Abstract
Background
Ginsenosides have been shown to exert beneficial pharmacological effects on the central nervous, cardiovascular, and endocrine systems. We sought to determine whether total ginsenosides (TG) inhibit monocrotaline (MCT)-induced pulmonary hypertension and to elucidate the underlying mechanism.
Methods
MCT-intoxicated rats were treated with gradient doses of TG, with or without NG-nitro-l-arginine methyl ester. The levels of molecules involving the regulation of nitric oxide and mitogen-activated protein kinase pathways were determined.
Results
TG ameliorated MCT-induced pulmonary hypertension in a dose-dependent manner, as assessed by the right ventricular systolic pressure, the right ventricular hypertrophy index, and pulmonary arterial remodeling. Furthermore, TG increased the levels of pulmonary nitric oxide, endothelial nitric oxide synthase, and cyclic guanosine monophosphate. Lastly, TG increased mitogen-activated protein kinase phosphatase-1 expression and promoted the dephosphorylation of extracellular signal-regulated protein kinases 1/2, p38 mitogen-activated protein kinase, and c-Jun NH2-terminal kinase 1/2.
Conclusion
TG attenuates MCT-induced pulmonary hypertension, which may involve in part the regulation of nitric oxide and mitogen-activated protein kinase pathways.
Keywords: endothelial nitric oxide synthase, mitogen-activated protein kinases, nitric oxide, pulmonary hypertension, total ginsenoside
1. Introduction
Pulmonary hypertension is a progressive disease associated with increased constriction and remodeling of the pulmonary vasculature, ultimately leading to right heart failure. However, the pathological mechanism of pulmonary hypertension at the molecular level remains unclear. Nitric oxide (NO) is produced from l-arginine (l-arg) by endothelial NO synthase (eNOS) and causes smooth muscle relaxation by the activation of soluble guanylate cyclase, followed by the accumulation of cyclic guanosine monophosphate (cGMP) [1]. Evidence suggests that impaired NO production may lead to pulmonary hypertension [2]. Furthermore, daily treatment with an NO donor attenuates monocrotaline (MCT)-induced pulmonary hypertension and pulmonary vascular remodeling [3].
The mitogen-activated protein kinases (MAPKs)—including three members p38 MAPK, c-Jun NH2-terminal kinase 1/2 (JNK1/2), and extracellular signal-regulated protein kinase 1/2 (ERK1/2)—are a family of central signaling molecules that respond to numerous stimuli by phosphorylating a variety of substrates including transcription factors, enzymes, and other kinases, thereby orchestrating cellular proliferation, differentiation, survival, apoptosis, and inflammation. Recent evidence supports the notion that MAPKs may be involved in the remodeling of vasculature [4], [5].
Ginsenosides are the main active ingredients in ginseng (Panax ginseng Meyer), a well-known and popular herbal medicine used in China. To date, at least 30 different ginsenosides have been extracted from the roots [6], stems, or leaves of P. ginseng [7]. Several cell culture and animal studies show that ginsenosides confer beneficial effects on the cardiovascular system through various mechanisms including adjusting blood pressure, modifying vasomotor function, stimulating NO production, and influencing ion channels [8], [9]. We have shown previously that total ginsenosides (TG) can inhibit vascular smooth muscle cell proliferation, carotid neointimal hyperplasia, and ventricular hypertrophy both in vivo and in vitro [10], [11], [12]. However, little is known regarding the effect of TG on pulmonary hypertension. Therefore, we sought to investigate whether TG alleviates MCT-induced pulmonary hypertension in rats and to explore whether TG affects signaling pathways previously implicated in pulmonary hypertension.
2. Materials and methods
2.1. Materials
TG extracted from P. ginseng Meyer leaves and stems was received from Professor Rui Zhao (Beijing Naturally Occurring Drugs Research Institute, China) and quantified in our laboratory as described previously [13]. Briefly, TG was refluxed with 70% ethanol and then separated by column chromatography with D101 resin (Xi'an Jiaotong University School of Medicine, China). For quality control, TG chemical fingerprints were established on a Phenomenex ODS column using high-performance liquid chromatography at 203 nm. Ten ginsenosides (Rg1, Re, Rb1, Rb2, Rc, Rg2, Rb3, Rg3, Rf, and Rd) were identified from TG by comparing retention times with authentic compounds. The final product consisted of 21.60% Re, 18.24% Rg2, 15.36% Rb2, 13.65% Rd, 8.4% Rc, 5.26% Rb1, 5.20% Rg1, 4.64% Rb3, 2.15% Rg3, and 1.97%Rf, accounting for 96.47% of the TG, and other minor ginsenosides.
MCT was obtained from Sigma Chemical Co. (St. Louis, MO, USA). l-Arg and NG-nitro-L-arginine methyl ester (l-NAME, an eNOS inhibitor) were purchased from Alexis Biochemicals Company (Lausanne, Switzerland). Bicinchoninic acid protein assay kit was purchased from Merck (Whitehouse Station, NJ, USA). The NO detection kit was purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China), and the cGMP assay kit was purchased from Amersham (UK). The reverse transcription-polymerase chain reaction (RT-PCR) kit was purchased from TaKaRa (Dalian, China). The primer of eNOS was synthesized by TaKaRa Biological Engineering Company (TaKaRa, Dalian, China). Antibodies recognizing eNOS, phospho-ERK1/2 (p-ERK1/2), phospho-p38MAPK (p-p38MAPK), phospho-JNK1/2 (p-JNK1/2), mitogen-activated protein kinase phosphatase-1 (MKP-1), and horseradish peroxidase-conjugated secondary antibody were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA), and the catalog numbers were sc-650, sc-23759-R, sc-17852-R, sc-6254, and sc-370, respectively. All other chemicals were of reagent grade.
2.2. Animals and experimental design
All animal procedures were in accordance with the Regulations of Experimental Animal Administration issued by the State Committee of Science and Technology of the People's Republic of China. The protocol was approved by the Committee on Animal Research and Ethics of Xi'an Jiaotong University (Xi'an, China).
8 to 12-wk-old male Sprague–Dawley rats weighing 180–220 g were purchased from the Fourth Military Medical University, Xi'an, China. Two animals per cage were housed under constant temperature (25°C) and humidity (50%) and maintained on a 12-h light/dark cycle. Animals were maintained on standard rodent chow, and food and water were available ad libitum. Prior to the initiation of the experiment, rats were acclimatized to the environmental conditions for 1 wk.
All rats were given a single intraperitoneal injection (i.p.) of MCT (60 mg/kg) except for the control group (an equal volume of 0.9% saline). Each group consisted of 8–10 rats. Rats injected with MCT were randomly divided into seven groups: (1) MCT group—rats were administered with vehicle (i.p.); (2–4) TG group—rats were administered (i.p.) with TG at 20, 40, and 80 mg/kg/d (TG was dissolved in 0.9% saline, and the concentration of TG was 0.4%, 0.8%, and 1.6% (w/v), respectively); (5) l-arg group—rats were given l-arg (200 mg/kg/d, i.p.); (6) TG + L-N group—rats were given intragastric (i.g.) administrations of l-NAME at 20 mg/kg/d and i.p. administration of TG at 40 mg/kg/d; (7) l-a + L-N group—rats were administered with l-NAME (20 mg/kg/d, i.g.) and l-arg (200 mg/kg/d, i.p.). Control group rats were given i.p. with vehicle. All treatments continued for 18 d. TG doses were chosen according to our preliminary experimental results and previous reports [10], [14].
2.3. Right ventricular systolic pressure and right ventricular hypertrophy index measurements
Eighteen days after MCT/saline injections, all surviving rats were anesthetized with sodium pentobarbital solution (40 mg/kg), and right ventricular systolic pressure (RVSP) was measured by right heart catheterization. The right jugular vein was isolated, and a small polyethylene catheter (PE-50 tube; American Health & Medical Supply International Corp., Scarsdale, New York, USA) was inserted through the right jugular vein via a small transverse cut and then advanced into the right ventricle (RV) under the guidance of the pressure waveform. The catheters were filled with heparinized saline (10 U/ml heparin in 0.9% saline). The other end of the catheter was connected to a biosignal acquisition processor, and RVSP was directly measured using PowerLab monitoring hardware and software (AD Instruments, Colorado Springs, CO, USA). Next, rats were killed by cervical dislocation under anesthesia. The chest was opened, and the whole lungs and hearts were excised.
The lungs and body were weighed, and the lung weight (LW)/body weight (BW) was calculated. The left lungs were removed for histological analysis, and the right lungs were excised and divided by the upper and lower lobes. The lower lobe of each group was used for NO and cGMP measurement, and the upper lobe was frozen in liquid nitrogen for real-time PCR and Western blot analysis.
The RV and left ventricle (LV) plus septum (LV + S) were weighed, and the weight ratio of RV/(LV + S) was calculated to assess the right ventricular hypertrophy index (RVHI).
2.4. Assessment of pulmonary artery remodeling
Left lungs were fixed in 4% paraformaldehyde, paraffin-embedded, and sectioned at 5 μm. Hematoxylin and eosin (HE) and elastic Van Gieson (EVG) stainings were performed according to common histopathological procedures. Pulmonary vascular remodeling was evaluated by determining wall thickness (WT) according to the method described by Barth et al [15]. External diameters (EDs) and WTs of at least 15 pulmonary arteries (50–100 μm in diameter) in each rat in control, MCT, TG 40 mg/kg, l-arg 200 mg/kg, TG + L-N, and l-a + L-N groups were assessed under 400× magnification using a computerized morphometric system (Qwin; Leica, Wetzlar, Germany) by two pathologists blinded to treatment category. A semiquantitative histological assessment of medial wall thickness was calculated as: WT (%) = (2 × WT/ED) × 100%.
2.5. NO and cGMP levels measurement
One-half of the lower lobe of each right lung was used for NO measurement. Lungs were cut into small pieces and then homogenized in ice-cold buffer. The homogenate was then centrifuged at 2,000g for 8 min at 4°C. The supernatant was transferred to a cold microcentrifuge tube, and protein concentrations were determined using a bicinchoninic acid protein assay. NO levels were determined spectrophotometrically by measuring total nitrate plus nitrite (NO3– + NO2–) with an NO detection kit according to the manufacturer's instructions. Briefly, nitrate was enzymatically converted into nitrite by nitrate reductase, and nitrite was quantified using Griess reagent at an absorbance of 550 nm. Results were expressed as micromoles per gram of protein.
The remaining half of the lower lobe of each right lung was used for cGMP measurement via radioimmunoassays using a cGMP assay kit according to the manufacturer's instructions. Each tissue sample was homogenized in 1 mL 0.1M HCl. The homogenate was centrifuged at 3,000g for 10 min at 4°C, and the supernatant was evaporated under a stream of N2 gas. The radioactivity of the supernatant was measured to calculate the cGMP level in the sample, and the amount (pmol) in pulmonary tissues (1 mg) was calculated.
2.6. Gene expression analysis
Total RNA was extracted from lung tissue using TRIzol (MRC Company, Cincinnati, USA). RT-PCR was performed with an RT-PCR kit according to the manufacturer's instructions, and carried out in an iCycler iQ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA) with SYBR Green PCR Master Mix (ABI Company, Warrington, UK). The primers were designed with Express Software (ABI Co., Foster, CA, USA). The following primers were used: eNOS (GenBank accession no. NM_021838) forward, 5′-GGC ATC ACC AGG AAG ACT T-3′; reverse, 5′-CAC ACG CTT CGC CAT CAC-3′ (97 bp product); β-actin (GenBank accession no. NM_031144) forward: 5′-TGA CAG GAT GCA GAA GGA GA-3′, reverse: 5′-TAG AGCCAC CAA TCC ACA CA-3′ (104 bp product). The results (Ct values) of the target gene were normalized with β-actin of the same sample, and expressed relative to controls.
2.7. Western blot analysis
Proteins were extracted from the lung tissues. The protein samples (5–10 μg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred on polyvinylidene difluoride nylon membranes. The membranes were incubated with primary antibodies to eNOS, p-ERK1/2, p-p38 MAPK, p-JNK1/2, and MKP-1 at 4°C overnight, followed by incubation with a horseradish peroxidase-conjugated secondary antibody. An enhanced chemiluminescence system (ECL Plus; GE Healthcare) was used to detect immunoblots, and bands were visualized and quantified with a lumino-analyzer (LAS-1000; Fujifilm, Tokyo, Japan). The signal intensity was normalized to β-actin expression.
2.8. Statistical analysis
All data were expressed as mean ± standard deviation. Statistical comparisons between groups were performed using a one-way analysis of variance test followed by the Student t test using SPSS version 13.0 software (SPSS Inc., Chicago, IL, USA). The level of statistical significance was set at p < 0.05.
3. Results
3.1. TG attenuates MCT-induced pulmonary hypertension
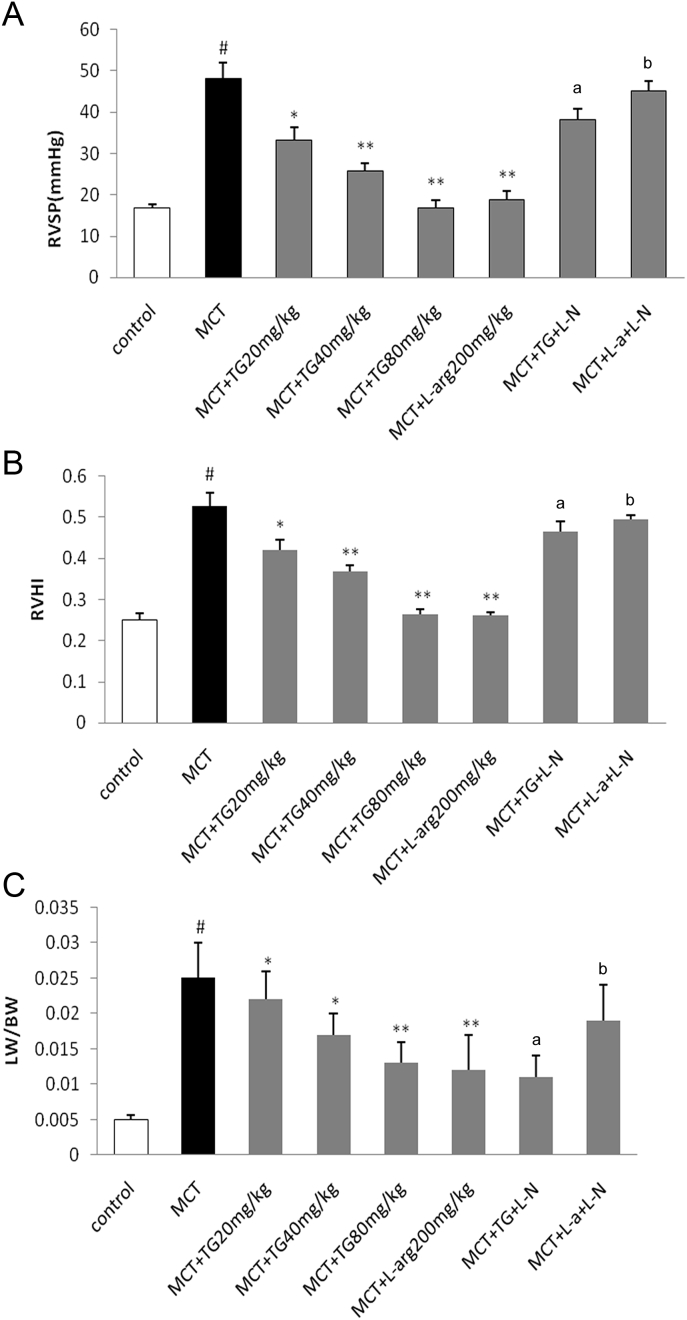
MCT-induced pulmonary hypertension in rats was validated via measurement of RVSP, RVHI, and LW/BW. All these parameters were significantly higher in the MCT group when compared with the control group. Administration of TG (20, 40, or 80 mg/kg/d) significantly prevented the increases of RVSP, RVHI, and LW/BW in a dose-dependent manner. l-Arg also reduced RVSP, RVHI, and LW/BW. The effects of both TG and l-arg were markedly offset by l-NAME (Fig. 1).
Fig. 1.
Effects of TG on (A) RVSP, (B) RVHI, and (C) LW/BW in MCT-induced pulmonary hypertension rats. Eighteen days after treatment with TG, RVSP was measured by right heart catheterization. RVHI was calculated as the weight ratio of RV/(LV + S) and used to describe the degree of right ventricular hypertrophy. LW/BW was calculated as the weight ratio of LW/BW. Results are represented as mean ± SD. a Statistical significance at p < 0.05 TG + L-N versus TG 40 mg/kg group. b Statistical significance at p < 0.05 L-a + L-N versus l-arg 200 mg/kg group. * Statistical significance at p < 0.05 versus MCT group. ** Statistical significance at p < 0.01 versus MCT group. # Statistical significance at p < 0.05 versus control group. BW, body weight; l-a, l-arginine; L-N, NG-nitro-l-arginine methyl ester; LW, lung weight; MCT, monocrotaline; RVHI, right ventricular hypertrophy index; RVSP, right ventricular systolic pressure; TG, total ginsenosides.
3.2. TG attenuates MCT-induced pulmonary arterial remodeling
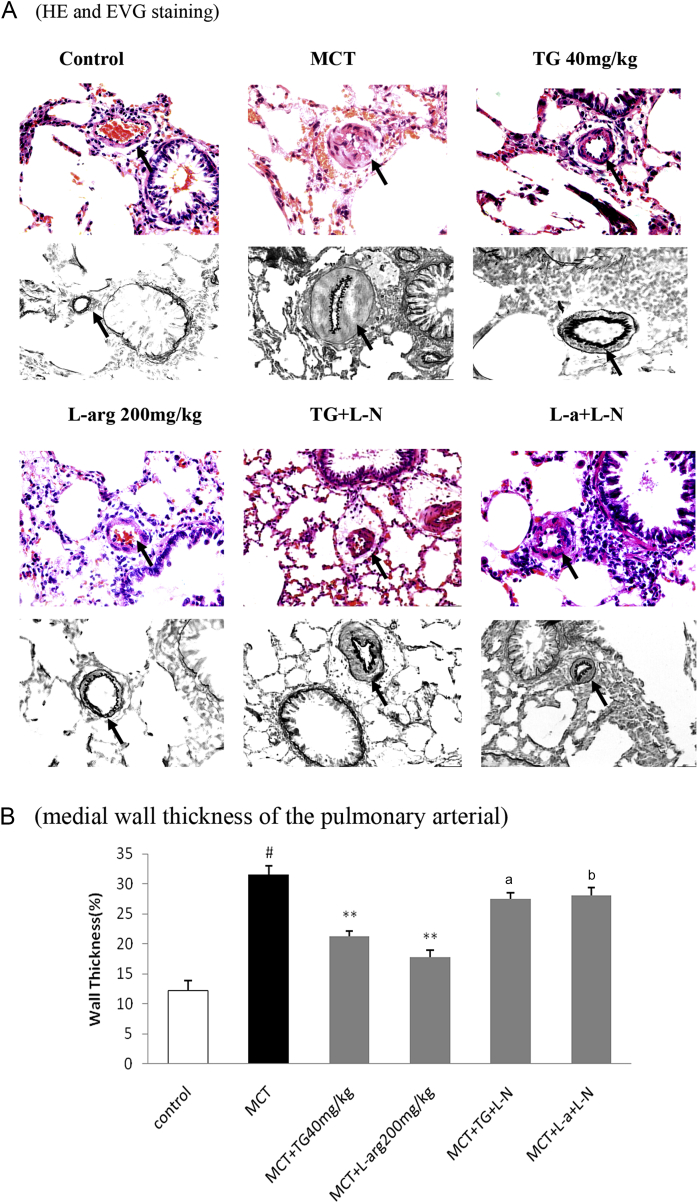
Histological analysis revealed that MCT caused a marked medial hypertrophy in pulmonary arteries, a widening of alveolar septa, muscularization in the distal arterioles, and marked periarteritis of vascular arteries. Treatment with TG 40 (mg/kg) or l-arg (200 mg/kg) significantly attenuated the MCT-induced medial wall thickening of pulmonary arteries with a diameter of 50–100 μM. The effects of TG and l-arg were partly reversed by l-NAME (Fig. 2).
Fig. 2.
Effects of TG on pulmonary arterial remodeling in MCT-induced pulmonary hypertension rats. (A) Histological findings of the pulmonary arterial (arrows, 400×). Top, HE staining; bottom, EVG staining. HE and EVG stainings were performed according to common histopathological procedures. (B) A semiquantitative histological assessment of medial wall thickness was calculated as: WT (%) = (2 × WT/ED) × 100%. MCT markedly increased the percent medial wall thickness of the pulmonary arterial, and TG and l-arg attenuated MCT-induced medial wall thickening. The effects of TG and l-arg were inhibited by l-NAME. Results are represented as mean ± SD. * Statistical significance at p < 0.05 versus MCT group. # Statistical significance at p < 0.05 versus control group. ED, external diameter; EVG, elastic Van Gieson; HE, hematoxylin and eosin; l-NAME, NG-nitro-l-arginine methyl ester; MCT, monocrotaline; TG, total ginsenosides; WT, wall thickness.
3.3. Effects of TG on NO, cGMP, and eNOS levels
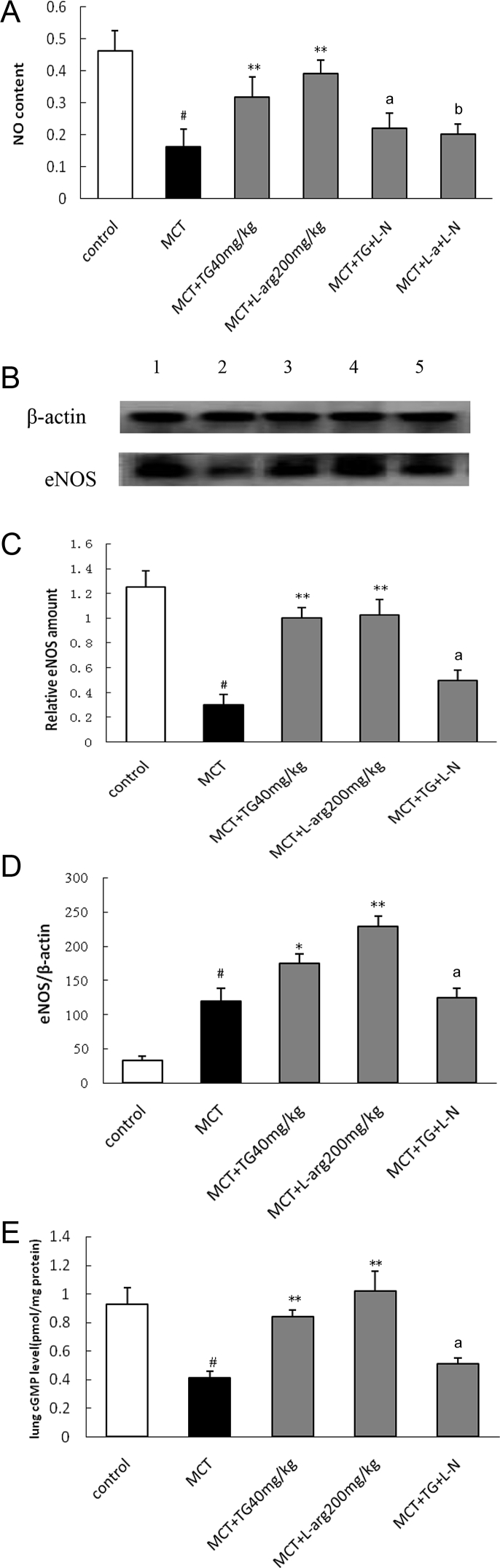
As shown in Fig. 3, the levels of NO, cGMP, and eNOS protein expressions decreased significantly, but the expression level of eNOS mRNA increased in the MCT group. Treatment with TG 40 mg/kg and l-arg 200 mg/kg increased the levels of NO and cGMP (Figs. 3A and 3E) and elevated the levels of eNOS mRNA and protein expressions in lung tissues, whereas these effects were partly blunted by l-NAME (Figs. 3B–3D).
Fig. 3.
Effects of TG on NO, eNOS, and cGMP in pulmonary tissues in MCT-induced pulmonary hypertension rats. (A) NO levels were determined spectrophotometrically by measuring total nitrate plus nitrite (NO3– + NO2–). NO level in lung tissues decreased in MCT group compared with control group (p < 0.05). Notably, TG 40 mg/kg administration caused a significant increase in NO production (p < 0.05), whereas these effects of TG were blocked by l-NAME. (B) The expressions of eNOS in lung tissues were determined by Western blot; lanes 1, 2, 3, 4, and 5 represent control, MCT, TG 40 mg/kg, l-arg 200 mg/kg, and TG + L-N, respectively. MCT significantly downregulated the expressions of eNOS protein, TG 40 mg/kg restored eNOS protein expressions in MCT-injured lungs, which were blocked by l-NAME. (C) Bands were quantified using a lumino-analyzer, and the data of eNOS protein expressions were expressed as fold increases normalized to β-actin expression. (D) The eNOS mRNA levels were analyzed using quantitative real-time PCR, and relative expression levels were calculated by comparison with the internal β-actin control. The eNOS mRNA expression increased significantly in MCT group, and TG 40 mg/kg and l-arg 200 mg/kg could further enhance the eNOS mRNA expression. (E) The levels of cGMP in lung tissues were determined using the radioimmunoassays. The cGMP levels in the MCT group were significantly low compared with control group (p < 0.05). TG 40 mg/kg treatment could increase cGMP level (p < 0.05) in pulmonary tissues, whereas these effects of TG were blocked by l-NAME. Results are presented as mean ± SD. a Statistical significance at p < 0.05 TG + L-N versus TG 40 mg/kg group. b Statistical significance at p < 0.05 l-a + L-N versus l-a 200 mg/kg group. * Statistical significance at p < 0.05 versus MCT group. ** Statistical significance at p < 0.01 versus MCT group. # Statistical significance at p < 0.05 versus control. cGMP, cyclic guanosine monophosphate; eNOS, endothelial nitric oxide synthase; l-a, l-arginine; l-NAME, NG-nitro-l-arginine methyl ester; MCT, monocrotaline; PCR, polymerase chain reaction; TG, total ginsenosides.
3.4. Effects of TG on p-ERK1/2, p-p38MAPK, p-JNK1/2, and MKP-1 protein expressions
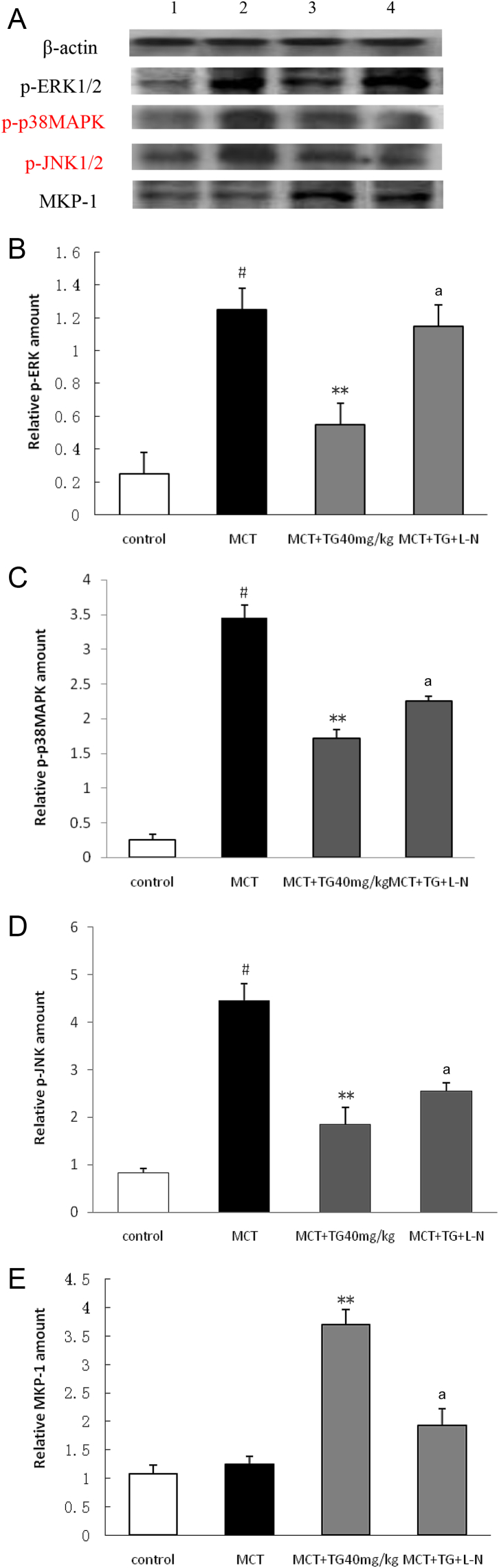
Western blot analysis showed that p-ERK1/2, p-p38MAPK, and p-JNK1/2 protein expressions in the MCT group exhibited a significant increase compared with that in the control group, but MKP-1 protein expression showed a slight increase (p > 0.05). TG treatment obviously decreased the protein expressions of p-ERK1/2, p-p38MAPK, and p-JNK1/2, and increased the MKP-1 protein expression, whereas these effects of TG were also partly abolished by l-NAME (Fig. 4).
Fig. 4.
Effects of TG on p-ERK1/2, p-p38MAPK, p-JNK1/2, and MKP-1 protein expressions in pulmonary tissues in MCT-induced pulmonary hypertension rats. Eighteen days after treatment with TG, the protein expressions of p-ERK1/2, p-p38MAPK, p-JNK1/2, and MKP-1 in lungs were determined by Western blot. (A) Lanes 1, 2, 3, and 4 represent control, MCT, TG (40 mg/kg), and TG + L-N, respectively. Bands were quantified using a lumino-analyzer, and the data of (B) p-ERK1/2, (C) p-p38MAPK, (D) p-JNK1/2, and (E) MKP-1 expressions were expressed as fold increases normalized to β-actin expression. Results are represented as mean ± SD. a Statistical significance at p < 0.05 TG + L-N versus TG 40 mg/kg group. * Statistical significance at p < 0.05 versus MCT group. ** Statistical significance at p < 0.01 versus MCT group. # Statistical significance at p < 0.05 versus control group. L-N, NG-nitro-l-arginine methyl ester; MCT, monocrotaline; MKP-1, mitogen-activated protein kinase phosphatase-1; p38MAPK, p38 mitogen-activated protein kinase; p-ERK1/2, phospho-ERK1/2; p-JNK1/2, phospho-JNK1/2; TG, total ginsenosides.
4. Discussion
Despite the marked progress has been achieved in the past decade in the treatment of group I pulmonary hypertension [16], [17], current practices provide merely symptom relief. MCT-induced pulmonary hypertension is a well-established model, which is very similar to human pulmonary hypertension [18]. In this study, the increase of RVSP, RVHI, and LW/BW in the MCT group indicated that the pulmonary hypertension model was successfully established.
We found that administration of TG (20, 40, 80 mg/kg/d) significantly decreased RVSP, RVHI, and LW/BW in MCT-induced pulmonary hypertension rats. We also found that the WT of small pulmonary arteries of TG-treated rats was significantly decreased compared with that in the MCT group. Such ameliorated effects of TG were markedly neutralized by l-NAME. Our results indicate that TG exerted a protective effect on pulmonary hypertension, which might occur by reducing pulmonary arterial pressure, improving pulmonary vascular remodeling, and regulating the NO signal transduction pathway.
To prove if the protective effect of TG involves NO signal transduction pathway, we measured the levels of NO, cGMP, and eNOS in lung tissues. A previous study showed that MCT selectively damages pulmonary endothelium, reduces NO production and secretion, and decreases cGMP levels in lung tissues [19]. In our study, NO production, cGMP levels, and eNOS protein expression significantly decreased in the MCT group, but the level of eNOS mRNA expression was higher than those of the control group, in agreement with other studies that showed that levels of eNOS mRNA increased in MCT-treated rats although eNOS protein levels decreased [20], [21]. This disparity raises the possibility that even though there was stimulation of eNOS transcription in this model of pulmonary hypertension, there were also factors that interfered with the translation of the eNOS message and/or augmented degradation of the enzyme. Ginseng extract administration was previously shown to enhance NO production, improve vessel wall thickening, alleviate hypertension, and stimulate nongenomic Akt-mediated eNOS activation in spontaneously hypertensive rats [22]. Similarly, our results also showed that TG administration increased NO and cGMP production, and upregulated eNOS mRNA and protein expressions, whereas these effects were blunted by l-NAME. Taken together, it appears to be possible that TG could suppress MCT-induced pulmonary hypertension in rats at least partly by promoting NO and cGMP formation through upregulation of eNOS protein expression.
Increased MAPK activation has been associated with remodeling of pulmonary arteries in MCT-induced pulmonary hypertension rats [5], [23], [24]. MKP-1, a dual specificity phosphatase induced by growth factors, may be a negative feedback mechanism in the control of MAPKs activity in vascular smooth muscle cells [25]. Here, we found that the expressions of p-ERK1/2, p-p38MAPK, and p-JNK1/2 in pulmonary arteries significantly increased in the MCT group. Furthermore, TG treatment could obviously decrease p-ERK, p-p38MAPK, and p-JNK1/2 expressions and upregulate MKP-1 expression, which might be the cause for downregulation of ERK, p38MAPK, and JNK phosphorylation. These results strongly suggested that the molecular mechanism for the effect of TG on pulmonary vascular remodeling might also involve the suppression of the MAPKs signal pathway. In addition, NO has been shown to attenuate endothelin-1-induced activation of ERK1/2 in vascular smooth muscle cells in a cGMP-dependent manner [26]. cGMP was reported to regulate ERK1/2 and p38MAPK among the MAPKs [27]. The present results also confirmed that l-NAME could partly blunt the inhibitory effect of TG on the MAPKs signal pathway, demonstrating the existence of the crosstalk between the NO and MAPK signal pathways in regulation of the development of pulmonary hypertension.
In conclusion, our study demonstrates that TG treatment can attenuate pulmonary hypertension when administered early, in part by the regulation on the NO–cGMP and MAPK signal pathways. Our results strongly support the notion that TG has a promising therapeutic potential for pulmonary hypertension.
Conflicts of interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgments
The study was supported by programs from the National Natural Science Foundation of China (81472038, 81370899, 81222026, 81170741, and 81071440/H0601) and also by programs from the New Century Excellent Talents from the Ministry of Education (NCET-11-0437), China.
Contributor Information
Hongzhi Sun, Email: sunhongzhi@mail.xjtu.edu.cn.
Shufang Wu, Email: shufangw@hotmail.com.
References
- 1.Klinger J.R., Abman S.H., Gladwin M.T. Nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188:639–646. doi: 10.1164/rccm.201304-0686PP. [DOI] [PubMed] [Google Scholar]
- 2.Hampl V., Herget J. Role of nitric oxide in the pathogenesis of chronic pulmonary hypertension. Physiol Rev. 2000;80:1337–1372. doi: 10.1152/physrev.2000.80.4.1337. [DOI] [PubMed] [Google Scholar]
- 3.Mathew R., Gloster E.S., Sundararajan T., Thompson C.I., Zeballos G.A., Gewitz M.H. Role of inhibition of nitric oxide production in monocrotaline-induced pulmonary hypertension. J Appl Physiol (1985) 1997;82:1493–1498. doi: 10.1152/jappl.1997.82.5.1493. [DOI] [PubMed] [Google Scholar]
- 4.Lu J., Shimpo H., Shimamoto A., Chong A.J., Hampton C.R., Spring D.J., Yada M., Takao M., Onoda K., Yada I. Specific inhibition of p38 mitogen-activated protein kinase with FR167653 attenuates vascular proliferation in monocrotaline-induced pulmonary hypertension in rats. J Thorac Cardiovasc Surg. 2004;128:850–859. doi: 10.1016/j.jtcvs.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Yu M.Q., Liu X.S., Wu H.X., Xiang M., Xu Y.J. ERK1/2 promotes cigarette smoke-induced rat pulmonary artery smooth muscle cells proliferation and pulmonary vascular remodeling via up-regulating cycline1 expression. J Huazhong Univ Sci Technol Med Sci. 2013;33:315–322. doi: 10.1007/s11596-013-1117-8. [DOI] [PubMed] [Google Scholar]
- 6.Fuzzati N., Gabetta B., Jayakar K., Pace R., Peterlongo F. Liquid chromatography-electrospray mass spectrometric identification of ginsenosides in Panax ginseng roots. J Chromatogr A. 1999;854:69–79. doi: 10.1016/s0021-9673(99)00463-x. [DOI] [PubMed] [Google Scholar]
- 7.Li K.K., Yang X.B., Yang X.W., Liu J.X., Gong X.J. New triterpenoids from the stems and leaves of Panax ginseng. Fitoterapia. 2012;83:1030–1035. doi: 10.1016/j.fitote.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Kim J.H. Cardiovascular Diseases and Panax ginseng: a review on molecular mechanisms and medical applications. J Ginseng Res. 2012;36:16–26. doi: 10.5142/jgr.2012.36.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahn H.Y., Hong S.Y., Kim J.Y., Kwon O. Panax ginseng extract rich in ginsenoside protopanaxatriol offers combinatorial effects in nitric oxide production via multiple signaling pathways. Springerplus. 2013;2:96. doi: 10.1186/2193-1801-2-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu X.F., Deng J., Yang D.L., Gao Y., Gong Q.H., Huang X.N. Total ginsenosides suppress the neointimal hyperplasia of rat carotid artery induced by balloon injury. Vascul Pharmacol. 2011;54:52–57. doi: 10.1016/j.vph.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Huang J., Li L.S., Yang D.L., Gong Q.H., Deng J., Huang X.N. Inhibitory effect of ginsenoside Rg1 on vascular smooth muscle cell proliferation induced by PDGF-BB is involved in nitric oxide formation. Evid Based Complement Alternat Med. 2012;2012 doi: 10.1155/2012/314395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin N., Gong Q.H., Wei L.W., Wu Q., Huang X.N. Total ginsenosides inhibit the right ventricular hypertrophy induced by monocrotaline in rats. Biol Pharm Bull. 2008;31:1530–1535. doi: 10.1248/bpb.31.1530. [DOI] [PubMed] [Google Scholar]
- 13.Yi X.Q., Li T., Wang J.R., Wong V.K., Luo P., Wong I.Y., Jiang Z.H., Liu L., Zhou H. Total ginsenosides increase coronary perfusion flow in isolated rat hearts through activation of PI3K/Akt-eNOS signaling. Phytomedicine. 2010;17:1006–1015. doi: 10.1016/j.phymed.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Zheng G.Q., Cheng W., Wang Y., Wang X.M., Zhao S.Z., Zhou Y., Liu S.J., Wang X.T. Ginseng total saponins enhance neurogenesis after focal cerebral ischemia. J Ethnopharmacol. 2011;133:724–728. doi: 10.1016/j.jep.2010.01.064. [DOI] [PubMed] [Google Scholar]
- 15.Barth P.J., Kimpel C., Roy S., Wagner U. An improved mathematical approach for the assessment of the medial thickness of pulmonary arteries. Pathol Res Pract. 1993;189:567–576. doi: 10.1016/S0344-0338(11)80368-7. [DOI] [PubMed] [Google Scholar]
- 16.Olschewski H., Ghofrani A., Wiedemann R., Rose F., Enke B., Gessler T., Voswinckel R., Kohstall M., Grimminger F., Seeger W. Pulmonary hypertension. Internist (Berl) 2002;43(1498):1501–1509. doi: 10.1007/s00108-002-0761-z. [DOI] [PubMed] [Google Scholar]
- 17.Galiè N., Torbicki A., Barst R., Dartevelle P., Haworth S., Higenbottam T., Olschewski H., Peacock A., Pietra G., Rubin L.J. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. Rev Esp Cardiol. 2005;58:523–566. doi: 10.1157/13074846. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Arroyo J.G., Farkas L., Alhussaini A.A., Farkas D., Kraskauskas D., Voelkel N.F., Bogaard H.J. The monocrotaline model of pulmonary hypertension in perspective. Am J Physiol Lung Cell Mol Physiol. 2012;302:L363–L369. doi: 10.1152/ajplung.00212.2011. [DOI] [PubMed] [Google Scholar]
- 19.Mathew R., Yuan N., Rosenfeld L., Gewitz M.H., Kumar A. Effects of monocrotaline on endothelial nitric oxide synthase expression and sulfhydryl levels in rat lungs. Heart Dis. 2002;4:152–158. doi: 10.1097/00132580-200205000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Tyler R.C., Muramatsu M., Abman S.H., Stelzner T.J., Rodman D.M., Bloch K.D., McMurtry I.F. Variable expression of endothelial NO synthase in three forms of rat pulmonary hypertension. Am J Physiol. 1999;276:L297–L303. doi: 10.1152/ajplung.1999.276.2.L297. [DOI] [PubMed] [Google Scholar]
- 21.Resta T.C., Gonzales R.J., Dail W.G., Sanders T.C., Walker B.R. Selective upregulation of arterial endothelial nitric oxide synthase in pulmonary hypertension. Am J Physiol. 1997;272:H806–H813. doi: 10.1152/ajpheart.1997.272.2.H806. [DOI] [PubMed] [Google Scholar]
- 22.Hong S.Y., Kim J.Y., Ahn H.Y., Shin J.H., Kwon O. Panax ginseng extract rich in ginsenoside protopanaxatriol attenuates blood pressure elevation in spontaneously hypertensive rats by affecting the Akt-dependent phosphorylation of endothelial nitric oxide synthase. J Agric Food Chem. 2012;60:3086–3091. doi: 10.1021/jf204447y. [DOI] [PubMed] [Google Scholar]
- 23.Zeng Z., Li Y., Jiang Z., Wang C., Li B., Jiang W. The extracellular signal-regulated kinase is involved in the effects of sildenafil on pulmonary vascular remodeling. Cardiovasc Ther. 2010;28:23–29. doi: 10.1111/j.1755-5922.2009.00115.x. [DOI] [PubMed] [Google Scholar]
- 24.Henriques-Coelho T., Oliveira S.M., Moura R.S., Roncon-Albuquerque R., Jr., Neves A.L., Santos M., Nogueira-Silva C., La Fuente Carvalho F., Brandão-Nogueira A., Correia-Pinto J. Thymulin inhibits monocrotaline-induced pulmonary hypertension modulating interleukin-6 expression and suppressing p38 pathway. Endocrinology. 2008;149:4367–4373. doi: 10.1210/en.2008-0018. [DOI] [PubMed] [Google Scholar]
- 25.Bokemeyer D., Lindemann M., Kramer H.J. Regulation of mitogen-activated protein kinase phosphatase-1 in vascular smooth muscle cells. Hypertension. 1998;32:661–667. doi: 10.1161/01.hyp.32.4.661. [DOI] [PubMed] [Google Scholar]
- 26.Bouallegue A., Daou G.B., Srivastava A.K. Nitric oxide attenuates endothelin-1-induced activation of ERK1/2, PKB, and Pyk2 in vascular smooth muscle cells by a cGMP-dependent pathway. Am J Physiol Heart Circ Physiol. 2007;293:H2072–H2079. doi: 10.1152/ajpheart.01097.2006. [DOI] [PubMed] [Google Scholar]
- 27.Marathe N., Rangaswami H., Zhuang S., Boss G.R., Pilz R.B. Pro-survival effects of 17β-estradiol on osteocytes are mediated by nitric oxide/cGMP via differential actions of cGMP-dependent protein kinases I and II. J Biol Chem. 2012;287:978–988. doi: 10.1074/jbc.M111.294959. [DOI] [PMC free article] [PubMed] [Google Scholar]