Abstract
Background
During the aging process, skin shows visible changes, characterized by a loss of elasticity and the appearance of wrinkles due to reduced collagen production and decreased elasticity of elastin fibers. Panax ginseng Meyer has been used as a traditional medicine for various diseases due to its wide range of biological activities including skin protective effects. Ginsenosides are the main components responsible for the biological activities of ginseng. However, the protective activities of an enzymatic preparation of red ginseng against human skin aging have not been investigated.
Methods
The efficacy of an enzyme-treated powder complex of red ginseng (BG11001) in preventing human skin aging was evaluated by oral administration to 78 randomized individuals. All patients were requested to take three daily capsules containing either 750 mg of BG11001 or a placebo vehicle for 24 wk; at the end of the testing period, skin roughness, elasticity, and skin water content were measured.
Results
BG11001 significantly reduced the average roughness of eye wrinkles and the Global Photo Damage Score compared with the placebo, although there were no significant differences in arithmetic roughness average between the groups. In addition, gross elasticity and net elasticity values increased, and transepidermal water loss level decreased, indicating improved skin elasticity and moisture content.
Conclusion
In conclusion, enzyme-treated red ginseng extract significantly improved eye wrinkle roughness, skin elasticity, and moisture content. Moreover, enzyme-treated red ginseng extract would be useful substance as a bio-health skin care product.
Keywords: elasticity, enzyme-treated red ginseng, human skin, Panax ginseng, wrinkle
1. Introduction
Aging is a dynamic and highly complex process defined by multiple physiological changes over time. All bodily systems are subject to the aging process and the integumentary system is not an exception. The human skin covers the whole outer body, which makes the skin the largest organ of the integumentary system. The skin has multiple layers of ectodermal tissue and guards the underlying muscles, bones, ligaments, and internal organs. During the process of aging, the skin changes show the most visible signs characterized by decreased elasticity, increased roughness, uneven skin tone with dark spots, and the formation of wrinkles [1]. Wrinkles on the face are the most dominantly recognized signs of skin aging [2]. Over time, the epidermis becomes thinner, even though the number of cell layers remains unchanged. Not only does the dermal layer thin, but also less collagen is produced, and the changes in the connective tissue reduce the skin's strength and elasticity [3], [4]. These changes in the structure of the skin cause the skin to wrinkle and slacken. Facial skin sites like the corners of the eyes are especially susceptible to wrinkle formation, which is also popularly known as “crow's feet”.
The maintenance of younger-looking skin is constantly desired by a large proportion of the world's population. Therefore, many institutes and cosmetic and pharmaceutical companies have been trying to develop functional cosmetic materials from natural substances such as herbs, roots, essential oils, and flowers. Panax ginseng has a history of medical use for over 5,000 yr. Panax derived from Greek word “Panakos” presents Pan—meaning “all” and akos meaning “cure”. Ginseng is said to mean “wonder of the world.” Like the literal meaning of its name, Panax ginseng Meyer has been used as a traditional medicine for various diseases with wide range of biological activities, including anti-inflammatory [5], antioxidant [6], antitumor [7], and antistress effects [8]. In recent studies, researchers investigated the protective effects of Panax ginseng, against the UVB-irradiation on epidermal keratinocytes and dermal fibroblasts. They found that ginseng recovered the UVB-induced decrease in antiapoptotic gene expression in the human keratinocytes and dermal fibroblast, indicating that ginseng can protect cells from apoptosis caused by strong UVB radiation [9]. Another study showed that ginseng extract induced type I collagen production in human dermal fibroblast cells by activation of Smad signaling, suggesting ginseng as a potential candidate as a wrinkle-reducing agent by topical application [10].
Ginsenosides are the pharmacologically active components and are responsible for the biological functions in ginseng. Among more than 50 isolated ginsenosides, major ones (Rb1, Rb2 , Rc, Rd, Re, Rg1, and Rf) constitute more than 80% of the total ginsenosides and the minor ginsenosides (F1, F2, Rg3, Rh1, Rh2 compound Y, compound Mc, and compound K) are present at low concentrations in ginseng [11]. Many studies show that the minor ginsenosides have pharmacologically active than major ones because absorption of major ginsenosides by the gastrointestinal tract is quite poor [12]. As a result, the minor ginsenosides have been demonstrated to be pharmaceutically active and excellent potential drug candidates [13]. However, hydrolysis of sugar moieties to convert major ginsenosides to minor forms by digestive enzymes in gastrointestinal tract are quite low even though the minor ginsenosides are more easily absorbed into the bloodstream [12]. Since it is possible to transform into minor ginsenosides by enzyme treatment, enzyme-treated red ginseng has been shown to have strong antiwrinkle activity and reduced toxicity in in vitro and animal studies [14]. Also, our previous studies demonstrated that enzyme-treated ginseng protected UVB-induced skin damage through the regulation of procollagen type I and matrix metalloproteinase (MMP)-1 expression in hairless mice [15], [16]. However, the protective activity of the enzymatic preparation of red ginseng against human skin aging has not been investigated. In this study, we investigated whether enzyme-treated powder complex of red ginseng (BG11001) prevents human skin aging by reducing skin wrinkles and enhancing elasticity.
2. Materials and methods
2.1. Preparation of enzyme-treated powder complex of red ginseng (BG11001)
Enzyme-treated extract of red ginseng was prepared following a patented protocol [Korea patent no. 10-2011-0091287 (in private), in press] [15]. Red ginseng powder with 10 times volume of distilled water was mixed for 2 h using a homo-mixer and then enzyme treated for 24 h at 55°C. Enzyme-treated red ginseng was heated up to 90°C, cooled down to 10–15°C, and then centrifuged. Supernatant was concentrated, added 10 times volume of 50% ethanol, and extracted for 40 min at 85°C. Finally, the same amount of malt dextrin was added, spray dried and used as raw material of BG11001.
For thin layer chromatography analysis of the ginsenoside compositions, the total ginsenosides were spotted together with the standard 20(S)-protopanaxdiol (PPD) or 20(S)-protopanaxtriol samples on thin layer chromatography plate (silica gel 60 F254, Merck) containing CHCl3-MeOH-H2O (65:35:10, v/v), thereafter stained by spraying with 30% H2SO4, followed by heating at 105°C.
2.2. Study design
This study was designed as a randomized, double-blind study to assess the effects of 250 mg of oral BG11001 given thrice a day for 24 wk of the trial period in patients with cutaneous photoaging. The compositions of the BG11001 and placebo tablets are shown in Table 1.
Table 1.
Compositions of the BG11001 and placebo tablets
| Ingredient | Tablet (mg) | Percentage (%) |
|---|---|---|
| BG11001 tablet | ||
| BG11001 | 250 | 41.7 |
| Microcrystalline cellulose | 88.4 | 14.7 |
| Dextrin | 150 | 25 |
| Maltitol syrup powder | 96 | 16 |
| Magnesium stearate | 9 | 1.5 |
| Silicon dioxide | 3 | 0.5 |
| HPMC | 3.3 | 0.55 |
| Glycerol fatty acid ester | 0.3 | 0.05 |
| Total | 600 | 100 |
| Placebo tablet | ||
| Microcrystalline cellulose | 210 | 35 |
| Lactose powder | 331.5 | 55.25 |
| Caramel coloring | 1.32 | 0.22 |
| Gardenia yellow pigment | 0.78 | 0.13 |
| Maltitol syrup powder | 43.5 | 7.25 |
| Magnesium stearate | 9.3 | 1.55 |
| HPMC | 3.3 | 0.55 |
| Glycerol fatty acid ester | 0.3 | 0.05 |
| Total | 600 | 100 |
HPMC, hydroxypropylmethylcellulose.
2.3. Global photodamage score
Patients' periorbital wrinkles were evaluated based on a global photodamage score (0, none; 1, none/mild; 2, mild; 3, mild/moderate; 4, moderate; 5, moderate/severe; 6, severe; 7, very severe) at Wk 0 (baseline). If the investigators' evaluations differed, low-grade efficacy and high-grade adverse effect were selected. The patients' periorbital wrinkles were classified into eight grades.
2.4. Participants
Ninety-eight healthy Asian women, aged between 40 and 60 yr, clinically diagnosed with a global photodamage score of 2–6 according to the Jung score of photoaging of facial skin were recruited and included in this study after written informed consent. Those who experienced any esthetic procedure like peeling, laser, intense pulsed light, dermabrasive therapies, or have used any antiaging cream or nutritional supplement within the past 3 mo could not participate in the study. Participants were requested not to expose themselves to sunlight during the trial. They were also requested not to use lotions, creams, or other products on the face and forearms. Participants agreed to follow these instructions during the trial period. On completion of the abovementioned enrollment process of the participants in the study, the study coordinator allocated them into each group.
2.5. Groups
Participants were randomly allocated into two groups which were of the same size and matched on the basis of global photodamage score and age. A computer-generated table of random numbers was prepared in advance and was later used to randomly allocate participants into one of the two groups. Participants in the BG11001 experimental group were supplemented with “enzyme-treated powder complex of red ginseng (BG11001)” capsules and those in the placebo control group with placebo capsules. Those who used any nutritional supplement other than the test material during the trial period were dropped from the trial.
The trial started in the summer of 2012 and was completed in the winter of 2013. The protocol has been reviewed and approved by the Ethics Committee (Oriental Hospital of Se-Myung University, Jecheon, South Korea) and was approved in June 2012. The study was carried out in accordance to the Declaration of Helsinki (1964) changed in Tokyo (2004) and Seoul (2008).
2.6. Treatment
All patients were requested to take three capsules daily for 24 wk. The capsules were prepared to contain either 250 mg of BG11001 or the excipients of lactose powder, microcrystalline cellulose, and powered maltitol syrup. Participants were instructed to take one capsule in the morning, another in the afternoon, and the other in the evening with a glass of water. Placebo and BG11001 capsules were identical in color, taste, odor, and packaging and their content was blinded to the participants and investigator.
2.7. Blinding
Only the study coordinator knew the group allocation and prepared the capsules for both groups accordingly. Both the participants and the investigator were blinded to the information on the group allocation until the end of the study. Allocation concealment was maintained using an opaque envelope. Group allocation information was opened to the participant at the end of the trial period.
2.8. Compliance
Those participants whose compliance was under 80% were dropped from the study.
2.9. Serology
All parameters were measured at baseline and after 24 wk of supplementation. Blood samples were collected from fasting participants at baseline and after a 24-wk supplementation. To evaluate the safety of oral administration of BG11001, creatinine, albumin, total protein, total cholesterol, high-density lipoprotein-cholesterol, low-density lipoprotein-cholesterol, and glutamic-oxalacetic transaminase, glutamic-pyruvic transaminase, gamma-glutamyltransferase of serum were determined. Other parameters analyzed were white blood cells, red blood cells, mean corpuscular volume, hemoglobin, hematocrit, and platelet count. Specific gravity, pH, protein, glucose, blood (red blood cells), human chorionic gonadotropin of urine were also investigated.
2.10. Noninvasive measurements of the skin
All measurements were performed under standardized conditions, i.e., room temperature of 22 ± 2°C and a relative humidity level of 45 ± 5%. An acclimatization time of at least 30 min was respected before measurements started.
Microrelief (roughness) of the skin was measured with a skin visiometer SV 600 (Courage-Khazaka, Cologne, Germany). Investigated roughness parameters were R3 (average roughness) and R5 (arithmetic average roughness). Elastic properties of the skin were measured with a Cutometer (Courage-Khazaka, Cologne, Germany). Investigated elastic parameters were R2 (gross elasticity) and R5 (net elasticity). Hydration, transepidermal water loss (TEWL), elastic properties, and microrelief of the skin were evaluated on the crow's feet area at baseline and after 24 wk of supplementation with the following noninvasive methods. Hydration level and TEWL of the skin surface was measured with the Corneometer CM 825 (Courage-Khazaka, Cologne, Germany) and Tewameter TM300 (Courage-Khazaka, Cologne, Germany). Elastic properties of the skin were measured with the Cutometer (Courage-Khazaka, Cologne, Germany).
2.11. Statistical analysis
Results were determined using PRISM Statistical Analysis System (Version 5, GraphPad, La Jolla, CA, USA). Differences between groups were evaluated with Student t test and differences within groups were analyzed with analysis of covariance. A value of p < 0.05 was considered significant.
3. Results
3.1. Ginsenoside composition changes of red ginseng powder by enzyme treatment
When red ginseng powder was enzyme treated, ginsenoside compositions were changed which showed significantly increased F2, Rd+Re, F1+Rh1, and C-K (Fig. 1 and Table 2). Even if enzyme-treated red ginseng contained relatively high amounts of ginsenosides-Rd and Re, ginsenosides Re as a 20(S)-protopanaxtriol type saponin [17] was almost unhydrolyzed by the enzyme. Ginsenoside-Rd is an intermediate product hydrolyzed from PPD type saponin (e.g., ginsenosides-Rb1, -Rb2, and Rc), thus, it has a high possibility to switch to other saponins. Ginsenoside C-K and F1+Rh1 were also increased in enzyme-treated red ginseng, but the portion of the total amount from those ginsenosides was very small. In the case of ginsenosides-F2, it is also hydrolyzed from a PPD-based major saponin, but the F2 content of the enzyme-treated red ginseng was relatively high and with content variation (coefficient of variation) of each batch was quite low (11% in lab scale and 5.7% in scale-up). In addition, our previous studies demonstrated that enzyme-treated ginseng (index material: ginsenosides F2) protected UVB-induced skin damage through the regulation of procollagen type I and MMP-1 expression in hairless mice [15], [16]. Therefore, ginsenoside-F2 is considered as an index material of enzyme-treated red ginseng (BG11001).
Fig. 1.

Thin liquid chromatography profiles of crude red ginseng ginsenosides before and after treatment of enzyme. CK, compound K; PPD, 20(S)-protopanaxdiol (PPD); PPT, 20(S)-protopanaxtriol.
Table 2.
Changes in ginsenoside composition
| Ginsenoside | Content (%) |
Changes (%) | ||
|---|---|---|---|---|
| Before | After | |||
| C-K | — | 6.41 | ↑ | 6.41 |
| F1+Rh1 | — | 7.26 | ↑ | 7.26 |
| F2 | — | 40.78 | ↑ ↑ | 40.78 |
| Rg1 | 20.22 | — | ↓ ↓ | 20.22 |
| Rf | 12.60 | — | ↓ ↓ | 12.60 |
| Rd+Re | 14.51 | 27.17 | ↑ ↑ | 12.66 |
| Rc | 25.27 | 18.38 | ↓ | 6.89 |
| Rb1 | 25.27 | — | ↓ ↓ | 25.27 |
C-K, compound K.
3.2. Demographics of participants
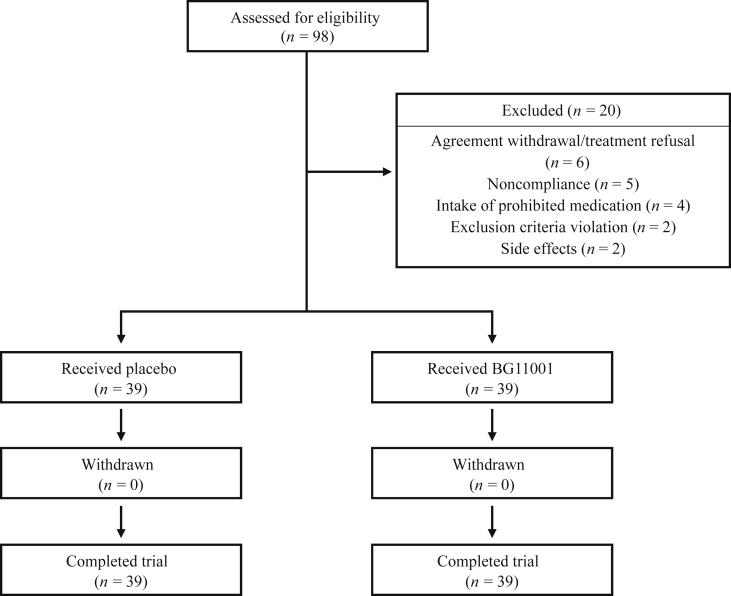
In total, 98 women were enrolled and randomized into two groups—placebo and BG11001. Twenty out of the initial 98 enrolled women were excluded from subsequent analysis for the following reasons: (1) agreement withdrawal/treatment refusal (n = 6); (2) noncompliance (n = 5); (3) intake of prohibited medication (n = 4); (4) exclusion criteria violation (n = 2); (5) side effects (n = 2; right anterior cruciate ligament rupture and finger fracture); and (6) agreement acquisition violation (n = 1). Thus, the number for final data analysis was 78 (BG11001: n = 39, placebo: n = 39) (Fig. 2). In the treatment group (n = 39), the average age was 51.16 ± 4.41 yr, and the average weight was 56.71 ± 7.07 kg; in the placebo group (n = 39), the average age was 51.14 ± 3.83 yr, and average weight was 59.26 ± 7.91 kg. The demographic data of the participants are summarized in Table 3. The Student t test revealed no significant difference in initial age or weight. Laboratory evaluations revealed no significant abnormalities in complete blood count and chemistry (Table 4).
Fig. 2.
Study flowchart of the participants describing trial progress.
Table 3.
Demographics of the participants in each group
| BG11001 | Placebo | p | |
|---|---|---|---|
| No. enrolled | 49 | 49 | |
| No. completed | 39 | 39 | |
| Average age (yr) | 51.16 ± 7.07 | 51.15 ± 3.83 | 0.981 |
| Average weight (kg) | 56.71 ± 7.07 | 59.26 ± 7.91 | 0.095 |
Data are presented as mean ± SD.
Table 4.
Serology data
| BG11001 |
p | Placebo |
p | |||
|---|---|---|---|---|---|---|
| Before |
After 24 wk |
Before |
After 24 wk |
|||
| Average ± SD | Average ± SD | |||||
| Hematology | ||||||
| WBC | 5.92 ± 1.37 | 5.90 ± 1.33 | 0.940 | 5.79 ± 1.39 | 5.87 ± 1.45 | 0.564 |
| RBC | 4.31 ± 0.28 | 4.39 ± 0.25 | 0.002** | 4.31 ± 0.27 | 4.33 ± 0.26 | 0.271 |
| Hemoglobin | 13.26 ± 0.85 | 13.57 ± 0.84 | 0.002** | 13.22 ± 0.90 | 13.36 ± 0.89 | 0.048* |
| Hematocrit | 39.99 ± 2.31 | 40.82 ± 2.23 | 0.001** | 40.02 ± 2.31 | 40.26 ± 2.12 | 0.204 |
| Platelets | 239.08 ± 46.76 | 247.98 ± 48.90 | 0.041* | 247.51 ± 60.29 | 246.41 ± 65.58 | 0.793 |
| Blood chemistry | ||||||
| ALP | 225.18 ± 68.63 | 251.12 ± 72.89 | 0.000** | 215.14 ± 53.37 | 240.96 ± 65.18 | 0.000** |
| AST | 23.04 ± 5.31 | 26.31 ± 13.42 | 0.066 | 23.76 ± 8.31 | 24.45 ± 8.43 | 0.430 |
| ALT | 20.00 ± 8.14 | 24.80 ± 16.11 | 0.011* | 22.24 ± 14.52 | 23.33 ± 14.61 | 0.375 |
| γ-GTP | 22.49 ± 18.34 | 25.12 ± 22.09 | 0.086 | 21.06 ± 22.45 | 18.90 ± 19.84 | 0.033* |
| Total protein | 7.16 ± 0.31 | 7.16 ± 0.34 | 0.885 | 7.24 ± 0.31 | 7.15 ± 0.32 | 0.042* |
| Albumin | 4.45 ± 0.17 | 4.49 ± 0.22 | 0.077 | 4.49 ± 0.20 | 4.46 ± 0.18 | 0.261 |
| Creatinine | 0.85 ± 0.12 | 0.80 ± 0.11 | 0.004** | 0.82 ± 0.08 | 0.81 ± 0.13 | 0.425 |
| MCV | 92.84 ± 3.31 | 92.91 ± 3.17 | 0.682 | 92.95 ± 3.50 | 92.98 ± 3.54 | 0.858 |
| Glucose | 85.94 ± 9.05 | 91.55 ± 18.37 | 0.029* | 87.69 ± 11.48 | 91.53 ± 19.29 | 0.048* |
| Total cholesterol | 206.14 ± 37.72 | 203.43 ± 35.72 | 0.471 | 195.06 ± 24.11 | 192.49 ± 24.63 | 0.031* |
| HDL | 61.57 ± 15.55 | 61.29 ± 15.89 | 0.806 | 61.98 ± 13.90 | 61.39 ± 14.84 | 0.686 |
| LDL | 127.20 ± 34.03 | 129.31 ± 34.68 | 0.490 | 116.14 ± 26.03 | 113.86 ± 29.75 | 0.402 |
| Triglycerid | 150.39 ± 93.85 | 134.53 ± 77.14 | 0.219 | 124.49 ± 73.69 | 115.27 ± 58.02 | 0.349 |
*p < 0.05, **p < 0.01 by Student’s t-test for comparison between before and 24 wk treated group.
ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; GTP, guanosine-5′-triphosphate; HDL, high density lipoprotein; LDL, low density lipoprotein; MCV, mean corpuscular volume; RBC, red blood cells; Std., standard deviation; WBC, white blood cells.
3.3. Analysis of BG110001 effect on eye wrinkles in human skin
Comparisons of eye wrinkles before and after 8 wk, 16 wk, and 24 wk of BG11001 consumption between the treatment group and the placebo group, as measured with the SV600 visiometer and Global Photo Damage Score (GPDS) are shown in Table 5, Table 6, Table 7. At 8 wk, value of average roughness (R3) for eye wrinkles was reduced 0.007 ± 0.009 AU in the treatment group and 0.003 ± 0.011 AU in the placebo group. Those reductions were similarly maintained after 16 wk (0.005 AU in the treatment group and 0.003 AU in the placebo group) and 24 wk (0.006 AU in the treatment group and 0.004 AU in the placebo group), respectively (Table 5). Those R3 values were significantly decreased within the treatment group, but not in the placebo group. Therefore, each improvement of R3 values in the treatment group at each time point showed statistical significance after adjustment with baseline. However, the value of arithmetic roughness average (R5) did not show improvement by BG11001. When the R5 values were compared within the group, there were meaningful decreases by statistics. But, after adjustment with baseline, we could not find any statistical difference (Table 6).
Table 5.
Average roughness (R3) value changes
| BG11001 |
Placebo |
p1) | p2) | ||
|---|---|---|---|---|---|
| Arbitrary units (Average ± SD) | |||||
| SV600 (R3) | Baseline | 0.037 ± 0.012 | 0.040 ± 0.013 | 0.280 | |
| After 8 wk | 0.030 ± 0.008 | 0.037 ± 0.012 | 0.004 | 0.004** | |
| Difference3) | −0.007 ± 0.009 | −0.003 ± 0.011 | 0.095 | ||
| P4) | 0.000∗∗ | 0.057 | |||
| After 16 wk | 0.032 ± 0.008 | 0.037 ± 0.011 | 0.008 | 0.014* | |
| Difference5) | −0.005 ± 0.011 | −0.003 ± 0.010 | 0.226 | ||
| P4) | 0.003∗∗ | 0.106 | |||
| After 24 wk | 0.031 ± 0.007 | 0.036 ± 0.010 | 0.010 | 0.017* | |
| Difference6) | −0.006 ± 0.011 | −0.004 ± 0.009 | 0.382 | ||
| P4) | 0.004∗∗ | 0.018∗ | |||
∗p < 0.05 by Student t-test for comparison with placebo group.
∗∗p < 0.01 by Student t-test for comparison with placebo group.
Compared between groups: p-value by Student t-test.
Compared between groups: p-value by analysis of covariance (adjustment with baseline).
After 8 wk – baseline.
Compared within groups: p-value by paired t-test.
After 16 wk – baseline.
After 24 wk – baseline.
Table 6.
Arithmetic roughness average (R5) value changes
| BG11001 |
Placebo |
p1) | p2) | ||
|---|---|---|---|---|---|
| Arbitrary units (Average ± SD) | |||||
| SV600 (R5) | Baseline | 0.017 ± 0.008 | 0.022 ± 0.009 | 0.039 | |
| After 8 wk | 0.014 ± 0.006 | 0.016 ± 0.007 | 0.074 | 0.927 | |
| Difference3) | −0.004 ± 0.006 | −0.005 ± 0.008 | 0.407 | ||
| p4) | 0.000∗∗ | 0.000∗∗ | |||
| After 16 wk | 0.015 ± 0.006 | 0.017 ± 0.008 | 0.079 | 0.722 | |
| Difference5) | −0.003 ± 0.008 | −0.004 ± 0.010 | 0.530 | ||
| p4) | 0.026∗ | 0.016∗ | |||
| After 24 wk | 0.015 ± 0.006 | 0.016 ± 0.007 | 0.305 | 0.259 | |
| Difference6) | −0.003 ± 0.007 | −0.005 ± 0.008 | 0.139 | ||
| p4) | 0.020∗ | 0.000∗∗ | |||
∗p < 0.05 by Student t-test for comparison with placebo group.
∗∗p < 0.01 by Student t-test for comparison with placebo group.
Compared between groups: p-value by Student t-test.
Compared between groups: p-value by analysis of covariance (adjustment with baseline).
After 8 wk – baseline.
Compared within groups: p-value by paired t-test.
After 16 wk – baseline.
After 24 wk – baseline.
Table 7.
Global photodamage score value changes
| BG11001 |
Placebo |
p1) | p2) | ||
|---|---|---|---|---|---|
| Arbitrary units (Average ± SD) | |||||
| GPDS | Baseline | 3.31 ± 1.15 | 3.44 ± 1.25 | 0.639 | |
| After 8 wk | 3.15 ± 1.04 | 3.41 ± 1.21 | 0.318 | 0.262 | |
| Difference3) | −0.15 ± 0.75 | −0.03 ± 0.54 | 0.386 | ||
| p4) | 0.205 | 0.767 | |||
| After 16 wk | 3.13 ± 1.01 | 3.33 ± 1.13 | 0.400 | 0.397 | |
| Difference5) | −0.18 ± 0.64 | −0.10 ± 0.60 | 0.586 | ||
| p4) | 0.026∗ | 0.016∗ | |||
| After 24 wk | 3.05 ± 1.03 | 3.38 ± 1.14 | 0.178 | 0.062 | |
| Difference6) | −0.26 ± 0.60 | −0.05 ± 0.61 | 0.135 | ||
| p4) | 0.010∗ | 0.599 | |||
∗p < 0.05 by Student t-test for comparison with placebo group.
∗∗p < 0.01 by Student t-test for comparison with placebo group.
Compared between groups: p by Student t-test.
Compared between groups: p by analysis of covariance (adjustment with baseline).
After 8 wk – baseline.
Compared within groups: p by paired t-test.
After 16 wk – baseline.
After 24 wk – baseline.
Global photodamage was scored using a randomized and double-blinded method. Even if GPDS might be judged subjectively, only BG11001 significantly decreased the scores. Decreased scores were observed from 8 wk of treatment and tended to decline until 24 wk (Table 7).
3.4. Observation of improved skin elasticity
The values of gross elasticity (R2) and net elasticity (R5) were measured with a Cutometer (Table 8, Table 9). After BG11001 treatment, R2 values were increased in each time point, from 0.89 ± 0.05 AU at baseline to 0.91 ± 0.04 AU at 24 wk, but not in the placebo group. Within the treatment group, R2 values were statistically different after 16 wk and 24 wk. Also, when we compared R2 values between groups using analysis of covariance, after adjustment with baseline, the gross skin elasticity was statistically improved by enzyme-treated red ginseng extract (Table 8). Then, net elasticity (R5) was measured and showed that R5 values were significantly increased within the treatment group. In addition, statistics of comparison between the treatment and placebo group showed that BG11001 improved net skin elasticity (Table 9).
Table 8.
Skin elasticity (R2) value changes
| BG11001 |
Placebo |
p1) | p2) | ||
|---|---|---|---|---|---|
| Arbitrary units (Average ± SD) | |||||
| Cutometer (R2) | Baseline | 0.89 ± 0.05 | 0.86 ± 0.07 | 0.185 | |
| After 8 wk | 0.90 ± 0.04 | 0.86 ± 0.05 | 0.000 | 0.000** | |
| Difference3) | 0.01 ± 0.05 | −0.01 ± 0.08 | 0.118 | ||
| p4) | 0.058 | 0.485 | |||
| After 16 wk | 0.91 ± 0.05 | 0.87 ± 0.07 | 0.002 | 0.003** | |
| Difference5) | 0.02 ± 0.04 | −0.00 ± 0.50 | 0.040* | ||
| p4) | 0.006∗∗ | 0.732 | |||
| After 24 wk | 0.91 ± 0.04 | 0.87 ± 0.07 | 0.003 | 0.006** | |
| Difference6) | 0.02 ± 0.05 | −0.00 ± 0.07 | 0.108 | ||
| p4) | 0.011∗ | 0.946 | |||
∗p < 0.05 by Student t-test for comparison with placebo group.
∗∗p < 0.01 by Student t-test for comparison with placebo group.
Compared between groups: p by Student t-test.
Compared between groups: p by analysis of covariance (adjustment with baseline).
After 8 wk – baseline.
Compared within groups: p by paired t-test.
After 16 wk – baseline.
After 24 wk – baseline.
Table 9.
Skin elasticity (R5) value changes
| BG11001 |
Placebo |
p1) | p2) | ||
|---|---|---|---|---|---|
| Arbitrary units (Average ± SD) | |||||
| Cutometer (R5) | Baseline | 0.68 ± 0.18 | 0.62 ± 0.14 | 0.127 | |
| After 8 wk | 0.75 ± 0.19 | 0.66 ± 0.15 | 0.021 | 0.071 | |
| Difference3) | 0.07 ± 0.12 | 0.04 ± 0.08 | 0.160 | ||
| p4) | 0.001∗∗ | 0.006∗∗ | |||
| After 16 wk | 0.79 ± 0.21 | 0.67 ± 0.17 | 0.009 | 0.033* | |
| Difference5) | 0.11 ± 0.19 | 0.05 ± 0.16 | 0.138 | ||
| p4) | 0.001∗∗ | 0.045∗ | |||
| After 24 wk | 0.78 ± 0.22 | 0.65 ± 0.16 | 0.004 | 0.014* | |
| Difference6) | 0.10 ± 0.16 | 0.03 ± 0.16 | 0.043 | ||
| p4) | 0.000∗∗ | 0.246 | |||
∗p < 0.05 by Student t-test for comparison with placebo group.
∗∗p < 0.01 by Student t-test for comparison with placebo group.
Compared between groups: p by Student t-test.
Compared between groups: p by analysis of covariance (adjustment with baseline).
After 8 wk – baseline.
Compared within groups: p by paired t-test.
After 16 wk – baseline.
After 24 wk – baseline.
3.5. Changes of the moisture content of the stratum corneum and TEWL
The hydration level of the stratum corneum was measured using a corneometer (Table 10). During the treatment of BG11001 for 24 wk, hydration level was decreased from 8 wk treatment. However, decreased hydration level was maintained until 24 wk in the treatment group. In addition, the degrees of decrease were much smaller than those in the placebo group. Then, the measurement of TEWL was performed with a Tewameter (Table 11). After 24 wk of BG11001 consumption, TEWL values were decreased in the treatment group, even at 8 wk of treatment; however, TEWL values were increased in the placebo group in each time point for 24 wk. Also, the statistics of comparison between groups at the 24 wk time point, showed that BG11001 significantly prevented TEWL.
Table 10.
Skin hydration value changes
| BG11001 |
Placebo |
p1) | p2) | ||
|---|---|---|---|---|---|
| Arbitrary units (Average ± SD) | |||||
| Corneo-meter | Baseline | 72.23 ± 10.20 | 70.90 ± 11.77 | 0.692 | |
| After 8 wk | 67.79 ± 9.57 | 65.46 ± 9.67 | 0.222 | 0.083 | |
| Difference3) | −4.44 ± 8.46 | −5.44 ± 8.54 | 0.149 | ||
| p4) | 0.058 | 0.485 | |||
| After 16 wk | 65.67 ± 10.34 | 63.43 ± 9.29 | 0.074 | 0.008** | |
| Difference5) | −6.56 ± 8.65 | −7.46 ± 11.68 | 0.036 | ||
| p4) | 0.282 | 0.071 | |||
| After 24 wk | 67.49 ± 10.49 | 63.89 ± 10.87 | 0.090 | 0.005** | |
| Difference6) | −4.74 ± 9.19 | −7.01 ± 12.96 | 0.005 | ||
| p4) | 0.172 | 0.013∗ | |||
∗p < 0.05 by Student t-test for comparison with placebo group.
∗∗p < 0.01 by Student t-test for comparison with placebo group.
Compared between groups: p by Student t-test.
Compared between groups: p-value by analysis of covariance (adjustment with baseline).
After 8 wk – baseline.
Compared within groups: p-value by paired t-test.
After 16 wk – baseline.
After 24 wk – baseline.
Table 11.
Transepidermal water loss value changes
| BG11001 |
Placebo |
p1) | p2) | ||
|---|---|---|---|---|---|
| Arbitrary units (Average ± SD) | |||||
| Tewa-meter | Baseline | 14.29 ± 2.60 | 14.94 ± 3.25 | 0.332 | |
| After 8 wk | 13.67 ± 2.47 | 15.16 ± 3.98 | 0.051 | 0.062 | |
| Difference3) | −0.62 ± 1.79 | 0.22 ± 2.47 | 0.091 | ||
| p4) | 0.039 | 0.580 | |||
| After 16 wk | 13.68 ± 2.62 | 14.96 ± 3.73 | 0.083 | 0.109 | |
| Difference5) | −0.61 ± 0.96 | 0.02 ± 2.56 | 0.154 | ||
| p4) | 0.000∗∗ | 0.958 | |||
| After 24 wk | 13.75 ± 2.92 | 15.30 ± 3.12 | 0.027 | 0.030* | |
| Difference6) | −0.53 ± 1.81 | 0.36 ± 2.57 | 0.081 | ||
| p4) | 0.073 | 0.391 | |||
∗p < 0.05 by Student t-test for comparison with placebo group.
∗∗p < 0.01 by Student t-test for comparison with placebo group.
Compared between groups: p by Student t-test.
Compared between groups: p-value by analysis of covariance (adjustment with baseline).
After 8 wk – baseline.
Compared within groups: p-value by paired t-test.
After 16 wk – baseline.
After 24 wk – baseline.
4. Discussion
The present study demonstrated that 24-wk oral administration of enzyme-treated powder complex of red ginseng (BG11001) has substantial benefits over the placebo in the improvement of eye wrinkle roughness, elasticity, and skin moisture content. The administration of BG11001 significantly reduced average roughness (R3) of eye wrinkles and GPDS score, compared with a placebo; although, there was no significant difference in arithmetic roughness average (R5) between both groups. In addition, gross elasticity (R2) and net elasticity (R5) values were increased and TEWL level was decreased, indicating improved skin elasticity and moisture content. However, hydration levels of the stratum corneum were decreased even in the treatment group. To interpret this result, we needed to consider the weather conditions during the study. When the study was started at early July, the weather was humid (average humidity: 78.5%) and warm (average temperature: 23.4°C) (Korea Meteorological Administration). However, as the treatment proceeded, the weather became drier (average humidity: 69.4% at the 24 wk time point) and colder (average temperature: −5.2°C at the 24 wk time point), meaning that skin could have lost moisture and elasticity more than the baseline time point [18]. Therefore, with considering the weather conditions, results should be reinterpreted, as eye wrinkle roughness was remarkably improved by showing the average roughness (R3) value and GPDS score. Even though values of arithmetic roughness average (R5) were not decreased enough to show any statistical difference, BG11001 prevented worsening of the eye wrinkle formation despite the cold and dry weather conditions. With the same point of view, skin elasticity (R2 and R5) and moisture content (TEWL) after treatment was substantially improved, and decreased skin hydration was prevented by BG11001, even with poor weather conditions for skin.
The primary cause for eye wrinkles, such as crow's feet, is exposure to sunlight. Sunlight ages the skin, and it also encourages people to squint, wrinkling the skin around their faces. In UV-damaged skin, increased MMP-1 triggers collagen degradation, especially collagen type I which is a source of structural support in the dermis [19]. Also, UV irradiation increased the expression of activator protein-1, which in turn interferes with the synthesis of collagen type I by blocking transforming growth factor-β1 and procollagen type I [21], [20]. In a previous study, Panax ginseng restored decreased expression of transforming growth factor-β1 and TIMP-1 (Tissue Inhibitor of Metalloproteinase-1) and increased expression of MMP-1. Panax ginseng also markedly attenuated MMP-1 expression. Those regulations finally increased collagen type I (or procollagen type I) expression [15], [16], [22]. Also, other studies observed that Panax ginseng induces collagen type I synthesis through activation of Smad or peroxisome proliferator-activated receptor-delta signaling [10], [23]. In addition, enzyme-treated extract of red ginseng reversed the inhibitory effects of UV irradiation on filaggrin and profilaggrin production in both the epidermis and dermis in mice [15]. These results are important because filaggrin in stratum corneum are incorporated into lipid envelopes and release free amino acids to assist in water retention [26], [24], [25]. Another previous study reported that dietary supplementation of red ginseng protected skin from UV-induced dryness with an accumulation of ceramides via elevated expression of serine palmitoyltransferase, a key enzyme involved in de novo ceramide synthesis [27]. With all these previous results, we could suggest that BG11001 improved human eye wrinkle roughness by the regulation of collagen type I expression, and BG11001 was also able to increase skin elasticity and maintain moisture content by inhibition of filaggrin expression and elevation of serine palmitoyltransferase expression.
In this study, we used oral administration of BG11001 despite the possibility of oral intake delaying the effect on skin because the substance should be absorbed and metabolized. However, there are more benefits than topical application. Firstly, the aged do not absorb topical substances more rapidly than the young, probably less [28]. Secondly, when Panax ginseng is administrated orally, skin care effects would be better with its wide range of biological activities. For example, it promotes skin cell regeneration by a wound healing effect [29] and also has the effect of boosting blood circulation as well as detoxifying the blood [31], [30]. Thirdly, with oral intake we did not need to be worried about topical skin irritation. Furthermore, there was no clinically significant adverse effects of BG11001 during the study.
In conclusion, enzyme-treated red ginseng extract significantly improved eye wrinkle roughness, skin elasticity, and moisture content. Moreover, enzyme-treated red ginseng extract would be useful substance as a bio-health skin care product.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This research was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (iPET, 810006-03-3-SB110), Korea.
References
- 1.Farage M.A., Miller K.W., Elsner P., Maibach H.I. Characteristics of the aging skin. Adv Wound Care (New Rochelle) 2013;2:5–10. doi: 10.1089/wound.2011.0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Proksch E., Proksch E., Schunck M., Zague V., Segger D., Degwert J., Oesser S. Oral intake of specific bioactive collagen peptides reduces skin wrinkles and increases dermal matrix synthesis. Skin Pharm Physiol. 2014;27:113–119. doi: 10.1159/000355523. [DOI] [PubMed] [Google Scholar]
- 3.Takema Y., Yorimoto Y., Kawai M., Imokawa G. Age-related changes in the elastic properties and thickness of human facial skin. Br J Dermatol. 1994;131:641–648. doi: 10.1111/j.1365-2133.1994.tb04975.x. [DOI] [PubMed] [Google Scholar]
- 4.Takema Y., Yorimoto Y., Kawai M. The relationship between age-related changes in the physical properties and development of wrinkles in human facial skin. J Soc Cosmet Chem. 1995;46:163–173. [Google Scholar]
- 5.Hong C.E., Lyu S.Y. Anti-inflammatory and anti-oxidative effects of Korean red ginseng extract in human keratinocytes. Immune Netw. 2001;11:42–49. doi: 10.4110/in.2011.11.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae H.M., Kim S.S., Cho C.W., Yang D.C., Ko S.K., Kim K.T. Antioxidant activities of ginseng seeds treated by autoclaving. J Ginseng Res. 2012;36:411–417. doi: 10.5142/jgr.2012.36.4.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yun T.K. Experimental and epidemiological evidence on nonorgan specific cancer preventive effect of Korean ginseng and identification of active compounds. Mutat Res. 2003;523–524:63–74. doi: 10.1016/s0027-5107(02)00322-6. [DOI] [PubMed] [Google Scholar]
- 8.Kim C.S., Jo Y.J., Park S.H., Kim H.J., Han J.Y., Hong J.T., Cheong J.H., Oh K.W. Anti-stress effects of ginsenoside Rg3-standardized ginseng extract in restraint stressed animals. Biomol Ther. 2010;18:219–225. [Google Scholar]
- 9.Lee H., Lee J.Y., Song K.C., Kim J., Park J.H., Chun K.H., Hwang G.S. Protective effect of processed Panax ginseng, sun ginseng on UVB-irradiated human skin keratinocyte and human fibroblast. J Ginseng Res. 2012;36:68–77. doi: 10.5142/jgr.2012.36.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee J., Jung E., Lee J., Huh S., Kim J., Park M., So J., Ham Y., Jung K., Hyun C.G. Panax ginseng induces human Type I collagen synthesis through activation of Smad signaling. J Ethnopharmacol. 2007;109:29–34. doi: 10.1016/j.jep.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Lü J.M., Yao Q., Chen C. Ginseng compounds: an update on their molecular mechanisms and medical applications. Curr Vasc Pharmacol. 2009;7:293–302. doi: 10.2174/157016109788340767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karikura M., Miyase T., Tanizawa H., Taniyama T., Takino Y. Studies on absorption, distribution, excretion and metabolism of ginseng sapoinins VII. Comparison of the decomposition modes of ginsenodise-Rb1 and –Rb2 in the digestive tract of rats. Chem Pham Bull. 1991;39:2357–2361. doi: 10.1248/cpb.39.2357. [DOI] [PubMed] [Google Scholar]
- 13.Park C.S., Yoo M.H., Noh K.H., Oh D.K. Biotransformation of ginsenosides by hydrolyzing the sugar moieties of ginsenosides using microbial glycosidases. Appl Microbiol Biotech. 2010;87:9–19. doi: 10.1007/s00253-010-2567-6. [DOI] [PubMed] [Google Scholar]
- 14.Lee H.S., Kim M.R., Park Y., Park H.J., Chang U.J., Kim S.Y., Suh H.J. Fermenting red ginseng enhances its safety and efficacy as a novel skin care anti-aging ingredient: in vitro and animal study. J Med Food. 2012;15:1015–1023. doi: 10.1089/jmf.2012.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang E., Sun Z.W., Lee T.H., Shin H.S., Park S.Y., Lee D.G., Cho B.G., Sohn H., Kwon O.W., Kim S.Y. Enzyme-processed Korean Red Ginseng extracts protects against skin damage induced by UVB irradiation in hairless mice. J. Ginseng Res. 2013;37:425–434. doi: 10.5142/jgr.2013.37.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang E., Lee T.H., Park S.Y., Yi T.H., Kim S.Y. Enzyme-modified Panax ginseng inhibits UVB-induced skin aging through the regulation of procollagen type I and MMP-1 expression. Food Func. 2014;5:265–274. doi: 10.1039/c3fo60418g. [DOI] [PubMed] [Google Scholar]
- 17.Yuan C.S., Wang C.Z., Wicks S.M., Qi L.W. Chemical and pharmacological studies of saponins with a focus on American ginseng. J Ginseng Res. 2010;34:160–167. doi: 10.5142/jgr.2010.34.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tagami H. Functional characteristics of the stratum corneum in photoaged skin in comparison with those found in intrinsicaging. Arch Dermatol Res. 2008;300:S1–S6. doi: 10.1007/s00403-007-0799-9. [DOI] [PubMed] [Google Scholar]
- 19.Ichihashi M., Ueda M., Budiyanto A., Bito T., Oka M., Fukunaga M., Tsuru K., Horikawa T. UV-induced skin damage. Toxicol. 2003;189:21–39. doi: 10.1016/s0300-483x(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 20.Varani J., Spearman D., Perone P., Fligiel S.E., Datta S.C., Wang Z.Q., Shao Y., Kang S., Fisher G.J., Voorhees J.J. Inhibition of type I procollagen synthesis by damaged collagen in photoaged skin and by collagenase-degraded collagen in vitro. Am J Pathol. 2001;158:931–942. doi: 10.1016/S0002-9440(10)64040-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pittelkow M.R., Coffey R.J., Jr., Moses H.J. Keratinocytes produce and are regulated by transforming growth factors. Ann N Y Acad Sci. 1988;548:211–224. doi: 10.1111/j.1749-6632.1988.tb18809.x. [DOI] [PubMed] [Google Scholar]
- 22.Song K.C., Chang T.S., Lee H., Kim J., Park J.H., Hwang G.S. Processed Panax ginseng, sun ginseng increases type I collagen by regulating MMP-1 and TIMP-1 expression in human dermal fibroblasts. J Ginseng Res. 2012;36:61–67. doi: 10.5142/jgr.2012.36.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwok H.H., Yue P.Y., Mak N.K., Wong R.N. Ginsenoside Rb1 induces type I collagen expression through peroxisome proliferator-activated receptor-delta. Biochem Pharmacol. 2012;84:532–539. doi: 10.1016/j.bcp.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Kambayashi H., Yamashita M., Odake Y., Takada K., Funasaka Y., Ichihashi M. Epidermal changes caused by chronic low-dose UV irradiation induce wrinkle formation in hairless mouse. J Dermatol Sci. 2001;27:S19–S25. doi: 10.1016/s0923-1811(01)00113-x. [DOI] [PubMed] [Google Scholar]
- 25.Biniek K., Levi K., Dauskardt R.H. Solar UV radiation reduces the barrier function of human skin. Proc Natl Acad Sci USA. 2012;109:17111–17116. doi: 10.1073/pnas.1206851109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ovaere P., Lippens S., Vandenabeele P., Declercq W. The emerging roles of serine protease cascades in the epidermis. Trends Biochem Sci. 2009;34:453–463. doi: 10.1016/j.tibs.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 27.Kim H., Oh I., Park K.H., Kim N.M., Do J.H., Cho Y. Stimulatory effect of dietary red ginseng on epidermal hydration and ceramide levels in ultraviolet-irradiated hairless mice. J Med Food. 2009;12:746–754. doi: 10.1089/jmf.2008.1185. [DOI] [PubMed] [Google Scholar]
- 28.Kligman A.M., Balin A.K. Aging of human skin. In: Balin A.K., Kligman A.M., editors. Aging and the skin. Raven Press; New York: 1989. pp. 1–42. [Google Scholar]
- 29.Kim W.K., Song S.Y., Oh W.K., Kaewsuwan S., Tran T.L., Kim W.S., Sung J.H. Wound-healing effect of ginsenoside Rb from leaves of Panax ginseng via cyclic AMP-dependent protein kinase pathway. Eur J Pharmacol. 2013;702:285–293. doi: 10.1016/j.ejphar.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 30.Kang J., Lee N., Ahn Y., Lee H. Study on improving blood flow with Korean red ginseng substances using digital infrared thermal imaging and Doppler sonography: randomized, double blind, placebo-controlled clinical trial with parallel design. J Tradit Chin Med. 2013;33:39–45. doi: 10.1016/s0254-6272(13)60098-9. [DOI] [PubMed] [Google Scholar]
- 31.Gum S.I., Jo S.J., Ahn S.H., Kim S.G., Kim J.T., Shin H.M., Cho M.K. The potent protective effect of wild ginseng (Panax ginseng C.A. Meyer) against benzo[alpha]pyrene-induced toxicity through metabolic regulation of CYP1A1 and GSTs. J Ethnopharmacol. 2007;112:568–576. doi: 10.1016/j.jep.2007.05.014. [DOI] [PubMed] [Google Scholar]