Abstract
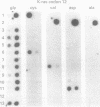
AIMS: To investigate the prevalence of K-ras codon 12 point mutations in ampullary neoplasms, to explore their clinical usefulness, and to test whether the detection of these mutations could be used to identify ampullary malignancies at an early stage. METHODS: Forty one tumour specimens from 28 patients with ampullary neoplasms were analysed for activating point mutations in K-ras codon 12 using a sensitive polymerase chain reaction (PCR) based assay. RESULTS: Eleven (39%) of the 28 primary tumours harboured point mutations in K-ras. Mutations were identified in seven (41%) of the 17 carcinomas and four (36%) of the 11 adenomas. Four of the possible six permutations in codon 12 were found in these 11 samples. This spectrum of mutations is different from pancreatic carcinoma but resembles that of colorectal neoplasms. Cytological brush specimens were available in 11 cases, and in all of these specimens, the K-ras status in the primary tumour and brush specimens was identical. CONCLUSIONS: K-ras codon 12 point mutations occur in about 40% of ampullary neoplasms at a relatively early stage in tumorigenesis. The pattern of mutations in these tumours resembles that of the adenoma-carcinoma sequence in the colorectum. These results indicate that ampullary neoplasms can be detected at an early stage by searching for genetic alterations in the K-ras oncogene in cytological brush specimens.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allema J. H., Reinders M. E., van Gulik T. M., van Leeuwen D. J., Verbeek P. C., de Wit L. T., Gouma D. J. Results of pancreaticoduodenectomy for ampullary carcinoma and analysis of prognostic factors for survival. Surgery. 1995 Mar;117(3):247–253. doi: 10.1016/s0039-6060(05)80197-7. [DOI] [PubMed] [Google Scholar]
- Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988 May 20;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Baczako K., Büchler M., Beger H. G., Kirkpatrick C. J., Haferkamp O. Morphogenesis and possible precursor lesions of invasive carcinoma of the papilla of Vater: epithelial dysplasia and adenoma. Hum Pathol. 1985 Mar;16(3):305–310. doi: 10.1016/s0046-8177(85)80018-6. [DOI] [PubMed] [Google Scholar]
- Bos J. L., Fearon E. R., Hamilton S. R., Verlaan-de Vries M., van Boom J. H., van der Eb A. J., Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. 1987 May 28-Jun 3Nature. 327(6120):293–297. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- Bos J. L. ras oncogenes in human cancer: a review. Cancer Res. 1989 Sep 1;49(17):4682–4689. [PubMed] [Google Scholar]
- Caldas C., Hahn S. A., Hruban R. H., Redston M. S., Yeo C. J., Kern S. E. Detection of K-ras mutations in the stool of patients with pancreatic adenocarcinoma and pancreatic ductal hyperplasia. Cancer Res. 1994 Jul 1;54(13):3568–3573. [PubMed] [Google Scholar]
- Crist D. W., Cameron J. L. The current status of the Whipple operation for periampullary carcinoma. Adv Surg. 1992;25:21–49. [PubMed] [Google Scholar]
- Fearon E. R. K-ras gene mutation as a pathogenetic and diagnostic marker in human cancer. J Natl Cancer Inst. 1993 Dec 15;85(24):1978–1980. doi: 10.1093/jnci/85.24.1978. [DOI] [PubMed] [Google Scholar]
- Gouma D. J., Obertop H., Vismans J., Willebrand D., Soeters P. B. Progression of a benign epithelial ampullary tumor to adenocarcinoma. Surgery. 1987 Apr;101(4):501–504. [PubMed] [Google Scholar]
- Hruban R. H., van Mansfeld A. D., Offerhaus G. J., van Weering D. H., Allison D. C., Goodman S. N., Kensler T. W., Bose K. K., Cameron J. L., Bos J. L. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993 Aug;143(2):545–554. [PMC free article] [PubMed] [Google Scholar]
- Huibregtse K., Smits M. E. Endoscopic management of diseases of the pancreas. Am J Gastroenterol. 1994 Aug;89(8 Suppl):S66–S77. [PubMed] [Google Scholar]
- Lerut J. P., Gianello P. R., Otte J. B., Kestens P. J. Pancreaticoduodenal resection. Surgical experience and evaluation of risk factors in 103 patients. Ann Surg. 1984 Apr;199(4):432–437. doi: 10.1097/00000658-198404000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monson J. R., Donohue J. H., McEntee G. P., McIlrath D. C., van Heerden J. A., Shorter R. G., Nagorney D. M., Ilstrup D. M. Radical resection for carcinoma of the ampulla of Vater. Arch Surg. 1991 Mar;126(3):353–357. doi: 10.1001/archsurg.1991.01410270099016. [DOI] [PubMed] [Google Scholar]
- Motojima K., Tsunoda T., Kanematsu T., Nagata Y., Urano T., Shiku H. Distinguishing pancreatic carcinoma from other periampullary carcinomas by analysis of mutations in the Kirsten-ras oncogene. Ann Surg. 1991 Dec;214(6):657–662. doi: 10.1097/00000658-199112000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motojima K., Tsunoda T., Kanematsu T., Nagata Y., Urano T., Shiku H. Distinguishing pancreatic carcinoma from other periampullary carcinomas by analysis of mutations in the Kirsten-ras oncogene. Ann Surg. 1991 Dec;214(6):657–662. doi: 10.1097/00000658-199112000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordback I. H., Hruban R. H., Cameron J. L. Second primary lesions in the biliary tree after successful resection of ampullary carcinoma. Surgery. 1992 Jul;112(1):111–115. [PubMed] [Google Scholar]
- Scarpa A., Capelli P., Zamboni G., Oda T., Mukai K., Bonetti F., Martignoni G., Iacono C., Serio G., Hirohashi S. Neoplasia of the ampulla of Vater. Ki-ras and p53 mutations. Am J Pathol. 1993 Apr;142(4):1163–1172. [PMC free article] [PubMed] [Google Scholar]
- Scarpa A., Zamboni G., Achille A., Capelli P., Bogina G., Iacono C., Serio G., Accolla R. S. ras-family gene mutations in neoplasia of the ampulla of Vater. Int J Cancer. 1994 Oct 1;59(1):39–42. doi: 10.1002/ijc.2910590109. [DOI] [PubMed] [Google Scholar]
- Seifert E., Schulte F., Stolte M. Adenoma and carcinoma of the duodenum and papilla of Vater: a clinicopathologic study. Am J Gastroenterol. 1992 Jan;87(1):37–42. [PubMed] [Google Scholar]
- Sivak M. V. Clinical and endoscopic aspects of tumors of the ampulla of Vater. Endoscopy. 1988 Aug;20 (Suppl 1):211–217. doi: 10.1055/s-2007-1018178. [DOI] [PubMed] [Google Scholar]
- Slebos R. J., Boerrigter L., Evers S. G., Wisman P., Mooi W. J., Rodenhuis S. A rapid and simple procedure for the routine detection of ras point mutations in formalin-fixed, paraffin-embedded tissues. Diagn Mol Pathol. 1992 Jun;1(2):136–141. [PubMed] [Google Scholar]
- Tada M., Omata M., Kawai S., Saisho H., Ohto M., Saiki R. K., Sninsky J. J. Detection of ras gene mutations in pancreatic juice and peripheral blood of patients with pancreatic adenocarcinoma. Cancer Res. 1993 Jun 1;53(11):2472–2474. [PubMed] [Google Scholar]
- Tada M., Omata M., Ohto M. Clinical application of ras gene mutation for diagnosis of pancreatic adenocarcinoma. Gastroenterology. 1991 Jan;100(1):233–238. doi: 10.1016/0016-5085(91)90606-l. [DOI] [PubMed] [Google Scholar]
- Tsao J. I., Rossi R. L., Lowell J. A. Pylorus-preserving pancreatoduodenectomy. Is it an adequate cancer operation. Arch Surg. 1994 Apr;129(4):405–412. doi: 10.1001/archsurg.1994.01420280081010. [DOI] [PubMed] [Google Scholar]
- Van Laethem J. L., Vertongen P., Deviere J., Van Rampelbergh J., Rickaert F., Cremer M., Robberecht P. Detection of c-Ki-ras gene codon 12 mutations from pancreatic duct brushings in the diagnosis of pancreatic tumours. Gut. 1995 May;36(5):781–787. doi: 10.1136/gut.36.5.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Warshaw A. L., Gu Z. Y., Wittenberg J., Waltman A. C. Preoperative staging and assessment of resectability of pancreatic cancer. Arch Surg. 1990 Feb;125(2):230–233. doi: 10.1001/archsurg.1990.01410140108018. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Sawabu N., Ohta H., Satomura Y., Yamakawa O., Motoo Y., Okai T., Takahashi H., Wakabayashi T. Identification of K-ras oncogene mutations in the pure pancreatic juice of patients with ductal pancreatic cancers. Jpn J Cancer Res. 1993 Sep;84(9):961–965. doi: 10.1111/j.1349-7006.1993.tb00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Es J. M., Polak M. M., van den Berg F. M., Ramsoekh T. B., Craanen M. E., Hruban R. H., Offerhaus G. J. Molecular markers for diagnostic cytology of neoplasms in the head region of the pancreas: mutation of K-ras and overexpression of the p53 protein product. J Clin Pathol. 1995 Mar;48(3):218–222. doi: 10.1136/jcp.48.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]