Abstract
Oocyte developmental competence in superstimulated cows is dependent in part on the duration of the FSH coasting. FSH coasting refers to superstimulation with FSH (2 days of endogenous FSH following follicle ablation and 3 days of FSH injections) followed by no FSH for a specific duration. The optimal duration varies among individuals. FSH coasting appears to modulate the transcriptome of different follicular compartments, which cooperate as a single functional unit. However, the integrative effects of FSH coasting on different follicular compartments remain ambiguous. Meta-analysis of three independent transcriptome studies, each focused on a single cell type (granulosa, cumulus, and oocyte) during FSH coasting, allowed the identification of 12 gene clusters with similar time-course expression patterns in all three compartments. Network analysis identified HNF4A (involved in metabolic functions) and ELAVL1 (an RNA-binding protein) as hub genes regulated respectively upward and downward in the clusters enriched at the optimal coasting time, and APP (involved in mitochondrial functions) and COPS5 (a member of the COP9 signalosome) as hub genes regulated respectively upwards and downwards in the clusters enriched progressively throughout the coasting period. We confirmed the effects on HNF4A downstream targets (TTR, PPL) and other hub genes (ELAVL1, APP, MYC, and PGR) in 30 cows with RT-quantitative PCR. The correlation of hub gene expression levels with FSH coasting indicated that a combination of these genes could predict oocyte competence with 83% sensitivity, suggesting that they are potential biomarkers of follicle differentiation. These findings could be used to optimize FSH coasting on an individual basis.
Keywords: ovarian follicle, oocyte competence, transcriptomics, meta-analysis, FSH coasting
assisted reproductive technologies have been used successfully in a wide range of research and clinical applications. However, the success rate remains low, despite increasing research efforts (19). Better understanding of ovarian physiology and of the acquisition of oocyte developmental competence in particular is needed to improve the efficiency of fertility treatments in the clinical setting and in livestock production.
Ovarian follicles are composed of different compartments, each consisting of a distinctive cell type. Although functionally divergent, the compartments cooperate as a single functional unit to optimize folliculogenesis and to ensure that the oocyte acquires developmental competence. This acquisition is an intricate process that requires the activities of numerous endocrine and paracrine factors, which elicit specific gene expression profiles and hence functional properties in the different cell types (16, 31). A developmentally competent oocyte thus results from the proper coordination of different follicular compartments (10).
Follicle somatic cell gene expression patterns associated with oocyte developmental competence have been described (3, 12, 16). However, despite known interactions between compartments, gene expression profiles have been studied in specific cell types, apparently to maximize technical coherence. Such an approach overlooks the possibility that some pathway active in the follicle as a whole ultimately determines oocyte competence (27). This underscores the need to perform integrative meta-analyses of different cell transcriptomes to gain a fuller understanding of follicle physiology.
Other than a report by Wigglesworth et al. (31) in which mouse cumulus and mural granulosa cells were compared, meta-analyses of different ovarian tissue transcriptomes are rare. The Ovarian Kaleidoscope database (OKdb) provides information about listed genes, such as expression in different ovarian cell types or association with various ovarian functions (http://okdb.appliedbioinfo.net/) (15). However, complete understanding of ovarian physiology requires the integration of gene expression profiles generated under a broad range of physiological conditions. We recently generated an interactive web interface named GranulosaIMAGE, which provides dynamic expression profiles of genes of interest and all isoforms thereof in granulosa cells at different stages of folliculogenesis (http://emb-bioinfo.fsaa.ulaval.ca/granulosaIMAGE/). We now need to provide equally comprehensive overviews of cumulus and oocyte transcriptome dynamics to identify putative pathways common to all follicular compartments.
We have shown that FSH starvation (coasting) after ovarian superstimulation with this hormone affects bovine oocyte competence in a time-dependent manner, the optimal coasting period appearing to be about 44 h on average (20). We subsequently analyzed the effect of FSH coasting for 20, 44, 68, and 92 h on the transcriptomic profiles of granulosa cells (21), cumulus cells (5) and germinal-vesicle breakdown (GVBD) oocytes (18) using same technological platform. In the present study, we performed a meta-analysis of our previous studies in an attempt to identify one or more gene networks active in all three follicular compartments and correlated with FSH coasting time and acquisition of oocyte developmental competence.
MATERIAL AND METHODS
Data Retrieval
Microarray gene expression data from three earlier publications [(GSE79760) (5), (GSE40916) (21), (GSE38345) (18)] were retrieved from the ELMA database and pooled together for meta-analysis. All datasets were generated using the EmbryoGENE bovine transcriptome microarray (24). Each experiment focused on a specific cell-type within the ovarian follicle: granulosa cells (21), cumulus cells (5), and the GVBD oocyte (18). Each cell type was analyzed at 20, 44, 68, and 92 h of FSH coasting after ovarian superstimulation with this hormone. FSH coasting refers to a protocol of ovarian superstimulation in which cows are given 5 days of FSH support (2 days of endogenous FSH following ablation of dominant follicle and 3 days of exogenous FSH injections) (20). In all the experiments included in this study animals were stimulated with same protocol using 40 mg NIH Folltropin-V given at 12 h intervals for 3 days. Briefly, on day 1 the dominant follicle is ablated and a new wave of follicular development starts under endogenous FSH release. The cows then receive FSH injections twice daily on days 3, 4, and 5. Following last FSH injection, the follicles are allowed to develop without FSH support for 20, 44, 68, or 92 h, the period called FSH starvation or FSH coasting. Interestingly, the time when the maximum oocyte quality is achieved varies slightly among individuals. In earlier experiments this time has been observed to be between 44 and 68 h post-FSH withdrawal (20). Here, the duration of FSH coasting when there is maximum oocyte competence achieved is referred to as “optimal coasting.” In the experiments included in this meta-analysis, ovarian follicles were allowed to develop either for 20, 44, 68, or 92 h post-FSH withdrawal to determine the gene dynamics during this period. The gene dynamics during this whole period of four days are referred to as “throughout coasting.” The microarray data was exported after “log2 transformation” and “normalization within” each array.
Time-course Meta-analysis using Fuzzy Clustering
We performed time-course analysis through gene clustering (Fig. 1). The details of the procedures are described below.
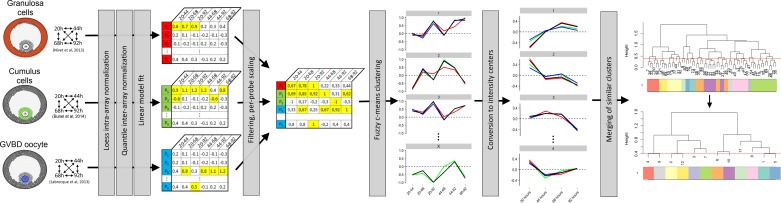
Fig. 1.
Overview of the bovine ovarian transcriptome meta-analysis strategy. We analyzed 9 microarray gene expression datasets with fuzzy clustering to chart time-course patterns of gene expressions with similar dynamics in 3 different follicle cell types (granulosa, cumulus, and oocyte) postsuperstimulation FSH coasting. GVBD, germinal-vesicle breakdown.
Normalization and identification of differentially expressed genes.
Datasets were normalized by intra-array loess normalization and interarray quantile normalization within each direct contrast. Differential expression was then assessed for all noncontrol probes for all direct contrasts, yielding three 38,853 × 6 matrices of differentials and P values, one for each cell type. Genes whose expression level had more than doubled (log2 differential > 1) and had a P value < 0.05 were considered differentially expressed. Both normalization and linear fit procedures were carried out with the limma package (28). Individual channels were considered separately using the ANOVA functionality from the MAANOVA package to identify genes that varied in expression versus all conditions (31a).
Fuzzy clustering.
To avoid the complexities raised by different numbers of arrays within each experiment (66 for granulosa cells, 72 for the other cell types) as well as dye effects and within-array variation, fuzzy c-means clustering (8) was applied to the expression differentials rather than intensity values, using P values as weights. For the purposes of clustering, each probe-tissue combination was considered as a separate entity. This allows us to identify genes that may have similar expression profiles in two but not all three types of cell. Probes with at least one limma-calculated absolute differential >0.5 were kept for the clustering. To avoid clustering of probes along cell-type lines, per probe differentials were scaled to the [−1,1] interval. Fuzzy clustering was carried out using the latest version of the Mfuzz bioconductor package (17) (http://www.bioconductor.org/packages/release/bioc/html/Mfuzz.html). Intensity centers were then calculated for all clusters using the weights provided by Mfuzz. These intensity centers were then subjected to hierarchical clustering. Clusters with a Euclidean distance <0.4 were merged using a pairwise iterative algorithm until all clusters were sufficiently distinct.
Function enrichment.
The set of all gene ontology annotations for Bos taurus, Homo sapiens, and Mus musculus was downloaded from the Gene Ontology Consortium (http://geneontology.org) and associated with the probes on the EmbryoGENE microarray. Probes targeting a known bovine gene were associated with the corresponding Gene Ontology (GO) terms, whereas novel transcribed element probes were associated with human or mouse ontology terms if the sequence targeted by the microarray showed a significant homology to a human or mouse protein. GO enrichment of clusters was then performed using the weighted scoring algorithm developed by Alexa et al. (2) and made available through the topGO bioconductor package (1). Pathway overrepresentation assessment was based on comparison of the KEGG (http://www.genome.jp/kegg/) annotation associated with each cluster to the hypergeometric distribution.
Network-based Meta-analysis
NetworkAnalyst was used to perform network-based meta-analysis (33). The lists of upregulated and downregulated genes in clusters 1, 2, 6, 8, 9, and 12 were submitted separately. The pathway construction was restricted to contain the original seed proteins only, which is called zero-order analysis in NetworkAnalyst (33).
Reverse-transcriptase Quantitative PCR
Total RNA of granulosa cells obtained from 90 randomly selected cows other than those included in the meta-analysis was reverse-transcribed using a q-Script FlexTM cDNA synthesis kit (Quanta Biosciences, Gaithersburg, MD) with oligo dT (20) primers following the manufacturer's recommendations. The primers used for real-time RT-PCR were designed using the IDT PrimerQuestTM tool (available on the Integrated DNA technologies website) from sequences obtained using the UMD3.1/bosTau5 assembled version of the bovine genome. To confirm the specificity of each pair of primers, we performed electrophoresis on a standard 1.2% agarose gel for each amplified fragment, and the sequenced PCR template was then used to prepare a standard curve, which was included in each reaction. Primer sequences and annealing temperatures are shown in Table 1. Real-time PCR was performed using LightCycler 480 SYBR Green I Master and the LightCycler 480TM (Roche Diagnostics, Laval, QC, Canada). The PCR conditions used for all genes were as follows: denaturing cycle for 10 min at 95°C; 50 PCR cycles (denaturing, 95°C for 10 s; annealing for 10 s; extension, 72°C for 20 s), a melting curve (95°C for 1 s, 65°C for 1 s, and a step cycle starting at 72°C up to 97°C at 0.11°C/s), and a final cooling step at 4°C. The data were normalized through geNORM (29), and the most stable reference genes were identified by the stepwise exclusion of the least stable gene and recalculating the M values. ACTB, GAPDH were the most stable genes with M values < 1.5 as recommended by the software. One-way ANOVA with the Bonferroni posttest or conventional t-test was performed on normalized data using GraphPad Prism version 5.00 (GraphPad Software, San Diego, CA). Primer sequences and annealing temperatures are shown in Table 1. A receiver operating characteristic (ROC) curve analysis was performed using SAS Software (SAS Institute, Cary, NC) to evaluate the diagnostic accuracy of different candidate genes for prediction of blastocyst percentage (oocyte competence) and follicular differentiation (time of FSH coasting). A backward linear discriminant analysis was performed to determine the sensitivity and specificity values for the combinations of different biomarkers.
Table 1.
List of primers used to perform quantitative PCR experiments
| Gene Symbol | Primer Sequence |
Product Size, bp | Annealing Temperature, °C | GenBank Accession Number | |
|---|---|---|---|---|---|
| GAPDH | Fwd: | CCAACGTGTCTGTTGTGGATCTGA | 218 | 57 | NM_001034034.2 |
| Rev: | GAGCTTGACAAAGTGGTCGTTGAG | ||||
| ACTB | Fwd: | ATCGTCCACCGCAAATGCTTCT | 102 | 57 | NM_173979.3 |
| Rev: | GCCATGCCAATCTCATCTCGTT | ||||
| TTR | Fwd: | CTCCTTTGTCTCGCTGGACT | 229 | 57 | NM_173967.3 |
| Rev: | ATTTGTCCTCTGTGGTGAGCC | ||||
| PPL | Fwd: | TGAGCTATGTCCCTCAGCCT | 448 | 57 | XM_002697888.5 |
| Rev: | CTCTGACGGTGCGTTCTCTT | ||||
| ELAVL1 | Fwd: | ACCAGAACAAAAACGTGGCG | 363 | 57 | NM_001076454.2 |
| Rev: | GGTAGCCGTTCAGACTTGCT | ||||
| APP | Fwd: | ATGCAGAACTAGACCACCGC | 440 | 57 | NM_001076796.1 |
| Rev: | CAGCTAAACCCCCACGTTCA | ||||
| PGR | Fwd: | CCTAAGCCAGAGAATCACTTT | 262 | 57 | NM_001205356.1 |
| Rev: | CCAAGAATACTGGATGAGAGTT | ||||
| MYC | Fwd: | ATAGACGTGTTGCAGAAGAG | 225 | 57 | NM_001038214.1 |
| Rev: | CTTAAAGATCCAGCCAAGGT | ||||
Fwd, forward; Rev, reverse.
RESULTS
The time-course meta-analysis of the three independent transcriptome studies was performed using the fuzzy clustering method (17) (Fig. 1). RT-quantitative (q)PCR was then performed to validate the hub gene status as revealed by the meta-analysis, and correlations with FSH coasting time and the potential for predicting successful embryo development (the percentage of embryos reaching the blastocyst stage) were evaluated.
Time-course Meta-analysis Using Gene Clustering
Nine microarray datasets were included in this analysis. After initial data processing, 21,816 probes (9,149 for granulosa, 5,691 for cumulus, and 6,976 for oocytes) were kept for submission to fuzzy k-mean determination. These represented a total of 8,677 known genes identified in a single tissue (cell) type and 2,667 novel transcribed regions. Novel transcribed regions associated with a single tissue outnumbered those associated with more than one tissue (1,585 vs. 1,082), whereas the opposite was the case for known genes (3,712 vs. 4,965).
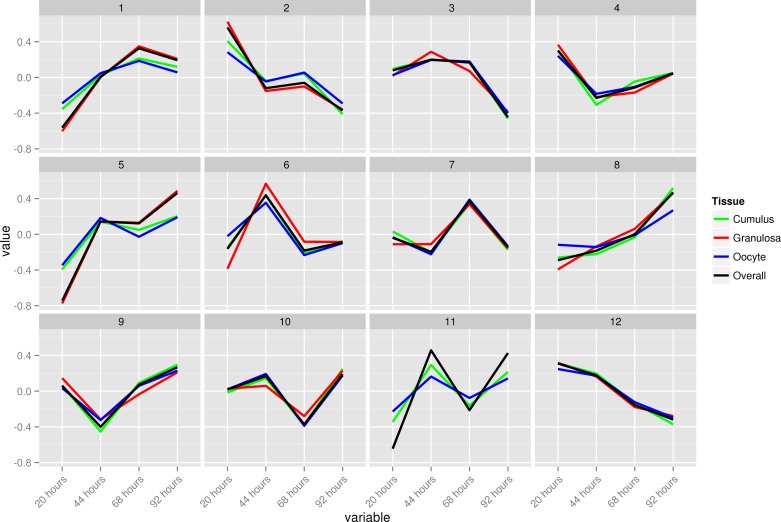
After similar clusters were merged, 12 clusters representing a variety of differential expression profiles were retained (Fig. 2). In all clusters, the majority of transcripts were associated with a single cell type. However, in eight of the clusters, at least 5% of the transcripts were associated with more than one tissue, 19% and 10% in clusters 2 and 8, respectively, with granulosa and cumulus cells accounting for all of the commonality (Fig. 3). Gene summaries representing the relative change in expression level by cluster, intensity centers, tissue composition and overlap, and functional enrichment results were also generated. Due to space constraints only cluster 1 gene summary is presented here (Fig. 4).
Fig. 2.
Time-course dynamics of bovine ovarian follicle cell transcriptomes during 4 days of FSH coasting. Differentially expressed probes in different cell types (21,816) were submitted to fuzzy k-means clustering (9,149 from granulosa, 5,691 from cumulus, and 6,976 from oocytes). The 12 clusters obtained after merging represent overall ovarian transcriptome dynamics.
Fig. 3.
Cord diagrams representing the distribution of differentially expressed bovine genes among the 3 ovarian follicular cell types (red = cumulus, green = granulosa, violet = oocyte) for the 12 clusters presented in Fig. 2. Each circle represents a cluster, and the length of each color bar in a circle represents the number of genes contributed by that cell type in that specific cluster. From these it can be appreciated that different follicular cells (as coded by different colors in the circle) contribute different number of genes in each cluster. Since the numbers of genes in different clusters are different, so the intercircle comparison should not be made for these diagrams. Moreover, genes common in 2 or more cell types are represented as blue lines connecting the sharing colour bars. The more blue lines in a circle, the more genes are shared between different cells types for that cluster.
Fig. 4.
Gene summary of cluster 1 representing the relative change in expression level, intensity centers, tissue composition, and gene overlap as well as functional enrichment results.
Network-based Meta-analysis
Meta-analysis of genes associated with oocyte competence.
The time-course meta-analysis of three follicular tissues revealed that cluster 6 contained probes upregulated exclusively after 44 h of FSH coasting [the optimal time for oocyte developmental potential (20)]. The opposite expression pattern (i.e., downregulation at 44 h) was noted in cluster 9. These clusters were therefore analyzed subsequently in greater detail.
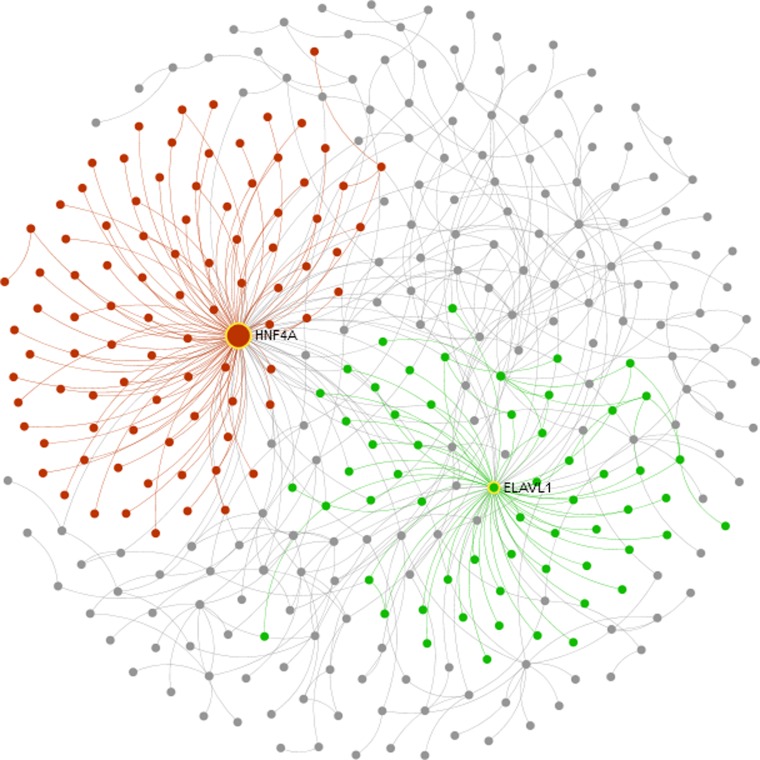
Network-based meta-analysis of genes in clusters 6 and 9 was performed in NetworkAnalyst (33). In terms of network topology measures, HNF4A (encoding hepatocyte nuclear factor 4 alpha, involved in gluconeogenesis and lipid metabolism) was identified as the most significantly upregulated hub gene (degree = 226, betweenness = 17,843; red network in Fig. 5), while ELAVL1 was identified as the most downregulated hub gene (degree = 222, betweenness = 5,811.3; green network in Fig. 5). ELAV like-1 is an RNA-binding protein that may influence RNA stability and translation.
Fig. 5.
Network analysis of bovine genes expressed differentially in association with the FSH coasting time found optimal (44 h) for ensuring oocyte developmental competence. Clusters 6 and 9 contain, respectively, genes that were upregulated and downregulated only at 44 h. Zero-order protein-protein interaction networks in NetworkAnalyst identified HNF4A (red) and ELAVL1 (green) as the most significant hub genes in clusters 6 and 9, respectively.
Meta-analysis of genes associated with FSH coasting time.
The gene clusters that either increased or decreased in activity throughout the FSH coasting period were analyzed. Based on the time-course meta-analysis, clusters 1 and 8 contained probes that were upregulated (with minor deviations from the overall trend) in response to FSH coasting, while probes in clusters 2 and 12 were unequivocally downregulated.
Network analysis of highly connected hub proteins was carried out in NetworkAnalyst (33) separately for upregulated (clusters 1 and 8) and downregulated (clusters 2 and 12) genes. Due to the large numbers, only the probes that were expressed differentially in more than one individual experiment were included. In terms of network topology measurement, APP (amyloid precursor protein) was the most upregulated through coasting extension (degree = 36, betweenness = 529.4; red network in Fig. 6A), followed by MYC (v-myc myelocytomatosis virus-related oncogene, degree = 22, betweenness = 198.17; blue network). APP suppresses mitochondrial function and MYC is a transcription factor.
Fig. 6.
Network analysis of genes belonging to clusters associated with differentiation in bovine ovarian follicles. Clusters 1 and 8 contain genes that were upregulated progressively during the 4 days of FSH coasting, while clusters 2 and 12 contain genes that were downregulated over the same time period. Zero-order protein-protein interaction networks in NetworkAnalyst identified APP (red) followed by MYC (blue) as the most significant hub genes in clusters 1 and 8 (A) and COPS5 (turquoise) and ELAVL1 (green) in clusters 2 and 12 (B).
Next, COPS5 (constitutive photomorphogenic homolog subunit 5) was identified as the most significantly downregulated hub gene through coasting extension (degree = 33, betweenness = 950.5). COPS5 is a member of the COP9 signalosome involved in intracellular signaling. It should be noted that PGR, known for its role in ovarian function, was found downstream of COPS5 and was included in validation experiments instead of COPS5, which is relatively less known in ovarian physiology. The second most downregulated hub gene in these clusters was ELAVL1 (degree = 60, betweenness = 676.8), which has also been identified in oocyte competence clusters (Fig. 6B).
Validation of Meta-analysis Gene Expression Patterns
Quantitation of candidate gene transcripts and correlation thereof with FSH coasting time.
Granulosa cells from 30 cows selected at a commercial embryo production facility were used to corroborate the time-course meta-analysis results. Due to the nature of the facility operations, granulosa cells were the only biological material available for this purpose. They were collected after 30 and 44 h of FSH coasting. It was fortunate that the corresponding yields of blastocysts at these FSH coasting times were obtainable. The genes included in the RT-qPCR analysis were HNF4A, APP, MYC, and ELAVL1, the most significant hub genes identified in the network-based analysis. Targets downstream from HNF4A included TTR (transthyretin), PPL (periplakin), and the progesterone receptor (PGR).
HNF4A mRNA was not detected in granulosa cells collected at the time of ovum pickup, although it was detectable in RNA samples from whole ovarian tissue during validation experiments (data not shown). Expression patterns of other candidate genes were consistent with the meta-analysis results (Fig. 7). HNF4A downstream targets TTR (P = 0.04) and PPL (P = 0.0001) were upregulated at 44 h compared with 30 h of FSH coasting, along with APP (P = 0.01) and MYC (P = 0.0006). In contrast, ELAVL1 (P = 0.03) and PGR (P = 0.003) were increasingly downregulated as coasting time increased.
Fig. 7.
Whisker plots of the abundance of selected mRNA transcripts measured in granulosa cells of 30 cows by RT-qPCR. Identified by time-course meta-analysis, the 6 genes were expressed differentially in association with oocyte competence (A) and FSH coasting time (B). Values were normalized relative to endogenous GAPD and ACTB transcripts. These results provide visual confirmation that changes in gene expression associated with FSH coasting are consistent with those associated with developmental competence. Significant differences were calculated by the Mann-Whitney test. Statistical differences between different groups are marked with asterisks (the higher the number of asterisks, the greater the statistical difference is).
All of the genes analyzed by qRT-PCR correlated well with FSH coasting time (Fig. 8): TTR (r = 0.37, P = 0.04), PPL (r = 0.79, P = 0.0001), ELAVL1 (r = −0.39, P = 0.03), MYC (r = 0.64, P = 0.0001), APP (r = 0.45, P = 0.01), and PGR (r = −0.45, P = 0.01).
Fig. 8.
Analysis of the correlations of hub gene candidate expression levels in ovarian tissues of cows with postsuperstimulation FSH coasting time.
Evaluation of putative biomarkers of oocyte competence and follicle differentiation.
ROC analysis was performed to predict follicular differentiation and oocyte competence during FSH coasting using the transcript abundance of candidate genes. The oocytes in this analysis were defined as highly competent when the blastocyst development rate was 65% or higher, and oocytes with 35% or less blastocyst rates were considered less competent. Although the number of cows included in this analysis is limited (n = 30), MYC alone could be used as a marker of FSH coasting time to predict follicle differentiation with 87% sensitivity and 74% specificity (Fig. 9A), while the combination of TTR, PPL, ELAVL1, APP, and MYC predicted high competence of oocytes (>65% reaching the blastocyst stage) with 83% sensitivity and 80% specificity (Fig. 9B).
Fig. 9.
Receiver operating characteristic (ROC) analysis of the potential of differentially expressed genes as biomarkers of follicle differentiation in cows during postsuperstimulation FSH coasting. A: prediction of differentiation with 87% sensitivity by MYC; B: prediction of oocyte developmental competence (blastocyst yield) with 83% sensitivity by TTR, PPL, ELAVL1, APP, and MYC.
DISCUSSION
Folliculogenesis requires the concerted action of numerous intrinsic and extrinsic factors that determine the functional properties of and interactions between different follicular cell types and thereby the acquisition of oocyte developmental competence (10). Using the gene clustering approach, the present study reveals associations between bovine systems-level gene regulation and follicular states during FSH coasting and correlated these with blastocyst yield. Our results have implications for strategies designed to improve fertility through individualization of the coasting interval in FSH-superstimulated cows.
Based on network meta-analysis of genes belonging to the clusters (i.e., 6 and 9) that were enriched specifically at the coasting time associated with high competence, HNF4A was identified as the most significant hub gene in all three follicular cell types. This gene is expressed in liver, adipose, pancreatic, and intestinal tissues and is an important metabolic regulator involved in gluconeogenesis (23), lipid metabolism (34), and inflammation (4). Although we could not detect HNF4A transcripts in granulosa cells using qRT-PCR, we did confirm the abundance of HNF4A downstream targets (TTR and PPL), which suggests that this transcription factor has an active role in ovarian follicle differentiation. Although a more detailed analysis regarding the expression of HNF4A in the hours after FSH withdrawal would be interesting, we suppose that a minute change in the RNA quantity of this transcription factor is sufficient to affect downstream targets and hence cellular functions.
Follicular metabolism must produce sufficient energy to ensure the completion of various functions including fertilization and embryo development (26). Disruption of metabolic activities like glycolysis (11) or beta-oxidation (22, 25) during in vitro maturation decreases oocyte meiotic maturation rate in many species. Disruption of fatty acid metabolism and homoeostasis also influences oocyte competence (32). It is therefore plausible that HNF4A, which interacts with several genes involved in lipid metabolism and homoeostasis (34), is involved in the regulation of follicle metabolic activities. Hnf4α knockout is lethal in mice (14), and conditional knockout in hepatic cells has been shown to depress genes involved in lipogenesis (Srebf1, Acc, Fas, Dgat2), de novo cholesterol biosynthesis (Hmgcr, Hmgcs), cholesterol esterification (Acat2), and fatty acid uptake (CD36) (34). The corresponding bovine genes had similar expression patterns in the present study. We therefore hypothesize that FSH coasting optimizes fatty acid metabolism in follicle tissue through HNF4A expression to produce sufficient energy for proper ovarian function.
Besides the genes expressed differentially at the 44 h point of FSH coasting, other clusters (1, 2, 8, and 12) of genes that were either depressed or stimulated over the 4 days of FSH coasting might also shed light on the optimal poststimulation rest period and the associated physiological status of the follicle. Network analysis revealed in particular upregulation of APP and MYC and downregulation of PGR and ELAVL1, genes that have important roles in ovarian function. APP has been associated with mitochondrial dysfunction, which may compromise oocyte quality, fertilization, and development into viable offspring (26). Overexpression of MYC, in addition to causing ovarian cancer (30), affects follicle growth, oocyte quality, and embryo development (7, 9). Decreased expression of PGR (progesterone receptor), known for its role in oocyte competence acquisition and ovulation (6), likely decreases oocyte quality. Different lines of evidence suggest that proper FSH coasting improves oocyte quality by optimizing several interconnected pathways such as follicular metabolism, mitochondrial function, growth, and atresia. The expression dynamics of these genes not only demonstrate a functional effect on ovarian follicles but also depict collectively a state of follicular maturity that may be targeted by optimizing the FSH coasting interval for each individual.
To find a functional link, we determined the degree of correlation between FSH coasting and gene expression patterns analyzed with qRT-PCR. On the basis of 30 cows, we show that the correlation was significant for some genes. ROC analysis shows that MYC alone could be used to estimate follicle maturity with 87% sensitivity. In combination, these genes were biomarkers of oocyte developmental potential with >80% sensitivity. These findings indicate that a combination of genes may be more useful as a biomarker of competence.
Conclusions
In summary, our analysis suggests that systems of gene clusters common to three different follicular compartments exist and that although they contain unrelated genes, combinations thereof may be superior predictors of oocyte quality. Network meta-analyses of gene clusters reveal metabolic and mitochondrial pathways that affect oocyte developmental competence in a time-dependent manner. Variations in follicle maturity could account for individual differences in the optimal FSH coasting time, which could be determined through specific gene expression patterns. This study therefore provides valuable insight into the molecular mechanisms by which oocyte competence is acquired during FSH coasting. These findings have practical implications, since suitable classification of ovarian follicular states could eventually guide the optimization of FSH coasting time for each individual and thereby enhance oocyte competence.
GRANTS
This study was supported by grants from NSERC-CRD and the EmbryoGENE Network of Canada (to M.-A. Sirard and D. R. Khan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
D.R.K. and M.-A.S. conception and design of research; D.R.K., D.A.L., and É.F. performed experiments; D.R.K., D.A.L., and É.F. analyzed data; D.R.K. and M.-A.S. interpreted results of experiments; D.R.K. prepared figures; D.R.K. drafted manuscript; D.R.K., D.A.L., É.F., C.V., P.B., and M.-A.S. approved final version of manuscript; D.A.L., É.F., C.V., P.B., and M.-A.S. edited and revised manuscript.
ACKNOWLEDGMENTS
We thank previous students in our lab Anne-Laure Nivet, Rémy Labrecque, and Audrey Bunel, whose work has been used to perform this meta-analysis.
REFERENCES
- 1.Alexa A, Rahnenfuhrer J. topGO: Enrichment analysis for Gene Ontology http://www.bioconductor.org/packages/release/bioc/html/topGO.html. [Google Scholar]
- 2.Alexa A, Rahnenfuhrer J, Lengauer T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics 22: 1600–1607, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Assidi M, Dufort I, Ali A, Hamel M, Algriany O, Dielemann S, Sirard MA. Identification of potential markers of oocyte competence expressed in bovine cumulus cells matured with follicle-stimulating hormone and/or phorbol myristate acetate in vitro. Biol Reprod 79: 209–222, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Babeu JP, Boudreau F. Hepatocyte nuclear factor 4-alpha involvement in liver and intestinal inflammatory networks. World J Gastroenterol 20: 22–30, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bunel A, Nivet AL, Blondin P, Vigneault C, Richard FJ, Sirard MA. Cumulus cell gene expression associated with pre-ovulatory acquisition of developmental competence in bovine oocytes. Reprod Fertil Dev 26: 855–865, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Conti M, Hsieh M, Park JY, Su YQ. Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol 20: 715–723, 2006. [DOI] [PubMed] [Google Scholar]
- 7.Douville G, Sirard MA. Changes in granulosa cells gene expression associated with growth, plateau and atretic phases in medium bovine follicles. J Ovarian Res 7: 1757–2215, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Futschik ME, Carlisle B. Noise-robust soft clustering of gene expression time-course data. J Bioinformat Comput Biol 3: 965–988, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Girard A, Dufort I, Douville G, Sirard MA. Global gene expression in granulosa cells of growing, plateau and atretic dominant follicles in cattle. Reprod Biol Endocrinol 13: 015–0010, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gougeon A. Regulation of ovarian follicular development in primates: facts and hypotheses. Endocr Rev 17: 121–155, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Gutnisky C, Morado S, Dalvit GC, Thompson JG, Cetica PD. Glycolytic pathway activity: effect on IVM and oxidative metabolism of bovine oocytes. Reprod Fertil Dev 25: 1026–1035, 2013. [DOI] [PubMed] [Google Scholar]
- 12.Hamel M, Dufort I, Robert C, Leveille MC, Leader A, Sirard MA. Genomic assessment of follicular marker genes as pregnancy predictors for human IVF. Mol Hum Reprod 16: 87–96, 2010. [DOI] [PubMed] [Google Scholar]
- 14.Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol 21: 1393–1403, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsueh AJ, Rauch R. Ovarian Kaleidoscope database: ten years and beyond. Biol Reprod 86: 192, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khan DR, Guillemette C, Sirard MA, Richard FJ. Characterization of FSH signalling networks in bovine cumulus cells: a perspective on oocyte competence acquisition. Mol Hum Reprod 21: 688–701, 2015. [DOI] [PubMed] [Google Scholar]
- 17.Kumar L, MEF. Mfuzz: a software package for soft clustering of microarray data. Bioinformation 2: 5–7, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Labrecque R, Vigneault C, Blondin P, Sirard MA. Gene expression analysis of bovine oocytes with high developmental competence obtained from FSH-stimulated animals. Mol Reprod Dev 80: 428–440, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Lonergan P, Rizos D, Gutierrez-Adan A, Fair T, Boland MP. Oocyte and embryo quality: effect of origin, culture conditions and gene expression patterns. Reprod Domest Anim 38: 259–267, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Nivet AL, Bunel A, Labrecque R, Belanger J, Vigneault C, Blondin P, Sirard MA. FSH withdrawal improves developmental competence of oocytes in the bovine model. Reproduction 143: 165–171, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Nivet AL, Vigneault C, Blondin P, Sirard MA. Changes in granulosa cells' gene expression associated with increased oocyte competence in bovine. Reproduction 145: 555–565, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Paczkowski M, Silva E, Schoolcraft WB, Krisher RL. Comparative importance of fatty acid beta-oxidation to nuclear maturation, gene expression, and glucose metabolism in mouse, bovine, and porcine cumulus oocyte complexes. Biol Reprod 88: 111, 2013. [DOI] [PubMed] [Google Scholar]
- 23.Rhee J, Inoue Y, Yoon JC, Puigserver P, Fan M, Gonzalez FJ, Spiegelman BM. Regulation of hepatic fasting response by PPARgamma coactivator-1alpha (PGC-1): requirement for hepatocyte nuclear factor 4alpha in gluconeogenesis. Proc Natl Acad Sci USA 100: 4012–4017, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robert C, Nieminen J, Dufort I, Gagne D, Grant JR, Cagnone G, Plourde D, Nivet AL, Fournier E, Paquet E, Blazejczyk M, Rigault P, Juge N, Sirard MA. Combining resources to obtain a comprehensive survey of the bovine embryo transcriptome through deep sequencing and microarrays. Mol Reprod Dev 78: 651–664, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Lazo L, Brisard D, Elis S, Maillard V, Uzbekov R, Labas V, Desmarchais A, Papillier P, Monget P, Uzbekova S. Fatty acid synthesis and oxidation in cumulus cells support oocyte maturation in bovine. Mol Endocrinol 28: 1502–1521, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schatten H, Sun QY, Prather R. The impact of mitochondrial function/dysfunction on IVF and new treatment possibilities for infertility. Reprod Biol Endocrinol 12: 1477–7827, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sirard MA. Toward building the cow folliculome. Anim Reprod Sci 149: 90–97, 2014. [DOI] [PubMed] [Google Scholar]
- 28.Smyth GK. Limma: linear models for microarray data. In: Bioinformatics and Computational Biology Solutions Using R and Bioconductor, edited by Gentleman R, Carey V, Huber W, Irizarry, Dudoit S. New York: Springer, 2005, p. 397–420. [Google Scholar]
- 29.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: 18, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Duan W, Li X, Liu J, Li D, Ye L, Qian L, Yang A, Xu Q, Liu H, Fu Q, Wu E, Ma Q, Shen X. PTTG regulates the metabolic switch of ovarian cancer cells via the c-myc pathway. Oncotarget 6: 40959–40969, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wigglesworth K, Lee KB, Emori C, Sugiura K, Eppig JJ. Transcriptomic diversification of developing cumulus and mural granulosa cells in mouse ovarian follicles. Biol Reprod 5: 121756, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.Wu H, Sheppard K, Churchill G, Kerr K, Cui X. maanova: Tools for analyzing Micro Array experiments https://www.bioconductor.org/packages/release/bioc/html/maanova.html. [Google Scholar]
- 32.Wu LL, Dunning KR, Yang X, Russell DL, Lane M, Norman RJ, Robker RL. High-fat diet causes lipotoxicity responses in cumulus-oocyte complexes and decreased fertilization rates. Endocrinology 151: 5438–5445, 2010. [DOI] [PubMed] [Google Scholar]
- 33.Xia J, Gill EE, Hancock RE. NetworkAnalyst for statistical, visual and network-based meta-analysis of gene expression data. Nat Protoc 10: 823–844, 2015. [DOI] [PubMed] [Google Scholar]
- 34.Yin L, Ma H, Ge X, Edwards PA, Zhang Y. Hepatic hepatocyte nuclear factor 4alpha is essential for maintaining triglyceride and cholesterol homeostasis. Arterioscler Thromb Vasc Biol 31: 328–336, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]