Abstract
Dientamoeba fragilis is a single-celled protozoan, closely related to the trichomonads. Reported worldwide as causing human gastrointestinal symptoms, D. fragilis is very common and is second only to Blastocystis spp. Dientamoebiasis equals or exceeds the incidence of giardiasis. This minireview includes diagnostic options, clinical relevance, therapy, an animal model, the confirmed cyst stage, and sequencing data. The development of a rodent model, fulfilling Koch's postulates, and the confirmation of a cyst stage have clarified transmission routes, including fecal-oral transmission. The prevalence of D. fragilis varies between 0% to over 82%; results depend on the geographic location, group studied, and diagnostic methods used.
INTRODUCTION
Dientamoeba fragilis is a flagellate protozoan parasite of the human gastrointestinal (GI) tract that has remained somewhat controversial regarding various aspects of life cycle and pathogenicity. However, numerous reports have been and continue to be published regarding an association between this organism and human illness. Unfortunately, in some areas, its pathogenicity tends to be ignored. It is also recognized that some laboratory diagnostic methods are quite insensitive in terms of organism recognition and identification. The use of new diagnostic approaches has enhanced the detection of D. fragilis in clinical specimens and supports its potential role in human disease. This review will provide information that will help to clarify the significance of D. fragilis as a human pathogen and will update information on the biology and life cycle of this neglected gastrointestinal flagellate pathogen. Studies have identified emerging species of intestinal protozoa, such as D. fragilis and Blastocystis spp., that are relevant to global public health and how they too might emerge as important gastrointestinal pathogens in the coming years (1).
D. fragilis was first seen in 1909 by Charles Wenyon after the examination of his own fecal specimen; however, the organism was not described until 1918 by Margaret Jepps and Clifford Dobell (2, 3). They indicated that the organism was an amoeba with a binucleate structure, which was described as fragile and disintegrating quickly outside the body. Thus, the name Dientamoeba fragilis was proposed. In 1940, Dobell recognized the close morphological similarities between D. fragilis and Histomonas meleagridis, the ameboflagellate parasite of turkeys. He suggested that D. fragilis was a flagellate, although he was unable to demonstrate actual flagella (4).
A key scientific advance was made in 1934 when Tyzzer reported that H. meleagridis is transmitted in the eggs of Heterakis, the cecal worm of chickens and turkeys, a fact that has relevance to the life cycle of D. fragilis (4, 5). On the basis of electron microscopy studies, D. fragilis has been reclassified as an ameboflagellate rather than an ameba and is closely related to Histomonas and Trichomonas spp. (6). It has a cosmopolitan distribution, and past surveys demonstrate incidence rates of 0.4% (patients with gastrointestinal discomfort) to 82.9% (children infected with gastrointestinal protozoa) (Tables 1 and 2).
TABLE 1.
Prevalence of D. fragilis infections in various studies throughout the United States
| Prevalence (%) | Fecal specimens/site | No. of patients | Methoda | Area | Reference |
|---|---|---|---|---|---|
| 2.4 | Parasitology diagnostic laboratory | 14,203 | LM | USA | 61 |
| 4 | Parasitology diagnostic laboratory | 13,194 | LM (C, TS) | Los Angeles, CA | 62 |
| 52 | Adults, semi-communal group | 81 | LM | Los Angeles, CA | 63 |
| 21.1 | Children, dental, general pediatric clinics | 104 | LM | Los Angeles, CA | 63 |
| 1.3 | Homosexual men | 150 | LM | San Francisco, CA | 64 |
| 1.1 | Homosexual men with diarrhea | 274 | LM | Chicago, IL | 65 |
| 1.6 | Public Health laboratories | 3,500 (mean/lab) | LM (C, TS) | USA | 66 |
| 2.3 | Pediatric refugees | 87 | LM | USA | 67 |
| 0.4 | Patients with GI discomfort | 2,604 | LM (WM, TS) | Rocky Mountain region | 68 |
| 5 | Internationally adopted children | 1,042 | LM (TS) | USA | 69 |
| 1.1 | Refugees (worldwide) tested in California | 1,232 | LM (C, TS) | Santa Clara | 70 |
C, concentration; LM, light microscopy; TS, trichrome stain; WM, wet mount.
TABLE 2.
Prevalence of D. fragilis infections in representative studies in areas other than the United States
| Prevalence (%) | Fecal specimens/site | No. of patient | Methoda | Area | Reference |
|---|---|---|---|---|---|
| 16.8 | Outbreak of GI complaints | 125 | LM | Australia | 71 |
| 1.5 | Patients with diarrhea | 260 | CULT | Australia | 72 |
| 0.9 | Patients with diarrhea | 6,750 | LM | Australia | 73 |
| 13 | Patients with enteric protozoa | 25,914 | LM | Australia | 74 |
| 5.2 | Patients with GI complaints | 750 | qPCR | Australia | 28 |
| 5.5 | Parasitology diagnostic laboratory | 472 | MT-PCR | Australia | 42 |
| 6.3 | Patients suspected of parasitic GI infection | 448 | LM, TFT | Belgium | 75 |
| 13.6 | Patients from very poor areas | 88 | PCR | Brazil | 51 |
| 18.4 | Patients from very poor areas | 38 | PCR | Brazil | 51 |
| 14.6 | Health practice patients (2002–2004) | 3,719 | LM | British Isles | 76 |
| 16.9 | Health practice patients (2005–2007) | 2,491 | LM | British Isles | 76 |
| 4.2 | Parasitology diagnostic laboratory | 43,029 | LM | Canada | 77 |
| 2.9 | Parasitology diagnostic laboratory | 9,376 | LM | Canada | 78 |
| 33.7 | Parasitology diagnostic laboratory | 2,777 | LM | Canada | 79 |
| 43 | Specimens submitted to Statens Serum Institut | 22,000b | qPCR | Denmark | 45 |
| 82.9 | Children infected with GI protozoa, included Giardia and/or other mixed GI protozoa; all symptomatic | 123 | LM | Germany | 80 |
| 21.4 | Patients, clinical suspicion of GI parasites | 491 | q-PCR | Italy | 46 |
| 60.6 | General pediatric population, symptomatic and asymptomatic; single stool/patient | 249 | LM (direct wet mounts only), qPCR | Lebanon | 81 |
| 32 | Patients with GI complaints | 397 | qPCR, LM | The Netherlands | 43 |
| 23 | Children (4–16 yr old) referred to secondary med center | 220 | SLP | The Netherlands | 82 |
| 62 | Symptomatic pediatric patients | 163 | qPCR | The Netherlands | 44 |
| 3 | Patients with GI disorders | 1,350 | LM | New Zealand | 83 |
| 6.3 | Hospitalized children, acute GI disease | 176 | Multiplex qPCR | Portugal | 47 |
LM, light microscopy; MT-PCR, multiplex tandem real-time PCR; qPCR, quantitative real-time PCR; SLP, standard laboratory procedures; TS, trichrome stain; TFT, triple feces test.
Stool specimens, not individual patients.
The published higher incidence figures have been reported for mental institution inmates, missionaries, and Native Americans in Arizona. D. fragilis tends to be common in some pediatric populations, and incidence figures in some studies are higher for patients younger than 20 years of age.
LIFE CYCLE AND MORPHOLOGY
The life cycle and mode of transmission of D. fragilis were always speculative; however, newer information has clarified some of the morphology issues. Transmission via helminth eggs, such as those of Ascaris and Enterobius spp., has been postulated (6, 7) (Fig. 1). The cyst stage has recently been confirmed, thus also confirming the fecal-oral transmission of D. fragilis (8) (Table 3). The precyst and cyst forms continue to be investigated in terms of transmission potential.
FIG 1.
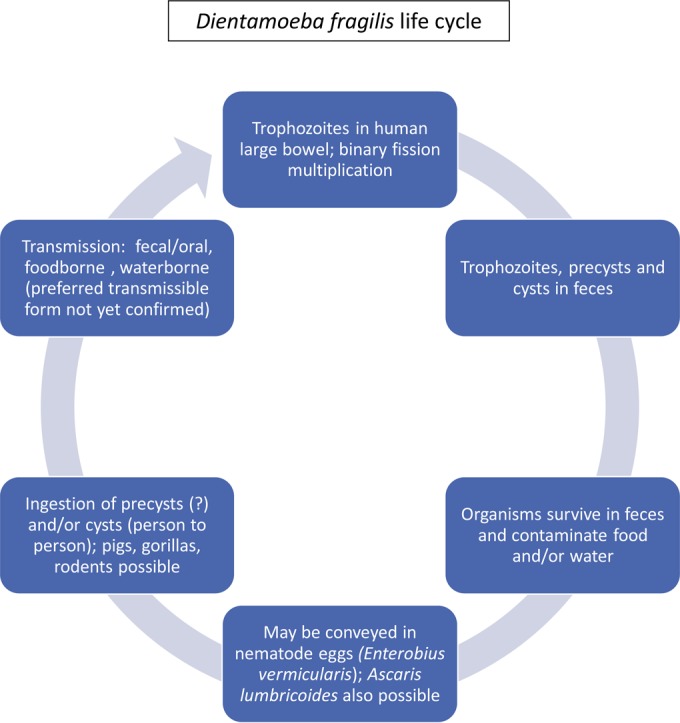
Life cycle of Dientamoeba fragilis (30).
TABLE 3.
Morphological characteristics: trophozoites and cysts of Dientamoeba fragilis
| Dientamoeba fragilis characteristic | Shape and size | Motility | No. of nuclei and visibility | No. of flagella (usually difficult to see) | Other features |
|---|---|---|---|---|---|
| Trophozoites | Shaped like amebae; 5–15 μm; usual range, 9–12 μm | Usually nonprogressive; pseudopodia are angular, serrated, or broad lobed and almost transparent | Percentage may vary, but 40% of organisms have 1 nucleus and 60% have 2 nuclei; not visible in unstained preparations; no peripheral chromatin; karyosome is composed of a cluster of 4–8 granules | No visible flagella | Cytoplasm finely granular and may be vacuolated with ingested bacteria, yeasts, and other debris; may be great variation in size and shape on a single smear |
| Cysts | Generally oval to round; ∼5–8 μm; inner organism about 5 μm; inner, outer cyst walls | Nonmotile | 2; essentially the same shape and size as nuclei seen in the trophozoite stages | No visible flagella | Distinct cyst wall; inner cyst wall irregular, located directly adjacent to encysted parasite; peritrophic space exists between outer cyst wall and encysted parasite. Koch's postulates fulfilled with mice/rats; fecal-oral cycle established |
Trophozoite.
The trophozoite is characterized as having one nucleus (20% to 40%) or two nuclei (60% to 80%) (2). The nuclear chromatin is usually fragmented into three to five granules, and there is normally no peripheral chromatin on the nuclear membrane (Fig. 2, first two rows). In some organisms, the nuclear chromatin arrangement tends to mimic that of Endolimax nana, Entamoeba hartmanni, or even Chilomastix mesnili, particularly if the organisms are overstained. The cytoplasm is usually vacuolated and may contain ingested debris as well as some large, uniform granules. The cytoplasm can also appear uniform and clean with few inclusions. When many vacuoles are present, this probably represents degeneration and may be seen in fecal specimens that have not undergone immediate fixation. There can also be considerable size (5 to 15 μm) and shape (oval to round) variation among organisms, even on a single stained fecal smear. Trophozoite movement is by cytoplasmic streaming of pseudopodia, which is similar to that seen with the amebae (9). Most standard parasitology texts will contain discussion related to the overall morphological characteristics of the intestinal protozoa and specific comments on how they may mimic one another.
FIG 2.
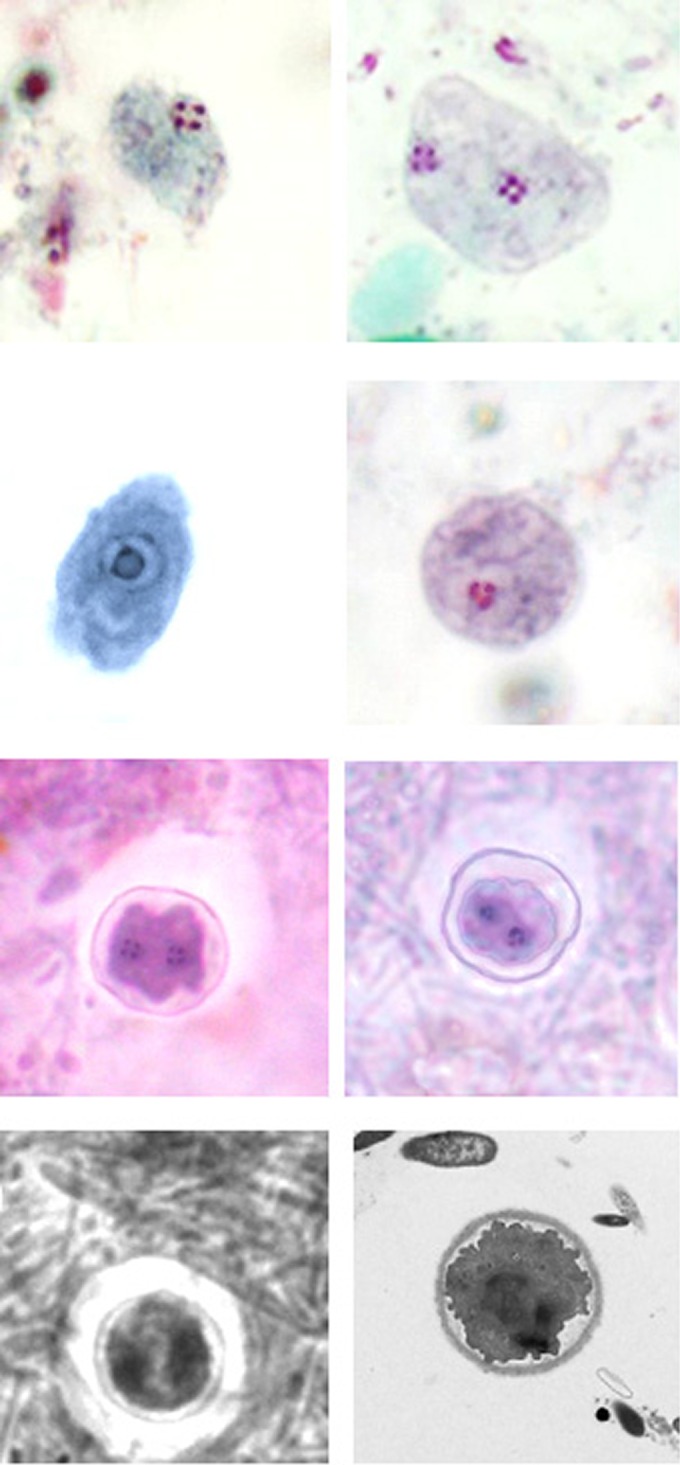
Dientamoeba fragilis trophozoites and cysts. Upper row, trichrome stain: left, trophozoite with single fragmented nucleus; right, trophozoite with two fragmented nuclei. Second row: left, trophozoite with single nucleus that has not yet fragmented—can mimic Endolimax nana trophozoite (iron hematoxylin stain); right, trophozoite with single fragmented nucleus—three chromatin dots visible. Third row, trichrome stain: cysts showing two nuclei and the cyst wall—note that the organism is somewhat shrunken within the cyst wall. Bottom row: left, black and white image showing the cyst with two nuclei and cyst wall (also note the zone of clearance around the cyst); right, transmission electron micrograph of cyst showing the cyst wall and the encysted organism. (Images in the third and bottom rows are courtesy of Damien Stark, St. Vincent's Hospital, New South Wales, Australia, reproduced with permission).
Precysts.
Precysts have recently been described by Stark et al. (10); however, previous publications have described these stages in the past (2, 11, 12). These stages range from 3.5 to 5 μm in diameter, have one or two nuclei, and contain finely granular and uniform cytoplasm (12). Although these stages appear to survive unfavorable environmental conditions, their infectivity remains unconfirmed.
The precystic forms of D. fragilis are more frequently seen than the cyst forms and have a prevalence of up to 5% in clinical samples (10). This precystic stage is characterized by a compact spherical shape with a reduction in size of up to 50% from “normal” trophozoites. These forms range in size from 4 to 5 μm. The cytoplasm is darkly stained, indicating a denser structure than what is found in normal trophozoites. The cytoplasm is homogeneous and rarely contains any inclusions (4).
Cysts.
Although cysts were thought not to exist in humans but only in animal hosts, early reports suggest that a human cyst stage does occur. Kofoid described a cyst form in 1923 (11), and additional reports were published in 1928 (13) and 1948 (14). Based on the rodent model of this infection, D. fragilis cyst forms were identified in the fecal specimens of infected animals (8). Cyst forms were then reported from human clinical fecal specimens in 2014 from two separate laboratories in Australia and the United States (10).
Electron microscopy reveals various organelles within the cyst, including an axostyle, flagellar axonemes, and a costa. External flagella are absent. Observation of flagellar components only within the cyst and not in the trophozoite stage provides support for the suggestion that D. fragilis has adapted to life in the gut by losing the flagella and adopting an amebic appearance and style of locomotion in the gut (9). However, the flagella may not be lost, but the organism no longer has the ability to express them externally like other flagellates (8).
Precysts and cysts are extremely difficult to identify and tend to be quite rare (<5%) compared with the cyst numbers of other protozoa (10). The cysts have a distinct cyst wall (∼5 μm in diameter) with a clear zone around the cyst. A space is present between the cyst wall and the organism enclosed within the cyst wall. The nuclear structure was morphologically identical to that found in D. fragilis trophozoites. All of the cysts that were seen contained two nuclei, with each nucleus containing a large central karyosome with a delicate nuclear membrane. No chromatin is visible on the nuclear membrane, and the nucleus is often fragmented into distinct granules of chromatin. These “true” cysts are rarely encountered in clinical samples, which probably accounts for the limited number of descriptive reports (Fig. 2, bottom two rows).
Transmission.
Although there is evidence to suggest transmission via helminth eggs, such as those of Ascaris lumbricoides and Enterobius vermicularis, the confirmation of precyst and cyst forms from human fecal specimens provides another possible mode of transmission. While the precyst and cyst forms are rare in human specimens, they may play a more important role in epidemiological possibilities that include risks related to potential waterborne transmission.
Implication of possible helminth vectors is based on the fact that the organism most closely related to D. fragilis, Histomonas meleagridis, has a helminth vector (15, 16). Although a number of reports support this hypothesis of transmission, other reports find no association between helminth vectors and infections with D. fragilis. Reviewing all of the data on each side of the argument suggests that, while E. vermicularis may be able to transmit D. fragilis within its eggs, E. vermicularis apparently is not required for transmission.
Studies related to the detection of D. fragilis DNA from the sterilized surface of E. vermicularis eggs, as well as D. fragilis DNA within these eggs, certainly supports the role of E. vermicularis in D. fragilis transmission (5, 16). However, the presence of DNA within the eggs is not confirmation that viable D. fragilis organisms were present (5).
Taxonomy.
While D. fragilis was first thought to be an ameba, after many years of study using light microscopy, the organism was placed into a new family with Histomonas in 1953, the Dientamoebidae (4, 12, 17). With the introduction of electron microscopy studies, confirmation was obtained that D. fragilis was closely related to flagellates (18). Key features of the uninucleated and binucleated trophozoites included the demonstration of a persistent internuclear spindle of microtubules in the binucleate stage and a well-developed parabasal filament in the two stages (19). Additional studies also showed strong common antigenic characteristics with Histomonas, while D. fragilis was quite different from Entamoeba histolytica and Entamoeba invadens (20). Using molecular techniques and studies using protein sequences, the information also confirms the close relationship between D. fragilis and H. meleagridis (21, 22). Currently, D. fragilis is in the phylum Parabasalia, class Tritrichomonadea, order Tritrichomonadida, family Dientamoebidae, genus Dientamoeba, and species Dientamoeba fragilis (2).
CLINICAL DISEASE
Although Jepps and Dobell described D. fragilis as a nonpathogen, this organism has been associated with a wide range of symptoms (2, 5, 6, 8, 9). Reports range from patients who are asymptomatic to those with symptoms that include intermittent diarrhea, abdominal pain, nausea, anorexia, malaise, fatigue, poor weight gain, and unexplained eosinophilia. Approximately half of the patients have eosinophilia (23–25). The most common symptoms in patients infected with this parasite appear to be intermittent diarrhea, abdominal pain, and fatigue. In some patients, the organism and the symptoms persist or reappear until appropriate treatment is initiated.
Eleven pediatric patients, seven of whom had peripheral eosinophilia and a history of recent travel, were diagnosed with D. fragilis infection and reported symptoms of anorexia, intermittent vomiting, abdominal pain, and diarrhea. Based on findings in these patients, which included bovine protein allergy and eosinophilic colitis, D. fragilis should be included in the differential diagnosis of chronic diarrhea and eosinophilic colitis. The identification of this pathogen requires clinical awareness of epidemiologic risk factors and presenting complaints as well as proper laboratory permanent staining procedures that are essential for correct identification. Long-term parasite carriage by rodents and prolonged shedding of cysts, together with elevated levels of calprotectin in the stool, confirm the capacity of this organism to cause disease. Increases in fecal calprotectin have been reported in patients suffering from intestinal disorders, such as inflammatory bowel disease (26). This information definitely suggests that dientamoebiasis should be considered in the differential diagnosis of gastrointestinal diseases, including inflammatory bowel disease (8, 27, 28).
Since the late 1920s, hundreds of published studies and patient case reports have provided support for the potential pathogenicity of D. fragilis (6, 26–28). Based on the majority of reports, patients infected with D. fragilis complained of chronic or acute symptoms. Chronic symptoms are common with up to a third of patients exhibiting persistent diarrhea. Numerous studies have successfully demonstrated parasite clearance coupled with complete resolution of clinical symptoms following treatment with various antiparasitic compounds (Tables 1 and 2). Additional information can be seen below in the section Genetic Diversity.
DIAGNOSIS
Routine diagnostic procedures.
Clinicians should include infection with D. fragilis in their differential diagnosis of patients presenting with abdominal pain, diarrhea, unexplained flatulence, nausea, and vomiting. Diagnosis of D. fragilis infection depends on proper collection and processing techniques (a minimum of three fecal specimens) (29–35). Although the survival time for this parasite has been reported as 24 to 48 h, morphological characteristics will not be preserved if the specimen is not examined immediately or immediately preserved in a suitable fixative soon after defecation. It is particularly important that stained smears of stool material (trichrome, iron-hematoxylin) be examined with an oil immersion objective (100×). These organisms have been recovered in formed stool; therefore, a permanent stained smear must be prepared and examined for every stool sample submitted for a routine ova and parasite (O&P) examination. If the laboratory is accredited by the College of American Pathologists, the permanent stain is a mandatory part of the O&P procedure. Organisms seen in direct wet mounts may appear as refractile, round forms; the nuclear structure cannot be seen without examination of the permanent stained smear. It has also been confirmed that molecular methods are far more sensitive than wet mounts (36). With the recent confirmation of the cyst stage, one needs to take into account the more shrunken appearance of this form compared with the trophozoite.
Key points—laboratory identification of D. fragilis using routine methods.
(i) A minimum of three specimens within 10 days, one collected every other day (stool), should be submitted for the diagnosis of Dientamoeba infections. (ii) Although a cyst stage has been confirmed, trophozoites and cysts will still be difficult to see on a wet preparation. Consequently, it is mandatory that a permanent stained smear be included in the ova and parasite examination. Trophozoites with either one or two nuclei can be found in the same specimen; there may also be tremendous size variation among the organisms seen in a single smear. (iii) Trophozoite forms have been recovered from formed stool, hence the need to perform the permanent stained smear on specimens other than liquid or soft stools. (iv) Remember, the cyst form will appear shrunken with two cyst walls; often, there will be a large clear area surrounding the cyst (permanent stain). (v) Organisms with a single nucleus can easily be confused with Endolimax nana or Entamoeba hartmanni, which are considered nonpathogenic.
Antigen detection.
Although rapid fecal immunoassays (enzyme immunoassays, fluorescent antibody, rapid cartridge formats) for antigen detection are not yet available commercially within the United States, antigen detection tests have been developed using the immunofluorescence format (37). Studies using the enzyme immunoassay method are also under way. It is anticipated that these assays will soon be available since preliminary results look very promising. The potential for detection of DNA from feces is also being developed; certainly, these rapid, specific, and sensitive tests would be extremely helpful within the diagnostic laboratory setting (6).
Antibody detection.
Using an indirect immunofluorescence assay, Chan et al. (38) found that serum samples from three patients with confirmed D. fragilis infections had positive titers of 1:80, and 12 matched controls had positive titers ranging from 1:20 to 1:160. Of the 189 healthy children, 172 (91%) were positive at a serum dilution of 1:10 or higher. The specificity of this assay was reinforced by immunoblotting 20 representative serum samples against D. fragilis; in all 17 indirect immunofluorescence-positive serum samples, a 39-kDa protein band of D. fragilis was identified. In this study, findings over a 5-year period indicated that D. fragilis was the most common protozoan, followed closely by Giardia lamblia and more distantly by Cryptosporidium parvum.
Culture.
The approach to in vitro culture is not new; however, some excellent improvements have recently been developed (39, 40). Currently, no axenic cultures of D. fragilis exist. D. fragilis will grow quite well in xenic cultures with support flora consisting mostly of Escherichia coli. Slight variations in the species of prokaryotic support flora present within D. fragilis cultures are unlikely to exhibit any significant effect on growth. However, other protozoa, such as E. histolytica, G. lamblia, and Trichomonas vaginalis, have been routinely grown in axenic cultures for many years; this approach avoids the possible interference of bacteria present in other systems. A temperature of 42°C and a microaerophilic atmosphere are also optimum for growth. Compared to other media, Loeffler's slope medium led to much better growth of D. fragilis (40). A modified Earle's balanced salt solution containing cholesterol, ferric ammonium citrate, and rice starch is considered a superior liquid overlay that can be used along with Loeffler's serum slope for culture of D. fragilis under anaerobic conditions. Studies have shown successful cultivation from feces stored at room temperature for up to 24 h but only up to 10 h for refrigerated feces. Culture methods for intestinal parasites are difficult and time-consuming with many variables; quality control requirements are mandatory. Use of these methods is normally limited to experienced parasitology laboratories. While D. fragilis can be cultured, long-term culture is difficult to achieve, and overall sensitivity varies tremendously (41).
Molecular testing.
Molecular assays have been developed to provide rapid, sensitive, and specific simultaneous detection and identification of multiple diarrhea-causing protozoan parasites that infect humans (42–44, 84). Studies also highlight the lack of sensitivity demonstrated by microscopy, and thus, molecular methods are considered the diagnostic methods of choice for enteric protozoan parasites. However, until all potential human protozoan pathogens are included in the molecular panels, they will remain highly sensitive but will fail to detect all possible pathogens (28, 42–50, 84). Although molecular procedures detect a high percentage of intestinal protozoa in pediatric patients with gastrointestinal symptoms, interpretation and determination of the clinical relevance of a positive PCR result in this population may remain somewhat difficult. With increased detection rates at a lower workload using algorithms, the potential to expand additional parasite targets combined with fully automated DNA isolation and molecular high-throughput screening could eventually replace microscopy with molecular options. When conventional PCR and real-time PCR (qPCR) were compared with microscopy for the detection of D. fragilis, conventional PCR had a sensitivity of 88.9% and a specificity of 100%, while qPCR was 100% sensitive and specific (49). However, this assay was later found to cross-react with other trichomonads; thus, in routine diagnostic testing, specificity may be an issue. In addition to conventional PCR and qPCR, a number of nested PCR assays have also been reported.
The diagnostic approach is in transition from single pathogen detection to a multiplex approach, allowing simultaneous detection and identification of multiple parasites. Based on the patient population (children, immunocompromised patients, travelers, and potential outbreaks), various targets can be used within a routine diagnostic laboratory. Epidemiologic monitoring and evaluation of control policies may become possible using automation associated with these newer multiplex approaches (BD Max enteric parasite panel [Becton, Dickinson and Company, Sparks, MD], BioFire FilmArray gastrointestinal panel [bioMérieux, Marcy l 'Etoile, France], and the xTAG gastrointestinal pathogen panel [Luminex, Inc., Austin, TX]) (48). Often, the parasitic targets are included with relevant bacterial and viral targets as multiple targets within the multiplex approach. However, current panels do not include D. fragilis. Although no commercially available molecular methods are currently cleared for D. fragilis by the Food and Drug Administration, expanded parasite panels are expected to include Dientamoeba as a target in the near future.
GENETIC DIVERSITY
There are two major D. fragilis genotypes, with genotype 1 being the most common and genotype 2 (Bi/PA strain) (6, 27, 51, 52). Although minor (∼2%), these distinctions are based on 18S rRNA sequence differences (49, 53, 54). The internal transcribed spacer (ITS) region of the rRNA operon has been studied in the two genotypes of D. fragilis. While extensive variation between copies of the sequence within the same strain has been seen, the overall significance of this finding is somewhat unclear (55).
Differences in the clinical outcomes of parasitic infections with D. fragilis probably indicate parasite genetic diversity. The presence of D. fragilis in asymptomatic individuals certainly raises the possibility of multiple lineages, some of which may be nonpathogenic for humans. Genetic analyses of three D. fragilis housekeeping genes provide a clear distinction between the two known genotypes (56). High-resolution melting curve studies found that four profiles (subtypes) were present. One of these profiles (profile 1) was predominant (50%). Profile 2 was present on 20%. Profiles 3 and 4 were present on 16.7% and 13.4%, respectively. No mixed profiles were detected among the samples (57). At this time, it remains unclear whether D. fragilis may or may not represent a species complex. A recent publication involves the identification of 6,595 transcripts of D. fragilis, data that provide new insights on the organism metabolism, kinome, degradome, and potential mechanisms of pathogenicity (39).
TREATMENT
Clinical improvement has been observed in adults receiving tetracycline; symptomatic relief has been observed in children receiving diiodohydroxyquin, metronidazole, or tetracycline. Current recommendations include iodoquinol, paromomycin, or combination therapy. However, no large-scale double-blind randomized placebo controlled trials testing the efficacy of antimicrobial agents against D. fragilis have been undertaken. Since symptomatic relief has been observed to follow appropriate therapy, D. fragilis is probably pathogenic in infected individuals who are symptomatic. Although limited studies have been undertaken regarding the efficacy of various therapies, information continues to support the fact that the elimination of this organism from symptomatic patients leads to clinical improvement. Current recommendations include iodoquinol, paromomycin, or metronidazole (30).
Although there are a number of reports of susceptibility testing of potential therapeutic drugs for D. fragilis, these studies do not use axenic culture. Thus, the presence of bacterial flora within the testing system complicates the interpretation of test results. In cases of treatment failure, these findings may be related to developing drug resistance, poor treatment compliance, or inadequate drug dosage (58).
Data on the associations between antimicrobial use and potential risk of enteric protozoal infection are rare. However, a retrospective study was conducted on 9,945 Danish patients between 2008 and 2011. The authors found that exposure to metronidazole conferred a decreased risk of D. fragilis infection as did other antimicrobials not normally used for this parasitic infection, including broad-spectrum penicillin, fluoroquinolones, and macrolides. However, mebendazole exposure was associated with an increased risk of D. fragilis infection (59).
EPIDEMIOLOGY AND PREVENTION
As reported for many of the intestinal protozoa, D. fragilis is worldwide in distribution. It is suspected that the true incidence of this infection is considerably higher than reported, particularly since many laboratories do not yet emphasize diagnostic methods, such as the permanent stained smear that would confirm the diagnosis (9) (Tables 1 and 2).
Since fecal-oral transmission has now been documented, preventive measures would tend to be those related to other intestinal pathogenic protozoa. With transmission occurring from the ingestion of certain helminth eggs and/or cyst forms, the use of hygiene and sanitary measures to prevent contamination with fecal material would be appropriate. There is speculation that D. fragilis may be infrequently recovered and identified; low incidence or absence from survey studies may be due to poor laboratory techniques and a general lack of knowledge about the organism (6, 29, 30). A study in 2012 confirmed that pigs are a natural host and harbor genotypes found in humans; thus, there is potential for zoonotic transmission (60). However, human to human transmission is generally considered the most common route of infection.
Biography

Lynne S. Garcia, former Manager of the UCLA Clinical Microbiology Laboratory, is currently the Director of LSG & Associates, providing training, teaching, and consultation for diagnostic medical parasitology and health care administration. She has given over 400 presentations (international, national, and local) and published over 175 manuscripts, book chapters, and articles. She is the author of Diagnostic Medical Parasitology (6th edition, 2016) and Practical Guide to Diagnostic Parasitology (2nd edition, 2009), ASM Press, Washington, DC. She is the editor in chief of Clinical Microbiology Procedures Handbook (3rd edition, 2010). She is the editor in chief of Clinical Laboratory Management (2nd edition, 2013). She is a reviewer for nine journals. She consults for the CAP Microbiology Resource Committee, is chair of the NCCLS Parasitology Subcommittee, and is a Fellow of The American Academy of Microbiology. Lynne is the 2009 recipient of the ASM bioMérieux Sonnenwirth Award for Leadership in Clinical Microbiology.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Turkeltaub JA, McCarty TR III, Hotez PJ. 2015. The intestinal protozoa: emerging impact on global health and development. Curr Opin Gastroenterol 31:38–44. doi: 10.1097/MOG.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 2.Jepps MW, Dobell C. 1918. Dientamoeba fragilis n.g., n. sp.: a new intestinal amoeba from man. Parasitology 10:352–367. [Google Scholar]
- 3.Beaver PC, Jung RC, Cupp EW. 1984. Clinical parasitology, 9th ed Lea & Febiger, Philadelphia, PA. [Google Scholar]
- 4.Dobell C. 1940. Researches on intestinal protozoa in monkeys and man. X. The life history of Dientamoeba fragilis: observations, experiments and speculations. Parasitology 32:417–461. [Google Scholar]
- 5.Clark CG, Roser D, Stensvold CR. 2014. Transmission of Dientamoeba fragilis: pinworm or cysts? Trends Parasitol 30:136–140. doi: 10.1016/j.pt.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Johnson EH, Windsor JJ, Clark CG. 2004. Emerging from obscurity: biological, clinical, and diagnostic aspects of Dientamoeba fragilis. Clin Microbiol Rev 17:553–570. doi: 10.1128/CMR.17.3.553-570.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roser D, Nejsum P, Carlsgart AJ, Nielsen H, Stensvold CR. 2013. DNA of Dientamoeba fragilis detected within surface-sterilized eggs of Enterobius vermicularis. Exp Parasitol 133:57–61. doi: 10.1016/j.exppara.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 8.Munasinghe VS, Vella NG, Ellis JT, Windsor PA, Stark D. 2013. Cyst formation and faecal-oral transmission of Dientamoeba fragilis – the missing link in the life cycle of an emerging pathogen. Int J Parasitol 43:879–883. doi: 10.1016/j.ijpara.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Windsor JJ, Johnson EH. 1999. Dientamoeba fragilis: the unflagellated human flagellate. Br J Biomed Sci 56:293–306. [PubMed] [Google Scholar]
- 10.Stark D, Garcia LS, Barratt JL, Phillips O, Roberts T, Marriott D, Harkness J, Ellis JT. 2014. Description of Dientamoeba fragilis cyst and precystic forms from human samples. J Clin Microbiol 52:2680–2683. doi: 10.1128/JCM.00813-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kofoid CA. 1923. Amoeba and man. University of California Press, Berkeley, CA. [Google Scholar]
- 12.Wenrich D. 1936. Studies on Dientamoeba fragilis (protozoa). I. Observations with special reference to nuclear structure. J Parasitol 22:76–83. [Google Scholar]
- 13.Greenway D. 1928. Dientamoeba fragilis en la Argentina. Arch Argent Enferm Apar Dig 3:897. [Google Scholar]
- 14.Piekarski G. 1948. Zur frage der crystenbildung bei Dientamoeba fragilis. Zeitshrift Hyg Infektion 127:496–500. [PubMed] [Google Scholar]
- 15.Barratt JL, Harkness J, Marriott D, Ellis JT, Stark D. 2011. The ambiguous life of Dientamoeba fragilis: the need to investigate current hypotheses on transmission. Parasitology 138:557–572. doi: 10.1017/S0031182010001733. [DOI] [PubMed] [Google Scholar]
- 16.Ogren J, Dienus O, Lofgren S, Iveroth P, Matussek A. 2013. Dientamoeba fragilis DNA detection in Enterobius vermicularis eggs. Pathog Dis 69:157–158. doi: 10.1111/2049-632X.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chatton E. 1953. Famille des Dientamoebidae, nov. In Grasse PP. (ed), Traite de zoologie, Masson et Cie., Paris, France. [Google Scholar]
- 18.Banik GR, Birch D, Stark D, Ellis JT. 2012. A microscopic description and ultrastructural characterisation of Dientamoeba fragilis: an emerging cause of human enteric disease. Int J Parasitol 42:139–153. doi: 10.1016/j.ijpara.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Bird RG, Sargeaunt P, Upton CP. 1970. Uni- and binucleate trophozoites of Dientamoeba fragilis. Trans R Soc Trop Med Hyg 64:18. [PubMed] [Google Scholar]
- 20.Dwyer DM. 1974. Analysis of the antigenic relationships among Trichomonas, Histomonas, Dientamoeba and Entamoeba. 3. Immunoelectrophoresis technics. J Protozool 21:139–145. [DOI] [PubMed] [Google Scholar]
- 21.Silberman JD, Clark CG, Sogin ML. 1996. Dientamoeba fragilis shares a recent common evolutionary history with the trichomonads. Mol Biochem Parasitol 76:311–314. doi: 10.1016/0166-6851(95)02516-2. [DOI] [PubMed] [Google Scholar]
- 22.Noda S, Mantini C, Meloni D, Inoue J, Kitade O, Viscogliosi E, Ohkuma M. 2012. Molecular phylogeny and evolution of parabasalia with improved taxon sampling and new protein markers of actin and elongation factor-1α. PLoS One 7:e29938. doi: 10.1371/journal.pone.0029938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preiss U, Ockert G, Broemme S, Otto A. 1991. On the clinical importance of Dientamoeba fragilis infections in childhood. J Hyg Epidemiol Microbiol Immunol 35:27–34. [PubMed] [Google Scholar]
- 24.Gray TJ, Kwan YL, Phan T, Robertson G, Cheong EY, Gottlieb T. 2013. Dientamoeba fragilis: a family cluster of disease associated with marked peripheral eosinophilia. Clin Infect Dis 57:845–848. doi: 10.1093/cid/cit389. [DOI] [PubMed] [Google Scholar]
- 25.Cuffari C, Oligny L, Seidman EG. 1998. Dientamoeba fragilis masquerading as allergic colitis. J Pediatr Gastroenterol Nutr 26:16–20. doi: 10.1097/00005176-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Costa F, Mumolo M, Bellini M, Romano MR, Ceccarelli L, Arpe P, Sterpi C, Marchi S, Maltinti G. 2003. Role of faecal calprotectin as non-invasive marker of intestinal inflammation. Dig Liv Dis 35:642–647. doi: 10.1016/S1590-8658(03)00381-5. [DOI] [PubMed] [Google Scholar]
- 27.Barratt JL, Harkness J, Marriott D, Ellis JT, Stark D. 2011. A review of Dientamoeba fragilis carriage in humans: several reasons why this organism should be considered in the diagnosis of gastrointestinal illness. Gut Microbes 2:3–12. doi: 10.4161/gmic.2.1.14755. [DOI] [PubMed] [Google Scholar]
- 28.Stark D, Barratt J, Roberts T, Marriott D, Harkness J, Ellis J. 2010. A review of the clinical presentation of dientamoebiasis. Am J Trop Med Hyg 82:614–619. doi: 10.4269/ajtmh.2010.09-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clinical and Laboratory Standards Institute. 2005. Procedures for the recovery and identification of parasites from the intestinal tract; approved standard. CLSI document M28-A2. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 30.Garcia LS. 2016. Diagnostic medical parasitology, 6th ed ASM Press, Washington, DC. [Google Scholar]
- 31.Garcia LS. 2009. Practical guide to diagnostic medical parasitology, 2nd ed ASM Press, Washington, DC. [Google Scholar]
- 32.Garcia LS, Voge M. 1980. Diagnostic clinical parasitology. I. Proper specimen collection and processing. Am J Med Technol 46:459–466. [PubMed] [Google Scholar]
- 33.Garcia LS. (ed). 2010. Clinical microbiology procedures handbook, 3nd ed ASM Press, Washington, DC. [Google Scholar]
- 34.Isenberg HD. (ed). 1995. Essential procedures for clinical microbiology. American Society for Microbiology, Washington, DC. [Google Scholar]
- 35.Garcia LS. (ed). 2003. Selection and use of laboratory procedures for diagnosis of parasitic infections of the gastrointestinal tract. Cumitech 30A. ASM Press, Washington, DC. [Google Scholar]
- 36.Ogren J, Dienus O, Lofgren S, Einemo IM, Iveroth P, Matussek A. 2015. Dientamoeba fragilis prevalence coincides with gastrointestinal symptoms in children less than 11 years old in Sweden. Eur J Clin Microbiol Infect Dis 34:1995–1998. doi: 10.1007/s10096-015-2442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan FT, Guan MX, Mackenzie AM. 1993. Application of indirect immunofluorescence to detection of Dientamoeba fragilis trophozoites in fecal specimens. J Clin Microbiol 31:1710–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan F, Stewart N, Guan M, Robb I, Fuite L, Chan I, Diaz-Mitoma F, King J, MacDonald N, MacKenzie A. 1996. Prevalence of Dientamoeba fragilis antibodies in children and recognition of a 39 kDa immunodominant protein antigen of the organism. Eur J Clin Microbiol Infect Dis 15:950–954. doi: 10.1007/BF01690516. [DOI] [PubMed] [Google Scholar]
- 39.Barratt JL, Cao M, Stark DJ, Ellis JT. 2015. The transcriptome sequence of Dientamoeba fragilis offers new biological insights on its metabolism, kinome, degradome and potential mechanisms of pathogenicity. Protist 166:389–408. doi: 10.1016/j.protis.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 40.Barratt JL, Banik GR, Harkness J, Marriott D, Ellis JT, Stark D. 2010. Newly defined condition for the in vitro cultivation and cryopreservation of Dientamoeba fragilis: new techniques set to fast track molecular studies on this organism. Parasitology 137:1837–1878. [DOI] [PubMed] [Google Scholar]
- 41.Clark CG, Diamond LS. 2002. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev 15:329–341. doi: 10.1128/CMR.15.3.329-341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stark D, Al-Qassab SE, Barratt JL, Stanley K, Roberts T, Marriott D, Harkness J, Ellis JT. 2011. Evaluation of multiplex tandem real-time PCR for detection of Cryptosporidium spp., Dientamoeba fragilis, Entamoeba histolytica, and Giardia intestinalis in clinical stool samples. J Clin Microbiol 49:257–262. doi: 10.1128/JCM.01796-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bruijnestejn van Coppenraet LE, Wallinga JA, Ruijs GJ, Bruins MJ, Verweij JJ. 2009. Parasitological diagnosis combining an internally controlled real-time PCR assay for the detection of four protozoa in stool samples with a testing algorithm for microscopy. Clin Microbiol Infect 15:869–874. doi: 10.1111/j.1469-0691.2009.02894.x. [DOI] [PubMed] [Google Scholar]
- 44.Maas L, Dorigo-Zetsma JW, de Groot CJ, Bouter S, Plotz FB, van Ewijk BE. 2014. Detection of intestinal protozoa in paediatric patients with gastrointestinal symptom by multiplex real-time PCR. Clin Microbiol Infect 20:545–550. [DOI] [PubMed] [Google Scholar]
- 45.Roser D, Simonsen J, Nielsen HV, Stensvold CR, Molbak K. 2013. Dientamoeba fragilis in Denmark: epidemiological experience derived from four years of routine real-time PCR. Eur J Clin Microbiol Infect Dis 32:1303–1310. doi: 10.1007/s10096-013-1880-2. [DOI] [PubMed] [Google Scholar]
- 46.Calderaro A, Gorrini C, Montecchini S, Peruzzi S, Piccolo G, Rossi S, Gargiulo F, Manca N, Dettori G, Chezzi C. 2010. Evaluation of a real-time polymerase chain reaction assay for the detection of Dientamoeba fragilis. Diagn Microbiol Infect Dis 67:239–245. doi: 10.1016/j.diagmicrobio.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 47.Julio C, Furtado C, Rocha R, Escobar C, Brito MJU, Oleastro M. 2015. Detection of Dientamoeba fragilis in Portuguese children with acute gastroenteritis between 2011 and 2013. Parasitology 142:1398–1403. doi: 10.1017/S0031182015000906. [DOI] [PubMed] [Google Scholar]
- 48.van Lieshout L, Verweij JJ. 2010. Newer diagnostic approaches to intestinal protozoa. Curr Opin Infect Dis 23:488–493. doi: 10.1097/QCO.0b013e32833de0eb. [DOI] [PubMed] [Google Scholar]
- 49.Stark D, Beebe N, Marriott D, Ellis J, Harkness J. 2005. Detection of Dientamoeba fragilis in fresh stool specimens using PCR. Int J Parasitol, 35:57–62. doi: 10.1016/j.ijpara.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 50.ten Hove RJ, van Esbroeck M, Vervoort T, van den Ende J, van Lieshout L, Verweij JJ. 2009. Molecular diagnostics of intestinal parasites in returning travelers. Eur J Clin Microbiol Infect Dis 28:1045–1053. doi: 10.1007/s10096-009-0745-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.David EB, Guimaraes S, de Oliveira AP, Goulart de Oliveira-Sequeira TC, Nogueira Bittencourt G, Morael Nardi AR, Martins Ribolla PE, Bueno Franco RM, Branco N, Tosini F, Bella A, Pozio E, Caccio SM. 2015. Molecular characterization of intestinal protozoa in two poor communities in the state of Sao Paulo, Brazil. Parasit Vectors 8:103. doi: 10.1186/s13071-015-0714-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Windsor JJ, Clark CG, Macfarlane L. 2004. Molecular typing of Dientamoeba fragilis. Br J Biomed Sci 61:153–155. [DOI] [PubMed] [Google Scholar]
- 53.Johnson JA, Clark CG. 2000. Cryptic genetic diversity in Dientamoeba fragilis. J Clin Microbiol 38:4653–4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peek R, Reedecker FR, van Gool T. 2004. Direct amplification and genotyping of Dientamoeba fragilis from human stool specimens. J Clin Microbiol 42:631–635. doi: 10.1128/JCM.42.2.631-635.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Windsor JJ, Macfarlane L, Clark CG. 2006. Internal transcribed spacer dimorphism and diversity in Dientamoeba fragilis. J Eukaryot Microbiol 53:188–192. doi: 10.1111/j.1550-7408.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- 56.Stensvold CR, Clark CG, Roser D. 2013. Limited intra-genetic diversity in Dientamoeba fragilis housekeeping genes. Infect Genet Evol 18:284–286. doi: 10.1016/j.meegid.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 57.Hussein EM, Al-Mohammed HI, Hussein AM. 2009. Genetic diversity of Dientamoeba fragilis isolates of irritable bowel syndrome patients by high-resolution melting-curve analysis. Parasitol Res 105:1053–1060. doi: 10.1007/s00436-009-1515-9. [DOI] [PubMed] [Google Scholar]
- 58.Nagata N, Marriott D, Harkness J, Ellis JT, Stark D. 2012. Current treatment options for Dientamoeba fragilis infections. Int J Parasitol Drugs Drug Resist 2:204–215. doi: 10.1016/j.ijpddr.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Roser D, Simonsen J, Nielsen HV, Stensvold CR, Molbak K. 2015. History of antimicrobial use and the risk of Dientamoeba fragilis infection. Eur J Clin Microbiol Infect Dis 34:1145–1151. doi: 10.1007/s10096-015-2334-9. [DOI] [PubMed] [Google Scholar]
- 60.Caccio SM, Sanella AR, Manuali E, Tosini F, Sensi M, Crotti D, Pozio E. 2012. Pigs as natural hosts of Dientamoeba fragilis genotypes found in humans. Emerg Infect Dis 18:836–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kean BH, Malloch CL. 1966. The neglected ameba: Dientamoeba fragilis. A report of 100 “pure” infections. Am J Digest Dis 11:735–746. [DOI] [PubMed] [Google Scholar]
- 62.Garcia LS, Brewer TC, Bruckner DA. 1979. A comparison of the formalin-ether concentration and trichrome-stained smear methods for the recovery and identification of intestinal protozoa. Am J Med Technol 45:932–935. [PubMed] [Google Scholar]
- 63.Millet V, Spencer MJ, Chapin M, Stewart M, Yatabe JA, Brewer T, Garcia LS. 1983. Dientamoeba fragilis, a protozoan parasite in adult members of a semicommunal group. Dig Dis Sci 28:335–339. doi: 10.1007/BF01324950. [DOI] [PubMed] [Google Scholar]
- 64.Ortega HB, Borchardt KA, Hamilton R, Ortega P, Mahood J. 1984. Enteric pathogenic protozoa in homosexual men from San Francisco. Sex Transm Dis 11:59–63. doi: 10.1097/00007435-198404000-00001. [DOI] [PubMed] [Google Scholar]
- 65.Peters CS, Sable R, Janda WM, Chittom AL, Kocka FE. 1986. Prevalence of enteric parasites in homosexual patients attending an outpatient clinic. J Clin Microbiol 24:684–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grendon JH, Digiacomo RF, Frost FJ. 1991. Dientamoeba fragilis detection methods and prevalence: a survey of state public health laboratories. Public Health Rep 106:322–325. [PMC free article] [PubMed] [Google Scholar]
- 67.Meropol SB. 1995. Health status of pediatric refugees in Buffalo, NY. Arch Pediatr Adolesc Med 149:887–892. [DOI] [PubMed] [Google Scholar]
- 68.Church C, Neill A, Schotthoefer AM. 2010. Intestinal infections in humans in the Rocky Mountain region, United States. J Parasitol 96:194–196. doi: 10.1645/GE-2229. [DOI] [PubMed] [Google Scholar]
- 69.Staat MA, Rice M, Donauer S, Mukkada S, Holloway M, Cassedy A, Kelley J, Salisbury S. 2011. Intestinal parasite screening in internationally adopted children: importance of multiple stool specimens. Pediatrics 128:e613–e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang AH, Perry S, Du JN, Agunblade A, Polesky A, Parsonnet J. 2013. Decreasing intestinal parasites in recent Northern California refugees. Am J Trop Med Hyg 88:191–197. doi: 10.4269/ajtmh.2012.12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walker JC, Bahr G, Ehl AS. 1985. Gastrointestinal parasites in Sydney. Med J Aust 143:80. [DOI] [PubMed] [Google Scholar]
- 72.Sawangjaroen N, Luke R, Prociv P. 1993. Diagnosis by faecal culture of Dientamoeba fragilis infections in Australian patients with diarrhoea. Trans R Soc Trop Med Hyg 87:163–165. doi: 10.1016/0035-9203(93)90472-3. [DOI] [PubMed] [Google Scholar]
- 73.Stark D, Beebe N, Marriott D, Ellis J, Harkness J. 2005. Prospective study of the prevalence, genotyping, and clinical relevance of Dientamoeba fragilis infections in an Australian population. J Clin Microbiol 43:2718–2723. doi: 10.1128/JCM.43.6.2718-2723.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fletcher S, Caprarelli G, Merif J, Andresen D, Hal SV, Stark D, Ellis J. 2014. Epidemiology and geographical distribution of enteric protozoan infections in Sydney, Australia. J Public Health Res 3:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vandenberg O, Peek R, Souayah H, Dediste A, Buset M, Scheen R, Retore P, Zissis G, van Gool T. 2006. Clinical and microbiological features of dientamoebiasis in patients suspected of suffering from a parasitic gastrointestinal illness: a comparison of Dientamoeba fragilis and Giardia lamblia infections. Int J Infect Dis 10:255–261. doi: 10.1016/j.ijid.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 76.Schuster H, Jackson RS. 2009. Prevalence of Dientamoeba fragilis among patients consulting complementary medicine practitioners in the British Isles. J Clin Pathol 62:182–184. doi: 10.1136/jcp.2008.059659. [DOI] [PubMed] [Google Scholar]
- 77.Yang J, Scholten T. 1977. Dientamoeba fragilis: a review with notes on its epidemiology, pathogenicity, mode of transmission and diagnosis. Am J Trop Med Hyg 26:16–22. [DOI] [PubMed] [Google Scholar]
- 78.Libman MD, Gyorkos TW, Kokoskin E, Maclean JD. 2008. Detection of pathogenic protozoa in the diagnostic laboratory: result reproducibility, specimen pooling, and competency assessment. J Clin Microbiol 46:2200–2205. doi: 10.1128/JCM.01666-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maier A, Krolik J, Majury A. 2014. Triage and protocol recommendations for the parasitology laboratory based on an epidemiological investigation of parasite diagnostics in Ontario laboratories. Can J Infect Dis Med Microbiol 25:305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Preiss U, Ockert G, Bromme S, Otto A. 1990. Dientamoeba fragilis infection, a cause of gastrointestinal symptoms in childhood. Klin Padiatr 202:120–123. doi: 10.1055/s-2007-1025503. [DOI] [PubMed] [Google Scholar]
- 81.Osman M, El Safadi D, Cian A, Benamrouz S, Nourisson C, Poirier P, Pereira B, Razakandrainibe R, Pinon A, Lambert C, Wawzyniak I, Dabboussi F, Delbac F, Favennec L, Hamze M, Viscogliosi E, Certad G. 2016. Prevalence and risk factors for intestinal protozoan infections with Cryptosporidium, Giardia, Blastocystis, and Dientamoeba among schoolchildren in Tripoli, Lebanon. PLoS Negl Trop Dis 10(3):e0004496. doi: 10.1371/journal.pntd.0004496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gijsbers CF, Benninga M, Buller H. 2011. Clinical and laboratory findings in 220 children with recurrent abdominal pain. Acta Paediatr 100:1028–1032. doi: 10.1111/j.1651-2227.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- 83.Oxner RB, Paltridge GP, Chapman BA, Cook HB, Sheppard PF. 1987. Dientamoeba fragilis: a bowel pathogen? N Z Med J 100:64–65. [PubMed] [Google Scholar]
- 84.Verweij JJ, Stensvold CR. 2014. Molecular testing for clinical diagnosis and epidemiological investigations of intestinal parasitic infections. Clin Microbiol Rev 27:371–418. doi: 10.1128/CMR.00122-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


