Abstract
Background. Extraosseous Ewing's sarcoma in the spinal epidural space is a rare malignancy, especially in adults. Case Presentation. A 40-year-old male presented with back pain and urinary hesitancy. MRI revealed a thoracic extradural mass with no osseous involvement. He underwent surgery for gross total resection of the mass, which was diagnosed as Ewing's sarcoma. He was subsequently treated with chemoradiotherapy. He remains disease-free 1 year after surgery. Review of the literature indicated only 45 previously reported cases of spinal epidural extraosseous Ewing's sarcoma in adults. Conclusions. Extraosseous Ewing's sarcoma in the spinal epidural space is a rare clinical entity that should be included in the differential for spinal epidural masses. Its treatment is multidisciplinary but frequently requires surgical intervention due to compressive neurologic symptoms. Gross total resection appears to correlate with improved outcomes.
1. Introduction
Ewing's sarcoma (ES) is a malignant bone tumor of childhood and adolescence that occurs primarily in the diaphysis of long bones, such as femur, tibia, fibula, and humerus, but may also occur in other bony structures and cartilage tissue. This tumor was named after James Ewing, who in the 1920s described this small round blue cell tumor as being a separate entity from other histologically similar malignancies, such as lymphoma or neuroblastoma. It is the second most common malignant bone tumor after osteosarcoma, with the highest incidence in the second decade of life [1, 2]. The American Cancer Society estimates that 225 new cases are diagnosed annually in North America [1].
ES is the main member of a group of tumors known as Ewing's Sarcoma Family Tumors (ESFTs), which also contains peripheral primitive neuroectodermal tumors (pPNET). ES and pPNET are small round blue cell tumors; they were originally described as different entities; however, they are now recognized to represent ends of the morphologic spectrum of the ESFTs due to their close molecular relationship [3–7]. Some authors even assume pPNET and ES to be the same tumor with variable neural differentiation, a view that has been recently supported by immunohistochemical and cytogenetic findings [3]. The ESFT now includes osseous Ewing's sarcoma, EES, pPNET and Askin's tumor [7–9].
Ewing's sarcoma has two forms: the more common osseous Ewing's sarcoma (OES) and the relatively rare extraosseous Ewing's sarcoma (EES). EES has been reported in various tissues, including the chest wall, larynx, kidney, and esophagus. EES was first described by Tefft et al. in 1969, when they reported four patients with paravertebral soft tissue tumors histologically resembling ES [10]. Angervall and Enzinger in 1975 were the first to name this entity EES when they reviewed 39 patients with malignant soft tissue paravertebral tumors not arising from bone but having similar histologic characteristics to OES [11].
Spinal epidural EES in adults is a rare presentation among those locations where EES may occur. Here, we present an adult patient we recently treated, who represents only the 46th case of adult spinal epidural EES in the literature. Neurosurgeons should be aware of this rare clinical entity, which often presents with myelopathic and radicular symptoms associated with an epidural mass on imaging studies. Our review sheds light on the diagnosis, management, and prognosis of these cases.
2. Case Presentation
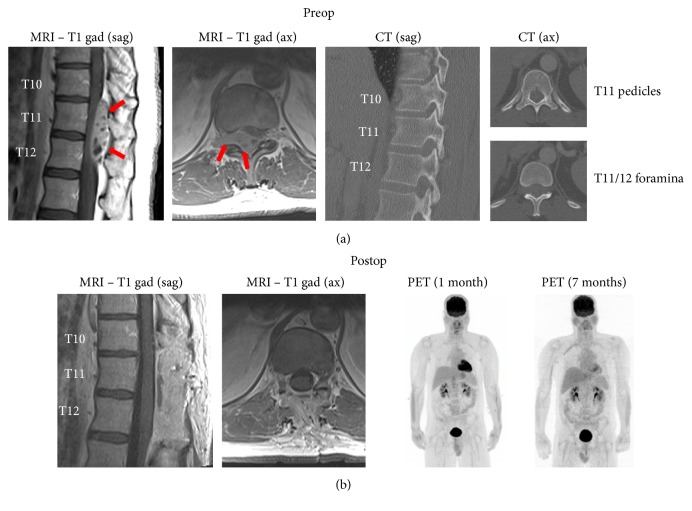
The patient is a 40-year-old male, previously healthy, who presented to the emergency department with several weeks of back pain and some urinary hesitancy lasting a few days. MRI of the thoracic spine indicated a heterogeneously enhancing extradural mass within the spinal canal at T10–T12, causing severe cord compression (Figure 1(a)). The mass was extended through the right neural foramina at T11-12 and T12-L1. CT did not suggest osseous involvement (Figure 1(a)). There were no other spinal lesions on MRI. CT of the chest, abdomen, and pelvis did not reveal any extraspinal sites suspicious for tumor growth. There were a number of somewhat enlarged periceliac lymph nodes of uncertain significance.
Figure 1.
Radiographic findings. (a) Preoperative MRI indicates a heterogeneously enhancing epidural mass (arrows) at T10–12 extending from the spinal canal into the right T11-12 foramen. CT shows that the osseous elements are intact. (b) Postoperative imaging shows T10–12 laminectomies and gross total resection of the lesion. PET imaging 1 and 7 months after resection shows no abnormal FDG uptake. Sag: sagittal, ax: axial, and gad: gadolinium.
The patient underwent a T10–12 laminectomy for gross total resection of the tumor (Figure 1(b)), with preservation of motor and sensory function, resolution of urinary hesitancy, and significant improvement in the back pain. Resection of the foraminal component of the tumor required ligation and amputation of the right T11 nerve root.
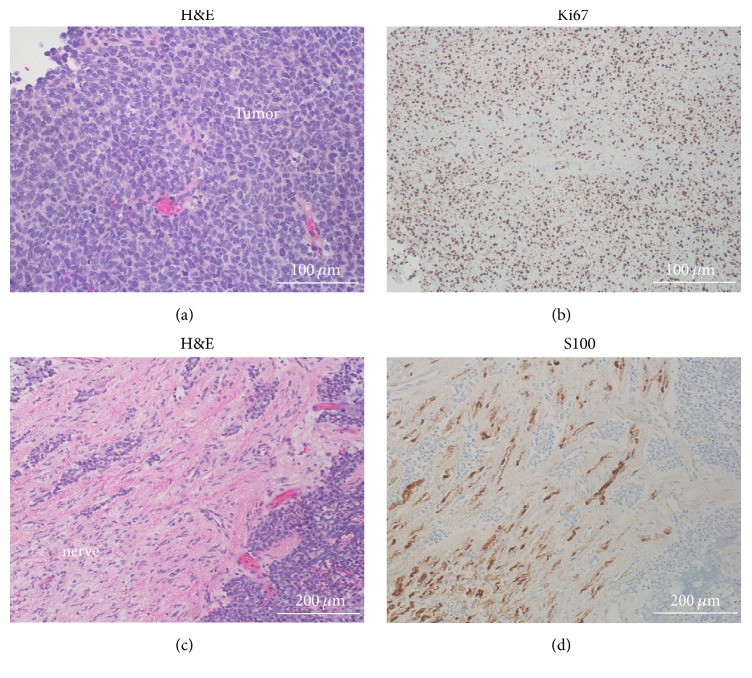
Pathologic examination indicated a small round blue cell neoplasm (Figure 2(a)) composed of primitive densely packed cells with a very high mitotic index (60–70% of cells positive for Ki67) (Figure 2(b)). Molecular studies showed the EWSR1 rearrangement, confirming the diagnosis of Ewing's sarcoma. The tumor itself was negative for S100/chromogranin/synaptophysin and CD45/CD20, thus ruling out the small round blue cell tumors: pPNET and lymphoma, respectively. Microscopic analysis of the resected right T11 nerve root showed tumor invasion through the perineurium (Figures 2(c) and 2(d)).
Figure 2.
Histologic findings. (a) H&E stain within the tumor shows the small round blue cell appearance. (b) Ki67 immunostaining indicates a very high mitotic index (60–70%). (c) H&E stain demonstrates tumor invasion through the perineurium and into the right T11 nerve root. (d) Tumor invasion in the T11 nerve root is demonstrated by tumor cells interspersed within S100-positive Schwann cells. The tumor itself was S100-negative. H&E: hematoxylin & eosin.
Postoperative MRI and PET scan did not reveal residual or metastatic tumor (Figure 1(b)). The previously noted periceliac lymph nodes did not show increased FDG uptake on PET scan. Six weeks after surgery he started adjuvant chemotherapy consisting of ifosfamide (supplemented with mesna), cyclophosphamide, doxorubicin, and irinotecan. Eleven weeks after surgery he began adjuvant radiotherapy (45 Gy in 25 doses). He has tolerated all treatments well. He has no evidence of disease on repeat PET scan and MRI of the thoracic spine one year after surgery.
3. Discussion
3.1. Epidemiology
Spinal epidural EES in adults represents a very small fraction of spinal epidural masses and a rare presentation among those locations where EES may occur. We performed literature searches on PubMed to identify reports of spinal EES. We identified 119 cases of spinal EES in the literature from 1969 to 2015. In 43 of these cases the tumor was intradural, while it was localized to the epidural space in 76 cases. Of the epidural EES cases, 31 cases were pediatric patients and 45 were adults (Table 1). Treatment of these patients commonly required a combination of surgery, chemotherapy, and radiotherapy. The case we present here is the 77th reported case of spinal epidural EES and only the 46th case of adult epidural EES in the literature.
Table 1.
Cases of adult primary spinal epidural EES/PNET tumors in the literature.
| Author | Year | Age (years)/sex (M or F) | Location/diagnosis | Treatment | Follow-up (months) | Outcome | CD99 | t(11:22) | Country∗ |
|---|---|---|---|---|---|---|---|---|---|
| Angervall and Enzinger [11] | 1975 | 20/M | T2–T5/EES | STR/RT/CT | 12 | DOD | NA | NA | Sweden |
| Angervall and Enzinger [11] | 1975 | 18/F | L5/EES | GTR/RT/CT | 6 | DOD | NA | NA | Sweden |
| Scheithauer and Egbert [29] | 1978 | 18/M | L1/EES | GTR/RT/CT | 16 | NED | NA | NA | USA |
| Scheithauer and Egbert [29] | 1978 | 27/F | T4–T6/EES | STR/RT/CT | 132 | NED | NA | NA | USA |
| Mahoney et al. [30] | 1978 | 23/M | S1/EES | Biopsy/RT/CT | 12 | DOD | NA | NA | USA |
| Fink and Meriwether [31] | 1979 | 19/M | L2-L3/EES | STR/RT/CT | 12 | NED | NA | NA | USA |
| N'Golet et al. [32] | 1982 | 29/M | T1–T3/EES | GTR/RT/CT | 6 | NED | NA | NA | France |
| N'Golet et al. [32] | 1982 | 47/F | L4/EES | GTR/RT/CT | 4 | DOD | NA | NA | France |
| Sharma et al. [33] | 1986 | 18/M | T10/EES | STR/RT/CT | 42 | DOD | NA | NA | India |
| Liu et al. [34] | 1987 | 26/F | L5-S1/PNET | STR/RT | 6 | NED | NA | NA | Taiwan |
| Christie et al. [35] | 1997 | 36/F | L2-L3/EES | STR/RT | 96 | DOD | NA | NA | Australia |
| Dorfmüller et al. [3] | 1999 | 18/M | L3-L4/PNET | GTR/RT/CT | 23 | NED | + | + | Austria |
| Kennedy et al. [36] | 2000 | 24/M | C1–C5/EES | STR/RT/CT | 13 | NED | NA | NA | Ireland |
| Shin et al. [37] | 2001 | 38/M | C5-C6/EES | STR/CT | 17 | NED | + | NA | South Korea |
| Shin et al. [37] | 2001 | 22/F | C7-T1/EES | STR/CT | 48 | NED | + | NA | South Korea |
| Morandi et al. [38] | 2001 | 22/F | T4-T5/EES | GTR/RT/CT | 66 | NED | + | NA | France |
| Morandi et al. [38] | 2001 | 25/F | L1-S2/EES | STR/CT | 7 | DOD | + | NA | France |
| Mukhopadhyay et al. [15] | 2001 | 29/F | C3–C5/EES | STR/RT/CT | 30 | NED | + | NA | India |
| Mukhopadhyay et al. [15] | 2001 | 18/M | T8-T9/EES | STR/RT/CT | 18 | NED | + | NA | India |
| Mukhopadhyay et al. [15] | 2001 | 22/M | L5-S1/EES | Biopsy/RT/CT | 15 | NED | + | NA | India |
| Mukhopadhyay et al. [15] | 2001 | 31/M | L3-L4/EES | STR/RT/CT | 32 | NED | + | NA | India |
| Gandhi et al. [39] | 2003 | 33/M | T5–T10/EES | GTR/RT/CT | 3 | NED | + | NA | Canada |
| Weber et al. [40] | 2004 | 26/M | L1-L2/PNET | GTR/RT/CT | 16 | NED | + | NA | Switzerland |
| Koudelova et al. [41] | 2006 | 28/F | L1-L2/PNET | STR/RT/CT | 24 | NED | NA | NA | Czech Republic |
| Isefuku et al. [5] | 2006 | 20/M | L5-S1/EES | STR/CT | 15 | DOD | + | + | Japan |
| Ozturk et al. [8] | 2007 | 18/M | C6-T1/EES | GTR/CT | 13 | NED | + | NA | Turkey |
| Lakhdar et al. [16] | 2008 | 24/F | C6-C7/EES | GTR/CT/RT | NA | NA | NA | NA | Morocco |
| Bozkurt et al. [42] | 2007 | 28/M | C3–C5/EES | GTR/RT/CT | 18 | NED | + | NA | Turkey |
| Feng et al. [43] | 2008 | 24/M | T8–T10/PNET | GTR/RT | 14 | NED | NA | NA | China |
| Musahl et al. [44] | 2008 | 27/M | S1-S2/PNET | GTR/RT/CT | 24 | NED | NA | NA | USA |
| Theeler et al. [9] | 2009 | 28/F | T6/NS | GTR/CT | 2 | NED | + | + | USA |
| Kiatsoontorn et al. [45] | 2009 | 25/M | T7/PNET | GTR/RT/CT | 6 | NED | + | NA | Japan |
| Jingyu et al. [19] | 2009 | 58y/M | T4/PNET | GTR Only | 25 | NED | + | NA | China |
| Duan et al. [4] | 2011 | 26/F | T4–T7/PNET | STR/RT/CT | 3 | NED | + | NA | China |
| Duan et al. [4] | 2011 | 34/M | T12/PNET | STR Only | 1 | NED | + | NA | China |
| Yasuda et al. [20] | 2011 | 37/F | T8-T9/EES | STR/RT/CT | 22 | DOD | + | + | Japan |
| Bostelmann et al. [46] | 2011 | 29/M | C7/EES | STR/RT/CT | 6 | DOD | + | NA | Germany |
| Saeedinia et al. [7] | 2012 | 44/F | S1–S3/NS | GTR/RT | 9 | NED | + | NA | Iran |
| Zhu et al. [21] | 2012 | 46/M | C3–C6/EES | STR/RT/CT | 12 | DOD | + | NA | China |
| Zhu et al. [21] | 2012 | 27/M | C1–C4/EES | GTR/RT/CT | 10 | NED | + | NA | China |
| Zhu et al. [21] | 2012 | 27/M | C7/EES | GTR/RT/CT | 24 | NED | + | NA | China |
| Zhu et al. [21] | 2012 | 24y/M | C5/EES | STR/RT/CT | 7 | DOD | + | NA | China |
| Kazanci et al. [12] | 2015 | 34/F | T4–T6/EES | GTR/RT/CT | 18 | NED | + | NA | Turkey |
| Kazanci et al. [12] | 2015 | 65/F | T7-T8/EES | GTR/RT/CT | 14 | NED | + | NA | Turkey |
| García-Moreno et al. [47] | 2015 | 45/F | C6-T3/EES | STR/RT/CT | 8 | NED | + | + | Spain |
| Present case | 2015 | 40/M | T10–T12/EES | GTR/RT/CT | 12 | NED | NA | + | USA |
M: male. F: female. EES: extraskeletal Ewing's sarcoma. PNET: peripheral neuroectodermal tumor. GTR: gross total resection. STR: subtotal (partial) resection. RT: radiotherapy. CT: chemotherapy. NED: no evidence of disease. DOD: dead of disease. NS: not specified.
∗Country: the country where the cases were reported and studied.
The review of the literature on combined pediatric and adult spinal EES/pPNET showed that the lumbar region is the most common site, followed by the thoracic and cervical spine, with the sacral region being the least common (5% of cases) [7, 12]. However, in our analysis of the 46 adult epidural EES/pPNET cases (including our case), we found that the most common site was the thoracic spine (17 cases), followed by lumbar (13 cases), cervical (13 cases), and sacral segments (3 cases).
Spinal EES in adults shows a predilection for males (61% of the cases), with a male : female ratio of 1.6 : 1, similar to that of OES [13, 14]. The average age at diagnosis was 29 years in contrast to 12 years for OES, and the oldest age reported was 65 years [12]. Interestingly, although it is stated that ES is rare in the Asian population [13, 14], we have found that half of the spinal EES patients in our study are Asians. However, no comprehensive epidemiological conclusions can be extracted due to the paucity of cases reported in the literature.
The mean diagnostic delay calculated from the previous cases is 4.5 months [7], which is explained by nonspecific symptoms at disease onset. The symptoms commonly include back and/or radicular pain in all patients, paresis in about 70%, sensory disturbances in 35%, and to lesser extent bladder and bowel dysfunction in about 12% of patients [7, 15]. One case presented with infection superimposed upon spinal epidural EES [16]. Distant metastases occurred in nearly 40% of the cases, either during or after diagnosis. Lung, spine, and brain were the most frequent sites of metastasis [7].
3.2. Histopathology
Ewing's sarcoma shows vague lobular proliferation of uniform small round blue cells with clear to lightly eosinophilic cytoplasm, evenly dispersed chromatin, and indistinct nucleoli. Peripheral PNET may specifically contain neuroblastic pseudorosettes termed Homer-Wright rosettes [3, 15, 17].
Immunohistochemical studies show that EES/PNETs strongly express cell surface glycoprotein CD99 (MIC2). This biomarker is considered one of the most accurate diagnostic tools and is positive in more than 90% of EES/pPNET cases. However, it is not exclusively specific for these tumors [18].
Approximately 25% of EES/pPNETs demonstrate aberrant expression of keratins, typically considered an epithelial marker. Expression of at least two different neuroglial antigens, such as neuron-specific enolase (NSE), protein S100, chromogranin, or synaptophysin, is required to distinguish pPNET from ES, with the former typically showing more neuronal differentiation [3, 4, 6, 15, 17, 19]. The tumor of the patient presented here was negative for S100, chromogranin, and synaptophysin, suggesting that the EES diagnosis was favored over pPNET.
At the genetic level, more than 90% of ES/pPNETs contain the same t(11;22)(q24;q12) translocation. Other translocations occur in 5–10% of cases [13]. The t(11;22)(q24;q12) translocation results in the formation of a chimeric gene (EWSR1-FLI1), which has been found to act as an oncogenic transcription factor in ES and pPNET [5, 6, 20]. This translocation can be detected by fluorescent in situ hybridization (FISH) in the nuclei of neoplastic cells. In its latest guidelines, ESMO (European Society for Medical Oncology) recommends that molecular studies be done to confirm the diagnosis of ESFTs through detection of this stereotypical translocation by FISH or RT-PCR [13].
3.3. Imaging Studies
Imaging studies are quintessential in such cases. MRI plays a prominent role in diagnosis, determination of the anatomic relationships with surrounding structures, and preoperative surgical planning. Commonly, EES/pPNET tumors have hypo- or isointense signal on T1-weighted imaging and a hyperintense signal on T2-weighted imaging and enhance heterogeneously. However, these MRI findings are nonspecific. In 15 case reports documenting MRI features of spinal epidural EES, the tumors were dumbbell-shaped and extended from the central canal toward widened foramina. In 3 cases, scalloping of bone was seen [20, 21].
Some reports suggest that the combination of FDG-PET with conventional imaging is a superior and valuable tool for disease staging and detecting metastases [22, 23].
In a recent study, O'Neill et al. proposed the concept of targeted imaging, using 64Cu-radiolabeled anti-CD99 antibody to detect these tumors and potential metastases. They found higher sensitivity with this approach as compared to FDG-PET in preclinical models [24].
3.4. Treatment and Prognosis
Surgical intervention is considered the primary and main approach in the management of these cases, particularly to relieve cord compression symptoms, as well as for cytoreductive purposes. Our analysis of previous cases showed that gross total resection (GTR) correlates with a much better outcome and decrease in recurrence rate than subtotal (partial) resection. Of the reported 46 cases in this paper, 45% had a subtotal resection (STR), while 55% of the cases had a GTR. Although partial resection has an increased risk of recurrence, complete resection is often precluded by tumor infiltration to the surrounding neural and paraspinal tissues.
Evidence from the literature strongly supports the use of local RT and systemic chemotherapy for treatment of EES/pPNET. Chemotherapy regimens for OES are often followed in adults with EES/pPNET. In the past, a traditional regimen was VACA (vincristine, actinomycin, cyclophosphamide, and/or doxorubicin). The addition of ifosfamide and/or etoposide to that regimen was the subject of many studies. In 1998, Ferrari et al. reported that ifosfamide/etoposide added to the induction, and maintenance phase of chemotherapy along with VACA resulted in a significantly better outcome in terms of histologic response and overall survival (OS) [25]. Other studies showed that adding ifosfamide and/or etoposide resulted in significant higher 5-year progression-free survival (PFS) and OS in nonmetastatic ESFTs. However, it did not improve outcomes in metastatic cases [15, 26].
Currently, the guidelines for treatment of ESFTs consider VAC/IE as the preferred first-line regimen for localized disease, concurrently with radiotherapy (45 Gy in 25 fractions). Regimens such as VAdriaC (vincristine, adriamycin, and cyclophosphamide) are used to treat metastatic disease [27]. Most of the recent adult spinal epidural EES/pPNET cases we reviewed followed such protocols postoperatively. We found that neoadjuvant chemotherapy was not used in any of these cases, despite its frequent use in the treatment of OES and other forms of EES. We postulate that neoadjuvant therapy may be of limited use in spinal epidural EES, due to the superior need for surgical decompression of the spinal cord.
Patients who underwent combined chemoradiotherapy after GTR or STR had better 1-year survival rates than patients treated with surgery, chemotherapy, or radiotherapy alone (88% versus 70%, resp.) [7]. In our study, 34 (74%) patients received combined chemoradiotherapy after GTR or STR; 2 (4%) cases underwent surgery only while chemotherapy and radiotherapy were given alone after surgery to 6 (13%) and 4 (9%) cases, respectively.
The prognosis of adult spinal epidural EES/pPNET is poor compared to OES. A study at Dana-Farber Cancer Center concluded that age plays an important prognostic factor, as survival rates are reduced in older adults. Also, primary extraosseous tumor and metastatic disease at diagnosis were adverse prognostic factors, even though both chemotherapy and radiotherapy were administered to patients with those three risk factors [28]. Another study reported that the 2-year survival rate in all spinal EES/pPNET cases was only 50% [7]. Furthermore, the 5-year survival rate in spinal epidural EES/pPNET is considered poor compared to other malignancies within the ESFT family. The 5-year survival rate of EES has been between 38% and 67%; however, the 5-year survival rate in spinal EES/pPNET ranged between 0 and 37.5% [20] (see also the follow-up and outcome data in Table 1).
4. Conclusions
Although primary spinal epidural EES/pPNET in adults is extremely rare, it should be considered in the differential diagnosis of patients with a history of nonspecific back pain and/or radicular pain, especially if accompanied by abnormal neurological examination and an epidural mass on MRI. The disease has an aggressive course, as evidenced by a high incidence of metastases and low survival rates reported in the literature. Early recognition of the disease entity and definitive management is essential. A multidisciplinary approach is the best strategy to manage epidural EES/pPNET, with surgical excision often being the initial intervention, due to neurological symptoms arising from spinal cord compression. Surgical resection should be followed by a combination of adjuvant chemotherapy and radiotherapy to improve overall outcome.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Esiashvili N., Goodman M., Marcus R. B., Jr. Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: surveillance epidemiology and end results data. Journal of Pediatric Hematology/Oncology. 2008;30(6):425–430. doi: 10.1097/mph.0b013e31816e22f3. [DOI] [PubMed] [Google Scholar]
- 2.Riley R. D., Burchill S. A., Abrams K. R., et al. A systematic review of molecular and biological markers in tumours of the Ewing's sarcoma family. European Journal of Cancer. 2003;39(1):19–30. doi: 10.1016/S0959-8049(02)00500-2. [DOI] [PubMed] [Google Scholar]
- 3.Dorfmüller G., Würtz F. G., Umschaden H. W., Kleinert R., Ambros P. F. Intraspinal primitive neuroectodermal tumour: report of two cases and review of the literature. Acta Neurochirurgica. 1999;141(11):1169–1175. doi: 10.1007/s007010050414. [DOI] [PubMed] [Google Scholar]
- 4.Duan X. H., Ban X. H., Liu B., et al. Intraspinal primitive neuroectodermal tumor: imaging findings in six cases. European Journal of Radiology. 2011;80(2):426–431. doi: 10.1016/j.ejrad.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 5.Isefuku S., Seki M., Tajino T., et al. Ewing's sarcoma in the spinal nerve root: a case report and review of the literature. Tohoku Journal of Experimental Medicine. 2006;209(4):369–377. doi: 10.1620/tjem.209.369. [DOI] [PubMed] [Google Scholar]
- 6.Machado I., López-Guerrero J. A., Llombart-Bosch A. Biomarkers in the Ewing sarcoma family of tumors. Current Biomarker Findings. 2014;4:81–92. [Google Scholar]
- 7.Saeedinia S., Nouri M., Alimohammadi M., Moradi H., Amirjamshidi A. Primary spinal extradural Ewing's sarcoma (primitive neuroectodermal tumor): report of a case and meta-analysis of the reported cases in the literature. Surgical Neurology International. 2012;3, article 55 doi: 10.4103/2152-7806.96154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ozturk E., Mutlu H., Sonmez G., Vardar Aker F., Cinar Basekim C., Kizilkaya E. Spinal epidural extraskeletal Ewing sarcoma. Journal of Neuroradiology. 2007;34(1):63–67. doi: 10.1016/j.neurad.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Theeler B. J., Keylock J., Yoest S., Forouhar M. Ewing's sarcoma family tumors mimicking primary central nervous system neoplasms. Journal of the Neurological Sciences. 2009;284(1-2):186–189. doi: 10.1016/j.jns.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 10.Tefft M., Vawter G. F., Mitus A. Paravertebral ‘round cell’ tumors in children. Radiology. 1969;92(7):1501–1509. doi: 10.1148/92.7.1501. [DOI] [PubMed] [Google Scholar]
- 11.Angervall L., Enzinger F. M. Extraskeletal neoplasm resembling Ewing's sarcoma. Cancer. 1975;36(1):240–251. doi: 10.1002/1097-0142(197507)36:1<240::aid-cncr2820360127>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Kazanci A., Gurcan O., Gurcay A. G., Senturk S., Yildirim A. E., Kilicaslan A., et al. Primary ewing sarcoma in spinal epidural space: report of three cases and review of the literature. Primer Spinal Epidural Ewing Sarkoma. 2015;32(1):250–261. [Google Scholar]
- 13.Group ESESNW. Bone sarcomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2014;25(supplement 3):iii113–iii123. doi: 10.1093/annonc/mdu256. [DOI] [PubMed] [Google Scholar]
- 14.Maheshwari A. V., Cheng E. Y. Ewing sarcoma family of tumors. Journal of the American Academy of Orthopaedic Surgeons. 2010;18(2):97–107. doi: 10.5435/00124635-201002000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Mukhopadhyay P., Gairola M., Sharma M. C., Thulkar S., Julka P. K., Rath G. K. Primary spinal epidural extraosseous Ewing's sarcoma: report of five cases and literature review. Australasian Radiology. 2001;45(3):372–379. doi: 10.1046/j.1440-1673.2001.00942.x. [DOI] [PubMed] [Google Scholar]
- 16.Lakhdar F., Gana R., Laghmari M., Moufid F., Maaqili R., Bellakhdar F. Infected cervical epidural Ewing's sarcoma (case report) Journal of Neuroradiology. 2008;35(1):51–55. doi: 10.1016/j.neurad.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt D., Herrmann C., Jurgens H., Harms D. Malignant peripheral neuroectodermal tumor and its necessary distinction from Ewing's sarcoma: a report from the Kiel Pediatric Tumor Registry. Cancer. 1991;68(10):2251–2259. doi: 10.1002/1097-0142(19911115)68:10<2251::aid-cncr2820681025>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.Olsen S. H., Thomas D. G., Lucas D. R. Cluster analysis of immunohistochemical profiles in synovial sarcoma, malignant peripheral nerve sheath tumor, and Ewing sarcoma. Modern Pathology. 2006;19(5):659–668. doi: 10.1038/modpathol.3800569. [DOI] [PubMed] [Google Scholar]
- 19.Jingyu C., Jinning S., Hui M., Hua F. Intraspinal primitive neuroectodermal tumors: report of four cases and review of the literature. Neurology India. 2009;57(5):661–668. doi: 10.4103/0028-3886.57804. [DOI] [PubMed] [Google Scholar]
- 20.Yasuda T., Suzuki K., Kanamori M., et al. Extraskeletal Ewing's sarcoma of the thoracic epidural space: case report and review of the literature. Oncology Reports. 2011;26(3):711–715. doi: 10.3892/or.2011.1326. [DOI] [PubMed] [Google Scholar]
- 21.Zhu Q., Zhang J., Xiao J. Primary dumbbell-shaped Ewing's sarcoma of the cervical vertebra in adults: four case reports and literature review. Oncology Letters. 2012;3(3):721–725. doi: 10.3892/ol.2012.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawkins D. S., Schuetze S. M., Butrynski J. E., et al. [18F]fluorodeoxyglucose positron emission tomography predicts outcome for ewing sarcoma family of tumors. Journal of Clinical Oncology. 2005;23(34):8828–8834. doi: 10.1200/JCO.2005.01.7079. [DOI] [PubMed] [Google Scholar]
- 23.Treglia G., Salsano M., Stefanelli A., Mattoli M. V., Giordano A., Bonomo L. Diagnostic accuracy of 18F-FDG-PET and PET/CT in patients with Ewing sarcoma family tumours: a systematic review and a meta-analysis. Skeletal Radiology. 2012;41(3):249–256. doi: 10.1007/s00256-011-1298-9. [DOI] [PubMed] [Google Scholar]
- 24.O'Neill A. F., Dearling J. L., Wang Y., et al. Targeted imaging of ewing sarcoma in preclinical models using a 64Cu-labeled anti-CD99 antibody. Clinical Cancer Research. 2014;20(3):678–687. doi: 10.1158/1078-0432.ccr-13-1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferrari S., Mercuri M., Rosito P., et al. Ifosfamide and actinomycin-D, added in the induction phase to vincristine, cyclophosphamide and doxorubicin, improve histologic response and prognosis in patients with non metastatic Ewing's sarcoma of the extremity. Journal of Chemotherapy. 1998;10(6):484–491. doi: 10.1179/joc.1998.10.6.484. [DOI] [PubMed] [Google Scholar]
- 26.Meyer W. H., Kun L., Marina N., et al. Ifosfamide plus etoposide in newly diagnosed Ewing's sarcoma of bone. Journal of Clinical Oncology. 1992;10(11):1737–1742. doi: 10.1200/JCO.1992.10.11.1737. [DOI] [PubMed] [Google Scholar]
- 27.Miser J. S., Krailo M. D., Tarbell N. J., et al. Treatment of metastatic Ewing's sarcoma or primitive neuroectodermal tumor of bone: evaluation of combination ifosfamide and etoposide—a children's cancer group and pediatric oncology group study. Journal of Clinical Oncology. 2004;22(14):2873–2876. doi: 10.1200/jco.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 28.Baldini E. H., Demetri G. D., Fletcher C. D. M., Foran J., Marcus K. C., Singer S. Adults with Ewing's sarcoma/primitive neuroectodermal tumor: adverse effect of older age and primary extraosseous disease on outcome. Annals of Surgery. 1999;230(1):79–86. doi: 10.1097/00000658-199907000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheithauer B. W., Egbert B. M. Ewing's sarcoma of the spinal epidural space: report of two cases. Journal of Neurology, Neurosurgery & Psychiatry. 1978;41(11):1031–1035. doi: 10.1136/jnnp.41.11.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahoney J. P., Ballinger W. E., Jr., Alexander R. W. So-called extraskeletal Ewing's sarcoma. Report of a case with ultrastructural analysis. American Journal of Clinical Pathology. 1978;70(6):926–931. doi: 10.1093/ajcp/70.6.926. [DOI] [PubMed] [Google Scholar]
- 31.Fink L. H., Meriwether M. W. Primary epidural Ewing's sarcoma presenting as a lumbar disc protrusion. Case report. Journal of Neurosurgery. 1979;51(1):120–123. doi: 10.3171/jns.1979.51.1.0120. [DOI] [PubMed] [Google Scholar]
- 32.N'Golet A., Pasquier B., Pasquier D., Lachard A., Couderc P. Extraskeletal Ewing's sarcoma of the epidural space. A report of two new cases with literature review. Archives d'Anatomie et de Cytologie Pathologiques. 1982;30(1):10–13. [PubMed] [Google Scholar]
- 33.Sharma B. S., Khosla V. K., Banerjee A. K. Primary spinal epidural Ewing's sarcoma. Clinical Neurology and Neurosurgery. 1986;88(4):299–302. doi: 10.1016/s0303-8467(86)80050-6. [DOI] [PubMed] [Google Scholar]
- 34.Liu H.-M., Yang W. C., Garcia R. L., Noh J. M., Malhotra V., Leeds N. E. Intraspinal primitive neuroectodermal tumor arising from the sacral spinal nerve root. The Journal of Computed Tomography. 1987;11(4):350–354. doi: 10.1016/0149-936x(87)90071-3. [DOI] [PubMed] [Google Scholar]
- 35.Christie D. R. H., Bilous A. M., Carr P. J. A. Diagnostic difficulties in extraosseous Ewing's sarcoma: a proposal for diagnostic criteria. Australasian Radiology. 1997;41(1):22–28. doi: 10.1111/j.1440-1673.1997.tb00463.x. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy J. G., Eustace S., Caulfield R., Fennelly D. J., Hurson B., O'Rourke K. S. Extraskeletal Ewing's sarcoma: a case report and review of the literature. Spine. 2000;25(15):1996–1999. doi: 10.1097/00007632-200008010-00022. [DOI] [PubMed] [Google Scholar]
- 37.Shin J. H., Lee H. K., Rhim S. C., Cho K.-J., Choi C. G., Suh D. C. Spinal epidural extraskeletal Ewing sarcoma: MR findings in two cases. American Journal of Neuroradiology. 2001;22(4):795–798. [PMC free article] [PubMed] [Google Scholar]
- 38.Morandi X., Riffaud L., Haegelen C., Lancien G., Kerbrat P., Guegan Y. Extraosseous Ewing's sarcoma of the spinal epidural space. Neurochirurgie. 2001;47(1):38–44. [PubMed] [Google Scholar]
- 39.Gandhi D., Goyal M., Belanger E., Modha A., Wolffe J., Miller W. Primary epidural Ewing's sarcomas: case report and review of literature. Canadian Association of Radiologists Journal. 2003;54(2):109–113. [PubMed] [Google Scholar]
- 40.Weber D. C., Rutz H. P., Lomax A. J., et al. First spinal axis segment irradiation with spot-scanning proton beam delivered in the treatment of a lumbar primitive neuroectodermal tumour. Clinical Oncology. 2004;16(5):326–331. doi: 10.1016/j.clon.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Koudelova J., Kunesova M., Koudela K., Jr., Matejka J., Novak P., Prausova J. Peripheral primitive neuroectodermal tumor—PNET. Acta Chirurgiae Orthopaedicae et Traumatologiae Cechoslovaca. 2006;73(1):39–44. [PubMed] [Google Scholar]
- 42.Bozkurt G., Ayhan S., Turk C. C., Akbay A., Soylemezoglu F., Palaoglu S. Primary extraosseous Ewing sarcoma of the cervical epidural space. Case illustration. Journal of Neurosurgery: Spine. 2007;6(2, article 192) doi: 10.3171/spi.2007.6.2.192. [DOI] [PubMed] [Google Scholar]
- 43.Feng J. F., Liang Y. M., Bao Y. H., Pan Y. H., Jiang J. Y. Multiple primary primitive neuroectodermal tumours within the spinal epidural space with non-concurrent onset. Journal of International Medical Research. 2008;36(2):366–370. doi: 10.1177/147323000803600222. [DOI] [PubMed] [Google Scholar]
- 44.Musahl V., Rihn J. A., Fumich F. E., Kang J. D. Sacral intraspinal extradural primitive neuroectodermal tumor. Spine Journal. 2008;8(6):1024–1029. doi: 10.1016/j.spinee.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 45.Kiatsoontorn K., Takami T., Ichinose T., et al. Primary epidural peripheral primitive neuroectodermal tumor of the thoracic spine—case report. Neurologia Medico-Chirurgica. 2009;49(11):542–545. doi: 10.2176/nmc.49.542. [DOI] [PubMed] [Google Scholar]
- 46.Bostelmann R. L. M., Steiger H. J., Eicker S., Cornelius J. F. Rapid progressive primary extraosseous Ewing sarcoma of the cervical intra- and epidural space. Proceedings of the 62nd Annual Meeting of the German Society of Neurosurgery (DGNC), Joint Meeting with the Polish Society of Neurosurgeons (PNCH); May 2011; Hamburg, Germany. [Google Scholar]
- 47.García-Moreno R., Bernal-García L. M., Pineda-Palomo M., Botana-Fernández M., Gilete-Tejero I. J., Cabezudo-Artero J. M. Epidural extraskeletal Ewing sarcoma. Case report and literature review. Neurocirugia. 2015;26(3):151–156. doi: 10.1016/j.neucir.2014.10.004. [DOI] [PubMed] [Google Scholar]