Abstract
Background
The findings of currently available studies are not consistent with regard to the association between the risk of cancer and ginseng consumption. Therefore, we aimed to evaluate this association by conducting a meta-analysis of different studies.
Methods
To systematically evaluate the effect of ginseng consumption on cancer incidence, six databases were searched, including PubMed, Ovid Technologies, Embase, The Cochrane Library, China National Knowledge Infrastructure, and Chinese VIP Information, from 1990 to 2014. Statistical analyses based on the protocol employed for a systematic review were conducted to calculate the summary relative risks (RRs) and 95% confidence intervals (CIs).
Results
We identified nine studies, including five cohort studies, three case-control studies, and one randomized controlled trial, evaluating the association between ginseng consumption and cancer risk; these studies involved 7,436 cases and 334,544 participants. The data from the meta-analysis indicated a significant 16% lower risk of developing cancer in patients who consumed ginseng (RR = 0.84, 95% CI = 0.76–0.92), with evidence of heterogeneity (p = 0.0007, I2 = 70%). Stratified analyses suggested that the significant heterogeneity may result from the incidence data for gastric cancer that were included in this study. Publication bias also showed the same result as the stratified analyses. In addition, subgroup analyses for four specific types of cancer (colorectal cancer, lung cancer, gastric cancer, and liver cancer) were also performed. The summary RRs for ginseng intake versus no ginseng consumption were 0.77 for lung cancer, 0.83 for gastric cancer, 0.81 for liver cancer, and 0.77 for colorectal cancer.
Conclusion
The findings of this meta-analysis indicated that ginseng consumption is associated with a significantly decreased risk of cancer and that the effect is not organ specific.
Keywords: cancer, ginseng, meta-analysis
1. Introduction
Cancer imposes a global threat to public health. According to the Global Cancer Statistics estimates, there were about 14.1 million new cancer cases and 8.2 million cancer deaths in 2012 [1]. Importantly, these numbers have rapidly increased with increased population growth and environmental pollution. Malignancy results from complex interactions among multiple genes, the intracellular environment, and neighboring tissues [2]. The basic theory of tumorigenesis suggests that the process starts with a normal cell that is transformed through the activation of proto-oncogenes and the suppression of tumor suppressor genes. After the transformation, the cell does not behave like a normal cell, but instead begins to exhibit the properties of a cancer cell. These transformed cells acquire the capability to proliferate uncontrollably through self-sufficiency in growth signals and are insensitive to antigrowth signals. In addition, they are able to evade apoptosis, eventually resulting in tumor growth. As the tumor continues to develop, its growth is aided by the development of new blood vessels that provide it with nutrients, thereby allowing it to sustain itself and even invade other tissues, resulting in metastasis that is ultimately lethal [2], [3], [4], [5].
Chemoprevention is defined as the use of natural, synthetic, or biological agents to prevent, suppress, and reverse the carcinogenic progression. It is ideally effective in prevention of the disease and should be nontoxic. Chemoprevention is characterized by the disruption of, or at least the delay of, multiple pathways and processes in the three stages of carcinogenesis, namely, initiation, promotion, and progression [6], [7]. Chemicals or biomolecules that inhibit the initiation stage are necessary for the preservation of DNA [8], [9]. In contrast to compounds that preserve DNA, compounds that affect the later stages of carcinogenesis (promotion and progression) are known for their ability to decrease the proliferative capacity of initiated cells. They interfere with cancer cell proliferation by downregulating the expression of the molecules involved in signal-transduction pathways, such as nuclear factor kappa-light-chain enhancer of activated B cells (NF-kB), mammalian target of rapamycin, and signal transducer and activator of transcription 3, and by inhibiting cytochrome P450 enzymes that modulate signal transduction to hormone-responsive elements [10]. In addition, suppressing agents are likely to reduce or delay the ability of cancer cells to acquire metastatic properties by promoting pathways leading to apoptosis [11] and inhibiting pathways leading to angiogenesis, epithelial mesenchymal transition, invasion, and dissemination [12].
Traditional herbal medicine used for thousands of years is advantageous in maintaining a balanced health status and help prevent further diseases in a safe and effective manner. Ginseng (Panax ginseng Meyer) is widely used and has been included in pharmacopoeias in China, Japan, Germany, France, Austria, and the United Kingdom. It is widely available as an over-the-counter drug and also commonly used as an adjuvant to increase human immunity [13], [14]. Furthermore, the protective effect of ginseng in cancer chemoprevention has been shown by extensive laboratory and preclinical studies [15]. Ginseng is chemoprophylactic and often acts on its cellular and molecular targets through various signaling pathways, thereby inhibiting the tumor by regulation of the cell cycle, induction of apoptosis, and inhibition of angiogenesis and invasion [16], [17]. The anticancer effects of ginseng involve modulation of diverse signaling pathways, including regulation of cell proliferation mediators (cyclin-dependent kinases and cyclins), growth factors (c-myc, epidermal growth factor receptor, and vascular endothelial growth factor), tumor suppressors (p53 and p21), oncogenes (MDM2), cell death mediators [B-cell lymphoma 2 (Bcl-2), B-cell lymphoma-extra large (Bcl-xL), X-linked inhibitor of apoptosis protein (XIAP), caspases, and death receptors], inflammatory response molecules (NF-κB and cyclooxygenase-2), and protein kinases (c-Jun N-terminal protein kinase, Akt, and adenosine monophosphate-activated protein kinase) [18]. During the past decade, although a series of epidemiologic studies had indicated that ginseng consumption affects cancer incidence, the results of the studies are inconsistent. Therefore, a quantitative analysis of the associations between ginseng consumption and risk of cancer was necessary to expound the existing inconsistency in the literature.
Thus, a meta-analysis aimed at reviewing and summarizing the relationship between ginseng consumption and risk of cancer was performed, and different relative risk (RR) ratios of cancer were determined by performing subgroup analyses.
2. Materials and methods
2.1. Search strategy
We conducted a literature search for relevant articles in PubMed, Ovid Technologies, Embase, Cochrane Library, China National Knowledge Infrastructure, and Chinese VIP Information for published papers from 1990 to July 2014 using the following search terms without language restrictions: “ginseng,” “fresh ginseng,” “white ginseng,” “red ginseng,” “ginseng supplement,” “ginseng or fresh ginseng or white ginseng or red ginseng or ginseng supplement,” “Neoplasms” (Mesh), “randomized controlled trial,” “cohort,” “case control,” “randomized controlled trial or cohort or case control,” “ginseng or fresh ginseng or white ginseng or red ginseng or ginseng supplement and neoplasms (Mesh) and randomized controlled trial or cohort or case control.”
In addition, the reference lists of the selected articles were also reviewed to identify other relevant articles. The research was conducting by two authors on their own account.
2.2. Study selection
The following criteria were chosen to identify the studies for this meta-analysis: studies comprising a randomized controlled trial or observation study with the exposure factor being ginseng intake, studies with risk of cancer being the end point of interest, and studies in which risk estimates were reported. If data were included more than once, the latest and complete research was chosen.
2.3. Data extraction and quality assessment
The data were charted as follows: first author, publication year, study design, region, study period, case and control, ginseng type and consumption, RR, and confounding factors of interest. The included studies were independently evaluated by two authors using the methodological quality assessment system in RevMan 5.3. Discrepancies in evaluation were resolved by a third author, and potential publication bias was examined using Begg's test. A linear regression approach was used to measure the funnel plot asymmetry on the natural logarithm scale of the RR ratios [19].
2.4. Statistical analysis
The main analyses were focused on the associations between consumption of ginseng and cancer incidence. In addition, the RR of lung cancer, colorectal cancer (cancer of the colon and rectum), gastric cancer (cancer of the stomach), and liver cancer was determined in relation to ginseng consumptions in a subgroup analysis.
All analyses were performed using Review Manager version 5.3 (Cochrane Collaboration software) and GRADE profiler version 3.6. All p values are two-sided and p < 0.1 was considered significant. Furthermore, the I2 index, a quantitative measure of inconsistency, across studies was calculated [20].
3. Results
3.1. Literature search
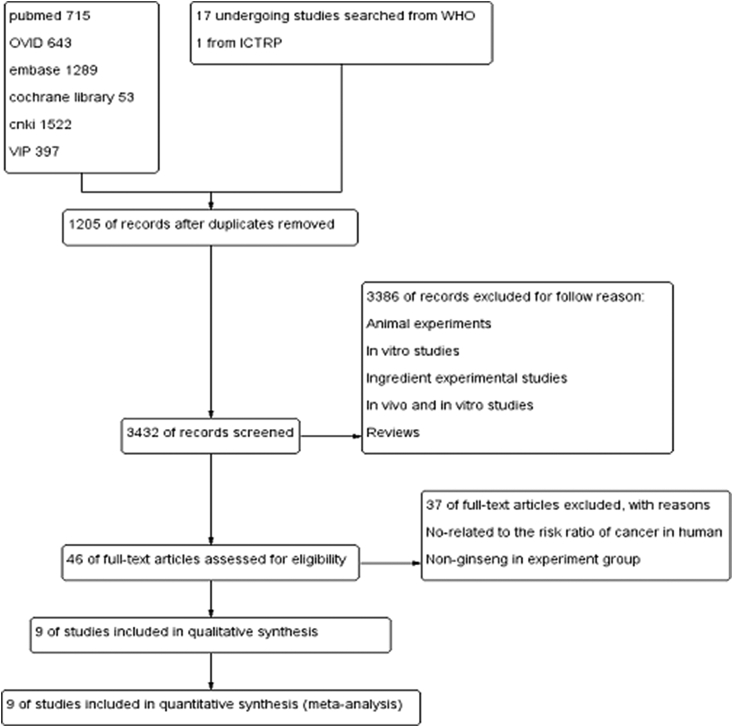
A flowchart showing the study selection process is presented in Fig. 1. In brief, a total of 4,619 publications and 18 studies that are currently underway were identified. A total of 1,205 duplicates were then removed. After screening the abstracts, we excluded the duplicates, studies involving animal experiments and in vitro analyses, ingredient experimental studies, and reviews, etc. for not meeting the inclusion criteria. After evaluating the full manuscripts of the 46 potentially relevant articles, 37 potentially relevant studies were excluded further because they were not related to the to the risk ratio of cancer in humans and nonginseng use in experimental group. Finally, nine studies were selected for analysis [21], [22], [23], [24], [25], [26], [27], [28], [29].
Fig. 1.
Flowchart of study selection. ICTRP, International Clinical Trials Registry Platform; WHO, World Health Organization.
3.2. Study characteristics
The included studies were identified with regard to ginseng consumption, and risk of cancer in this meta-analysis is presented in Table 1. The eight observation studies, including five cohort studies [21], [22], [23], [24], [25] and three case-control studies [26], [27], [28], were published between 1990 and 2014. Only one randomized controlled trial [29] was reported. Of these studies, four involved research on lung cancer [21], [23], [28], [29] and gastric cancer [21], [22], [28], [29], three assessed colorectal cancer [21], [23], [28], [29] and liver cancer [21], [28], [29], and one study each assessed prostate cancer [24], hematologic malignancies [25], and breast cancer [27].
Table 1.
Characteristics of studies included in the meta-analysis
| Study | Study design | Study population | Study period | Cases/control or cohort or RCT | Ginseng type and consumption | RR (95% CI) | Adjustments |
|---|---|---|---|---|---|---|---|
| Yun and Choi 1990 [26] | Case-control study | Seoul, Korean | 1987–1988 | 905/905 Men 48% |
Ginseng 562/905 vs. 674/905 | 0.83 (0.78,0.89) | Demographic characteristics (age, marital status, education, occupation, and income), lifestyle (cigarette smoking, alcohol consumption, and others), and ginseng consumption |
| Yun and Choi 1995 [28] | Case-control study | Seoul, Korean | 1988–1990 | 1,987/1,987 Men 54% |
Ginseng 1,066/1,987 vs. 1,382/1,987 | 0.77 (0.73, 0.81) | Sociodemographic characteristics, lifelong occupational history, smoking habits, drinking habits, and ginseng intake |
| Ginseng (colorectal cancer) 63/118 vs. 86/118 | 0.73 (0.60, 0.90) | ||||||
| Ginseng (lung cancer) 156/276 vs. 195/276 | 0.80 (0.70, 0.91) | ||||||
| Ginseng (gastric cancer) 158/300 vs. 224/300 | 0.71 (0.62, 0.80) | ||||||
| Ginseng (liver cancer) 156/264 vs. 179/264 | 0.79 (0.70, 0.90) | ||||||
| Yun and Choi 1998 [21] | Cohort study | Seoul, Korean | 1987–1992 | 137/4,450 age was over 40 yr, followed for 5 yr Men 51% |
Ginseng 75/137 vs. 3,167/4,450 | 0.77 (0.66, 0.90) | Demographic characteristics, lifelong occupation, smoking and drinking habits, history of diseases, ginseng intake, etc. |
| Ginseng (lung cancer) 10/24 vs. 3,167/4,405 | 0.59 (0.36, 0.94) | ||||||
| Ginseng (gastric cancer) 19/42 vs. 3,167/4,450 | 0.64 (0.46, 0.89) | ||||||
| Ginseng (liver cancer) 10/14 vs. 3,167/4,450 | 1.00 (0.72, 1.40) | ||||||
| Yun et al 2010 [29] | Randomized controlled trial | Hangzhou, Chinese | 1997–2008 | 325/318 age was between 40 and 69 yr with chronic atrophic gastritis 1 g of ginseng every wk for 3 yr and followed up for 8 yr Men 61% |
Red ginseng extract 8/24 vs. 317/616 | 0.65 (0.37, 1.15) | Demographic characteristics, lifelong occupation, smoking and alcohol drinking patterns, history of diseases, and history of ginseng intake |
| Ginseng (colorectal cancer) 1/2 vs. 324/641 | 0.99 (0.25, 3.96) | ||||||
| Ginseng (lung cancer) 2/8 vs. 323/635 | 0.49 (0.15, 1.64) | ||||||
| Ginseng (gastric cancer) 3/6 vs. 322/637 | 0.99 (0.44, 2.21) | ||||||
| Ginseng (liver cancer) 1/2 vs. 324/641 | 0.99 (0.25, 3.96) | ||||||
| Satia et al 2009 [23] | Cohort study | Western Washington State, American | 2000–2007 | 665/76,460 age was between 50 and 76 yr, followed for a mean of 5.0 y at least once a wk for a yr |
Ginseng (colorectal cancer) 29/428 vs. 6,309/76,084 | 0.82 (0.57, 1.16) | Duration in yr, frequency in d/wk, and usual dose of various supplements, including multivitamins, individual vitamin and mineral supplements, other mixtures, and herbal and specialty products |
| Ginseng (lung cancer) 43/665 vs. 6,322/76,460 | 0.78 (0.56, 1.05) | ||||||
| Kamangar et al 2007 [22] | Cohort study | Shanghai, Chinese | 1997–2004 | 21,318/52,134 Women aged between 40 and 70 yr followed for 4 yr |
Ginseng (gastric cancer) 56/153 vs. 21,318/73,452 At least five times a yr in the past 3 yr |
1.26 (1.02, 1.55) | Demographic characteristics, education and income, lifestyle and habits, diet, taken ginseng, and several other factors |
| Rebbeck et al 2007 [27] | Case-control study | Philadelphia and Delaware Counties in Pennsylvania; Camden County in New Jersey, American | 1999–2002 | 949/1,524 Women aged between 50 and 79 yr |
Ginseng (breast cancer) 72/949 vs. 164/1,524 | 0.71 (0.54, 0.92) | Demographic characteristics; family history of breast, endometrial, and ovarian cancer; contraceptive history; fertility history; menstrual and menopausal history; medical history; detailed gynecologic screening history; use of exogenous hormones; and use of other medications |
| Use in European Americans 41/677 vs. 84/905 | 0.65 (0.46, 0.94) | ||||||
| Use in African Americans 31/272 vs. 80/619 | 0.88 (0.60, 1.30) | ||||||
| At least three times a wk for 1 mo or more any time | |||||||
| Walter et al 2011 [25] | Cohort study | Western Washington State, American | 2000–2008 | 586/65,429 Men and women aged between 50 and 76 yr, followed for 6 yr Men 49% |
Ginseng (< 4 d/wk or < 3 yr, hematologic malignancies) 37/586 vs. 5,507/65,429 | 0.75 (0.55, 1.03) | For each vitamin, mineral, and specialty supplement taken at least once a wk for 1 yr, we ascertained intake from single supplements and multivitamins, including the duration in yr and frequency of use in d/wk during the 10-yr period prior to baseline. For individual vitamin and mineral supplements, we also ascertained the average dose taken each d. |
| Brasky et al 2011 [24] | Cohort study | Western Washington State, American | 2000–2008 | 1,602/33,637 Men aged between 50 and 76 yr (follow-up time was 6.1 yr) |
Ginseng (≥ 1 d/wk for ≥ 1 yr, prostate cancer) 76/1,602 vs. 1,821/33,637 10-yr average use |
0.88 (0.70, 1.10) | Specialty supplement use during the 10-yr period prior to baseline, in addition to use of vitamin and mineral supplements, inquired about current and past regular use (≥ 1 d/wk for ≥ 1 yr) of 18 specialty supplements including frequency of use (d/wk) and duration of use (yr) over the previous 10 yr. |
| Low use [< 4 d/wk or any use < 3 yr] 106/1,602 vs. 2,091/33,637 | 1.07 (0.88, 1.29) | ||||||
| High use [≥ 4 d/wk for ≥ 3 yr] 25/1,602 vs. 714/33,637 | 0.74 (0.49, 1.09) |
CI, confidence interval; RCT, randomized controlled trial; RR, relative risk.
3.3. Quality assessment of the included studies
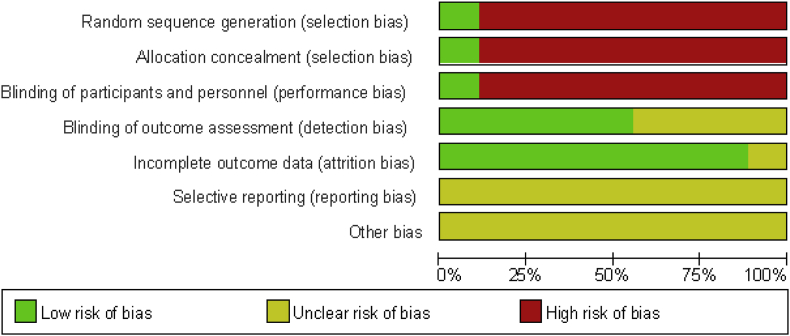
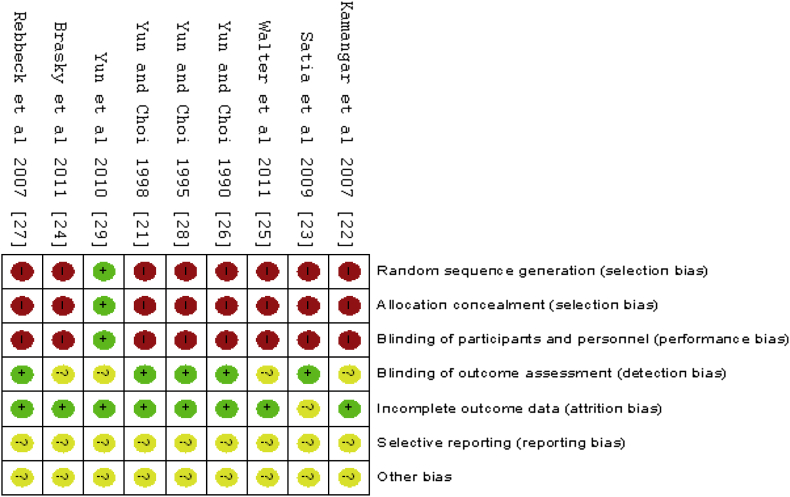
The Cochrane risk of bias tool was used to assess risk bias (Fig. 2, Fig. 3). Because eight of the studies were observation studies, the random sequence generation, allocation concealment, and blinding of participants and personnel all showed high risk. Most of the studies involved questionnaire analysis, and the participants were diagnosed in different hospitals by randomized doctors. Therefore, blinding of outcome assessment showed low risk. In addition, outcome data loss was less than 20%, and therefore, incomplete outcome data presented a low risk. Because the protocols of all the trials were not accessible, selective reporting was generally unclear. Therefore, the grading of recommendations assessment results is shown with a low quality of evidence (Table 2).
Fig. 2.
Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Fig. 3.
Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Table 2.
Grading of recommendations assessment results
| Quality assessment |
No of patients |
Effect |
Quality | Importance | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Ginseng | Control | Relative (95% CI) | Absolute | ||
| 9 | Observational studies (n = 8) and randomized controlled trial (n = 1) | Very serious1) | No serious inconsistency | No serious indirectness | No serious imprecision | Reduced effect for RR >> 1 or RR << 12) Dose–response gradient3) |
2,031/7,436 (27.3%) | 14% | RR 0.84 (0.76–0.92) | 22 fewer/1,000 (from 11 fewer to 34 fewer) | Low | Critical |
CI, confidence interval; RR, relative risk.
Most were observational studies.
Other factor may affect the results.
Some of studies described the dose-response.
3.4. Main analysis
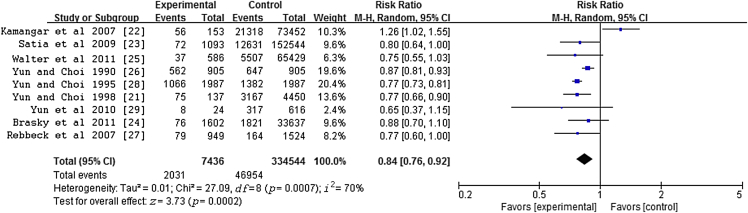
A total of nine studies were included in this meta-analysis to evaluate the association between ginseng intake and the risk of cancer. There was a significant 16% lower cancer risk associated with ginseng consumption in comparison with the risk associated with no ginseng consumption for all studies combined [RR = 0.84, 95% confidence interval (CI) = 0.76–0.92], with evidence of heterogeneity (p = 0.0007, I2 = 70%) in Fig. 4.
Fig. 4.
Meta-analysis of studies examining association between ginseng consumption and risk of cancer. CI, confidence interval.
3.5. Publication bias
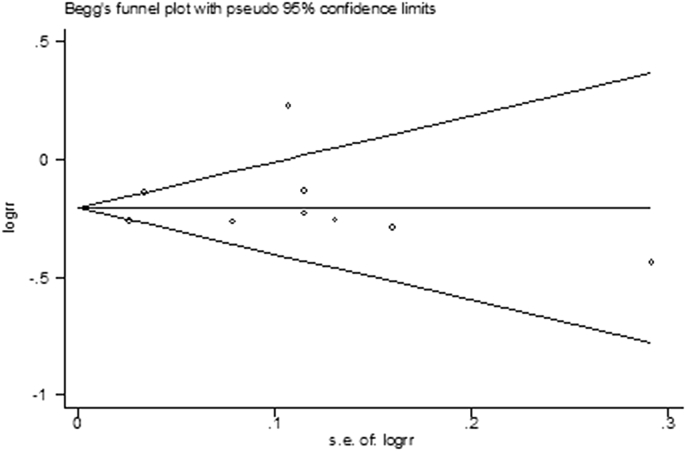
There was no significant combined publication bias in all studies assessing the relationship between ginseng intake and cancer incidence, as suggested by Begg's rank correlation test in Fig. 5 (p for Begg's test was 0.596). However, the study by Kamangar et al [22] may have had a high bias. When this study was excluded from this meta-analysis, we observed a significant 19% lower risk of developing cancer after ginseng consumption in comparison with the risk without ginseng consumption (RR = 0.81, 95% CI = 0.76–0.85), with low heterogeneity (p = 0.20, I2 = 28%).
Fig. 5.
Begg's funnel plot of ginseng consumption and risk of cancer incidence. RR, relative risk; s.e., standard error.
3.6. Subgroup and sensitivity analyses
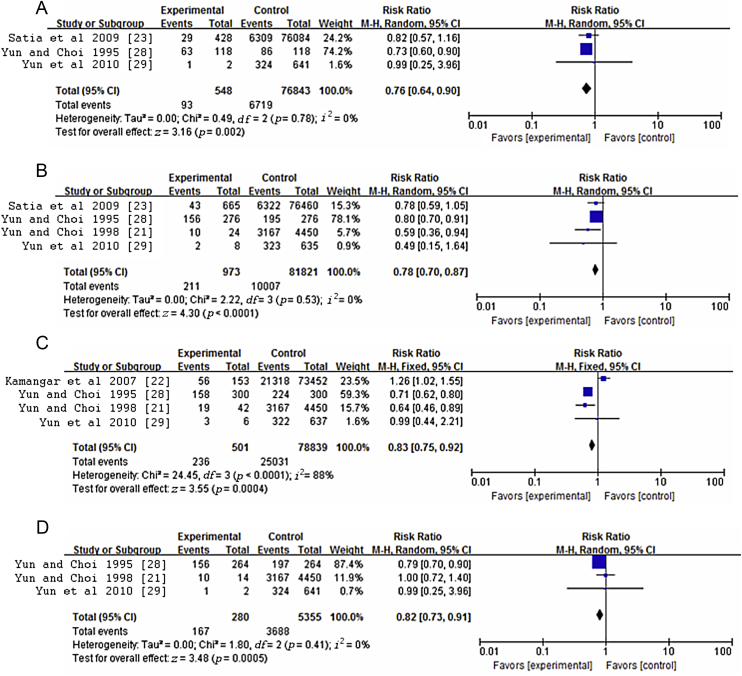
The subgroup analyses according to the type of cancer are presented in Table 3. When the analyses were stratified by cancer site, the summary RRs for ginseng intake versus no ginseng consumption were 0.77 for lung cancer, 0.83 for gastric cancer, 0.81 for liver cancer, and 0.77 for colorectal cancer. Evidence of heterogeneity was observed only in the gastric cancer subgroup (Fig. 6). In addition, sensitivity analysis indicated that the summary estimates (RR = 0.81, 95% CI = 0.76–0.85) showed low heterogeneity (p = 0.20, I2 = 28%) when the study by Kamangar et al [22] was excluded. Meanwhile, in the gastric cancer subgroup, the sensitivity analysis also indicated that when the Kamangar et al [22] study was excluded, the summary estimates changed (RR = 0.70, 95% CI = 0.62–0.79), with no significant heterogeneity (p = 0.59, I2 = 0%).
Table 3.
Subgroup analyses of the risk ratio of different kinds of cancer
| Group | Number of studies | Risk ratio (95% confidence interval) | pheterogeneity | I2, % |
|---|---|---|---|---|
| All | 9 | 0.84 (0.76, 0.92) | 0.0007 | 70 |
| Type of cancer | ||||
| Colorectal cancer | 3 | 0.76 (0.64, 0.90) | 0.78 | 0 |
| Lung cancer | 4 | 0.78 (0.70, 0.87) | 0.53 | 0 |
| Gastric cancer | 4 | 0.83 (0.75, 0.92) | < 0.0001 | 88 |
| Liver cancer | 3 | 0.82 (0.73, 0.91) | 0.41 | 0 |
| Breast cancer | 1 | 0.71 (0.54, 0.92) | — | — |
| Hematologic 35372 malignancies | 1 | 0.75 (0.55, 1.03) | — | — |
| Prostate cancer | 1 | 0.88 (0.70, 1.10) | — | — |
Fig. 6.
Meta-analysis of studies examining association between ginseng consumption and risk of (A) colorectal cancer, (B) lung cancer, (C) gastric cancer, and (D) liver cancer. CI, confidence interval.
4. Discussion
The present meta-analysis, based on the latest published results, is the first quantitative systematic analysis of the association between ginseng consumption and cancer risk in 7,436 cases and 334,544 participants. The meta-analysis of the studies identified indicated that ginseng consumption may be associated with a reduced risk of cancer. We found substantial heterogeneity in the association between ginseng consumption and cancer risk across studies. This is not surprising given the variation in study designs and characteristics of the study populations. However, the stratified analysis by cancer type showed significant heterogeneity only for gastric cancer. It indicated that gastric cancer may be the major source of heterogeneity. Furthermore, when we separated the population in Shanghai, China [22] from others in Seoul, Korea [21], [28], [29], the results indicated no significant inverse association between ginseng consumption and gastric cancer risk. Because the chemical components of ginseng differ by region, the inclusion of studies from different regions may have been responsible for the heterogeneity. Moreover, the exposure factor may be greater, because patient information obtained after diagnoses in an observation study could also result in systematic errors. However, the fact that large numbers of cases and controls were involved in this meta-analysis also means that the findings about association between ginseng consumption and the risk of cancer are more reliable. In addition, we found a significant association between ginseng intake and reduction of cancer incidence, which further strengthened our result.
The basic mechanism of cancer development is now known to result from an accumulation of genetic and epigenetic alterations in cells [30]. Although diagnosis and treatment are the major strategies for controlling cancer, the importance of cancer chemoprevention has gradually increased because advanced cancer is difficult to cure [31], [32].
Ginseng, a famous traditional Chinese medicine, has been used for thousands of years [33]. Its usefulness in cancer prevention and therapy has been shown by extensive preclinical and epidemiological studies [34], [35], [36]. The main active ingredients of ginseng are often thought to be ginsenosides. The anticancer effects of ginseng involve diverse molecular mechanisms of action, which in turn involve the regulation of most known modulators of carcinogenesis [19]. Because ginsenosides cause tumor cell death through various mechanisms, it may be difficult for cells to develop resistance to ginsenoside-induced death. Furthermore, the ability of ginsenosides to kill tumor cells and relative nontoxicity to normal cells make them attractive candidates for drug development [37]. The diverse properties of ginseng are attributable to the diversity in both chemical structure and biological activity.
The study by Kamangar et al [22] showed opposite effects. The types of ginseng used by the participants in their study were similar to those used in other studies. However, the group and its extension were selected from among patients who were also referred to other studies included in this meta-analysis. Therefore, the sex of the patients included was significantly different from others. The prevalence of sex differences might have led to greater bias in this group than in the general population because of the differences in lifestyle and genetic constitution of female participants. Therefore, further evaluation of the association between ginseng consumption and sex may be needed to clarify ginseng's role in cancer.
Several potential limitations of our meta-analysis should be considered while interpreting the results. First, most of the studies included were observational studies, and the presence of cohort and case-control studies may have introduced confounding factors and biases as a result of the different methods used in the studies. Second, studies included were mainly conducted in Korea, China, and the United States; therefore, the data should be extrapolated to other populations with caution. Third, the apparent protective effect of ginseng against cancer may be also attributable to genetic and other environmental factors. Finally, the articles included in our meta-analysis were published in journals. Unpublished studies and original data were not included. However, our meta-analysis also has several strengths. In particular, it allowed us to directly address the association between ginseng consumption and cancer risk in humans, avoiding the uncertainties derived from the use of animal data and mathematical models, and to assess the public health relevance of such a relation. Previous epidemiological studies have already identified several dietary and nutritional factors associated with the risk of various cancers, particularly those of the digestive tract. This weighs in favor of the capability of epidemiological studies included in this meta-analysis to assess the association of cancer with ginseng consumption.
5. Conclusion
To the best of our knowledge, this review is the first to systematically perform a quantitative evaluation of the chemopreventive effect of ginseng on the incidence of cancer, addressing the lack of this type of research. In summary, ginseng consumption was associated with a significantly lower risk of developing cancer. However, the findings should be interpreted with caution due to the low quality of the included trials. Rigorous multicenter, large-scale clinical trials should be carried out to reveal the exact effectiveness in the future.
Conflicts of interest
All contributing authors declare no conflicts of interest.
Acknowledgments
This work was supported by a grant from the National Natural Science Funds of China (Grant No. 81403119).
Contributor Information
Zeng-yong Jia, Email: zengyongjia_sq@163.com.
Xiao-bin Jia, Email: xiaobinjia_nj@126.com.
References
- 1.Torre L.A., Bray F., Siegel R.L., Ferlay J., Lortet-Tieulent J., Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal B.B., Van Kuiken M.E., Iyer L.H., Harikumar K.B., Sung B. Molecular targets of nutraceuticals derived from dietary spices: potential role in suppression of inflammation and tumorigenesis. Exp Biol Med (Maywood) 2009;234:825–849. doi: 10.3181/0902-MR-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar S., Weaver V.M. Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev. 2009;28:113–127. doi: 10.1007/s10555-008-9173-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noy R., Pollard J.W. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;47:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chanmee T., Ontong P., Konno K., Itano N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers (Basel) 2014;6:1670–1690. doi: 10.3390/cancers6031670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Flora S., Ferguson L.R. Overview of mechanisms of cancer chemopreventive agents. Mutat Res. 2005;591:8–15. doi: 10.1016/j.mrfmmm.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Steward W.P., Brown K. Cancer chemoprevention: a rapidly evolving field. Br J Cancer. 2013;109:1–7. doi: 10.1038/bjc.2013.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shu L., Cheung K.L., Khor T.O., Chen C., Kong A.N. Phytochemicals: cancer chemoprevention and suppression of tumor onset and metastasis. Cancer Metastasis Rev. 2010;29:483–502. doi: 10.1007/s10555-010-9239-y. [DOI] [PubMed] [Google Scholar]
- 9.Vanden Berghe W. Epigenetic impact of dietary polyphenols in cancer chemoprevention: lifelong remodeling of our epigenomes. Pharmacol Res. 2012;65:565–576. doi: 10.1016/j.phrs.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Gan F.F., Ling H., Ang X., Reddy S.A., Lee S.S., Yang H., Tan S.H., Hayes J.D., Chui W.K., Chew E.H. A novel shogaol analog suppresses cancer cell invasion and inflammation, and displays cytoprotective effects through modulation of NF-κB and Nrf2-Keap1 signaling pathways. Toxicol Appl Pharmacol. 2013;272:852–862. doi: 10.1016/j.taap.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Yager J.D., Davidson N.E. Estrogen carcinogenesis in breast cancer. N Engl J Med. 2006;354:270–282. doi: 10.1056/NEJMra050776. [DOI] [PubMed] [Google Scholar]
- 12.Din F.V., Valanciute A., Houde V.P., Zibrova D., Green K.A., Sakamoto K., Alessi D.R., Dunlop M.G. Aspirin inhibits mTOR signaling, activates AMP-activated protein kinase, and induces autophagy in colorectal cancer cells. Gastroenterology. 2012;142:1504–1515.e3. doi: 10.1053/j.gastro.2012.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ernst E. Panax ginseng: an overview of the clinical evidence. J Ginseng Res. 2010;34:259–263. [Google Scholar]
- 14.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yun T.K. Panax ginseng—a non-organ-specific cancer preventive? Lancet Oncol. 2001;2:49–55. doi: 10.1016/S1470-2045(00)00196-0. [DOI] [PubMed] [Google Scholar]
- 16.Qi L.W., Wang C.Z., Yuan C.S. American ginseng: potential structure-function relationship in cancer chemoprevention. Biochem Pharmacol. 2010;8:947–954. doi: 10.1016/j.bcp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Choi J.S., Chun K.S., Kundu J., Kundu J.K. Biochemical basis of cancer chemoprevention and/or chemotherapy with ginsenosides (Review) Int J Mol Med. 2013;32:1227–1238. doi: 10.3892/ijmm.2013.1519. [DOI] [PubMed] [Google Scholar]
- 18.Nag S.A., Qin J.J., Wang W., Wang M.H., Wang H., Zhang R. Ginsenosides as anticancer agents: in vitro and in vivo activities, structure-activity relationships, and molecular mechanisms of action. Front Pharmacol. 2012;3:25. doi: 10.3389/fphar.2012.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;4:1088–1101. [PubMed] [Google Scholar]
- 20.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yun T.K., Choi S.Y. Non-organ specific cancer prevention of ginseng: a prospective study in Korea. Int J Epidemiol. 1998;27:359–364. doi: 10.1093/ije/27.3.359. [DOI] [PubMed] [Google Scholar]
- 22.Kamangar F., Gao Y.T., Shu X.O., Kahkeshani K., Ji B.T., Yang G., Li H.L., Rothman N., Chow W.H., Zheng W. Ginseng intake and gastric cancer risk in the Shanghai Women's Health Study Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:629–630. doi: 10.1158/1055-9965.EPI-06-1009. [DOI] [PubMed] [Google Scholar]
- 23.Satia J.A., Littman A., Slatore C.G., Galanko J.A., White E. Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins And Lifestyle study. Cancer Epidemiol Biomarkers Prev. 2009;18:1419–1428. doi: 10.1158/1055-9965.EPI-09-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brasky T.M., Kristal A.R., Navarro S.L., Lampe J.W., Peters U., Patterson R.E., White E. Specialty supplements and prostate cancer risk in the VITamins And Lifestyle (VITAL) cohort. Nutr Cancer. 2011;63:573–582. doi: 10.1080/01635581.2011.553022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walter R.B., Brasky T.M., Milano F., White E. Vitamin, mineral, and specialty supplements and risk of hematologic malignancies in the prospective VITamins And Lifestyle (VITAL) study. Cancer Epidemiol Biomarkers Prev. 2011;20:2298–2308. doi: 10.1158/1055-9965.EPI-11-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yun T.K., Choi S.Y. A case-control study of ginseng intake and cancer. Int J Epidemiol. 1990;19:871–876. doi: 10.1093/ije/19.4.871. [DOI] [PubMed] [Google Scholar]
- 27.Rebbeck T.R., Troxel A.B., Norman S., Bunin G.R., DeMichele A., Baumgarten M., Berlin M., Schinnar R., Strom B.L. A retrospective case-control study of the use of hormone-related supplements and association with breast cancer. Int J Cancer. 2007;120:1523–1528. doi: 10.1002/ijc.22485. [DOI] [PubMed] [Google Scholar]
- 28.Yun T.K., Choi S.Y. Preventive effect of ginseng intake against various human cancers: a case-control study on 1987 pairs. Cancer Epidemiol Biomarkers Prev. 1995;4:401–408. [PubMed] [Google Scholar]
- 29.Yun T.K., Zheng S., Choi S.Y., Cai S.R., Lee Y.S., Liu X.Y., Cho K.J., Park K.Y. Non-organ-specific preventive effect of long-term administration of Korean red ginseng extract on incidence of human cancers. J Med Food. 2010;13:489–494. doi: 10.1089/jmf.2009.1275. [DOI] [PubMed] [Google Scholar]
- 30.Vogelstein B., Fearon E.R., Hamilton S.R., Kern S.E., Preisinger A.C., Leppert M., Nakamura Y., White R., Smits A.M., Bos J.L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 31.Slaughter D.P., Southwick H.W., Smejkal W. Field cancerization in oral stratified squamous epithelium; clinical implications of multicentric origin. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.Hong W.K., Sporn M.B. Recent advances in chemoprevention of cancer. Science. 1997;278:1073–1077. doi: 10.1126/science.278.5340.1073. [DOI] [PubMed] [Google Scholar]
- 33.Li C., Yan Z., Zhang L., Li Y. Research and implementation of good agricultural practice for traditional Chinese medicinal materials in Jilin Province, China. J Ginseng Res. 2014;38:227–232. doi: 10.1016/j.jgr.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bespalov V.G., Alexandrov V.A., Limarenko A.Y., Voytenkov B.O., Okulov V.B., Kabulov M.K., Peresunko A.P., Slepyan L.I., Davydov V.V. Chemoprevention of mammary, cervix and nervous system carcinogenesis in animals using cultured Panax ginseng drugs and preliminary clinical trials in patients with precancerous lesions of the esophagus and endometrium. J Korean Med Sci. 2001;16:S42–S53. doi: 10.3346/jkms.2001.16.S.S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J Korean Med Sci. 2001;16:28–37. doi: 10.3346/jkms.2001.16.S.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin H.R., Kim J.Y., Yun T.K., Morgan G., Vainio H. The cancer-preventive potential of Panax ginseng: a review of human and experimental evidence. Cancer Causes Control. 2000;11:565–576. doi: 10.1023/a:1008980200583. [DOI] [PubMed] [Google Scholar]
- 37.Wong A.S., Che C.M., Leung K.W. Recent advances in ginseng as cancer therapeutics: a functional and mechanistic overview. Nat Prod Rep. 2015;32:256–272. doi: 10.1039/c4np00080c. [DOI] [PubMed] [Google Scholar]