Abstract
Background
Ginsenoside Rg3 and arginine–fructose (Arg-Fru) are known as the hypotensive compounds of Panax ginseng; however, their efficacy on antihypertension has not been reported yet to our best knowledge. Thus, hypotensive components-enriched fraction of red ginseng (HCEF-RG) was prepared from fine root concentrate (FR) and their antihypertensive effects were investigated in spontaneously hypertensive rats (SHR).
Methods
Male SHRs were divided into six groups: control (Wistar Kyoto, SHR); FR 500; FR 1,000; HCEF-RG 500; and HCEF-RG 1,000; samples (mg/kg body weight) were orally administered every day for 8 wk. Blood pressure was monitored at 1 wk, 2 wk, 3 wk, 4 wk, 6 wk, and 8 wk by tail cuff method. At 8 wk after samples administration, mice were killed for the measurement of renin activity (RA), angiotensin-I converting enzyme inhibition, angiotensin II, and nitric oxide (NO) levels in plasma.
Results
HCEF-RG with four-fold more Rg3 and 24-fold more Arg-Fru contents was successfully prepared from reacted mixtures of FR and persimmon vinegar (12 times against FR, v/v) at 80°C for 18 h. Both FR 1,000 and HCEF-RG 1,000 showed lowered systolic blood pressure than SHR control group and HCEF-RG 1,000 group exhibited a significant decrease in diastolic blood pressure. RA was significantly lowered in all treated groups, while angiotensin II did not affect by FR and HCEF-RG treatment. However, angiotensin-I converting enzyme inhibition and NO in FR 1,000 and HCEF-RG 1,000 were significantly increased compared with SHR control group.
Conclusion
HCEF-RG is more effective and useful for alleviating hypertension than FR, implying the health benefit of Rg3 and Arg-Fru.
Keywords: arginine–fructose, blood pressure, ginsenoside Rg3, hypotensive components-enriched, Korean Red Ginseng
1. Introduction
The incidence of various age-related degenerative neurological and cardiovascular diseases continues to increase significantly as lifestyles change and the population ages. Hypertension is characterized by an increase in arterial blood pressure due to increased cardiac output or peripheral vasoconstriction. The incidence of hypertension as a disease is low; however, this disorder has been strongly associated with stroke, myocardial infarction, diabetes, and heart failure. Hypertension also leads to cardiac and vascular muscle hypertrophy, as well as arteriosclerosis [1]. The vascular complications of hypertension are exacerbated by concomitant vascular injuries such as hyperlipidemia or hyperglycemia, which are attributable to cell characteristics, expression of autocrine or paracrine growth hormones, and changes in growth hormone receptors [2].
Clinically, hypertension is defined as a condition in which the systolic blood pressure exceeds 120 mmHg and the diastolic pressure exceeds 80 mmHg. Blood pressure is mainly modulated by the renin–angiotensin systems (RAS) and the vasopressin system. Angiotensin-converting enzyme (ACE), which plays a key role in the RAS, converts angiotensin I to angiotensin II and the dipeptide histidine–leucine. To treat hypertension, ACE inhibitors such as enalapril, captopril, ramipril, and lisinopril have been developed and commercialized; however, these drugs are associated with side effects such as changes in taste, leukopenia, vascular edema, liver function abnormalities, and dry cough [3]. New antihypertensive drugs with established efficacy and safety are necessary to improve treatment regimens for patients that suffer from hypertension. Various in vitro and in vivo studies have examined the antihypertensive activities of different plants such as Gastrodia elata [4], Alisma canaliculatum [5], and Monascus [6].
The persimmon tree (Diospyros kaki) produces fruits in the fall season; as described in Oriental medicine, the fruits are characterized by a sweet and refreshing taste, with no poisonous substances. In particular, a colloidal fluid from unripe persimmon fruits has been used for treating hypertension [7]. In Japan, persimmon juice has been traditionally used to treat hypertension and prevent heart attacks [8]. The hypotensive effect of Panax ginseng is attributable to the saponin, ginsenoside Rg3, which could be converted by acidic hydrolysis from protopanaxadiol-type saponins and exhibits protective effects against hypertension [9], [10], [11], [12], [13], [14]. Furthermore, arginine–fructose (Arg-Fru) produced from arginine–fructose–glucose (Arg-Fru-Glc) by the Maillard reaction under acidic condition is known to be a specific active compound in Korean Red Ginseng [15]. Arg-Fru is absorbed into the small intestine after release from Arg-Fru-Glc by maltase and then participates in the nitric oxide (NO)-mediated vasodilation [16]. Therefore, if ginsenoside Rg3-Arg-Fru-enrichment materials can be produced, they will be useful in alleviating hypertension and consequently allowing the food industry to develop new hypotensive compounds-fortified products. Thus, in this study, we prepared a hypotensive components-enriched fraction from red ginseng (HCEF-RG) under various conditions with persimmon vinegar and evaluated the ability of HCEF-RG to improve the hypertension in spontaneously hypertensive rats (SHR).
2. Materials and methods
2.1. Materials
The red ginseng tail root (4-year-old, 2010) was provided by Cheon Ji Yang (Seoul, Korea) and Korean persimmon vinegar (pH 3.6) was purchased from Nong Hyup (Wanju, Korea). Other reagents were purchased from Sigma–Aldrich (St. Louis, MO, USA).
2.2. Preparation of HCEF-RG
The antihypertensive fractions with enriched ginsenoside Rg3 were made by modifying a method described by Ko et al [13]. To obtain the fine root concentrates (FR), red ginseng tail root (10 kg) was added to 70% ethanol (100 L, v/w) and extracted at 60 ± 2°C for 8 h. The extracts were centrifuged at 2,250g for 30 min and the supernatant was collected and then concentrated in a rotary evaporator at 60°C. FR was subjected to the preparation for antihypertensive fractions by mixing with persimmon vinegar (12 times, v/v) and then reacting in a water bath (WEB Multi-purpose Extraction Water Bath, Daihan, Korea) with a reflux condenser at 70°C, 80°C, and 90°C for 3 h, 6 h, 12 h, 18 h, 24 h, and 48 h.
2.3. Ginsenoside analysis by HPLC
Sep-Pak C18 cartridge (Waters, Milford, MA, USA) was used to pretreat the sample and then a C18 cartridge with 5 mL of 100% methanol and 15 mL of distilled water was gradually activated. The resulting samples (5 mL) were loaded onto the C18 cartridge, and gradually washed thoroughly with 15 mL of distilled water and 20 mL of 30% methanol. Next, crude saponin was used to rinse with 5 mL of 100% methanol. The fractions were filtered by a 0.22-μm membrane filter and the filtering fractions from methanol and distilled water were used for ginsenoside and Arg-Fru, respectively.
The Waters 486 Tunable Absorbance Detector HPLC system (Waters) equipped with an YMC-Pack Pro C18 column (4.6 mm × 250 mm, 5 μm; Waters) was used for ginsenoside separation. The detection wavelength was set at 203 nm and the solvent flow rate was held constant at 1.6 mL/min and the temperature of the column was set at 45°C. The mobile phase used for the separation consisted of solvent A (acetonitrile) and solvent B (water). A gradient elution procedure was used at 0 min, 15%; 0–14 min, 15–20%; 14–17 min, 20–39%; 17–57 min, 39–48%; 57–70 min, 48–70%; 70–80 min, 70–100%; 80–120 min, 100–60%; and 120–130 min, 60–15%. The injection volume was 20 μL. Standard ginsenoside materials (Embo Laboratory, Daejeon, Korea) were prepared in HPLC-grade methanol.
For the analysis of Arg-Fru, the Amperometric Detector HPLC system (Waters) equipped with an CarboPac PA-1 column (4 mm × 250 mm, 5 μm; Waters) was used. The solvent flow rate with the isocratic mobile phase (water: 250mM NaOH = 50:50, v/v) was held constant at 1.0 mL/min and the temperature of the column was set at 30°C. The injection volume was 5 μL and the working and reference electrode were Au and Ag/AgCl, respectively.
2.4. Animals and diets
The experiment was performed using 8-wk-old male Wistar Kyoto rats (WKY; normal group, male, 130–175 g body weight; Charles River Co., Kanawa, Japan) and SHR (hypertension group, male, 180–215 g body weight, Orient Bio Co. Ltd., Seoul, Korea). The animals were allowed to acclimate for a wk and then randomly divided into six groups (n = 8 each): WKY-control; SHR-control; SHR- FR 500; SHR- FR 1,000; SHR- HCEF-RG 500; and SHR- HCEF-RG 1,000. The animals were housed individually in stainless steel cages arranged in a randomized complete block design at a temperature of 23 ± 1°C and humidity of 53 ± 2% in a light-controlled room under a 12 h light–dark cycle. The animals had access to food (18% protein, 2018S; Harlan Laboratories Inc., Indianapolis, IN, USA) and water ad libitum. The control groups (WKY, SHR) were provided with sterile distilled water, and the other groups (FR, HCEF-RG) were given forced oral administration (500 mg/kg, 1,000 mg/kg bodyweight) using a disposable syringe for 8 wk. The weight and dietary intake were measured once per wk. At the end of the experimental period, the animals were anesthetized with isoflurane, nitrogen, and oxygen withholding food for 12 h, and blood samples were taken from the inferior vena cava to determine the levels of plasma biomarkers. The blood sample was centrifuged in a tube with EDTA coating at 1,006g for 10 min to remove plasma, and the resulting sample was stored at −80°C before analysis. The care and treatment of rats were approved by the Woojung Life Science Research Center Animal Care Committee (Suwon-si, Gyeonggi-do, Korea) (WJIACUC110818-03-04), and the procedures were in accordance with the Korean Guide for the Care and Use of Laboratory Animals.
2.5. Heart rate and blood pressure
Heart rate, systolic blood pressure, and diastolic blood pressure were measured at 1 wk, 2 wk, 3 wk, 4 wk, 6 wk, and 8 wk by electrosphygmomanometer (Letica LE 5002; Panlab, Barcelona, Spain). After stabilization for 15 min at 37°C in a mold, blood pressure was measured by the tail cuff method.
2.6. Renin activity
The plasma (200 μL) was added to 200 μL of precooled enzymatic inhibitor and was divided into two equal volumes of 200 μL each. Then the mixture was allowed to react for 1 h at 37°C and another mixture was allowed to react for 1 h at 4°C. Both mixtures (75 μL) were added to antibody coated tube and 100 μL of antigen 125I solution was added to the antibody coated tube containing each mixture. Then the resulting solution was put in a shaking water bath (280 rpm) at 25°C for 2 h. Plasma renin activity was calculated using 1470 WIZARD automatic gamma which was determined by counting the radioactivity (cpm) for 1 min.
2.7. Inhibitory activity of ACE
Sodium borate buffer (50mM) containing 100 μL of plasma and 300mM of NaCl was added to the 400 μL (hippuryl-L-histidyl-L-leucine, 7mM) of substrate and incubated for 30 min in 37°C and was then added to 500 μL of 1N HCl. The resulting solution was mixed with 3 mL of ethyl acetate, vortexed strongly for 60 s, and centrifuged at 11178g for 5 min. The supernatant (1 mL) was evaporated on an 80°C heating block, 500 μL of distilled water was added, and then the absorbance was read at 228 nm.
2.8. Angiotensin II levels
The plasma angiotensin II levels were measured using ELISA kit EA3501-1 (Assaypro, St Charles, MO, USA). The plasma sample (25 μL) was added to the plate coated with angiotensin II antibody, 25 μL of biotinylated angiotensin II solution was directly added to the plate, and then incubated for 2 h. After washing with 200 μL of wash buffer, the buffer was removed using a paper towel and the resulting contents were incubated with 50 μL of streptavidin–peroxidase conjugate for 50 min at room temperature. After adding 50 μL of chromogen substrate, they were incubated for 10 min and then the absorbance was read at 450 nm.
2.9. Nitric oxide concentration
The amount of nitrite in the supernatant was measured using a commercially available NO detection kit (iNtRON biotechnology, Seongnam, Korea). After filtering plasma through a 0.22 mm-size filter, 100 μL of N1 buffer was added to each well, and the plate was incubated at room temperature for 10 min. N2 buffer was then added and the plate was incubated at room temperature for 10 min. The absorbance of the content of each well was measured at 540 nm using a Sunrise absorbance reader (TECAN, Salzburg, Austria).
2.10. Statistical analysis
All experiments were performed in triplicate and the data are represented as the mean ± standard deviation. The significance of differences (p < 0.05) among the corresponding mean values was determined using one-way analysis of variance (ANOVA) followed by Duncan's multiple comparison test or Student t test (SPSS, version 17.0; SPSS Inc., Chicago, IL, USA).
3. Results and discussion
3.1. HCEF-RG preparation
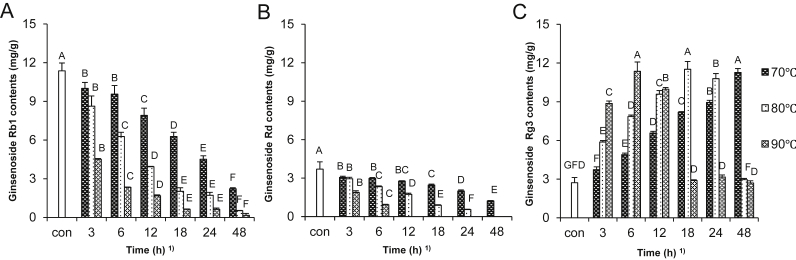
The contents of ginsenosides in the antihypertensive fractions prepared by various reaction conditions were investigated, as presented in Table 1. The contents of protopanaxadiol-type ginsenosides (Rb1, Rb2, Rc, and Rd) decreased with increasing reaction temperature and time. Furthermore, Rb2, Rc, and Rd were disappeared after 24 h and 6 h reaction time at 80°C and 90°C, respectively. Among protopanaxatriol groups, Rf contents decreased but the amounts of Rg3 and Rh2 increased with increasing reaction temperature and time. Moreover, Rg3 and Rh2 were significantly lowered after 24 h and 12 h reaction time at 80°C and 90°C, respectively. Even though there were the changes in the contents of each ginsenoside by treatment conditions, total ginsenoside levels decreased after treatment. However, the main concern in this study was the fortification of Rg3 contents as a hypotensive component. As can be seen in Fig. 1, the changes of Rb1 and Rd were closely related to formation of Rg3. The contents of Rb1 and Rd showed a time-dependent decrease at three temperatures, while Rg3 contents increased over time except at 90°C. Furthermore, the maximum Rg3 level was observed at 48 h at 70°C, 18 h at 80°C, and 6 h at 90°C. Kim et al [17] reported that Rb1 was converted to Rg3 when heat and acid treatments were administered concomitantly. At constant time and heat treatment at 0–130°C, the contents of Rc, Rd, and Re increased, although even these decreased at 105°C; however, the concentration of Rg2 and Rg3 increased with temperature. In addition, when ginseng root was treated at 37°C, 60°C, and 80°C using various acids including acetic acid, citric acid, lactic acid, tartaric acid, and HCl, Rg3 contents increased in all the treated groups, except for the 80°C HCl group. Treatment at 60°C with citric acid for 5 h resulted in a 3.6-fold increase in Rg3 content [12].
Table 1.
Ginsenoside contents in the hypotensive fractions from fine root concentrates treated with persimmon vinegar at various temperature and time1)
| Temp (°C) | Time (h) | Ginsenoside (mg/g) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Rb1 | Rb2 | Rc | Rd | Rf | Rg3 | Rh2 | Total | ||
| No treatment | 11.37 ± 0.61a | 5.58 ± 1.48a | 10.52 ± 1.13a | 3.70 ± 0.57a | 2.00 ± 0.41a | 2.72 ± 0.40j | 5.88 ± 0.63f | 40.96 ± 4.09a | |
| 70 | 3 | 10.01 ± 0.45b | 3.66 ± 0.18b | 9.11 ± 0.41b | 3.06 ± 0.09b | 1.66 ± 0.11b | 3.73 ± 0.21i | 6.24 ± 0.88f | 37.49 ± 1.90a |
| 6 | 9.56 ± 0.66b | 3.28 ± 0.25bc | 8.19 ± 0.19c | 2.98 ± 0.65bc | 1.67 ± 0.77b | 4.89 ± 0.12h | 6.88 ± 0.87f | 37.47 ± 1.30a | |
| 12 | 7.91 ± 0.55d | 2.70 ± 0.17cd | 6.24 ± 0.42d | 2.76 ± 0.03c | 1.68 ± 0.07b | 6.57 ± 0.13f | 10.00 ± 0.78d | 37.88 ± 1.75a | |
| 18 | 6.27 ± 0.32e | 2.13 ± 0.24de | 4.35 ± 0.44e | 2.46 ± 0.07d | 1.57 ± 0.06bc | 8.18 ± 0.47e | 9.86 ± 1.16d | 34.84 ± 1.51b | |
| 24 | 4.51 ± 0.27f | 1.25 ± 0.37f | 2.93 ± 0.32f | 1.99 ± 0.09e | 1.35 ± 0.06cd | 8.94 ± 0.16d | 10.74 ± 0.36d | 31.73 ± 0.72cd | |
| 48 | 2.22 ± 0.08gh | 0.32 ± 0.01g | 0.65 ± 0.03h | 1.22 ± 0.03g | 1.22 ± 0.10de | 11.27 ± 0.30a | 14.45 ± 0.59a | 31.36 ± 0.92cd | |
| 80 | 3 | 8.62 ± 0.79c | 3.05 ± 0.29bc | 6.57 ± 0.42d | 2.95 ± 0.11bc | 1.70 ± 0.11b | 5.87 ± 0.11g | 8.57 ± 0.51e | 37.35 ± 1.45a |
| 6 | 6.27 ± 0.33e | 1.94 ± 0.10e | 4.09 ± 0.34e | 2.33 ± 0.06d | 1.71 ± 0.17b | 7.85 ± 0.11l | 10.36 ± 0.55d | 34.57 ± 0.24b | |
| 12 | 3.93 ± 0.23f | 0.99 ± 0.14f | 2.02 ± 0.78g | 1.73 ± 0.08f | 1.51 ± 0.10bc | 9.59 ± 0.27c | 13.30 ± 0.71b | 33.09 ± 0.85bc | |
| 18 | 2.02 ± 0.27gh | 0.07 ± 0.06g | 0.33 ± 0.02h | 0.88 ± 0.35h | 1.02 ± 0.09ef | 11.51 ± 0.60a | 14.57 ± 0.31a | 30.43 ± 0.52d | |
| 24 | 1.71 ± 0.22gh | 0.00 ± 0.00g | 0.00 ± 0.00h | 0.55 ± 0.03i | 0.84 ± 0.13f | 10.79 ± 0.38b | 12.84 ± 0.23bc | 26.74 ± 0.91e | |
| 48 | 0.52 ± 0.20i | 0.00 ± 0.00g | 0.00 ± 0.00h | 0.00 ± 0.00j | 0.11 ± 0.10g | 2.96 ± 0.09j | 3.51 ± 0.01g | 7.12 ± 0.16f | |
| 90 | 3 | 0.45 ± 0.05f | 1.08 ± 0.08f | 2.01 ± 0.25g | 1.88 ± 0.13ef | 1.49 ± 0.09bc | 8.86 ± 0.18d | 10.71 ± 0.18d | 30.57 ± 0.38cd |
| 6 | 2.33 ± 0.23g | 0.00 ± 0.00g | 0.00 ± 0.00h | 0.93 ± 0.03h | 2.48 ± 0.15bc | 11.36 ± 0.71a | 15.44 ± 0.94a | 31.55 ± 1.51cd | |
| 12 | 1.68 ± 0.06h | 0.00 ± 0.00g | 0.00 ± 0.00h | 0.00 ± 0.00j | 0.93 ± 0.05f | 9.97 ± 0.13c | 11.93 ± 1.00c | 24.53 ± 1.03e | |
| 18 | 0.63 ± 0.02i | 0.00 ± 0.00g | 0.00 ± 0.00h | 0.00 ± 0.00j | 0.12 ± 0.11g | 2.90 ± 0.04j | 3.62 ± 0.08g | 7.29 ± 0.12f | |
| 24 | 0.63 ± 0.09i | 0.00 ± 0.00g | 0.00 ± 0.00h | 0.00 ± 0.00j | 0.04 ± 0.07g | 3.16 ± 0.16j | 4.20 ± 0.39g | 8.04 ± 0.49f | |
| 48 | 0.16 ± 0.14i | 0.00 ± 0.00g | 0.00 ± 0.00h | 0.00 ± 0.00j | 0.00 ± 0.00g | 2.70 ± 0.11j | 3.84 ± 0.30g | 6.71 ± 0.29f | |
Data are presented as mean ± SD.
Values with different superscripts within the same column are significantly different among samples at α = 0.05 level by Duncan's multiple range test.
Fig. 1.
(A) Changes of ginsenosides Rb1, (B) Rd, and (C) Rg3 in fine root concentrates treated with persimmon vinegar at various temperature and time. Data are expressed as mean ± SD. 1) Values with different superscripts in the same bar are significantly different among samples at α = 0.05 level by Duncan's multiple range test. Con, control.
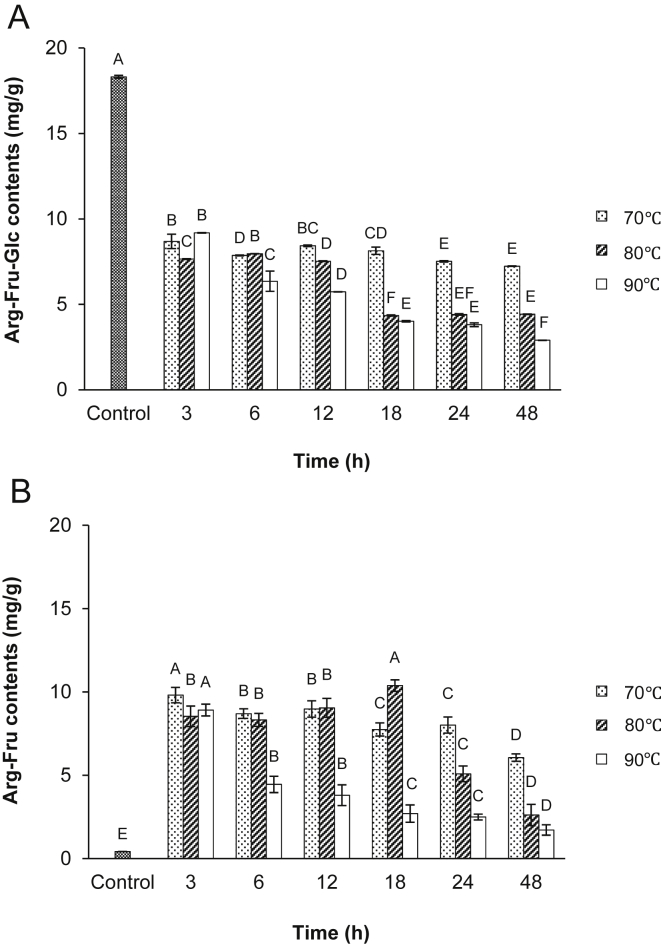
In Fig. 2, the contents of Arg-Fru-Glc decreased with increasing reaction temperature and time as compared with the control, while Arg-Fru increased as compared with control. Specially, Arg-Fru was fully released from Arg-Fru-Glc under acidic condition at 70°C for 3 h and at 80°C for 18 h, indicating 24-fold more levels than the control.
Fig. 2.
(A) Contents of arginine–fructose–glucose (Arg-Fru-Glc) and (B) arginine–fructose (Arg-Fru) in fine root concentrates treated with persimmon vinegar at various temperature and time. Data are expressed as mean ± SD. Values with different superscripts in the same bar are significantly different among samples at α = 0.05 level by Duncan's multiple range test.
Therefore, in this study, we determined the reaction condition of 18 h at 80°C with 12 times the amount of persimmon vinegar for Rg3 and Arg-Fru formation for enhancing hypotensive component in red ginseng considering the efficiency of reaction temperature and time. Then, HCEF-RG was compared for antihypertensive effects of FR using an SHR animal model system.
3.2. Heart rate and blood pressure
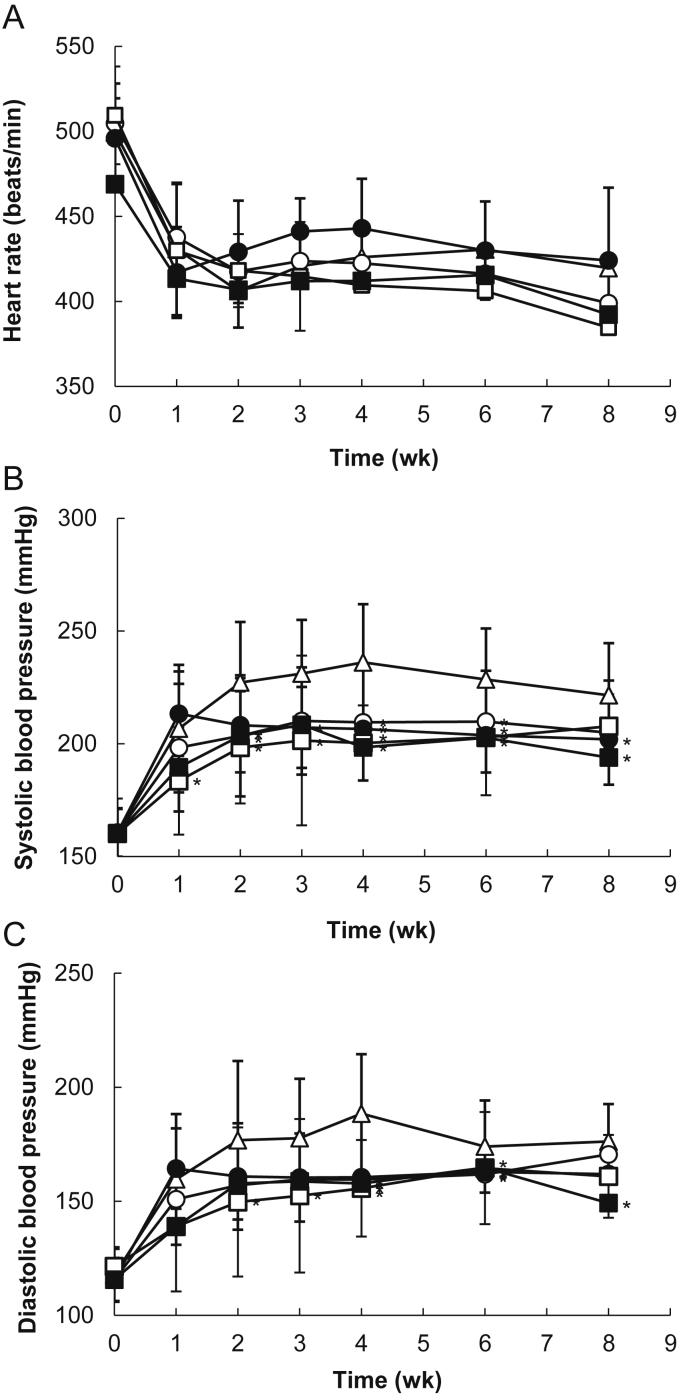
Fig. 3 shows the effect of the FR or HCEF-RG treatment, taken for 8 wk, on heart rate and systolic and diastolic blood pressure in SHR. The heart rate decreased in all of the groups after the 1st wk and then did not change from the range of 400–450 beats/min. The systolic blood pressure of all groups gradually increased until Week 4 and then decreased. After 8 wk of the study, the rats fed FR or HCEF-RG at 1,000 mg/kg body weight had significantly (p < 0.05) lower systolic blood pressure than the control group. The FR 500 group had a significantly greater decrease compared to the SHR control group after 2 wk but increased after 3 wk and significantly (p < 0.05) decreased until the 4–6 wk period. The last wk (Week 8) of the study indicated increased systolic blood pressure in the rats. The FR 1,000 group had significantly (p < 0.05) lower systolic blood pressure than the SHR control group for 8 wk and the decreased ratio of systolic blood pressure was 7% compared with the SHR control group. The systolic blood pressure remained significantly (p < 0.05) lower in the HCEF-RG 500 group compared to the SHR controls during the 6 wk, but increased after 8 wk. The systolic blood pressure remained significantly (p < 0.05) lower in the HCEF-RG 1,000 group compared to the SHR controls during the wk 4–8, and caused a progressive decrease in systolic blood pressure that was about 17% of the decrease ratio. As a result, the HCEF-RG 1,000 mg/kg body weight sample showed a stronger effect of decreased blood pressure compared with the other samples. All groups indicated an increase in diastolic blood pressure and a decrease after 4 wk. The HCEF-RG 1,000 group had significantly (p < 0.05) lower diastolic blood pressure than the SHR control group after 8 wk. The FR groups with 500 mg/kg and 1,000 mg/kg body weight samples had significantly lower diastolic blood pressure than the SHR controls after 4–6 wk. The HCEF-RG 500 group had significantly (p < 0.05) lower diastolic blood pressure than the SHR control group for 6 wk. The HCEF-RG 1,000 group had significantly (p < 0.05) lower diastolic blood pressure than the SHR control group for 2–6 wk. However, after 8 wk of study, the diastolic blood pressure was not significantly (p < 0.05) different. The results indicate that the magnitude of the diastolic blood pressure response of the HCEF-RG 1,000 mg/kg body weight group was significantly (p < 0.05) decreased compared to the SHR control group after 8 wk of study. The diastolic blood pressure was 149.2 ± 33.6 mmHg and the decrease ratio of the diastolic blood pressure was 11% compared with the SHR control group. In conclusion, the HCEF-RG 1,000 mg/kg body weight sample showed a decrease in blood pressure compared with the other samples.
Fig. 3.
(A) Changes of heart rate, (B) systolic blood pressure, and (C) diastolic blood pressure in spontaneously hypertensive rats (SHRs) treated with fine root concentrate (FR) and hypotensive component-enriched fraction of red ginseng (HCEF-RG) for 8 wk (SHR-control, ▵; FR500, ○; FR1,000, ●; HCEF-RG500, □; HCEF-RG1,000, ■). Wistar Kyoto (WKY)-control (normal control): WKY provided with sterile distilled water; SHR-control (hypertension control): SHR provided with sterile distilled water; FR groups (hypertension and FR treatment): SHR orally provided with fine root concentrations (500 mg/kg, 1,000 mg/kg body weight); HCEF-RG groups (hypertension and HCEF-RG treatment): SHR orally provided with hypotensive components-enriched fraction of red ginseng (500 mg/kg, 1,000 mg/kg body weight). Data are expressed as mean ± SD, n = 8. *p < 0.05 compared with SHR-control.
The earlier report showed that rats that received powder from Korean Red Ginseng had significantly (p < 0.05) lower blood pressure than the control group for 1–2 months [18], and that the crude saponin extracted from Korean Red Ginseng of 50 mg/kg and 100 mg/kg body weight of SHR decreased the systolic blood pressure (6% and 36%, respectively). After taking a capsule of Korea red ginseng, 0.5 g for 180 min, the patients with hypertension had significantly (p < 0.05) lower diastolic blood pressure than the placebo group. Additionally, Rg3-enriched red ginseng has also been reported to play an important role in decreasing blood pressure [19]. Total saponins and Rg3 are also involved in vasodilation; the mechanism is associated with the release of NO and the activation of Ca2+-dependent K+ channels in vascular smooth muscles [20]. Previous studies have demonstrated that an 8–9% decrease in blood pressure may induce a 21% decrease in heart attacks, as well as a 37% reduction in stroke [21]. In the present study, HCEF-RG of 1,000 mg/kg was administered to SHR, which is equivalent to a daily Rg3 intake of 4.04 mg. After 8 wk of treatment, a 17% decrease in systolic blood pressure, as well as an 11% reduction in diastolic blood pressure was observed. This response may be attributable to the Rg3/Arg-Fru-mediated NO release from vascular endothelial cells.
3.3. Renin activity, ACE, and angiotensin II
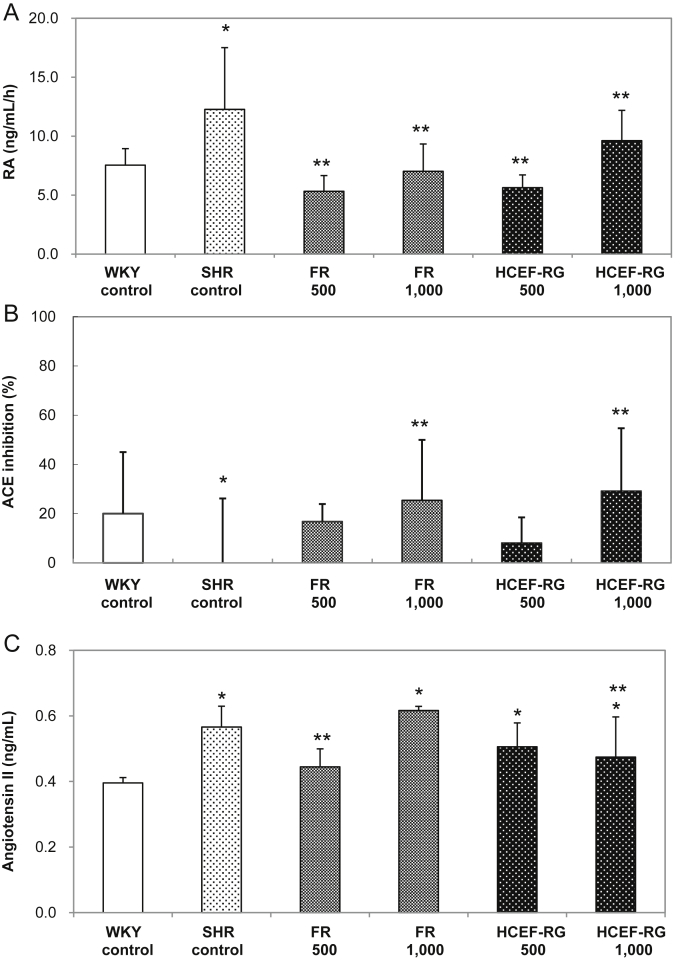
As can be seen in Fig. 4A, all samples had significantly lower renin activity than the SHR control group (p < 0.05). The FR and HCEF-RG groups had decreased levels when compared with the SHR controls. However, there were no significant differences in the concentration of samples between the FR and HCEF-RG groups and the SHR control group. In case of ACE-inhibiting activity (Fig. 4B), the FR and HCEF-RG groups had significantly decreased levels when compared with the SHR controls (p < 0.05); HCEF-RG 500 group (8% reduction) → FR 500 group (17% reduction) → FR 1,000 group (25% reduction) → HCEF-RG 1,000 group (29% reduction). Moreover, angiotensin II levels were dose-dependently reduced in the FR and HCEF-RG groups when compared to the SHR control groups (p < 0.05; Fig. 4C).
Fig. 4.
(A) Renin activity, (B) angiotensin I converting enzyme (ACE) inhibitory effects, and (C) angiotensin II levels in the plasma of spontaneously hypertensive rats (SHRs) treated with fine root concentrate (FR) and hypotensive component-enriched fraction of red ginseng (HCEF-RG) for 8 wk. WKY-control (normal control): WKY provided with sterile distilled water; SHR-control (hypertension control): SHR provided with sterile distilled water; FR groups (hypertension and FR treatment): SHR orally provided with fine root concentrations (500 mg/kg, 1,000 mg/kg body weight); HCEF-RG groups (hypertension and HCEF-RG treatment): SHR orally provided with hypotensive components-enriched fraction of red ginseng (500 mg/kg, 1,000 mg/kg body weight). Values are expressed as mean ± SD, n = 8. *p < 0.05 compared with Wistar Kyoto (WKY)-control. **p < 0.05 compared with SHR-control.
Several clinical results have shown that ACE-inhibiting substances not only directly inhibit hypertension by suppressing the activation of ACE but also effectively decrease chronic renal disease, arteriosclerosis, heart attack, and associated death [22], [23]. Kim et al [24] reported the ACE-inhibitory effects of ginseng in Korean traditional rice wine. Rg3 has been shown to inhibit the activation of ACE, thus contributing to the prevention of hypertension [25]. The Rg3 content in FR and HCEF-RG and the concentration-dependent effects of ACE inhibition were confirmed in this study; Rg3 enrichment further promoted ACE inhibition. In the case of the HCEF-RG 1,000 mg/kg group, which showed a 29% ACE inhibiting activity compared to the SHR control, a 16% inhibiting activity was observed for angiotensin II. These findings show that HCEF-RG 1,000 mg/kg acts on the overall hypotensive mechanism by decreasing renin activation, ACE, and angiotensin II in the RAS.
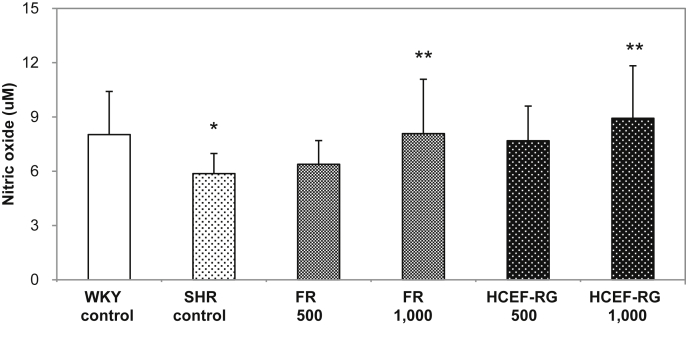
3.4. Nitric oxide production
Fig. 5 indicates that the FR 500 mg/kg and 1,000 mg/kg body weight samples had higher NO induction than the SHR control group. The FR 1,000 and HCEF-RG 1,000 groups had significantly (p < 0.05) higher induction of NO than the SHR control group.
Fig. 5.
Effects of fine root concentrate (FR) and the hypotensive component-enriched fraction of red ginseng (HCEF-RG) on plasma nitric oxide levels after oral administration in spontaneously hypertensive rats (SHRs) for 8 wk. WKY-control (normal control): WKY provided with sterile distilled water; SHR-control (hypertension control): SHR provided with sterile distilled water; FR groups (hypertension and FR treatment): SHR orally provided with fine root concentrations (500 mg/kg, 1,000 mg/kg body weight); HCEF-RG groups (hypertension and HCEF-RG treatment): SHR orally provided with hypotensive components-enriched fraction of red ginseng (500 mg/kg, 1,000 mg/kg body weight). Values are expressed as mean ± SD, n = 8. *p < 0.05 compared with Wistar Kyoto (WKY)-control. **p < 0.05 compared with SHR-control.
Red ginseng activates NO production, which results in a reduction in the arterial blood pressure [26]. It has previously been shown that the administration of red ginseng and white ginseng saponins have hypotensive effects in cat model [27]. Furthermore, saponins in Korean ginseng have been shown to induce an endothelium-dependent relaxation effect in rats, increase in cyclic GMP concentrations in tissues [28], and relaxation of vascular smooth muscle in the coronary arteries [29]. Thus, total saponins induce the release of NO from endothelial cells by increasing intracellular entry of Ca2+, which activates Ca2+-activated K+ channels within the vascular endothelial cell membranes, resulting in the influx of Ca2+ into endothelial cells and inducing the activation of endothelial nitric oxide synthase in endothelial cells to relax blood vessels [30]. In addition, Rg3 has been reported to suppress platelet aggregation and improve blood circulation [31]; endothelium-dependent relaxation attributable to Rg3 in total saponins followed a dose-dependent manner [32]. The endothelium-dependent relaxation potency of Rg3 was found to be 3.4-fold higher than that of protopanaxatriol ginsenosides, 6.2-fold higher than that of total saponins, and 79-fold higher than that of Rg1. In renovascular hypertensive rats, 100 mg/kg intravenous Korean ginseng saponins and nonsaponins, respectively, induced a 29 ± 7 mmHg and 11 ± 3 mmHg decrease in blood pressure, which was highly significant (p < 0.01). This reduction in blood pressure may be attributable to an increase in blood NO caused by an increase in the activity of NO synthase [33]. As a result, in this study, the administration of 1,000 mg/kg Rg3-Arg-Fru-enriched HCEF-RG to SHRs, which was equivalent to 4.04 mg/kg Rg3 and 4.48 mg/kg Arg-Fru, resulted in a 52% increase in NO production and decrease in blood pressure.
In conclusion, HCEF-RG was successfully prepared by persimmon vinegar treatment (12 times against fine root concentrates, v/v) at 80°C for 18 h. Systolic blood pressure and diastolic blood pressure in the SHR groups treated with HCEF-RG decreased significantly, compared with the SHR control group (p < 0.05). The SHR groups with HCEF-RG showed decreased activity in rennin and ACE and reduced angiotensin II levels. Moreover, the SHR group treated using HCEF-RG of 1,000 mg/kg body weight exhibited the highest NO concentration among SHR groups (p < 0.05). Therefore, the results indicate that HCEF-RG has great potential to be used as a new material for functional food applications and can have a significant effect on improving hypertensive conditions.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This work was supported by Convergence Technology Development Program of Agriculture, Industry and Commerce (2011) through the Korea Technology and Information Promotion Agency funded by Small and Medium Business Administration (S2042340) of Korea.
References
- 1.Alexander R.W. Hypertension and the pathogenesis of atherosclerosis. Hypertension. 1995;25:155–161. doi: 10.1161/01.hyp.25.2.155. [DOI] [PubMed] [Google Scholar]
- 2.Giles T.D. Lipid factors in the hypertension syndrome. J Cardiovasc Risk. 1997;4:257–259. [PubMed] [Google Scholar]
- 3.Doyle A.E. Elsevier; Amsterdam: 1984. Handbook of hypertension: clinical pharmacology of antihypertensive drugs. [Google Scholar]
- 4.Han C.K., Lee O.H., Kim K.I., Park J.M., Kim Y.C., Lee B.Y. Effect of powder, 50% ethanol and hot water extracts of Gastrodiae Rhizoma on serum lipids and blood pressure in SHR fed high-fat diet. J Korean Soc Food Sci Nutr. 2003;32:1095–1101. [Google Scholar]
- 5.Pak Y.B., Hong Y.G., Yang M.S. Effect of cumambrin A treatment on blood pressure in spontaneously hypertensive rats. Korean J Pharmacogn. 1999;30:226–230. [Google Scholar]
- 6.Rhyu M.R., Kim E.Y. The relations between antihypertensive effect and r-aminobutyric acid, mycelial weight and pigment of Monascus. Korean J Food Sci Technol. 2002;34:737–740. [Google Scholar]
- 7.Jeong B.S., Sin M.G. Yeong-rim Publishing Company; Seoul: 2003. Hyang-yak dictionary. [Google Scholar]
- 8.Uchida S., Ohta H., Niwa M., Mori A., Nonaka G., Nishioka I., Ozaki M. Prolongation of life span of stroke-prone spontaneously hypertensive rats ingesting persimmon tannin. Chem Pharm Bull. 1990;38:1049–1052. doi: 10.1248/cpb.38.1049. [DOI] [PubMed] [Google Scholar]
- 9.Park D., Bae D.K., Jeon J.H., Lee J., Oh N., Yang G., Yang Y.H., Kim T.K., Song J., Lee S.H. Immunopotentiation and antitumor effects of a ginsenoside Rg3-fortified red ginseng preparation in mice bearing H460 lung cancer cells. Environ Toxicol Pharmacol. 2011;31:397–405. doi: 10.1016/j.etap.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Yue P.Y.K., Wong D.Y.L., Wu P., Leung P., Mak N., Yeung H., Liu L., Cai Z., Jiang Z.H., Fan T. The angiosuppressive effects of 20 (R)-ginsenoside Rg3. Biochem Pharmacol. 2006;72:437–445. doi: 10.1016/j.bcp.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 11.Lee S.R., Park J.H., Choi K.J., Kim N.D. Inhibitory effects of ginsenoslde Rg3 on platelet aggregation and its mechanism of action. Korean J Ginseng Sci. 1997;21:132–140. [Google Scholar]
- 12.Bae E.A., Kim N.Y., Han M.J., Choo M.K., Kim D.H. Transformation of ginsenosides to compound K(IH-901) by lactic acid bacteria of human intestine. J Microbiol Biotechnol. 2003;13:9–14. [Google Scholar]
- 13.Ko S.K., Lee K.H., Hong J.K., Kang S.A., Sohn U.D., Im B.O., Han S.T., Yang B.W., Chung S.H., Lee B.Y. Change of ginsenoside composition in ginseng extract by vinegar process. Food Sci Biotechnol. 2005;14:509–513. [Google Scholar]
- 14.Sun C.P., Gao W.P., Zhao B.Z., Cheng L.Q. Conversion of protopanaxadiol type saponins to ginsenoside Rg3 by lemon. Nat Prod Commun. 2012;7:1155–1156. [PubMed] [Google Scholar]
- 15.Matsuura Y., Zheng Y., Takaku T., Kameda K., Okuda H. Isolation and physiological activities of a new amino acid derivatives from Korean Red Ginseng. J Trad Med. 1994;11:256–263. [Google Scholar]
- 16.Kumagai A. Studies on the non-saponin fraction of Korean Red Ginseng. Yakyong Ninjin. 1995:233–244. [Google Scholar]
- 17.Kim C.S., Choi K.J., Yang J.W., Kim S.B. Effect of preheating condition of raw ginseng on the yield and physical property of Korean Red Ginseng extract. Korean J Medicinal Crop Sci. 2000;8:146–150. [Google Scholar]
- 18.Joo I.W., Sung K.H., Park J.M., Lew J.H., Oh H.J. Effect of Korean Red Ginseng on blood pressure and aortic vascular (endothelial) histological changes in rats. J Ginseng Res. 2008;32:324–331. [Google Scholar]
- 19.Stavro P.M., Hana A.K., Vuksan V. The effect of Korean Red Ginseng extracts with escalating levels of ginsenoside Rg3 on blood pressure in individuals with high normal blood pressure or hypertension. Am J Hypertens. 2002;15:34–44. [Google Scholar]
- 20.Kim N.D., Kang S.Y., Kim M.J., Park J.H., Schini-Kerth V.B. The ginsenoside Rg3 evokes endothelium-independent relaxation in rat aorta: role of K+ channels. Eur J Pharmacol. 1999;367:51–57. doi: 10.1016/s0014-2999(98)00899-1. [DOI] [PubMed] [Google Scholar]
- 21.Hur M.H., Lee M.S., Yang H.J., Kim C., Bae I.L., Ernst E. Ginseng for reducing the blood pressure in patients with hypertension: a systematic review and meta-analysis. J Ginseng Res. 2010;34:342–347. [Google Scholar]
- 22.Bakris G.L. Angiotensin-converting enzyme inhibition to enhance vascular health-clinical and research models. Am J Hypertens. 2001;14:264–269. doi: 10.1016/s0895-7061(01)02152-5. [DOI] [PubMed] [Google Scholar]
- 23.Thurman J.M., Schrier R.W. Comparative effects of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on blood pressure and the kidney. Am J Med. 2003;114:588–598. doi: 10.1016/s0002-9343(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 24.Kim H.J., Lee J.C., Lee G.S., Jeon B.S., Kim N.M., Lee J.S. Manufacture and physiological functionalities of traditional ginseng liquor. J Ginseng Res. 2002;26:74–78. [Google Scholar]
- 25.Altaf R., Asmawi M.Z., Dewa A., Umar M.I. Sources and possible mechanisms of action of important phytoconstituents with cardiovascular properties. Afr J Pharmacy Pharmacol. 2012;6:563–580. [Google Scholar]
- 26.Han K., Shin I.C., Choi K.J., Yun Y.P., Hong J.T., Oh K.W. Korean ginseng water extract increase nitric oxide concentrations in exhaled breath. Nitric Oxide. 2005;12:159–162. doi: 10.1016/j.niox.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Kim N.D., Kim I.C. Studies on hypotensive mechanism of ginseng components. Korean J Pharmacogn. 1978;9:41–47. [Google Scholar]
- 28.Kim N.D., Kang S.Y., Schini V.B. Ginsenosides evoke endothelium-dependent vascular relaxation in rat aorta. Gen Pharmacol. 1994;25:1071–1077. doi: 10.1016/0306-3623(94)90121-x. [DOI] [PubMed] [Google Scholar]
- 29.Chang S.J., Suh J.S., Jeon B.H., Nam K.Y., Park H.K. Vasorelaxing effect by protopanaxatriol and protopanaxadiol of Panax ginseng in the pig coronary artery. Korean J Ginseng Sci. 1994;18:95–101. [Google Scholar]
- 30.Busse R., Fichtner H., Luckhoff A., Kohlhardt M. Hyperpolarization and increased free calcium in acetylcholine-stimulated endothelial cells. Am J Physiol. 1998;255:965–969. doi: 10.1152/ajpheart.1988.255.4.H965. [DOI] [PubMed] [Google Scholar]
- 31.Kim N.D. Ginsenosides-mediated vascular relaxation and its molecular mechanisms. J Ginseng Res. 2008;32:89–98. [Google Scholar]
- 32.Kim N.D., Kang S.Y., Kim M.J., Park J.H., Schini V.B. Ginsenoside Rg3 mediates endothelium-dependent relaxation in response to ginsenosides in rat aorta. Eur J Pharmacol. 1999;367:41–49. doi: 10.1016/s0014-2999(98)00898-x. [DOI] [PubMed] [Google Scholar]
- 33.Jeon B.H., Kim H.S., Chang S.J. Effect of saponin and non-saponin of Panax ginseng on the blood pressure in the renovascular hypertensive rats. J Ginseng Res. 1999;23:81–87. [Google Scholar]