Abstract
Background
Joboksansam, Korean bird wild ginseng, is an artificially cultivated wild ginseng germinated from bird feces. Although numerous pharmacologic activities of wild ginsengs have been reported, the beneficial effect of joboksansam in cancer has not been elucidated. In this study, we investigated the in vivo and in vitro anticancer activities of joboksansam powder.
Methods
To evaluate the in vivo anticancer activity of joboksansam, we established a xenograft mouse model bearing RMA cell-derived cancer. Direct cytotoxicity induced by joboksansam powder was also investigated in vitro using (3-4-5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide (MTT) assay. The inhibitory activity of this powder on the activation of cell survival signaling involving Akt and Src was examined with immunoblot analysis.
Results
Joboksansam powder displayed strong inhibitory activity against the increased tumor size, increased weight of total body and cancer tissues, and mortality of tumor-bearing mice. Joboksansam powder also suppressed the activation of survival regulatory enzymes Akt and Src, as assessed by phosphorylation levels in the immunoblot analysis of tumor tissues. Interestingly, the viability of RMA cells in vitro was directly decreased by joboksansam treatment.
Conclusion
Overall, our results strongly suggest that joboksansam powder has the potential to protect against cancer generation by direct cytotoxic effects on cancer cells resulting from suppression of cell survival signaling.
Keywords: anticancer activity, cytotoxicity, joboksansam, Panax ginseng, survival enzyme
1. Introduction
The root of Korean ginseng (Panax ginseng Meyer) has been used for 20 centuries in Korea, China, and Japan, and its beneficial activities, including antioxidative, anti-inflammatory, antidiabetic, antiobesity, and anticancer effects, have been widely demonstrated [1], [2]. Recent systemic studies have improved our understanding of the pharmacologic mechanisms and active components in P. ginseng roots. The efficacy value of wild ginseng was indicated to be higher than that of cultivated ginseng, although the production rate of wild ginseng was much lower. To increase the production rate of wild ginseng under cultivated conditions, we recently developed new cultivation methods for wild ginseng and obtained increased germination rates by preparing digested seeds in the feces of birds that eat ginseng berries, as in the natural process of the wild ginseng life cycle. Under these conditions, joboksansam displayed higher levels of germination than another artificially cultivated wild ginseng, sanyangsansam [3]. However, the pharmacologic value of joboksansam has not been fully elucidated, even though cultivation of this ginseng is very similar to the natural product. In this study, our aim was to test the pharmacologic activity of joboksansam by examining its anticancer activity in a xenograft mouse model bearing RMA (murine T cell lymphoma) cell-derived cancer.
2. Materials and methods
2.1. Materials
Joboksansam (10 yr old) was cultivated in Namyangju (Kyunggi, Korea). Sodium carboxymethylcellulose (Na CMC), dimethyl sulfoxide (DMSO), and (3-4-5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide (MTT) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Standard ginsenosides were purchased from Ambo Institute (Daejeon, Korea). Fetal bovine serum and RPMI 1640 were purchased from Gibco (Grand Island, NY, USA). RMA cells used in the present experiments were obtained from the American Type Culture Collection (Rockville, MD, USA). All other chemicals were from Sigma. Total or phospho-specific antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Plasmid constructs containing Src, AKT, and β-actin were used as reported previously [4], [5], [6].
2.2. Preparation of joboksansam powder
An ultrafine air mill (Turbo Mill, HKP-05; Korea Energy Technology, Seoul, Korea) was used to obtain ultrafine powder as reported previously [7], [8]. The coarse powders prepared previously were pulverized into ultrafine powder particles with sizes in the range of 0.1–50 μm in a milling chamber with a hot jacket (180°C). Feeding rate and circumferential velocity of the impeller in the grinding zone were fixed at 3 kg/h and 100 m/s, respectively. Simultaneously, a centrifugal air classification system separated the powders according to particle size. The ultrafine Joboksansam powder obtained was stored in a desiccator until use.
2.3. Cell culture
RAW264.7 and HEK293 cells were cultured in RPMI 1640 and Dulbecco's modified Eagle's medium, respectively, supplemented with 10% heat-inactivated fetal bovine serum and 1% antibiotics (penicillin and streptomycin) at 37°C under 5% CO2 [9].
2.4. Drug treatment
For in vivo experiments, joboksansam powder was suspended with 0.5% Na CMC. For in vitro cytotoxicity tests, joboksansam powder was suspended in 100% DMSO at a concentration of 100 mg/mL, and the solution was filtered prior to dilution with culture medium as reported previously [10], [11].
2.5. Xenograft mouse model experiments
All animal experiments were carried out in accordance with the National Research Council's Guidelines (IACUC, Korea) for the Care and Use of Laboratory Animals. The experimental protocol was approved by the Animal Experiments Committee of Sungkyunkwan University. C57BL/6 mice (female, 5 wk old; Orient-bio, Sungnam, Republic of Korea) were used as our xenograft animal model. Mice were housed individually on a 12-h day/12-h night cycle at 23–27°C and had access to food and water. Mice were randomly divided into two groups (n = 14/group): (1) a vehicle control group (n = 10), wherein animals received oral administration of 0.5% Na CMC; (2) a joboksansam powder treatment group (n = 14), wherein animals received oral administration of joboksansam powder (400 mg/kg). To produce tumors, each mouse was implanted with RMA cells (1 × 106 cells per animal) subcutaneously in the back next to the right hind leg. Joboksansam powder or vehicle was administered orally from Day 1 to Day 14. On the indicated days, the tumors were identified and measured with a standard caliper. Tumor volume was calculated as follows: tumor volume (mm3) = [tumor length (mm) × tumor width (mm)2]/2. Mice were sacrificed at Day 33.
2.6. Cell cytotoxicity
The cytotoxicity of joboksansam powder against RMA cells was evaluated using a conventional MTT assay as previously described [12]. Briefly, the cells were plated in 96-well plates at a density of 5 × 104 cells/well and treated with different concentrations of joboksansam powder (0, 200, 400, and 800 μg/mL) for 24 h. Absorbance was measured at 540 nm using a microplate reader.
2.7. Immunoblot analysis of total lysate from tumor
Total lysates prepared from cancer tissue were subjected to Western blot analysis of total or phospho-forms of Src and AKT as reported previously [13]. Total or phosphorylated forms of Akt, Src, and β-actin were visualized as described previously [14].
2.8. High-performance liquid chromatography analysis
For determination of ginsenosides in joboksansam powder, high-performance liquid chromatography (HPLC) was conducted as stated previously. The exact conditions of HPLC are described in Table 1.
Table 1.
Instrument and working conditions for ginsenoside analysis by high-performance liquid chromatography (HPLC)
| Instrument | Shimadzu LC-10AT HPLC system | ||
|---|---|---|---|
| Column | YMC AM303, 4.6 × 250 mm | ||
| Detector | UV/VIS detector (203 nm) | ||
| Solvent A | H2O | ||
| Solvent B | Acetonitrile | ||
| Injection volume | 20 μL | ||
| Flow rate | 1 mL/min | ||
| Gradient elution system | %A | %B | |
| Time (min) | 0 | 20 | 80 |
| 30 | 20 | 80 | |
| 35 | 35 | 65 | |
| 60 | 65 | 45 | |
| 65 | 20 | 80 | |
| 70 | 20 | 80 | |
UV/VIS, Ultraviolet/visible.
2.9. Statistical analysis
All data presented in this paper are the mean ± standard deviation of an experiment performed with 14 (Fig. 1) or three (Fig. 2, Fig. 3, Fig. 4) replicates. For statistical comparisons, the results were analyzed using analysis of variance/Scheffe post hoc test and Kruskal–Wallis/Mann–Whitney tests. A p < 0.05 was considered statistically significant. All statistical tests were carried out using the computer program SPSS (Version 22.0, 2013, IBM Corp., Armonk, NY, USA).
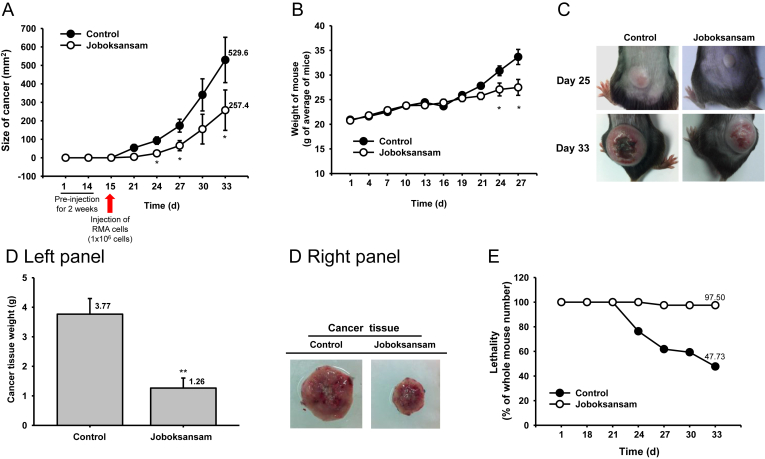
Fig. 1.
Anticancer effect of joboksansam powder in a xenograft mouse bearing RMA cell-derived cancer. Mice were injected with RMA cells (1 × 106 cells per mouse) subcutaneously into the back next to the right hind leg. Mice were then sorted into two groups (n = 14/group) and orally administered joboksansam (400 mg/kg) or vehicle. (A) Tumor size was measured on the indicated days until the experimental endpoint. (B) Weight of mice was measured on each indicated day. (C) Photographs of xenograft mice bearing RMA cell-derived cancer. (D) The cancer tissues were measured (left panel) and photographed (right panel) at 33 d. (E) Mortality of mice was determined on the indicated days. *p < 0.05, **p < 0.01 compared with control.
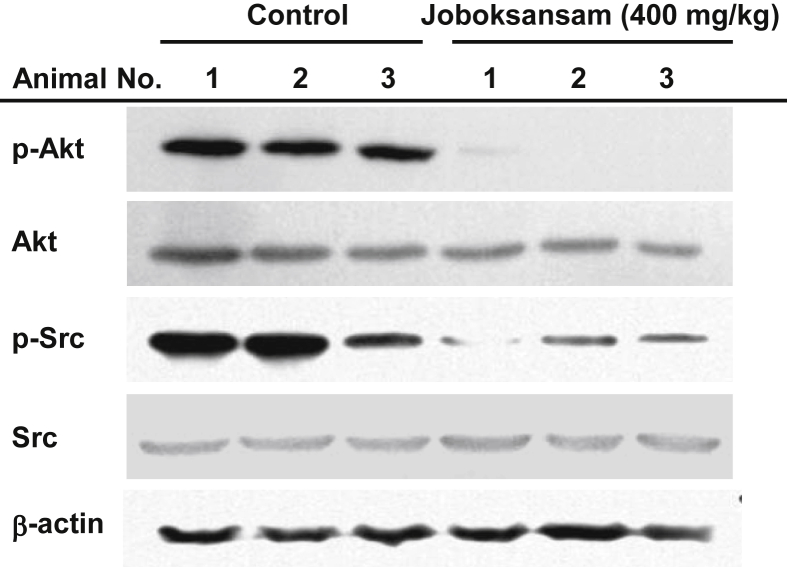
Fig. 2.
Effect of joboksansam powder on the phosphorylation level of survival signaling proteins. Levels of total and phosphorylated forms of Akt, Src, and β-actin in RMA tumor tissues were determined by immunoblot analysis.
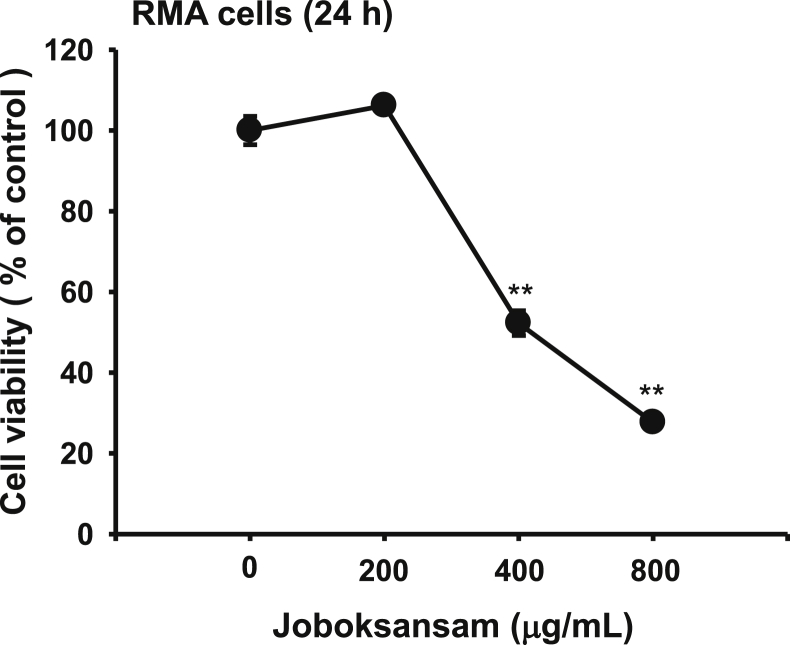
Fig. 3.
Effect of joboksansam on the viability of RMA cells. Viability of RMA cells treated with joboksansam for 24 h was determined by the MTT assay. **p < 0.01 compared with the control group. MTT, (3-4-5-Dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide.
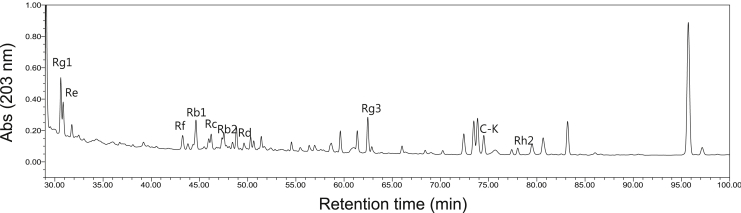
Fig. 4.
HPLC profile of joboksansam. Ginsenosides from joboksansam powder were identified by HPLC analysis. HPLC, High performance liquid chromatography.
3. Results and discussion
To determine whether joboksansam suppresses the development of cancer, we established a xenograft mouse model bearing RMA cell-derived cancer according to a previously published method [15]. Although RMA cells are a subline of the Raucher virus-induced T cell lymphoma RBL-5 (H-2b) [16], the mouse model exhibited a striking increase in tumor size and animal body weight (Fig. 1), as reported previously [15]. Interestingly, orally administered joboksansam powder (400 mg/kg) clearly suppressed the enhanced tumor size (Fig. 1A), suppressed the increased weight of total body and cancer tissues (Figs. 1B–1D), and decreased the mortality of tumor-bearing mice (Fig. 1E), implying that orally administered joboksansam might have anticancer activity. To date, numerous studies have demonstrated that wild and cultivated ginsengs, as well as processed ginsengs (red ginseng and fermented ginseng with Phellinus linteus), are good herbal medicines with anticancer activity [17], [18]. Because the mass and size of tumors were markedly decreased under our conditions, we next investigated whether the powder had an antiproliferative effect on cancer cells. For this purpose, we measured the activation levels of cell survival regulatory proteins such as Akt and Src [19], [20] by immunoblot analysis using phospho-specific antibodies that specifically recognize their active forms. As shown in Fig. 2, the levels of phospho-forms of Akt and Src were remarkably decreased in the joboksansam-treated group compared with the control group, implying that survival signaling might be decreased in joboksansam-treated tumor cells. As with other ginseng roots, these results strongly suggest that wild ginseng as well as joboksansam could be a useful herbal medicine for protection from tumorigenic responses. As cancer is one of the major diseases affecting human life span in most countries [21], our data indicate that long-term administration of ginseng might help lower the incidence of cancer diseases, as reported previously in the case of colorectal cancer [18].
Ginseng-derived anticancer activity is generally known to result from activation of the body's antitumor immune regulatory cells, including natural killer cells and macrophages [22], [23], [24]. In addition, specific components in ginseng or ginseng-derived preparations such as compound K, ginsenoside (G) Rh1, G-F2, G-Rg3, and G-Rp1 were shown to induce apoptosis of cancer cells and were proposed to contribute to the anticancer properties of ginseng [25], [26], [27], [28], [29]. Therefore, we examined whether the antiproliferative activity of joboksansam powder involves a direct cytotoxic effect using an in vitro cell proliferation assay. Intriguingly, treatment with joboksansam at 400 and 800 μg/mL suppressed the viability of RMA cells in a dose-dependent manner, implying that direct cytotoxicity induced by joboksansam might be one of the major factors contributing to its anticancer activity. Indeed, HPLC analysis clearly indicated that apoptosis-inducing ginsenosides [e.g., compound K (C–K), G-Rg3, and G-Rh2] are present in joboksansam powder (Table 2 and Fig. 4). The compounds in joboksansam powder that contribute to its active anticancer activity and the underlying antiproliferative mechanism have not yet been verified. Therefore, we will further investigate major anticancer components using activity-guided fractionation with in vitro cell proliferation assays and apoptosis-inducing patterns using apoptosis biomarkers such as AMP-activated protein kinase, Mcl-1, and caspases [30], [31].
Table 2.
Composition of ginsenosides in joboksansam powder
| Ginsenosides (mg/g dry weight) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rg1 | Re | Rf | Rb1 | Rb2 | Rc | Rd | Rg3 | Rh2 | C–K | total |
| 3.3 | 0.74 | 0.70 | 1.56 | 0.48 | 0.96 | 0.78 | 0.92 | 0.70 | 1.30 | 13.44 |
C–K, Compound K.
In summary, joboksansam powder displayed strong anticancer activity in a xenograft mouse model bearing RMA cell-derived cancer and exhibited direct suppression of RMA cell viability. Immunoblot analysis indicated that survival signaling pathways composed of AKT and Src are inhibited by joboksansam treatment. These results strongly imply that joboksansam might have a potential role in preventing the generation of cancer. Additional studies will focus on the active components in joboksansam as well as other pharmacologic effects such as anti-inflammatory activities.
Conflicts of interest
Authors declare no conflicts of interest.
Contributor Information
Jong-Hoon Kim, Email: jhkim1@chonbuk.ac.kr.
Jae Youl Cho, Email: jaecho@skku.edu.
References
- 1.Yang Y., Yang W.S., Yu T., Sung G.-H., Park K.W., Yoon K., Son Y.-J., Hwang H., Kwak Y.-S., Lee C.-M. ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean red ginseng water extract. J Ethnopharmacol. 2014;154:218–228. doi: 10.1016/j.jep.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Lee C.H., Kim J.H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38:161–166. doi: 10.1016/j.jgr.2014.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park KT, Park DJ, Cultivation method of ginseng sprout. Korea Patent 2013; 1013190820000.
- 4.Lee J.Y., Lee Y.G., Lee J., Yang K.J., Kim A.R., Kim J.Y., Won M.H., Park J., Yoo B.C., Kim S. Akt Cys-310-targeted inhibition by hydroxylated benzene derivatives is tightly linked to their immunosuppressive effects. J Biol Chem. 2010;285:9932–9948. doi: 10.1074/jbc.M109.074872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Y., Yang W.S., Yu T., Yi Y.S., Park J.G., Jeong D., Kim J.H., Oh J.S., Yoon K., Cho J.Y. Novel anti-inflammatory function of NSC95397 by the suppression of multiple kinases. Biochem Pharmacol. 2014;88:201–215. doi: 10.1016/j.bcp.2014.01.022. [DOI] [PubMed] [Google Scholar]
- 6.Kim H.G., Yang W.S., Sung G.H., Kim J.H., Baek G.S., Kim E., Yang S., Park Y.C., Sung J.M., Yoon D.H. IKK beta-targeted anti-inflammatory activities of a butanol fraction of artificially cultivated Cordyceps pruinosa fruit bodies. Evid Based Complement Alternat Med. 2014;2014:562467. doi: 10.1155/2014/562467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi K.O., Lee I., Paik S.Y., Kim D.E., Lim J.D., Kang W.S., Ko S. Ultrafine Angelica gigas powder normalizes ovarian hormone levels and has antiosteoporosis properties in ovariectomized rats: particle size effect. J Med Food. 2012;15:863–872. doi: 10.1089/jmf.2011.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piao J., Lee J.Y., Weon J.B., Ma C.J., Ko H.J., Kim D.D., Kang W.S., Cho H.J. Angelica gigas Nakai and Soluplus-based solid formulations prepared by hot-melting extrusion: oral absorption enhancing and memory ameliorating effects. PLoS One. 2015;10:e0124447. doi: 10.1371/journal.pone.0124447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hossen M.J., Jeon S.H., Kim S.C., Kim J.H., Jeong D., Sung N.Y., Yang S., Baek K.S., Kim J.H., Yoon D.H. In vitro and in vivo anti-inflammatory activity of Phyllanthus acidus methanolic extract. J Ethnopharmacol. 2015;168:217–228. doi: 10.1016/j.jep.2015.03.043. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y., Yu T., Jang H.J., Byeon S.E., Song S.Y., Lee B.H., Rhee M.H., Kim T.W., Lee J., Hong S. In vitro and in vivo anti-inflammatory activities of Polygonum hydropiper methanol extract. J Ethnopharmacol. 2012;139:616–625. doi: 10.1016/j.jep.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Hossen M.J., Kim S.C., Son Y.J., Baek K.S., Kim E., Yang W.S., Jeong D., Park J.G., Kim H.G., Chung W.J. AP-1-targeting anti-Inflammatory activity of the methanolic extract of Persicaria chinensis. Evid Based Complement Alternat Med. 2015;2015:608126. doi: 10.1155/2015/608126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim S.H., Park J.G., Lee J., Yang W.S., Park G.W., Kim H.G., Yi Y.S., Baek K.S., Sung N.Y., Hossen M.J. The dietary flavonoid Kaempferol mediates anti-inflammatory responses via the Src, Syk, IRAK1, and IRAK4 molecular targets. Mediators Inflamm. 2015;2015:904142. doi: 10.1155/2015/904142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu B., Xu Y., Li W.L., Zeng L. Proteomic analysis of differentially expressed skin proteins in iRhom2(Uncv) mice. BMB Rep. 2015;48:19–24. doi: 10.5483/BMBRep.2015.48.1.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang W.S., Ko J., Kim E., Kim J.H., Park J.G., Sung N.Y., Kim H.G., Yang S., Rho H.S., Hong Y.D. 21-O-Angeloyltheasapogenol E3, a novel triterpenoid saponin from the seeds of tea plants, inhibits macrophage-mediated inflammatory responses in a NF-kappaB-dependent manner. Mediators Inflamm. 2014;2014:658351. doi: 10.1155/2014/658351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Renukaradhya G.J., Sriram V., Du W., Gervay-Hague J., Van Kaer L., Brutkiewicz R.R. Inhibition of antitumor immunity by invariant natural killer T cells in a T-cell lymphoma model in vivo. Int J Cancer. 2006;118:3045–3053. doi: 10.1002/ijc.21764. [DOI] [PubMed] [Google Scholar]
- 16.Ljunggren H.G., Karre K. Experimental strategies and interpretations in the analysis of changes in MHC gene expression during tumour progression. Opposing influences of T cell and natural killer mediated resistance? J Immunogenet. 1986;13:141–151. doi: 10.1111/j.1744-313x.1986.tb01095.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee J.J., Kwon H.K., Jung I.H., Cho Y.B., Kim K.J., Kim J.L. Anti-cancer activities of ginseng extract Fermented with Phellinus linteus. Mycobiology. 2009;37:21–27. doi: 10.4489/MYCO.2009.37.1.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C.Z., Yuan C.S. Potential role of ginseng in the treatment of colorectal cancer. Am J Chin Med. 2008;36:1019–1028. doi: 10.1142/S0192415X08006545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baek K.S., Ahn S., Lee J., Kim J.H., Kim H.G., Kim E., Sung N.Y., Yang S., Kim M.S., Hong S. Immunotoxicological effects of aripiprazole: in vivo and in vitro studies. Korean J Physiol Pharmacol. 2015;19:365–372. doi: 10.4196/kjpp.2015.19.4.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim Y.R., Eom K.S. Simultaneous inhibition of CXCR4 and VLA-4 exhibits combinatorial effect in overcoming stroma-mediated chemotherapy resistance in mantle cell lymphoma cells. Immune Netw. 2014;14:296–306. doi: 10.4110/in.2014.14.6.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang G.D., Lai D.J., Burau K.D., Du X.L. Potential gains in life expectancy from reducing heart disease, cancer, Alzheimer's disease, kidney disease or HIV/AIDS as major causes of death in the USA. Public Health. 2013;127:348–356. doi: 10.1016/j.puhe.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Kang S., Min H. Ginseng, the 'immunity boost': the effects of Panax ginseng on immune system. J Ginseng Res. 2012;36:354–368. doi: 10.5142/jgr.2012.36.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byeon S.E., Lee J., Kim J.H., Yang W.S., Kwak Y.S., Kim S.Y., Choung E.S., Rhee M.H., Cho J.Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediators Inflamm. 2012;2012:732860. doi: 10.1155/2012/732860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim M.Y., Cho J.Y. 20S-Dihydroprotopanaxadiol, a ginsenoside derivative, boosts innate immune responses of monocytes and macrophages. J Ginseng Res. 2013;37:293–299. doi: 10.5142/jgr.2013.37.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H.S., Lim J.M., Kim J.Y., Kim Y., Park S., Sohn J. Panaxydol, a component of Panax ginseng, induces apoptosis in cancer cells through EGFR activation and ER stress and inhibits tumor growth in mouse models. Int J Cancer. 2016;138:1432–1441. doi: 10.1002/ijc.29879. [DOI] [PubMed] [Google Scholar]
- 26.Choi K., Choi C. Proapoptotic ginsenosides compound K and Rh enhance Fas-induced cell death of human astrocytoma cells through distinct apoptotic signaling pathways. Cancer Res Treat. 2009;41:36–44. doi: 10.4143/crt.2009.41.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim M.Y., Yoo B.C., Cho J.Y. Ginsenoside-Rp1-induced apolipoprotein A-1 expression in the LoVo human colon cancer cell line. J Ginseng Res. 2014;38:251–255. doi: 10.1016/j.jgr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J.H., Nao J.F., Zhang M., He P. 20(s)-ginsenoside Rg3 promotes apoptosis in human ovarian cancer HO-8910 cells through PI3K/Akt and XIAP pathways. Tumour Biol. 2014;35:11985–11994. doi: 10.1007/s13277-014-2497-5. [DOI] [PubMed] [Google Scholar]
- 29.Mao Q., Zhang P.H., Wang Q., Li S.L. Ginsenoside F(2) induces apoptosis in humor gastric carcinoma cells through reactive oxygen species–mitochondria pathway and modulation of ASK-1/JNK signaling cascade in vitro and in vivo. Phytomedicine. 2014;21:515–522. doi: 10.1016/j.phymed.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y.J., Lee S.H. Resveratrol and clofarabine induces a preferential apoptosis-activating effect on malignant mesothelioma cells by Mcl-1 down-regulation and caspase-3 activation. BMB Rep. 2015;48:166–171. doi: 10.5483/BMBRep.2015.48.3.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wi S.M., Lee K.Y. 5-Aminoimidazole-4-carboxamide riboside induces apoptosis through AMP-activated protein kinase-independent and NADPH oxidase-dependent pathways. Immune Netw. 2014;14:241–248. doi: 10.4110/in.2014.14.5.241. [DOI] [PMC free article] [PubMed] [Google Scholar]