Abstract
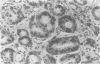
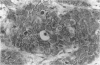
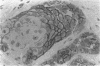
AIMS: To examine CD44H and CD44v3 expression in normal gastric and small bowel mucosa, normal and Barrett oesophagus, and oesophageal epithelial malignancies (squamous cell carcinoma and adenocarcinoma). METHODS: Ninety five specimens, comprised of 40 of normal oesophageal, gastric and small bowel mucosa, 22 of Barrett oesophagus (two with dysplastic changes), 20 of resected adenocarcinomas, and 13 of squamous cell carcinoma, were evaluated. The samples were fixed in formalin and subsequently stained with anti-CD44H and anti-CD44v3 monoclonal antibodies using the avidin-biotin peroxidase technique. RESULTS: In contrast to normal oesophagus, which showed positivity for both CD44 epitopes (CD44H and CD44v3) in the basal third of the epithelium, antral and intestinal subtypes of Barrett oesophagus expressed CD44H only, the distribution being focal in non-dysplastic and diffuse in dysplastic Barrett mucosa. Similarly, normal antral glands and small bowel epithelium were focally immunopositive for CD44H at the base of the crypts. All squamous cell carcinomas were diffusely positive for both isoforms, whereas 75% (15/20) of the adenocarcinomas expressed CD44H and 60% (12/20) expressed CD44v3. CONCLUSIONS: CD44H is expressed in the proliferating areas of both normal squamous epithelium and Barrett mucosa. CD44H expression seems to increase progressively in dysplasia and infiltrating carcinoma, similar to the process described in the stomach. CD44v3 expression, usually not observed in normal or neoplastic gastric mucosa, was present in normal squamous epithelium and oesophageal squamous cell carcinoma. CD44v3 immunoreactivity was also identified in 60% of adenocarcinomas. These findings suggest that CD44v3 may play a role in the development of oesophageal carcinoma of both squamous and glandular types.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett K. L., Jackson D. G., Simon J. C., Tanczos E., Peach R., Modrell B., Stamenkovic I., Plowman G., Aruffo A. CD44 isoforms containing exon V3 are responsible for the presentation of heparin-binding growth factor. J Cell Biol. 1995 Feb;128(4):687–698. doi: 10.1083/jcb.128.4.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S. B., Fawcett J., Jackson D. G., Collins I., Gatter K. C., Harris A. L., Gearing A., Simmons D. L. Normal human tissues, in addition to some tumors, express multiple different CD44 isoforms. Cancer Res. 1994 Aug 15;54(16):4539–4546. [PubMed] [Google Scholar]
- Günthert U., Hofmann M., Rudy W., Reber S., Zöller M., Haussmann I., Matzku S., Wenzel A., Ponta H., Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991 Apr 5;65(1):13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Hamilton S. R., Smith R. R. The relationship between columnar epithelial dysplasia and invasive adenocarcinoma arising in Barrett's esophagus. Am J Clin Pathol. 1987 Mar;87(3):301–312. doi: 10.1093/ajcp/87.3.301. [DOI] [PubMed] [Google Scholar]
- Harn H. J., Ho L. I., Chang J. Y., Wu C. W., Jiang S. Y., Lee H. S., Lee W. H. Differential expression of the human metastasis adhesion molecule CD44V in normal and carcinomatous stomach mucosa of Chinese subjects. Cancer. 1995 Mar 1;75(5):1065–1071. doi: 10.1002/1097-0142(19950301)75:5<1065::aid-cncr2820750503>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Hashimoto K., Higashiyama S., Asada H., Hashimura E., Kobayashi T., Sudo K., Nakagawa T., Damm D., Yoshikawa K., Taniguchi N. Heparin-binding epidermal growth factor-like growth factor is an autocrine growth factor for human keratinocytes. J Biol Chem. 1994 Aug 5;269(31):20060–20066. [PubMed] [Google Scholar]
- Heider K. H., Dämmrich J., Skroch-Angel P., Müller-Hermelink H. K., Vollmers H. P., Herrlich P., Ponta H. Differential expression of CD44 splice variants in intestinal- and diffuse-type human gastric carcinomas and normal gastric mucosa. Cancer Res. 1993 Sep 15;53(18):4197–4203. [PubMed] [Google Scholar]
- Heider K. H., Hofmann M., Hors E., van den Berg F., Ponta H., Herrlich P., Pals S. T. A human homologue of the rat metastasis-associated variant of CD44 is expressed in colorectal carcinomas and adenomatous polyps. J Cell Biol. 1993 Jan;120(1):227–233. doi: 10.1083/jcb.120.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M., Rudy W., Zöller M., Tölg C., Ponta H., Herrlich P., Günthert U. CD44 splice variants confer metastatic behavior in rats: homologous sequences are expressed in human tumor cell lines. Cancer Res. 1991 Oct 1;51(19):5292–5297. [PubMed] [Google Scholar]
- Jackson D. G., Bell J. I., Dickinson R., Timans J., Shields J., Whittle N. Proteoglycan forms of the lymphocyte homing receptor CD44 are alternatively spliced variants containing the v3 exon. J Cell Biol. 1995 Feb;128(4):673–685. doi: 10.1083/jcb.128.4.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J., Hyman R., Kincade P. W. CD44 and its interaction with extracellular matrix. Adv Immunol. 1993;54:271–335. doi: 10.1016/s0065-2776(08)60537-4. [DOI] [PubMed] [Google Scholar]
- Levine M. S., Caroline D., Thompson J. J., Kressel H. Y., Laufer I., Herlinger H. Adenocarcinoma of the esophagus: relationship to Barrett mucosa. Radiology. 1984 Feb;150(2):305–309. doi: 10.1148/radiology.150.2.6691080. [DOI] [PubMed] [Google Scholar]
- Mayer B., Jauch K. W., Günthert U., Figdor C. G., Schildberg F. W., Funke I., Johnson J. P. De-novo expression of CD44 and survival in gastric cancer. Lancet. 1993 Oct 23;342(8878):1019–1022. doi: 10.1016/0140-6736(93)92879-x. [DOI] [PubMed] [Google Scholar]
- Mulder J. W., Kruyt P. M., Sewnath M., Oosting J., Seldenrijk C. A., Weidema W. F., Offerhaus G. J., Pals S. T. Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet. 1994 Nov 26;344(8935):1470–1472. doi: 10.1016/s0140-6736(94)90290-9. [DOI] [PubMed] [Google Scholar]
- PUESTOW C. B., GILLESBY W. J., GUYNN V. L. Cancer of the esophagus. AMA Arch Surg. 1955 May;70(5):662–671. doi: 10.1001/archsurg.1955.01270110034006. [DOI] [PubMed] [Google Scholar]
- Screaton G. R., Bell M. V., Jackson D. G., Cornelis F. B., Gerth U., Bell J. I. Genomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exons. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12160–12164. doi: 10.1073/pnas.89.24.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. H., Antonioli D. A., Goldman H. Comparative features of esophageal and gastric adenocarcinomas: recent changes in type and frequency. Hum Pathol. 1986 May;17(5):482–487. doi: 10.1016/s0046-8177(86)80038-7. [DOI] [PubMed] [Google Scholar]
- Washington K., Gottfried M. R., Telen M. J. Expression of the cell adhesion molecule CD44 in gastric adenocarcinomas. Hum Pathol. 1994 Oct;25(10):1043–1049. doi: 10.1016/0046-8177(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Wielenga V. J., Heider K. H., Offerhaus G. J., Adolf G. R., van den Berg F. M., Ponta H., Herrlich P., Pals S. T. Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res. 1993 Oct 15;53(20):4754–4756. [PubMed] [Google Scholar]