Abstract
Twenty-seven species of the genus Onchocerca (Nematoda; Filarioidea) can cause a vector-borne parasitic disease called onchocercosis. Most Onchocerca species infect wild and domestic ungulates or the dog, and one species causes river blindness in humans mainly in tropical Africa. The European red deer (Cervus e. elaphus) is host to four species, which are transmitted by blackflies (simuliids) or biting midges (ceratopogonids). Two species, Onchocerca flexuosa and Onchocerca jakutensis, produce subcutaneous nodules, whereas Onchocerca skrjabini and Onchocerca garmsi live free in the hypodermal serous membranes. During the hunting season, September 2013, red deer (n = 25), roe deer (Capreolus c. capreolus, n = 6) and chamois (Rupicapra r. rupicapra, n = 7), all shot in the Grisons Region (Switzerland) were investigated for the presence of subcutaneous nodules which were enzymatically digested, and the contained Onchocerca worms were identified to species by light and scanning electron microscopy as well as by PCR/sequencing. In addition, microfilariae from skin samples were collected and genetically characterized. Neither nodules nor microfilariae were discovered in the roe deer and chamois. Adult worms were found in 24% of red deer, and all of them were identified as O. jakutensis. Two morphologically different microfilariae were obtained from five red deer, and genetic analysis of a skin sample of one red deer indicated the presence of another Onchocerca species. This is the first report of O. jakutensis in Switzerland with a prevalence in red deer similar to that in neighbouring Germany.
Keywords: Onchocerca jakutensis, Morphology, Cervus elaphus, Red deer, Subcutaneous nodule, Switzerland
Graphical abstract

Highlights
-
•
First description of Onchocerca jakutensis in red deer in Switzerland.
-
•
Six of 25 red deer (prevalence 24%) contained male and/or female Onchocerca worms.
-
•
Identification was done by morphology and DNA analysis.
-
•
A morphological description is given to link with the DNA analysis used.
-
•
Roe deer and chamois from the same area were not infected.
1. Introduction
Onchocercosis is a parasitic disease caused by any of the 27 species of the genus Onchocerca which are transmitted by simuliid or ceratopogonid insect vectors (Anderson, 2000). Most Onchocerca spp. infect wild and domestic ungulates, while Onchocerca lupi Rodonaja, 1967 infects dogs. The parasites cause lesions, inflammation of musculature and joints and lameness in ungulates, or eye disease in dogs (Deplazes et al., 2013). Furthermore, Onchocerca volvulus (Leuckart, 1893) Railliet and Henry, 1910 is the causative agent of river blindness of humans, mainly in tropical Africa, with currently over 37 million people being infected (WHO, 2015). Except for river blindness, onchocercosis in humans is rare. Only 25 cases have been described to date (reviewed in Uni et al., 2015), such as an unusual infection with Onchocerca jakutensis in a woman in Austria (Koehsler et al., 2007).
Worldwide seven Onchocerca species are known to parasitize Cervidae (Anderson, 2000). Publications from Europe describe four species in red deer (Cervus elaphus) in central Europe: Onchocerca flexuosa (Wedl, 1856), O. jakutensis (Gubanov, 1964) (Syn. Onchocerca tubingensis Bain and Schulz-Key, 1974), Onchocerca skrjabini (Ruchljadev, 1964) (Syn. Onchocerca tarsicola Bain and Schulz-Key, 1974) and Onchocerca garmsi Bain and Schulz-Key, 1976 (Schulz-Key et al., 1975). Studies have revealed Onchocerca infections in cervids in the Czech Republic and Slovakia (Dyková and Blazek, 1972), Hungary (Meszaros and Sugar, 1974), Yugoslavia (Brglez and Zeleznik, 1976), Romania (Dulceanu and Ghitescu, 1986), Germany (Schulz-Key, 1975), Poland (Demaskiewicz, 1993), Spain (Santín-Durán et al., 2001, San-Miguel et al., 2003) and Italy (Morandi et al., 2011).
Two species, O. flexuosa and O. jakutensis, produce large subcutaneous nodules up to several centimetres in diameter, whereas O. skrjabini and O. garmsi live free in the hypodermal serous membranes (Plenge-Bönig et al., 1994). The subcutaneous nodules of O. flexuosa are located between the shoulder blade and the lumbar region Brglez et al., 1967), in the region of the sacrum and rarely on the lateral abdomen or proximal parts of the extremities (Plenge-Bönig et al., 1994). The subcutaneous nodules of O. jakutensis are found in the region of the external thigh or in the caudal part of the back (Bain and Schulz-Key, 1974). The rare filarial species O. garmsi is located in the tissue above the sternum (Schulz-Key et al., 1975). Onchocerca skrjabini is found free between the serous membranes above the radio-carpal and tibio-tarsal joints or on the abductor tendon (Bain and Schulz-Key, 1974).
The microfilariae of O. garmsi and O. jakutensis are present with highest concentrations in the skin above the sternum, and also at the base of the ear. Microfilariae of O. skrjabini are predominantly found in the exterior part of the ear. They also appear in the nose region, on the scrotum and on the insides of the thigh (Schulz-Key et al., 1975), whereas preferred sites of O. flexuosa microfilariae are the inside of the thigh and the distal parts of the hind leg (Schulz-Key, 1975).
Little is known about the pathogenicity of the different species of Onchocerca in red deer. Dyková (1970) described a chronic sclerosing lymphadenitis caused by microfilariae of O. flexuosa. Later, she observed a microfilaria-induced myositis of the surrounding musculature and a dystrophic change of the hypodermal tissue (Dyková, 1972). Commichau (1982) reported a polyarthritis caused by an Onchocerca species in red deer.
The present investigation elucidates the presence of Onchocerca spp. in wild ruminants in Switzerland. The presence as well as the particular species is also of medical interest, as endemic zoonotic onchocercosis should be considered. Furthermore, the morphology of O. jakutensis as described by Bain and Schulz-Key (1974) versus Demaskiewicz (1993) differs in some parts. In such situations, DNA analysis has proven helpful and was used e.g. by Morandi et al. (2011) and in a zoonotic case (Koehsler et al., 2007). However, the morphology of the specimens identified in these molecular analyses remains uncertain. A detailed morphological description of the worms recovered here is therefore included in the present investigation to link with the results of DNA analyses.
2. Materials and methods
2.1. Origin of samples, sample preparation and morphological identification
The skins of 25 free-ranging red deer (Cervus e. elaphus), six roe deer (Capreolus c. capreolus) and seven chamois (Rupicapra r. rupicapra) were investigated for nodules of adult worms during the hunting season, September 2013. The animals were shot in the Grisons Region, Switzerland (Churer Rheintal, Prättigau, Heinzenberg, Albulatal, Hinterrheintal, Lenzerheide, Safiental, Domleschg and Misox). To isolate adult worms, the nodules were incubated for 8 h at 60 °C in subtilisin-enzyme solution (10% [v/v] Enzyrim OSA, Bauer Handels GmbH, Adetswil, Switzerland), buffered at pH 8.00 (9888 Titrisol Merck, Germany) and 2 drops of Mollescal-C (Bauer Handels GmbH) per 10 ml. After lysis specimens were fixed and stored in 70% ethanol. For morphological examination, adult worms were cleared in chloro-lactophenol. Drawings were done with a Leitz drawing tube. Scanning electron microscopy was performed at the Center for Microscopy and Image Analysis, University of Zurich using standard procedures. Specimens were dehydrated in 100% ethanol, followed by critical-point-drying and then coated with carbon or platinum.
In addition, 2 × 2 cm skin samples form the abdominal and from the metatarsal regions were collected from all investigated ungulates. The skin samples were treated as described by Schulz-Key (1975), with some modifications: They were cut into small pieces and incubated in petri dishes for 2–5 h at 37 °C in 20 ml of a saline solution containing 0.9% (w/v) NaCl and 0.1% (v/v) Mollescal-C (BASF, Ludwigshafen, Germany) as a preservative. After the incubation period, the skin shreds were removed, the petri dish set at a slight angle, and 8 droplets of the sediment microscopically investigated for the presence of microfilariae. The rest of the sediment from the samples containing microfilariae were kept frozen in 1.5 ml Eppendorf tubes for genetic analyses. Morphological identification was achieved using the keys given in Bain (1981) and Demaskiewicz (1993).
2.2. DNA extraction
About 2 cm long sections of single adult worms (n = 10 from four red deer) were washed 2 to 3 times in phosphate-buffered saline (PBS) at room temperature and transferred into 1.5 ml Eppendorf tubes containing 20–40 μl PBS. To disrupt the cell membranes, the tubes were frozen in liquid nitrogen, thawed in a heat block at 100 °C for 1 min and then rigorously vortexed for 1 min. This step was repeated three times. Worms were then triturated with a tissue homogenizer as described by Wenk et al. (2012). The tubes containing microfilariae were centrifuged for 5 min and the supernatant was reduced to 30 μl. Five μl were again examined for the presence of microfilariae, and the remaining 25 μl were minced in a tissue homogenizer. DNA isolation was done with the Qiagen (Hildesheim, Germany) Qiamp DNA mini kit according to the tissue protocol. DNA isolations were immediately frozen on dry ice and stored at −18 °C.
2.3. PCR
PCR was carried out according to Morales-Hojas et al. (2006), targeting the mitochondrial NADH-dehydrogenase gene. Amplifications were performed using the forward primer ND50vA (5′-TTG GTT GCC TAA GGC TAT GG-3′) and the reverse primer ND50vC (5′-CCC CTA GTA AAC AAC AAA CCA CA-3′) in a MyCycler thermocycler (Biorad, Cressier, Switzerland). Reactions were performed in a total volume of 20 μl, containing standard Taq-polymerase buffer (BioConcept, Alschwil, Switzerland), 200 μM each dNTP (NBL, UK), 0.4 μM each primer (Microsynth, Balgach, Switzerland), 1 unit Taq-DNA polymerase (BioConcept) and 2 μl of the DNA extract. Amplifications consisted of an initial denaturation step at 95 °C for 2 min, followed by 30 cycles of 30 s at 95 °C, 30 s at 58 °C and 30 s at 72 °C, with a final extension step of 5 min at 72 °C. Amplicons were analysed on 3% agarose gels, stained with GelRed™ (Chemie Brunschwig, Basel, Switzerland) and visualized with UV light. Negative controls containing H2O were run to control for contamination.
2.4. Sequencing and analysis
Amplicons were sequenced (Synergene, Schlieren, Switzerland), after purification with the minelute PCR purification kit (Qiagen) following the manufacturer’s instructions, using the PCR forward primer and 5 μl of the amplicons. Sequences were aligned with Multialin (Corpet, 1988) and then compared with GenBank entries.
3. Results
3.1. Prevalence of O. jakutensis
Of the 38 artiodactyls from the three species examined, Onchocerca specimens were found only in red deer. In six of the 25 red deer, subcutaneous nodules (n = 13) (Fig. 1) were collected containing female and male or only female O. jakutensis worms as identified by morphological and genetical analyses (prevalence 24%). Up to five nodules were found per deer. Nearly half of the collected adult Onchocerca worms contained granular lumps (Fig. 2) or were in a state of advanced decay to calcification. Microfilariae were observed in eight of ten tissue samples from five red deer from which adult O. jakutensis were recovered from only three.
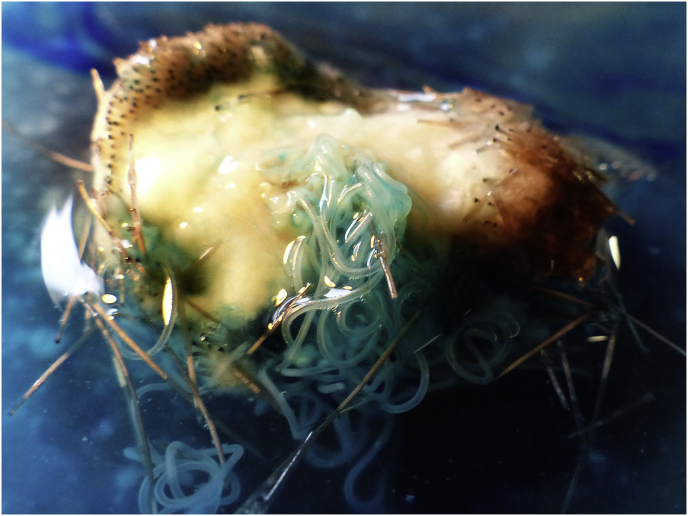
Fig. 1.
Subcutaneous nodule in red deer skin with partly freed O. jakutensis female (stained with methylene blue). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
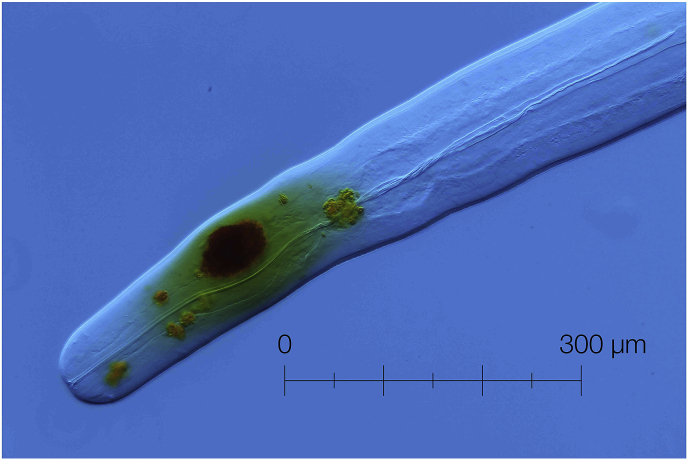
Fig. 2.
Anterior end of O. jakutensis female, with granular lumps in process of degeneration.
3.2. Morphology of O. jakutensis
The living worms are transparent opalescent, white-yellowish filarioids. Females are much longer than males. Mouth orifice 1.5 μm in diameter. Oesophagus divided in anterior muscular part and slightly longer posterior glandular part.
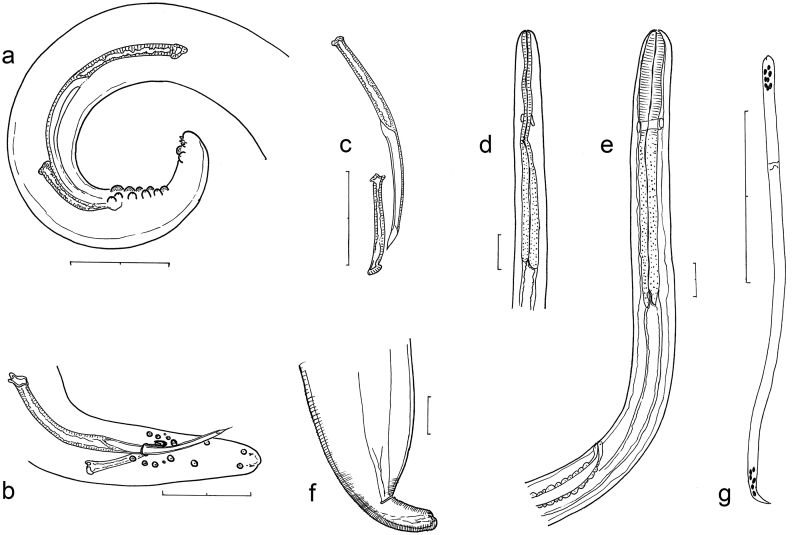
3.2.1. Male of O. jakutensis (Fig. 3a–d)
Fig. 3.
O. jakutensis male and female (bars: 100 μm): a. Tail of male with five pairs of pericloacal and two pair of closely spaced terminal papillae. b. Tail of another male with more distantly spaced papillae on tail end. Left spicule protruding. c. Spicules in ventral view. d. Head end of male. e. Head end of female with vulva. f. Posterior end of female with conical tail in ventral view, annulations indicated on sides. g. Microfilaria with terminal distribution of nuclei.
The description is given for the same specimen which was also used for PCR/DNA sequencing and SEM. (In addition measurements of another entire male and of the posterior part of a further male specimen are given in parentheses).
Body length 36 mm (40 mm), tapering gradually within about 6 mm towards anterior end, from a width of 180 μm (160 μm, 170 μm) at midbody to 90 μm (90 μm) at end of oesophagus and more rapidly tapering to 60 μm (50 μm, 50 μm) in diameter at cloaca. Cuticle with thin transverse annulations, 2.5 μm apart at midbody, narrowing towards both ends. Oesophagus length 670 μm (530 μm), glandular posterior part 360 μm (280 μm). Nerve ring close to end of muscular oesophagus at 250 μm (220 μm) from anterior end (Fig. 3d). Tail 130 μm (110 μm, 110 μm) long, coiled ventrad, flattened or slightly concave on ventral side (Fig. 3a).
Spicules unequal and dissimilar. Left spicule 275 μm (260 μm, 220 μm), right 90 μm (90 μm, 110 μm) long, both with well developed head. Proximal shaft cylindrical, distal part modified in both. Left spicule: distal part slightly longer than shaft, thinned to membrane with margins bent ventrad to form tube, very fine membrane forming pointed tip of spicule. Right spicule: distal half flattening to concave groove with strong knob on dorsal distal end. In protruded position, the right spicule serves as gubernaculum for the left spiculum. No gubernaculum present (Fig. 3b–c).
Two phasmids ventro-lateral near tip of tail with conspicuously protruding flaps. Five pairs of pericloacal papillae and 2 pairs close to tail tip, in proximity to phasmids (Fig. 3a–b). No unpaired precloacal papilla present (Fig. 4a,b). Considerable individual variation in placement and size of papillae (note in Fig. 3b the two very small papillae in pericloacal group and the distal pair on tail tip). In two of three specimens one pair of papillae set between cloacal group and group on tail tip.
Fig. 4.
a and b: Posterior end of O. jakutensis male with 5 pairs of pericloacal papillae without unpaired precloacal papilla.
3.2.2. Female of O. jakutensis (Fig. 3e–f)
Description is given of 4 entire females. In addition measurement of the specimen which was also used for PCR/sequencing is given in parentheses.
Body length 705–780 mm (810 mm). Oesophagus 600–800 μm (720 μm), glandular part slightly longer than anterior, muscular part. Vulva posterior to oesophagus, 1260–1500 μm (870 μm) from anterior end (Fig. 3e). Body diameter 310–320 μm (310 μm) at midbody, gradually tapering in anterior part to 120–130 μm (130 μm) diameter at end of oesophagus, tapering more rapidly posteriad to 65–105 μm (110 μm) diameter at anus. Tail nearly cylindrical, 90–165 μm (170 μm) long with rounded tip, bearing two flapped phasmids subapically (Fig 3f).
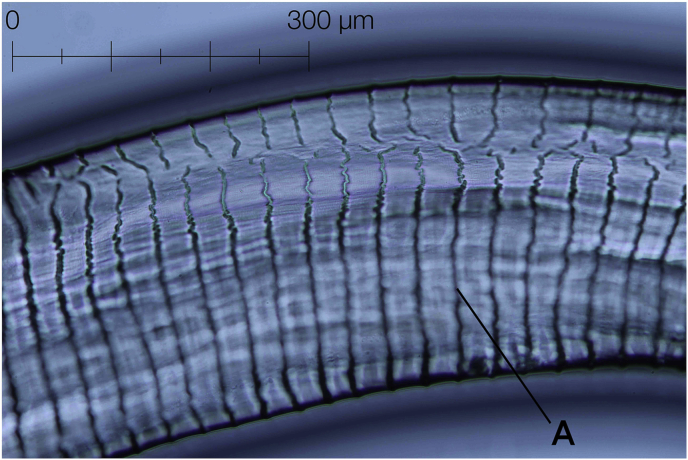
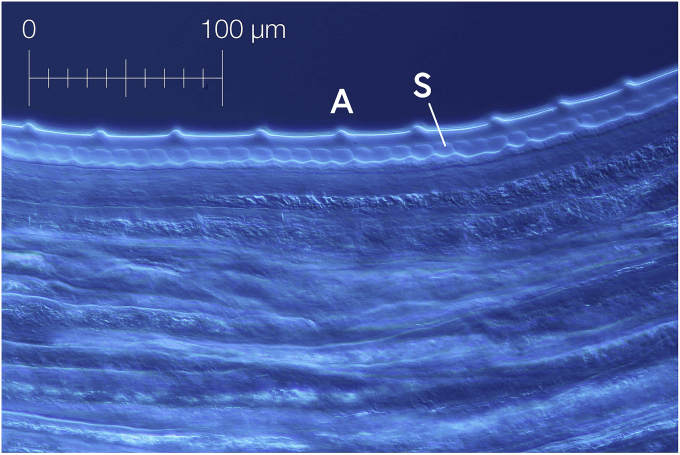
Outermost layer of cuticle thickened in intervals to conspicuous transverse annulations, which fade out towards lateral fields. Annulations rough in appearance, forming jagged lines (Fig. 5). Some single annuli bifurcated or interrupted towards lateral fields. Internal medullar part of cuticle forming transversely oriented, cushion-like striae, with the appearance of square cells in transverse sections. External annuli to striae in the ratio 1:4 (Fig. 6). External annulation smoothing out towards head and tail end of body, becoming smaller and narrower as in males (Fig. 3f).
Fig. 5.
External cuticular annulation (A) of O. jakutensis female with interruption over lateral field.
Fig. 6.
O. jakutensis female: Ratio of cuticular annulation (A) to medullar striae (S) 1:4.
3.2.3. Description of microfilariae from O. jakutensis from uterus
Microfilaria without sheath. Long and thin, specimens measured in oviduct close to vulva 260–270 μm long, 6 μm wide. Cuticle with fine transverse annulation, 0.5 μm apart (Fig. 7). Anterior end unevenly rounded with subapical protrusion, in some microfilariae slightly swollen (Fig. 3g). Tail tapering to fine tip. The distribution of terminal nuclei is depicted in Fig. 3g.
Fig. 7.
Microfilaria showing transverse annulation and irregular shape of swollen anterior end (magnitude of annulation and swelling might be exaggerated by artefact of fixation).
3.3. Sequences
PCR from adult worms (n = 10) yielded amplicons of around 440 bp. They were identical except for one sequence that differed at one nucleotide position from all other sequences (GenBank accession nrs. KU886066, KU886067). The sequences had a very high identity (399 of 401 nucleotides) with O. jakutensis sequences from Germany (Genbank accession: DQ523771) with <1% difference (two substitutions).
PCRs with DNA isolated from six microfilaria-containing samples originating from four red deer yielded an amplicon of the expected size in only one case. The 345 bp sequence of this amplicon (GenBank accession nr. KU886068) has 92–93% identity with many Onchocerca species, including O. jakutensis and O. flexuosa. To test whether nuclear pseudo-genes were amplified, the DNA sequences were translated, giving the same level of similarity at the corresponding amino acid sequences of these two species.
4. Discussion
The morphology of the described filarioids is in agreement with the characteristics of the genus Onchocerca as given by Bain (1981), i.e. a thickened cuticle in females with transverse annular ridges on the surface and striae beneath, and weakly developed body muscles. The specimens belong to the species O. jakutensis following the description given by Bain (1981) and Demaskiewicz (1993). i.e. oesophagus, shorter than 1000 μm, divided into a muscular and a posterior glandular part, vulva opening slightly posterior to the oesophagus, five pairs of pericloacal papillae, placed before, adjacent to and after the cloaca and two pairs of papillae in the vicinity of the tip of the tail, close to the two phasmids with protruding flaps. Also the size and morphology of the unequal spicules correspond with these descriptions. However, the specimens of O. jakutensis examined here differ in some respects from the morphological descriptions given in the literature. It is important to specify these differences, to integrate them into the taxon O. jakutensis and to link them with the DNA sequence data from the same material. Partial sequence of the mitochondrial NADH-dehydrogenase gene slightly differed from a corresponding GenBank entry (DQ523771).
Bain (1981) considered the ratio of cuticular annulae to striae in the deeper medulla to be of taxonomic value and used it in the key to the species. She reported a ratio of 1:3 for O. jakutensis. Re-describing the species Demaskiewicz (1993) noted “usually” a ratio of 1:3 but in the corresponding figure the ratio 1:4 was depicted. In their study, Morandi et al. (2011) reported a ratio 1:3 or 1:4. In the present material we observed a ratio 1:4 in all females. In males, both Bain and Schulz-Key (1974) and Bain (1981) reported the presence of an unpaired precloacal papilla, whereas this is not mentioned by Demaskiewicz (1993). In the material examined here, a precloacal papilla is absent (Fig. 4).
With up to 80 cm and more in length, O. jakutensis females are long nematodes, their mouth orifice, in contrast, is extremely small. With a diameter of 1.5 9 μm it is smaller than the diameter of its own microfilariae, with the obvious advantage of preventing the offspring from being swallowed by their parents, as was observed to occur in large numbers in another filarioid with a much larger mouth opening, Oswaldofilaria samfordensis, living in the serous membranes of agamid lizards (Manzanell, 1982).
Microfilariae found in the eight samples were of different sizes (235–270 μm x 6–10 μm or 380–440 μm × 18 μm), putatively corresponding to O. jakutensis and O. skrjabini (Schulz-Key, 1975). Genetic analysis of microfilariae in the present study was possible with one of the six samples, namely with the only sample that contained a considerable number of them. A sequence that was distinctly different from those reported from O. jakutensis was obtained. No corresponding sequences are available for two of the other Onchocerca species that have been described in the same hosts in Central Europe, i.e. O. skrjabini and O. garmsi. Thus, probably mixed infections occurred, as has been described in a study from neighbouring countries where prevalences in 94 investigated red deer were 96% for O. flexuosa, 82% for O. skrjabini, and 23% for O. jakutensis (Schulz-Key, 1975). Chamois and roe deer in the same area were apparently not infected.
Local hunters have been aware of the presence of subcutaneous nodules in red deer for some time, but misinterpreted them as consequences of tick bites. This is the first record of O. jakutensis in Switzerland, and extends its range of distribution from Jakutia in Eastern Russia (Gubanov, 1964, in Demaskiewicz, 1993), Kaukasus (Demaskiewicz, 1993), Germany (Bain and Schulz-Key, 1974, Plenge-Bönig et al., 1994), Italy (Morandi et al., 2011) and now also to Switzerland. Despite the relatively small amount of data, the prevalence of O. jakutensis is comparable to studies in Germany (Schulz-Key, 1975).
The Swiss red deer population is relatively young. Repopulation started from neighbouring Austria in 1872 (Haller et al., 2002), and this bottle-neck effect of the recolonisation could explain the small distribution range of the parasites and the lower number of species present.
Worldwide, 25 cases of a zoonotic onchocerosis are known. Given that Onchocerca spp. are transmitted by blackflies and biting midges, the importance of research on their prevalence and the rates of infection in Switzerland should be emphasised. The impact of this parasite on the deer population and eventually on humans has not been adequately studied.
Acknowledgements
The authors are grateful to SimplyScience, Zürich, for funding to support this work and its publication, and to the Center for Microscopy and Image Analysis, University of Zurich, for performing scanning electron microscopy. We thank Dr. Adrian Puntschart for establishing the PCR technology at the Bündner Kantonsschule Chur and providing training and assistance to the first author, and Jeannine Hauri for PCR work at the Institute of Parasitology, Vetsuisse Faculty, University of Zürich as well as Carlo Tuena for providing the foto of the red deer. This work was done in the frame of the scholarly Matura paper (‘Maturaarbeit’) of the first author.
Contributor Information
Felix Bosch, Email: felix.bosch@bluewin.ch.
Ralph Manzanell, Email: manzanell@hotmail.com.
Alexander Mathis, Email: alexander.mathis@uzh.ch.
References
- Anderson R.C. second ed. CABI Publishing; Wallingford, Oxon, UK: 2000. Nematode Parasites of Vertebrates: Their Development and Transmission; pp. 517–518. [Google Scholar]
- Bain O., Schulz-Key H. Les onchocerques du cerf européen: redescription d’ O. flexuosa (Wedl, 1856) et description d’ O. tubingensis n. sp. et tarsicola n. sp. Trop. Med. Parasitol. 1974;25:437–449. [PubMed] [Google Scholar]
- Bain O. Les espèces du genre Onchocerca: hypothèse sur son évolution et clé dichotomique des espèces. Ann. Parasitol. Hum. Comp. 1981;56:503–526. [PubMed] [Google Scholar]
- Brglez J., Hribar H., Rakovec R. Erster Fund der Onchocerca flexuosa (Wedl 1856) beim Hirsch (Cervus elaphus L.) in Jugoslawien. Z. Jagdwiss. 1967;13:153–155. [Google Scholar]
- Brglez J., Zeleznik Z. Pathogenesis of cutaneous filariasis in red deer. Z. Jagdwiss. 1976;22:112–115. [Google Scholar]
- Commichau C. Über eine durch Filarien verursachte Polyarthritis bei Rothirschen. Zbl. Vet. Med. B. 1982;29:532–539. [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic aAids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaskiewicz A.W. Redescription of Onchocerca jakutensis (Gubanov, 1964) (Nematoda, Filarioidea) Acta Parasitol. 1993;38:124–127. [Google Scholar]
- Deplazes P., Eckert J., von Samson-Himmelstjerna G., Zahner H. third ed. Enke Verlag; Stuttgart: 2013. Lehrbuch der Parasitologie für die Tiermedizin. [DOI] [PubMed] [Google Scholar]
- Dulceanu N., Ghitescu F. Incidence and some morphological aspects of subcutaneous onchocerciasis in deer (Cervus elaphus) Cercet. Agron. Mold. 1986;19:107–109. [Google Scholar]
- Dyková I. Lymph nodes of red deer infected with subcutaneous filariae Wehrdikmansia cervipedis (Wehr et Dikmans, 1935) and Onchocerca flexuosa (Wedl, 1856) Pathol. Vet. 1970;7:60–67. doi: 10.1177/030098587000700107. [DOI] [PubMed] [Google Scholar]
- Dyková I., Blazek K. Subcutaneous filariasis in red deer. Acta Vet. 1972;41:117–124. [Google Scholar]
- Dyková I. The incidence of microfilariae of Wehrdikmansia cervipedis (Wehr et Dikmans, 1935) and Onchocerca flexuosa (Wedl, 1956) in Cervus elaphus L. and the tissue reaction of the host. Acta Vet. 1972;41:197–201. [Google Scholar]
- Haller H., Kühn R., Fischlin A., Haller R. Der Rothirsch im Schweizerischen Nationalpark und dessen Umgebung – eine alpine Population von Cervus elaphus zeitlich und räumlich dokumentiert. Nationalpark-Forschung der Schweiz. 2002;91:23. [Google Scholar]
- Koehsler M., Soleiman A., Aspöck H., Auer H., Walochnik J. Onchocerca jakutensis filariasis in humans. Emerg. Infect. Dis. 2007;13:1749–1752. doi: 10.3201/eid1311.070017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzanell R. Oswaldofilaria spp. (Filarioidea, Nematoda) in Australian agamid lizards with a description of a new species and a redescription of O. chlamydosauri (Breinl) Ann. Parasitol. Hum. Comp. 1982;57:127–143. doi: 10.1051/parasite/1982572127. [DOI] [PubMed] [Google Scholar]
- Meszaros F., Sugar L. Proceedings of the 3rd International Congress of Parasitology. World Federation of Parasitologists; Munich: 1974. Investigations on the Onchocerca and Wehrdikmansia species living in Cervidae in Hungary; pp. 25–31. [Google Scholar]
- Morales-Hojas R., Cheke R.A., Post R.J. Molecular systematics of five Onchocerca species (Nematoda: Filarioidea) including the human parasite, O. volvulus, suggest sympatric speciation. J. Helminthol. 2006;80:281–290. [PubMed] [Google Scholar]
- Morandi F., Krueger A., Panarese S., Sarli G., Verin R., Nicoloso S., Benazzi C., Galuppi R. First description of nodular onchocercosis (Onchocerca jakutensis) in free-ranging Italian red deer (Cervus elaphus) J. Wildl. Dis. 2011;47:963–967. doi: 10.7589/0090-3558-47.4.963. [DOI] [PubMed] [Google Scholar]
- Plenge-Bönig A., Krömer M., Büttner M.W. Light and electron microscopy studies on Onchocerca jakutensis and O. flexuosa of red deer show different host-parasite interactions. Parasitol. Res. 1994;81:66–73. doi: 10.1007/BF00932419. [DOI] [PubMed] [Google Scholar]
- San-Miguel J.M., Álvarez G., Rodrìguez-Vigal C., Luzón M. Nodular onchocercosis of red deer in central Spain. Vet. Parasitol. 2003;114:75–79. doi: 10.1016/s0304-4017(03)00097-9. [DOI] [PubMed] [Google Scholar]
- Santín-Durán M., Alunda J.M., de la Fuente C., Hoberg E.P. Onchocercosis in red deer (Cervus elaphus) from Spain. J. Parasitol. 2001;86:1213–1215. doi: 10.1645/0022-3395(2001)087[1213:OIRDCE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Schulz-Key H. Untersuchungen über die Filarien der Cerviden in Süddeutschland 2. Die Filarien des Rothirsches (Cervus elaphus) Trop. Med. Parasitol. 1975;26:348–358. [PubMed] [Google Scholar]
- Schulz-Key H., Bain O., Wenk P. Untersuchungen über die Filarien der Cerviden in Süddeutschland. 4. Onchocerca garmsi Bain und Schulz-Key, 1976, eine subkutane Filarie des Rothirsches (Cervus elaphus) Trop. Med. Parasitol. 1975;27:229–232. [PubMed] [Google Scholar]
- Uni S., Fukuda M., Otsuka Y., Hiramatsu N., Yokobayashi K., Takahashi H., Murata S., Kusatake K., Morita E., Maruyama H., Hasegawa H., Shiwaku K., Ramli R., Azirun M.A., Takaoka H. New zoonotic cases of Onchocerca dewittei japonica (Nematoda: Onchocercidae) in Honshu, Japan. Parasit. Vectors. 2015;8:59. doi: 10.1186/s13071-015-0655-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk C., Kaufmann C., Schaffner F., Mathis A. Molecular characterization of Swiss Ceratopogonidae (Diptera) and evaluation of real-time PCR assays for the identification of Culicoides biting midges. Vet. Parasitol. 2012;184:258–266. doi: 10.1016/j.vetpar.2011.08.034. [DOI] [PubMed] [Google Scholar]
- WHO . 2015. Onchocerciasis.http://www.who.int/mediacentre/factsheets/fs374/en/ Fact sheet no. 373 (March 2015) (last accessed 10.02.16) [Google Scholar]