8 | 0.482 | 0.146 |
| γ-GT (IU/L) | 112.6 ± 71.2 | 79.1 ± 63.9 | 0.024 | 148.3 ± 235.1 | 166.0 ± 381.9 | 0.661 | 0.214 |
| LDH (IU/L) | 233.6 ± 113.8 | 201.7 ± 35.9 | 0.192 | 212.8 ± 33.0 | 207.3 ± 54.2 | 0.661 | 0.567 |
| TB (mg/dL) | 0.7 ± 0.2 | 0.7 ± 0.2 | 0.827 | 0.7 ± 0.2 | 0.6 ± 0.2 | 0.007 | 0.030 |
| Cholesterol (mg/dL) | 201.3 ± 37.7 | 183.2 ± 31.8 | 0.066 | 194.1 ± 45.9 | 180.1 ± 36.3 | 0.051 | 0.001 |
| ALP (IU/L) | 96.3 ± 64.0 | 86.7 ± 53.4 | 0.113 | 83.7 ± 22.0 | 87.9 ± 35.3 | 0.449 | 0.155 |
| Albumin (g/dL) | 4.5 ± 0.2 | 4.6 ± 0.6 | 0.540 | 4.6 ± 0.3 | 4.6 ± 0.4 | 0.326 | 0.399 |
| TP (g/dL) | 7.1 ± 0.3 | 7.1 ± 0.4 | 0.795 | 7.2 ± 0.5 | 7.2 ± 0.4 | 0.704 | 0.799 |
| Glucose (mg/dL) |
126.7 ± 71.1 |
124.4 ± 43.0 |
0.843 |
134.8 ± 70.0 |
125.4 ± 43.9 |
0.456 |
0.827 |
| BMI < 25 kg/m2 | |||||||
| KRG (n = 11) |
Placebo (n = 13) |
p∗ | |||||
| Pre |
Post |
p |
Pre |
Post |
p |
||
| BMI (kg/m2) | 22.8 ± 2.6 | 22.9 ± 1.2 | 0.965 | ||||
| AST (IU/L) | 38.6 ± 5.5 | 36.1 ± 14.9 | 0.668 | 54.1 ± 25.2 | 36.8 ± 19.2 | 0.054 | 0.519 |
| ALT (IU/L) | 69.5 ± 23.3 | 57.3 ± 55.0 | 0.501 | 76.6 ± 36.6 | 48.6 ± 24.6 | 0.083 | 0.593 |
| γ-GT (IU/L) | 97.4 ± 73.4 | 83.5 ± 47.2 | 0.236 | 65.1 ± 49.1 | 55.9 ± 49.9 | 0.545 | 0.645 |
| LDH (IU/L) | 220.5 ± 62.2 | 197.0 ± 40.1 | 0.393 | 218.1 ± 28.1 | 199.1 ± 30.0 | 0.026 | 0.897 |
| TB (mg/dL) | 0.7 ± 0.3 | 0.6 ± 0.2 | 0.413 | 0.6 ± 0.2 | 0.7 ± 0.2 | 0.592 | 0.585 |
| Cholesterol (mg/dL) | 185.0 ± 47.0 | 182.9 ± 27.1 | 0.822 | 147.9 ± 47.1 | 148.8 ± 30.9 | 0.911 | 0.067 |
| ALP (IU/L) | 80.9 ± 20.2 | 87.5 ± 22.1 | 0.109 | 93.4 ± 27.2 | 83.9 ± 18.4 | 0.129 | 0.059 |
| Albumin (g/dL) | 4.7 ± 0.4 | 4.9 ± 0.4 | 0.433 | 4.2 ± 0.4 | 4.2 ± 0.5 | 0.468 | 0.057 |
| TP (g/dL) | 7.2 ± 0.6 | 7.5 ± 0.6 | 0.259 | 6.9 ± 0.7 | 6.6 ± 0.7 | 0.139 | 0.037 |
| Glucose (mg/dL) | 129.5 ± 55.7 | 111.6 ± 33.1 | 0.124 | 124.5 ± 33.1 | 137.1 ± 50.4 | 0.536 | 0.168 |
Data are presented as mean ± SD.
∗ comparison between KRG and placebo.
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; KRG, Korean Red Ginseng; LDH, lactate dehydrogenase; TB, total bilirubin; TP, total protein; γ-GT, gamma-glutamyl transferase.
In the placebo group, the mean levels of AST (from 59.1 IU/L ± 37.2 IU/L to 47.1 IU/L ± 32.2 IU/L) and ALT (from 81.6 IU/L ± 32.2 IU/L to 65.1 IU/L ± 37.2 IU/L) improved after placebo therapy (p < 0.05). However, the levels of γ-GT, LDH, ALP, albumin, TP, and glucose did not show significant changes after 3 wk. There were significant changes in the levels of TB and cholesterol, but these were still within the normal range (Table 2).
3.3. Cytokines and adiponectin
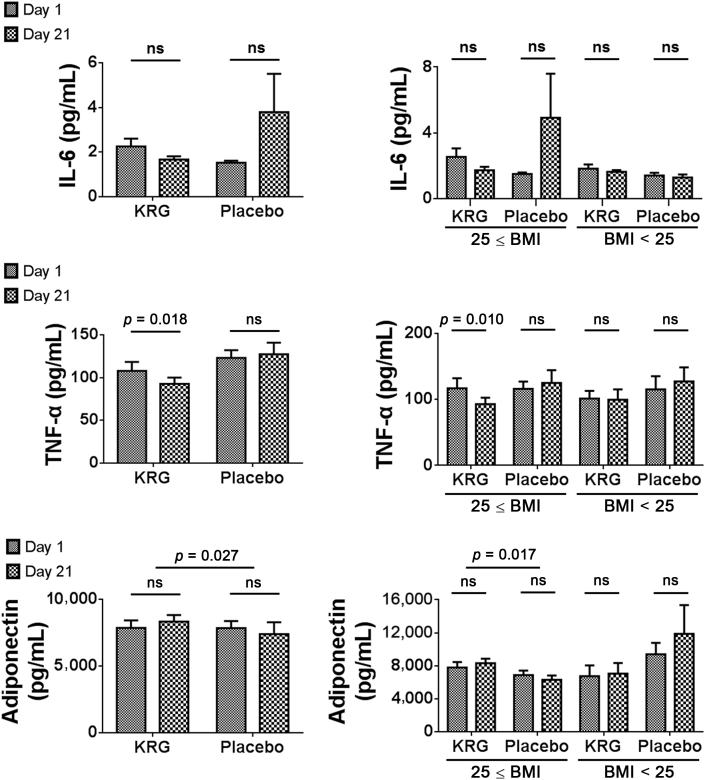
The mean IL-6 levels from baseline to after 3 wk changed as follows: from 2.3 ng/mL ± 1.9 ng/mL to 1.7 ng/mL ± 0.7 ng/mL (p = 0.141) in the KRG group and from 1.5 ng/mL ± 0.4 ng/mL to 3.8 ng/mL ± 8.0 ng/mL (p = 0.209) in the placebo group. The two groups did not show a statistically significant difference with regard to the change in IL-6 levels (p = 0.227; Fig. 3).
Fig. 3.
Changes in cytokines and adiponectin. There was a significant difference in the change in TNF-α and adiponectin levels between the KRG and placebo groups. The mean TNF-α and adiponectin levels in patients with a BMI of 25 kg/m2 or more showed particular improvement after KRG therapy. BMI, body mass index; IL-6, interleukin-6; KRG, Korean Red Ginseng; ns, not significant; TNF-α, tumor necrosis factor-α.
The mean TNF-α levels from baseline to after 3 wk changed as follows: from 108.0 pg/mL ± 54.8 pg/mL to 92.7 pg/mL ± 39.0 pg/mL (p = 0.018) in the KRG group and from 123.1 pg/mL ± 42.1 pg/mL to 127.5 pg/mL ± 62.2 pg/mL (p = 0.694) in the placebo group. There was a significant difference in the change in TNF-α levels between the KRG and placebo groups (p = 0.031; Fig. 3). The mean TNF-α levels in patients with a BMI of 25 kg/m2 or more showed particular improvement after KRG therapy (116.8 pg/mL ± 63.7 pg/mL to 92.6 pg/mL ± 41.6 pg/mL; p = 0.010). However, in patients with a BMI less than 25 kg/m2 who received KRG therapy, the mean TNF-α levels changed from 101.1 pg/mL ± 31.0 pg/mL to 99.5 pg/mL ± 41.1 pg/mL, which was not a statistically significant change (p = 0.824; Fig. 3).
The mean adiponectin levels from baseline to after 3 wk changed as follows: from 7,751.2 pg/mL ± 3,108.1 pg/mL to 8,197.3 pg/mL ± 2,714.5 pg/mL (p = 0.109) in the KRG group and from 7,711.6 pg/mL ± 3,041.3 pg/mL to 7,286.1 pg/mL ± 5,188.7 pg/mL (p = 0.717) in the placebo group. There was a significant difference in the change of adiponectin levels between the KRG and placebo groups (p = 0.027; Fig. 3). This was particularly noticeable in patients with a BMI of 25 kg/m2 or more, in whom the mean adiponectin levels changed from 7,799.6 pg/mL ± 3,003.3 pg/mL to 8,326.9 pg/mL ± 2,445.2 pg/mL (p = 0.186) in the KRG group and from 6,869.5 pg/mL ± 2,176.8 pg/mL to 6,306.5 pg/mL ± 2,052.0 pg/mL (p = 0.373) in the placebo group. There was a significant difference in the change of adiponectin levels in patients with a BMI of 25 kg/m2 or more within the KRG group from baseline to 3 wk of treatment (p = 0.017; Fig. 3).
3.4. Antioxidant activity
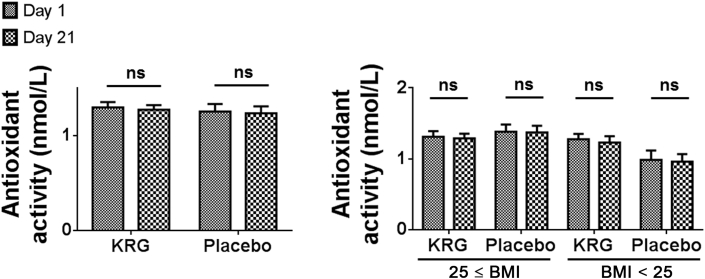
The mean antioxidant activity from baseline to after 3 wk changed as follows: from 1.3 ng/mL ± 0.3 ng/mL to 1.3 ng/mL ± 0.3 ng/mL (p = 0.467) in the KRG group and from 1.3 ng/mL ± 0.4 ng/mL to 1.3 ng/mL ± 0.4 ng/mL (p = 0.592) in the placebo group. No statistically significant difference in the change of antioxidant activity was observed between the two groups (p = 0.916; Fig. 4).
Fig. 4.
Changes in antioxidant activity. No statistically significant difference in the change of antioxidant activity was observed between the two groups. BMI, body mass index; KRG, Korean Red Ginseng; ns, not significant.
3.5. Fatigue Severity Scale
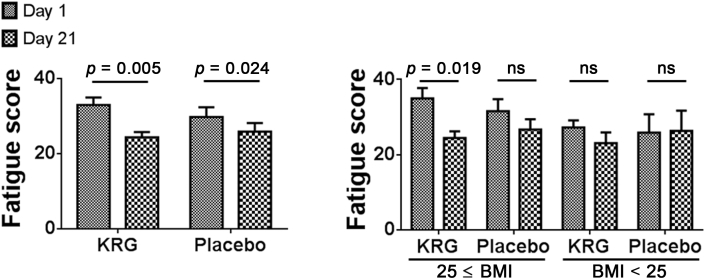
The mean fatigue scores from baseline to after 3 wk changed as follows: from 33.0 ± 11.6 to 24.3 ± 8.4 (p < 0.001) in the KRG group and from 29.8 ± 14.1 to 25.9 ± 12.5 (p = 0.024) in the placebo group. No statistically significant difference was found in the change of fatigue scores between the two groups (p = 0.221; Fig. 5). In patients with a BMI of 25 kg/m2 or more who received KRG treatment, the mean fatigue scores showed particular improvement (from 35.0 ± 13.2 to 24.5 ± 8.9; p = 0.003). However, no significant change was observed in patients with a BMI less than 25 kg/m2 in the KRG group, who had mean fatigue scores of 27.3 ± 5.3 at the baseline and 23.1 ± 8.0 at 3 wk (p = 0.166; Fig. 5).
Fig. 5.
Changes in Fatigue Severity Scale. In patients with a BMI of 25 kg/m2 or more who received KRG treatment, the mean fatigue scores showed particular improvement. BMI, body mass index; KRG, Korean Red Ginseng; ns, not significant.
4. Discussion
Our study demonstrated that fatigue severity significantly decreased between Day 0 and Day 21 in both the KRG and placebo groups. Interestingly, only overweight patients in the KRG group experienced a more significant decrease in their fatigue scores. A previous report suggested that P. ginseng Meyer is effective in treating idiopathic chronic fatigue patients [18]. Until now, however, no study has assessed the efficacy of P. ginseng Meyer in treating fatigue in overweight patients. Based on the results of this study, it is concluded that KRG is effective in treating fatigue in NAFLD patients, especially in overweight patients.
Outpatients follow-up in this study was performed by one clinician, who advised the patients to exercise regularly and eat healthily, and prescribed Legalon (mainly S. marianum) to all patients. Previously, lifestyle modifications involving diet and exercise reduced the body weight of NAFLD patients to 5% [19]. The NAFLD activity scores improved in 67% of NAFLD patients after 2 yr of exercise and diet modification [20]. S. marianum has been used in many countries to treat patients with various liver diseases [21], [22]. Our study also revealed the improvement in serum biochemical parameters (AST and ALT) for patients in the placebo group who received counseling for lifestyle modifications and Legalon. Taken together, lifestyle modifications and S. marianum might be recommended for NAFLD patients for the improvement of hepatitis.
In the KRG group, biochemical parameters such as AST, ALT, and γ-GT were significantly decreased after 3 wk of KRG treatment. We found that the decreases in ALT and γ-GT levels were more significant in patients with a BMI of 25 kg/m2 or more in the KRG group than among overweight patients in the placebo group. Previous research has shown that P. ginseng improves insulin resistance and decreases ALT levels in rats with liver fibrosis [23], [24]. Other studies have demonstrated that KRG extract decreases γ-GT levels in rats with alcohol-induced liver damage [25]. Results from previous studies and this study suggest that KRG alleviates hepatotoxicity, and is especially effective in overweight patients.
Adiponectin levels are related to metabolic syndrome and fatty liver disease [26]. Previous research has shown that treating adipocyte cells with P. ginseng extract increases adiponectin levels [27]. Similar results were found in studies using mice fed with a high-fat diet and treated with KRG. Increased adiponectin levels were observed in the serum of treated mice that were fed a high-fat diet [28]. Our study demonstrated that serum adiponectin levels increased in the KRG group, suggesting that KRG is an effective treatment for fatty liver disease.
KRG has been recognized as a natural substance with anti-inflammatory effects. Quan et al [29] demonstrated that streptozotocin-induced diabetic rats treated with 100 mg/kg of KRG showed lower serum TNF-α levels. Mice with acute liver failure have increased plasma TNF-α levels, which decrease after treatment with ginsenoside Rg1 [30]. In addition, it has been shown that ginsan (isolated from aqueous Korean P. ginseng extract) therapy can reduce TNF-α levels in healthy volunteers [31]. Based on these results, we suggest that KRG has significant anti-inflammatory activity.
It was reported that structures produced by adipocytes are homologous to adiponectin and TNF-α and are antagonistic to the insulin-modulating effects of these compounds [26], [32]. In this study, patients with a BMI of 25 kg/m2 or more showed a significant increase in serum adiponectin levels compared to patients with a BMI less than 25 kg/m2. In addition, overweight patients treated with KRG had significantly decreased TNF-α levels. In light of these findings, we suggest that KRG may be a useful therapeutic agent for treating fatty liver disease, especially in overweight patients.
We did not observe changes in the IL-6 level. However, IL-6 levels have been shown to decrease in lipopolysaccharide-treated THP-1 macrophages treated with Panax notoginseng [33]. Another study found that P. notoginseng saponins downregulated IL-6 in rats with liver fibrosis [33]. For the evaluation of exact mechanism of KRG, more research is required to further investigate the therapeutic effect of KRG on IL-6 levels.
One limitation of our study is that we did not obtain a liver biopsy from each patient. Instead, we selected patients who had been diagnosed with fatty liver and hepatitis by blood tests, a medical history, and abdominal ultrasound. We followed the clinical practice guidelines of the Korean Association for the Study of the Liver, which indicated that NAFLD can be diagnosed by abnormal findings on liver function tests, along with a medical history, serological testing, and abdominal ultrasonography [34].
In conclusion, KRG reduced TNF-α serum levels and increased adiponectin serum levels in patients with NAFLD. KRG has anti-inflammatory effects, and is especially effective in overweight patients. Based on these results, we conclude that it is effective to treat overweight patients with NAFLD with S. marianum, nutrition and exercise therapy, and KRG.
Conflicts of interest
The authors declare that there is no conflict of interest, including relevant financial interests, activities, relationships, affiliations, and any other conflict of interest as explicitly and implicitly expressed in the Editorial Policies for Authors.
Acknowledgments
We would like to thank C.W. Kim, Y.J. Lee (Department of Internal Medicine, Hallym University College of Medicine), who made this study possible. This research was supported by a grant from the Korea Society of Ginseng funded by Korea Ginseng Corporation (Korean Red Ginseng; 2011), by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2010-0021482), and Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ009859) Rural Development Administration, Republic of Korea. This research was supported by Hallym University Research Fund.
Footnotes
ClinicalTrials.gov: Trial Registration number NCT02331589 (http://clinicaltrials.gov/ct2/show/NCT02331589).
Authors' contributions
M.H., Y.H.L., and S.K. contributed equally to this study. M.H., Y.H.L., S.K. contributed to the analysis, interpretation, collection, and assembly of data, as well as to the drafting of this article; K.T.S. contributed to the conception and design, critical revision of the article for important intellectual content, and final approval of the article; other authors contributed to the provision of study materials or patients.
References
- 1.Brunt E.M. Nonalcoholic fatty liver disease: what the pathologist can tell the clinician. Dig Dis. 2012;30:61–68. doi: 10.1159/000341127. [DOI] [PubMed] [Google Scholar]
- 2.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., Ferrell L.D., Liu Y.C., Torbenson M.S., Unalp-Arida A. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 3.Review Team, LaBrecque D.R., Abbas Z., Anania F., Ferenci P., Khan A.G., Goh K.L., Hamid S.S., Isakov V., Lizarzabal M. World Gastroenterology Organisation global guidelines: nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Clin Gastroenterol. 2014;48:467–473. doi: 10.1097/MCG.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 4.Newton J.L., Jones D.E., Henderson E., Kane L., Wilton K., Burt A.D., Day C.P. Fatigue in non-alcoholic fatty liver disease (NAFLD) is significant and associates with inactivity and excessive daytime sleepiness but not with liver disease severity or insulin resistance. Gut. 2008;57:807–813. doi: 10.1136/gut.2007.139303. [DOI] [PubMed] [Google Scholar]
- 5.Morris G., Berk M., Walder K., Maes M. Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med. 2015;13:28. doi: 10.1186/s12916-014-0259-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marchesini G., Bugianesi E., Forlani G., Cerrelli F., Lenzi M., Manini R., Natale S., Vanni E., Villanova N., Melchionda N. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37:917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 7.Scalera A., Tarantino G. Could metabolic syndrome lead to hepatocarcinoma via non-alcoholic fatty liver disease? World J Gastroenterol. 2014;20:9217–9228. doi: 10.3748/wjg.v20.i28.9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarosz P.A., Davis J.E., Yarandi H.N., Farkas R., Feingold E., Shippings S.H., Smith A.L., Williams D. Obesity in urban women: associations with sleep and sleepiness, fatigue and activity. Womens Health Issues. 2014;24:e447–e454. doi: 10.1016/j.whi.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Hong S.H., Suk K.T., Choi S.H., Lee J.W., Sung H.T., Kim C.H., Kim E.J., Kim M.J., Han S.H., Kim M.Y. Anti-oxidant and natural killer cell activity of Korean Red Ginseng (Panax ginseng) and urushiol (Rhus vernicifera Stokes) on non-alcoholic fatty liver disease of rat. Food Chem Toxicol. 2013;55:586–591. doi: 10.1016/j.fct.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 10.Bang C.S., Hong S.H., Suk K.T., Kim J.B., Han S.H., Sung H., Kim E.J., Kim M.J., Kim M.Y., Baik S.K. Effects of Korean Red Ginseng (Panax ginseng), urushiol (Rhus vernicifera Stokes), and probiotics (Lactobacillus rhamnosus R0011 and Lactobacillus acidophilus R0052) on the gut-liver axis of alcoholic liver disease. J Ginseng Res. 2014;38:167–172. doi: 10.1016/j.jgr.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen L., Xiong Y., Wang D.Q., Howles P., Basford J.E., Wang J., Xiong Y.Q., Hui D.Y., Woods S.C., Liu M. Ginsenoside Rb1 reduces fatty liver by activating AMP-activated protein kinase in obese rats. J Lipid Res. 2013;54:1430–1438. doi: 10.1194/jlr.M035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barton D.L., Liu H., Dakhil S.R., Linquist B., Sloan J.A., Nichols C.R., McGinn T.W., Stella P.J., Seeger G.R., Sood A. Wisconsin Ginseng (Panax quinquefolius) to improve cancer-related fatigue: a randomized, double-blind trial, N07C2. J Natl Cancer Inst. 2013;105:1230–1238. doi: 10.1093/jnci/djt181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginseng helps cancer-related fatigue. Mayo Clin Health Lett. 2012;30:4. [PubMed] [Google Scholar]
- 14.Choi J.Y., Woo T.S., Yoon S.Y., Ike Campomayor Dela P., Choi Y.J., Ahn H.S., Lee Y.S., Yu G.Y., Cheong J.H. Red ginseng supplementation more effectively alleviates psychological than physical fatigue. J Ginseng Res. 2011;35:331–338. doi: 10.5142/jgr.2011.35.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katz P., Margaretten M., Trupin L., Schmajuk G., Yazdany J., Yelin E. Sleep disturbance, depression, obesity, and physical inactivity explain a significant portion of fatigue in rheumatoid arthritis. Arthritis Care Res. 2016;68:81–90. doi: 10.1002/acr.22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brainina K.h.Z., Ivanova A.V., Sharafutdinova E.N., Lozovskaya E.L., Shkarina E.I. Potentiometry as a method of antioxidant activity investigation. Talanta. 2007;71:13–18. doi: 10.1016/j.talanta.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Krupp L.B., LaRocca N.G., Muir-Nash J., Steinberg A.D. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 18.Kim H.G., Cho J.H., Yoo S.R., Lee J.S., Han J.M., Lee N.H., Ahn Y.C., Son C.G. Antifatigue effects of Panax ginseng C.A. Meyer: a randomised, double-blind, placebo-controlled trial. PloS One. 2013;8:e61271. doi: 10.1371/journal.pone.0061271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin Y.J., Kim K.M., Hwang S., Lee S.G., Ha T.Y., Song G.W., Jung D.H., Kim K.H., Yu E., Shim J.H. Exercise and diet modification in non-obese non-alcoholic fatty liver disease: analysis of biopsies of living liver donors. J Gastroenterol Hepatol. 2012;27:1341–1347. doi: 10.1111/j.1440-1746.2012.07165.x. [DOI] [PubMed] [Google Scholar]
- 20.Promrat K., Kleiner D.E., Niemeier H.M., Jackvony E., Kearns M., Wands J.R., Fava J.L., Wing R.R. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ball K.R., Kowdley K.V. A review of Silybum marianum (milk thistle) as a treatment for alcoholic liver disease. J Clin Gastroenterol. 2005;39:520–528. doi: 10.1097/01.mcg.0000165668.79530.a0. [DOI] [PubMed] [Google Scholar]
- 22.Shaker E., Mahmoud H., Mnaa S. Silymarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem Toxicol. 2010;48:803–806. doi: 10.1016/j.fct.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Niranjana Murthy H., Dandin V.S., Yoeup Paek K. Hepatoprotective activity of ginsenosides from Panax ginseng adventitious roots against carbon tetrachloride treated hepatic injury in rats. J Ethnopharmacol. 2014;158:442–446. doi: 10.1016/j.jep.2014.10.047. [DOI] [PubMed] [Google Scholar]
- 24.Zhang S.S., Wu Z.Y., Chen J.M., Guo Q.K., Li L., Wang Z.F., Gao Y., Ma Z.C. Study on the mechanism of action of total saponins of Panax notoginseng in ameliorating oxidative stress and insulin resistance in rats fed with high fat diet. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2014;34:56–61. [PubMed] [Google Scholar]
- 25.Seo S.J., Cho J.Y., Jeong Y.H., Choi Y.S. Effect of Korean Red Ginseng extract on liver damage induced by short-term and long-term ethanol treatment in rats. J Ginseng Res. 2013;37:194–200. doi: 10.5142/jgr.2013.37.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lihn A.S., Pedersen S.B., Richelsen B. Adiponectin: action, regulation and association to insulin sensitivity. Obes Rev. 2005;6:13–21. doi: 10.1111/j.1467-789X.2005.00159.x. [DOI] [PubMed] [Google Scholar]
- 27.Yeo C.R., Yang C., Wong T.Y., Popovich D.G. A quantified ginseng (Panax ginseng C.A. Meyer) extract influences lipid acquisition and increases adiponectin expression in 3T3-L1 cells. Molecules. 2011;16:477–492. doi: 10.3390/molecules16010477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song Y.B., An Y.R., Kim S.J., Park H.W., Jung J.W., Kyung J.S., Hwang S.Y., Kim Y.S. Lipid metabolic effect of Korean Red Ginseng extract in mice fed on a high-fat diet. J Sci Food Agric. 2012;92:388–396. doi: 10.1002/jsfa.4589. [DOI] [PubMed] [Google Scholar]
- 29.Quan H.Y., Kim do Y., Chung S.H. Korean Red Ginseng extract alleviates advanced glycation end product-mediated renal injury. J Ginseng Res. 2013;37:187–193. doi: 10.5142/jgr.2013.37.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao J., Shi Z., Liu S., Li J., Huang W. Ginsenosides Rg1 from Panax ginseng: a potential therapy for acute liver failure patients? Evid Based Complement Alternat Med. 2014;2014:538059. doi: 10.1155/2014/538059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho Y.J., Son H.J., Kim K.S. A 14-week randomized, placebo-controlled, double-blind clinical trial to evaluate the efficacy and safety of ginseng polysaccharide (Y-75) J Transl Med. 2014;12:283. doi: 10.1186/s12967-014-0283-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berg A.H., Combs T.P., Scherer P.E. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 33.Fan J.S., Liu D.N., Huang G., Xu Z.Z., Jia Y., Zhang H.G., Li X.H., He F.T. Panax notoginseng saponins attenuate atherosclerosis via reciprocal regulation of lipid metabolism and inflammation by inducing liver X receptor alpha expression. J Ethnopharmacol. 2012;142:732–738. doi: 10.1016/j.jep.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 34.Korean Association for the Study of the Liver (KASL) KASL clinical practice guidelines: management of nonalcoholic fatty liver disease. Clin Mol Hepatol. 2013;19:325–348. doi: 10.3350/cmh.2013.19.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]