Abstract
In patients with peri-implant mucositis and peri-implantitis, what is the efficacy of nonsurgical (i.e. referring to peri-implant mucositis and peri-implantitis) and surgical (i.e. referring to peri-implantitis) treatments with alternative or adjunctive measures on changing signs of inflammation compared with conventional nonsurgical (i.e. mechanical/ultrasonic debridement) and surgical (i.e. open flap debridement) treatments alone? After electronic database and hand search, a total of 40 publications (reporting on 32 studies) were finally considered for the qualitative and quantitative assessment. The weighted mean changes (WM)/ and WM differences (WMD) were estimated for bleeding on probing scores (BOP) and probing pocket depths (PD) (random effect model). Peri-implant mucositis: WMD in BOP and PD reductions amounted to −8.16 % [SE = 4.61] and −0.15 mm [SE = 0.13], not favouring adjunctive antiseptics/antibiotics (local and systemic) over control measures (p > 0.05). Peri-implantitis (nonsurgical): WMD in BOP scores amounted to −23.12 % [SE = 4.81] and −16.53 % [SE = 4.41], favouring alternative measures (glycine powder air polishing, Er:YAG laser) for plaque removal and adjunctive local antibiotics over control measures (p < 0.001), respectively. Peri-implantitis (surgical): WMD in BOP and PD reductions did not favour alternative over control measures for surface decontamination. WM reductions following open flap surgery (±resective therapy) and adjunctive augmentative therapy amounted to 34.81 and 50.73 % for BOP and 1.75 and 2.20 mm for PD, respectively. While mechanical debridement alone was found to be effective for the management of peri-implant mucositis, alternative/adjunctive measures may improve the efficacy over/of conventional nonsurgical treatments at peri-implantitis sites. Adjunctive resective and/or augmentative measures are promising; however, their beneficial effect on the clinical outcome of surgical treatments needs to be further investigated.
Review
Background
Peri-implant mucositis describes an inflammatory lesion that resides in the soft tissues compartment, while at peri-implantitis sites, this lesion has extended and also affects the implant supporting bone [1]. The 11th European Workshop on Periodontology has pointed to an “estimated weighted mean prevalence of peri-implant mucositis and peri-implantitis of 43 and 22 %, respectively” [2].
The main etiology of peri-implant mucositis refers to plaque accumulation [3, 4], and the conversion from mucositis to peri-implantitis was, particularly in the absence of a supportive maintenance care [5], positively correlated with the function time [2]. However, the presence of some independent systemic/patient-related (i.e. smoking) and local (i.e. residual cement, dimension of the keratinized tissue, surface roughness) risk indicators may increase the probability of the disease occurring [3].
According to the cause-related concept of therapy, professionally administered plaque removal is a key strategy for the prevention and management of peri-implant diseases [6]. In previous years, several alternative or adjunctive measures (e.g. local antibiotics, air polishing, laser application) have been proposed to improve the efficacy of nonsurgical treatment approaches [7–9]. At peri-implantitis sites, surgical protocols may involve different decontamination protocols, that may also be combined with resective (e.g. pocket elimination, bone re-contouring, implantoplasty) and/or augmentative approaches (e.g. bone substitutes or autografts with or without a supporting barrier membrane) [7, 10]. Accordingly, there is a need to identify the most effective interventions for the treatment of peri-implant diseases.
The aim of this systematic review was therefore to address the following focused question: in patients with peri-implant mucositis and peri-implantitis, what is the efficacy of nonsurgical (i.e. referring to peri-implant mucositis and peri-implantitis) and surgical (i.e. referring to peri-implantitis) treatments with alternative or adjunctive measures on changing signs of inflammation compared with conventional nonsurgical and surgical treatments alone?
Methods
This systematic review was structured and conducted according to the preferred reporting items of the PRISMA statement [11].
Focused question
The focused question serving for literature search was structured according to the PICO format [12]: “In patients with peri-implant mucositis and peri-implantitis, what is the efficacy of nonsurgical (i.e. referring to peri-implant mucositis and peri-implantitis) and surgical (i.e. referring to peri-implantitis) treatments with alternative or adjunctive measures on changing signs of inflammation compared with conventional nonsurgical and surgical treatments alone?”.
Population: patients with peri-implant mucositis and peri-implantitis based on case definitions used in respective publications
Intervention: alternative or adjunctive measures to nonsurgical and surgical treatments
Comparison: conventional measures for nonsurgical and surgical treatments
Outcome: changes in peri-implant mucosal inflammation
Search strategy
The PubMed database of the U.S. National Library of Medicine and the Web of Knowledge of Thomson Reuters were used as electronic databases to perform a systematic search for relevant articles published in the dental literature between 1992 up to April 30, 2015. A commercially available software program (Endnote X7, Thomson, London, UK) was used for electronic title management. Screening was performed independently by two authors (F.S. and A.S.). Disagreement regarding inclusion during the first and second stage of study selection was resolved by discussion.
The combination of key words (i.e. Medical Subject Headings MeSH) and free text terms included:
“treatment” OR “nonsurgical treatment” OR “non-surgical treatment” OR “surgical treatment” OR “regenerative treatment” OR “augmentative treatment” OR “resective treatment” OR “reconstructive treatment” OR “therapy” OR “nonsurgical therapy” OR “non-surgical therapy” OR “surgical therapy” OR “regenerative therapy” OR “augmentative therapy” OR “resective therapy” OR “reconstructive therapy” OR “antiseptic treatment” OR “antibiotic treatment” OR “adjunctive treatment” OR “antiseptic therapy” OR “antibiotic therapy” OR “adjunctive therapy”
AND
“peri-implant disease” OR “periimplant disease” OR “peri-implant infection” OR “periimplant infection” OR “mucositis” (MeSH) OR “peri-implant mucositis” OR “periimplant mucositis” OR “Periimplantitis” (MeSH) OR “peri-implantitis”.
Electronic search was complemented by a hand search of the following journals:
Clinical Implant Dentistry and Related Research; Clinical Oral Implants Research; International Journal of Oral and Maxillofacial Implants; Journal of Clinical Periodontology; Journal of Periodontology. Finally, the references of all selected full-text articles and related reviews were scanned. If required, the corresponding authors were contacted and requested to provide missing data or information.
Study inclusion and exclusion criteria
During the first stage of study selection, the titles and abstracts were screened and evaluated according to the following inclusion criteria:
English language.
Prospective randomized controlled (RCT), or non-randomized controlled (CCT) studies (split-mouth or parallel group designs) in humans comparing alternative (i.e. for biofilm removal) or adjunctive measures (i.e. for biofilm removal, or adjunctive antiseptic/antibiotic therapy, or regenerative/resective approaches) to conventional nonsurgical (i.e. mechanical/ultrasonic debridement) or surgical (i.e. open flap debridement) treatments.
Data on the clinical changes in mucosal inflammation (i.e. bleeding scores) and probing pocket depths (PD) following nonsurgical (referring to peri-implant mucositis and peri-implantitis) or surgical (referring to peri-implantitis) treatments in respective groups.
At the second stage of selection, all full-text articles identified during the first stage were acquired. During this procedure, the pre-selected publications were evaluated according to the following exclusion criteria:
Inclusion of less than five patients
Inadequate case definition [13]
Lack of clinical data on the changes in mucosal inflammation and PD
Quality assessment of selected studies
A quality assessment of all selected full-text articles was performed according to the Cochrane collaborations tool for assessing risk of bias (low, high, unclear) including the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and incomplete outcome data [14]. Quality assessment was performed in two different phases. In particular, during phase I, quality assessment was based on the published full-text article performed independently by both authors. In phase II, disagreements were resolved by discussion.
Data extraction and method of analysis
A data extraction template was generated and based on the study design, population, case definition, observation period, interventions, comparisons, and primary and secondary outcomes as well as the study quality. For data analysis, the bleeding index (BI)/bleeding on probing (BOP) and PD scores after respective healing periods were defined as primary outcomes. Secondary outcomes included changes in marginal bone levels, immunological/microbiological parameters and the resolution of peri-implant diseases, based on case definitions used in respective publications. RCTs and CCTs not implementing appropriate control measures but reporting on changes in primary outcomes were used for the estimation of the overall efficacy of treatments.
Heterogeneity between RCTs, meta-analysis (i.e. weighted mean changes/differences and 95 % confidence intervals, random effect model to account for potential methodological differences between studies), forest plots and publication bias (Egger’s regression to quantify the bias captured by funnel plots) were assessed using a commercially available software program (Comprehensive Meta-Analysis V2, Biostat, Englewood, NJ 07631 USA).
Results
Study selection
A total of 368 potentially relevant titles and abstracts were found during the electronic and manual search. During the first stage of study selection, 319 publications were excluded based on title and abstract. For the second phase, the complete full-text articles of the remaining 49 publications were thoroughly evaluated. A total of 19 papers had to be excluded at this stage because they did not fulfil the inclusion criteria of the present systematic review (Table 1).
Table 1.
Excluded clinical studies at the second stage of selection and the reason for exclusion
| Publication | Reason for exclusion |
|---|---|
| Lavigne et al. [63] | Experimental sites without BOP at baseline |
| Ciancio et al. [64] | Homecare plaque control protocol |
| Felo et al. [65] | Homecare plaque control protocol |
| Bach et al. [66] | Lack of clinical data defined for the present systematic review |
| Dörtbudak et al. [67] | Lack of a conventional control treatment |
| Khoury and Buchmann [68] | Changes in mucosal inflammation not reported |
| Roos-Jansaker et al. [69] | Observational study |
| Duarte et al. [70] | Observational study reporting on the same study population as [71] |
| Maximo et al. [71] | Observational study |
| Ramberg et al. [72] | Homecare plaque control protocol |
| Corbella et al. [73] | Prevention of peri-implant diseases |
| Heitz-Mayfield et al. [74] | Homecare plaque control protocol |
| Costa et al. [5] | Observational study |
| De Angelis et al. [75] | Lack of subgroup analyses |
| Salvi et al. [76] | Observational study |
| Deppe et al. [59] | Observational study |
| De Siena et al. [77] | Homecare plaque control protocol |
| McKenna et al. [78] | Case definition not reported |
| Flichy-Fernandez et al. [79] | Cross-over study design |
Finally, a total of 40 publications (reporting on 32 studies) were considered for the qualitative and quantitative assessment (Fig. 1).
Fig. 1.
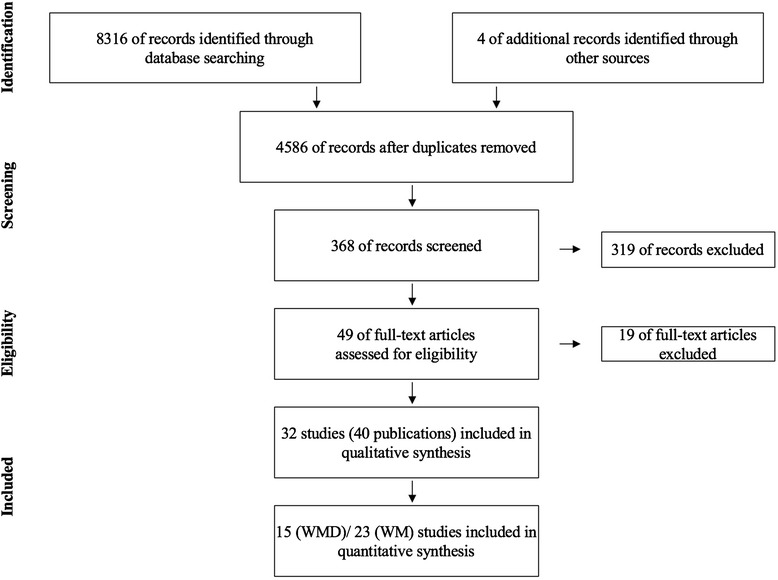
Flow diagram of literature search and inclusion
Quality and risk of bias assessment of selected studies
The review author’s judgement about each risk of bias item for each included RCT is presented in Table 2. In particular, the percentages across all included studies for high, low and unclear risk of bias items were 34.1, 54.8 and 11.1 %, respectively (Table 2).
Table 2.
Risk of bias summary for finally selected randomized studies
| Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | |
|---|---|---|---|---|---|
| Schenk et al. [16] | ? | − | − | − | + |
| Strooker et al. [22] | ? | − | − | − | + |
| Porras et al. [20] | ? | − | − | + | − |
| Büchter et al. [26] | + | − | − | ? | + |
| Romeo et al. [42, 57] | ? | − | − | − | + |
| Karring et al. [28] | ? | + | − | + | + |
| Schwarz et al. [25] | + | − | − | + | + |
| Renvert et al. [32] | + | + | − | + | + |
| Schwarz et al. [24] | + | − | − | + | + |
| Schwarz et al. [48, 51, 54] | + | − | − | + | + |
| Renvert et al. [29] | + | + | − | + | + |
| Renvert et al. [31] | + | ? | − | + | + |
| Renvert et al. [30] | + | − | − | + | + |
| Sahm et al.; John et al. [27, 33] | + | − | − | + | + |
| Thone-Mühling et al. [17] | − | ? | − | − | + |
| Hallström et al. [19] | + | ? | − | − | + |
| Schwarz et al. [49, 50, 52] | + | − | − | + | + |
| Aghanzadeh et al. [43] | ? | ? | − | + | + |
| Machtei et al. [23] | + | + | ? | + | + |
| Wohlfahrt et al. [55, 58] | + | + | − | + | + |
| deWaal et al. [38] | + | + | ? | + | + |
| McKenna et al. [78] | + | − | + | + | − |
| Schär et al.; Bassetti et al. [34, 37] | + | − | − | + | |
| deWaal et al. [39] | + | + | ? | + | + |
| Ji et al. [15] | + | ? | − | + | + |
| Papadopoulos et al. [41] | + | − | − | + | + |
| Riben Grundström et al. [21] | + | + | − | + | + |
+ low, − high, ? unclear
Subdivision of selected studies
All selected publications were subdivided according to differences in the treatment protocol:
o Nonsurgical treatment of peri-implant mucositis—alternative or adjunctive measures for biofilm removal (2 RCTs and 1 CCT) (Table 3)
o Nonsurgical treatment of peri-implant mucositis—adjunctive antispectic therapy (3 RCTs) (Table 4)
o Nonsurgical treatment of peri-implant mucositis—adjunctive antibiotic therapy (2 RCTs) (Table 5).
o Nonsurgical treatment of peri-implantitis—alternative measures for biofilm removal (6 RCTs) (Table 6)
o Nonsurgical treatment of peri-implantitis—adjunctive antiseptic therapy (1 RCT) (Table 7)
o Nonsurgical treatment of peri-implantitis—adjunctive antibiotic therapy (4 RCTs) (Table 8)
o Surgical treatment of peri-implantitis—alternative measures for surface decontamination (3 RCTs and 1 CCT) (Table 9)
o Surgical treatment of peri-implantitis—adjunctive resective therapy (1 RCT) (Table 10)
o Surgical treatment of peri-implantitis—adjunctive augmentative therapy (4 RCTs, 4 CCTs) (Table 11)
Table 3.
Included studies—nonsurgical treatment of peri-implant mucositis: alternative or adjunctive measures for biofilm removal
| Publication | Design | Population | Case definition | Period | Test | Control | Mean (SD) outcome |
|---|---|---|---|---|---|---|---|
| Ji et al. [15] | RCT, parallel | 24 patients | PD ≥4 mm, BOP + no radiographic bone loss compared with baseline (i.e. immediately after prosthesis installation) | 3 months | OHI + mechanical debridement (ultrasonic scaler with carbon fibre tips) + air abrasive device, glycine powder (sites with PD ≥4 mm) | OHI + mechanical debridement (ultrasonic scaler with carbon fibre tips) | Test |
| 33 implants | BI: 1.4 (0.57) (BL) to 1.1 (0.58) (3 months, subject level) | ||||||
| Molar/premolar sites | BI: 1.7 (0.93) (BL) to 1.1 (0.98) (3 months, implant level) | ||||||
| 1 implant system | Sites without bleeding: 29.3 % | ||||||
| PD: 3.6 (0.47) (BL) to 3.2 (0.48) mm (3 months, subject level) | |||||||
| Control | |||||||
| BI: 1.5 (0.65) (BL) to 1.0 (0.85) (3 months, subject level) | |||||||
| BI: 1.7 (1.0) (BL) to 0.9 (1.1) (3 months, implant level) | |||||||
| Sites without bleeding: 42 % | |||||||
| PD: 3.5 (0.5) (BL) to 3.1 (0.38) mm (3 months, subject level) | |||||||
| De Siena et al. [18] | CCT, parallel | 30 patients | BOP or spontaneous bleeding with local swelling | 6 months | OHI + mechanical debridement Teflon curettes, polishing) + air abrasive device, glycine powder | OHI + mechanical debridement Teflon curettes, polishing) | Test |
| No information on number and types of implants | PD ≤3.5 mm | PD: 3.0 (0.4) (BL) to 2.4 (0.5) mm (6 months, subject level) | |||||
| Bone loss ≤ 3.0 mm | 13 patients did not present bleeding at 6 months | ||||||
| Control | |||||||
| PD: 2.9 (0.4) (BL) to 3.0 (0.6) mm (6 months, subject level) | |||||||
| 9 patients did not present bleeding at 6 months | |||||||
| BI and PD scores sign. lower in the test group at 6 months | |||||||
| Riben Grundström et al. [21] | RCT, parallel | 37 patients | PD ≥4 mm, BOP + with or without suppuration | 12 months | OHI + air abrasive device, glycine powder | OHI + mechanical debridement (ultrasonic scaler with plastic coated tips) | Test |
| One implant per subject used | Bone loss ≤2 mm from implant shoulder | Repeated treatment at 3 and 6 months | Repeated treatment at 3 and 6 months | BOP: 43.9 (7.3) (BL) to 12.1 (3.8) % (12 months, subject level) | |||
| Test N = 19 | Number of diseased sites (pocket depth ≥4 mm with bleeding/suppuration) | ||||||
| Control N = 18 | 38 % (BL) to 8 % (12 months, subject level) | ||||||
| Control | |||||||
| BOP: 53.7 (7.9) (BL) to 18.6 (6.4) % (12 months, subject level) | |||||||
| Number of diseased sites (pocket depth ≥4 mm with bleeding/suppuration) | |||||||
| 52 % (BL) to 17 % (12 months, subject level) | |||||||
| No significant differences between groups for either reduction of | |||||||
| BOP or of diseased sites |
BI bleeding index, BL baseline, BOP bleeding on probing, CCT non-randomized controlled clinical study, OHI oral hygiene instructions, PD probing pocket depth, RCT randomized controlled clinical study
Table 4.
Included studies—nonsurgical treatment of peri-implant mucositis: adjunctive antiseptic therapy
| Publication | Design | Population | Case definition | Period | Test | Control | Mean (SD) outcome |
|---|---|---|---|---|---|---|---|
| Strooker et al. [22] | RCT Split-mouth design | 16 patients each with 4 mandibular implants and bar retained over denture | Not reported | 5 months | Supra-/subgingival scaling (carbon curettes) + polishing (rubber cup) + phosphoric acid gel (35 %) in sulcus for 1 min | Supra-/subgingival scaling (carbon curettes) + polishing (rubber cup) Once every month | Test (subject level) |
| Once every month | BOP: 30.5 (27.5) (BL) to 9.7 (10.97) % (5 months) | ||||||
| GI: 0.92 (0.75) (BL) to 0.34 (0.38) (5 months) | |||||||
| PD: 2.97 (0.68) (BL) to 2.34 (0.54) mm (5 months) | |||||||
| Control (subject level) | |||||||
| BOP: 29.2 (29.44) (BL) to 14.3 (22.47) % (5 months) | |||||||
| GI: 0.82 (0.8) (BL) to 0.57 (0.6) (5 months) | |||||||
| PD: 2.83 (0.57) (BL) to 2.48 (0.49) mm (5 months) | |||||||
| Sign. between group difference in mean GI values and colony-forming units at 5 months | |||||||
| Porras et al. [20] | RCT, parallel | 16 patients | Supra- and subgingival plaque | 3 months | OHI + mechanical cleansing (plastic scaler, rubber cups, polishing paste) + local irrigation CHX (0.12 %) and topical CHX gel application + 0.12 % CHX mouthrinse twice for 10 days | OHI + mechanical cleansing (plastic scaler, rubber cups, polishing paste) | mBI and BOP (%) scores: no sign. differences between groups at 1 and 3 months |
| 28 implants | PD ≤5 mm BOP + “incipient” radiographic lesion | PD values: | |||||
| 3 implant types (plasma-sprayed Ti/cp Ti (HA-coated Ti) | Test: 3.27 (0.81) (BL) to 2.71 (0.70) mm (3 months) | ||||||
| Control: 3.48 (0.61) (BL) to 2.55 (0.72) mm (3 months) | |||||||
| Changes in mean PD between test and control groups at 3 months were statistically significant (0.56 vs. 0.93 mm) | |||||||
| Microbiological improvements in both groups | |||||||
| Thone-Mühling et al. [17] | RCT, parallel | 11 patients | BOP + and/or GI ≥1 absence of radiographic bone loss during the last 2 years | 8 months | OHI + mechanical cleansing (plastic scaler and polyetheretherketone-coated ultrasonic instruments) + topical CHX gel application once + full mouth disinfection (deep scaling in one session + CHX disinfection of tongue and tonsils) + 0.2 % CHX mouthrinse 2×/day and tonsil spraying 1×/day for 14 days | OHI + mechanical cleansing (plastic scaler and polyetheretherketone-coated ultrasonic instruments) + full mouth scaling in one session | Test |
| 36 implants | BOP: 0.22 (0.11) (BL) to 0.16 (0.09) % (8 months) | ||||||
| 2 implant types | GI: 0.6 (0.24) (BL) to 0.44 (0.23) (8 months) | ||||||
| PD: 3.4 (0.68) (BL) to 2.82 (0.59) mm (8 months) | |||||||
| Control | |||||||
| BOP: 0.17 (0.19) (BL) to 0.17 (0.11) % (8 months) | |||||||
| GI: 0.62 (0.36) (BL) to 0.43 (0.37) (8 months) | |||||||
| PD: 3.49 (0.78) (BL) to 2.84 (0.64) mm (8 months) | |||||||
| Bacterial recolonization over time |
BL baseline, BOP bleeding on probing, GI modified gingival index, mBI modified bleeding index, OHI oral hygiene instructions, PD probing pocket depth, RCT randomized controlled clinical study
Table 5.
Included studies—nonsurgical treatment of peri-implant mucositis: adjunctive antibiotic therapy
| Publication | Design | Population | Case definition | Period | Test | Control | Mean (SD) outcome |
|---|---|---|---|---|---|---|---|
| Schenk et al. [16] | RCT Split-mouth design | 8 patients | PD >4 mm BOP on at least 1 site per implant ± mucosal hyperplasia no radiographic bone loss | 3 months | Supra-/subgingival scaling (steel curettes) + polishing (rubber cup) + locally delivered tetracycline HCl (25 %) fibre for 10 days +0.2 % CHX mouthrinse twice for 10 days | Supra-/subgingival scaling (steel curettes) + polishing (rubber cup) + +0.2 % CHX mouthrinse twice for 10 days | ΔBOP (3 months, subject level) |
| 24 implants | Test: −17 ± 25 % | ||||||
| 1 implant type (endossous part: titanium and zirconoxide/transmucosal part: titanium oxinitride) | Control: 15 ± 37 % | ||||||
| PD/CAL values without significant changes in both groups | |||||||
| No adverse events | |||||||
| Partial/complete fibre loss at three sites | |||||||
| Hallström et al. [19] | RCT, parallel | 45 patients | PD ≥4 mm BOP + and/or pus | 6 months | OHI + mechanical cleansing (titanium curettes + rubber cups + polishing paste) + Azithromycin® 500 mg day 1 and 250 mg days 2–4 | OHI + mechanical cleansing (titanium curettes + rubber cups + polishing paste) | Test |
| 3 implant systems | Radiographic bone loss ≤2 mm | BOP: 82.6 (24.4) (BL) to 27.3 (18.8) % (6 months, subject level) | |||||
| PD at worst site: 5.5 (0.8) (BL) to 4.1 (1.2) mm (6 months, subject level) | |||||||
| Control | |||||||
| BOP: 80.0 (25.0) (BL) to 47.5 (32.3) % (6 months, subject level) | |||||||
| PD at worst site: 5.7 (0.8) (BL) to 4.9 (1.1) mm (6 months, subject level) | |||||||
| Odds ratio of a positive treatment outcome (PD ≤ 4.0 mm and BOP ≤ 1) was 4.5:1 (test vs. control) | |||||||
| Comparable reductions in bacterial counts |
BL baseline, BOP bleeding on probing, CAL clinical attachment level, OHI oral hygiene instructions, PD probing pocket depth, RCT randomized controlled clinical study
Table 6.
Included studies—nonsurgical treatment of peri-implantitis: alternative measures for biofilm removal
| Publication | Design | Population | Case definition | Period | Test | Control | Mean (SD) outcome |
|---|---|---|---|---|---|---|---|
| Karring et al. [28] | 11 patients | PD ≥5 mm, BOP + bone loss >1.5 mm and exposed threads | 3 months | OHI + ultrasonic device with hydroxyapatite fluid polish | OHI + mechanical debridement (carbon fibre curettes) | Test | |
| 22 implants machined and medium-rough surfaces | BOP: 63.6 (BL) to 36.4 % (3 months, subject level) | ||||||
| PD: 5.8 (1.1) (BL) to 5.8 (1.2) mm (3 months, subject level) | |||||||
| Radiographic bone level changes: 0.1 (0.5) mm (3 months, subject level) | |||||||
| Control | |||||||
| BOP: 72.7 (BL) to 81.8 % (3 months, subject level) | |||||||
| PD: 6.2 (1.6) (BL) to 6.3 (2.2) mm (3 months, subject level) | |||||||
| Radiographic bone level changes: −0.2 (1.2) mm (3 months, subject level) | |||||||
| Absence of BOP at 7/11 (test) and 2/11 (control) sites | |||||||
| Schwarz et al. [25] | RCT, parallel | 20 patients | PD ≥4 mm, BOP + and pus | 6 months | OHI + Er:YAG laser device (cone-shaped glass fibre tip) at 12.7 J/cm2 | OHI + mechanical debridement (plastic curettes), 0.2 % CHX pocket irrigation and 0.2 % CHX gel | Test |
| 32 implants rough and medium-rough surfaces | Radiographic bone loss | BOP: 83.2 (17.2) (BL) to 31.1 (10.1) % (6 months, subject level) | |||||
| PD: 5.4 (1.2) (BL) to 4.6 (1.1) mm (6 months, subject level) | |||||||
| Control | |||||||
| BOP: 81.3 (19.0) (BL) to 58.3 (16.9) % (6 months, subject level) | |||||||
| PD: 5.5 (1.5) (BL) to 4.8 (1.4) mm (6 months, subject level) | |||||||
| BOP scores at 6 months were significantly lower in the test group | |||||||
| Schwarz et al. [24] | RCT, parallel | 20 patients | PD ≥4 mm, BOP + and pus | 12 months | OHI + Er:YAG laser device (cone-shaped glass fibre tip) at 12.7 J/cm2 | OHI + mechanical debridement (plastic curettes), 0.2 % CHX pocket irrigation and 0.2 % CHX gel | Test |
| 40 implants rough and medium-rough surfaces | Radiographic bone loss | Moderately deep sites | |||||
| BOP: 81.7 (6.7) (BL) to 35.0 (6.3) % (12 months, subject level) | |||||||
| PD: 4.5 (1.4) (BL) to 4.0 (0.1) mm (12 months, subject level) | |||||||
| Deep sites | |||||||
| BOP: 79.9 (4.8) (BL) to 55.0 (6.5) % (12 months, subject level) | |||||||
| PD: 5.9 (0.1) (BL) to 5.4 (0.1) mm (12 months, subject level) | |||||||
| Control | |||||||
| Moderately deep sites | |||||||
| BOP: 81.6 (5.2) (BL) to 53.3 (7.3) % (12 months, subject level) | |||||||
| PD: 4.4 (0.2) (BL) to 4.3 (0.1) mm (12 months, subject level) | |||||||
| Deep sites | |||||||
| BOP: 88.3 (3.5) (BL) to 66.6 (5.5) % (12 months, subject level) | |||||||
| PD: 5.9 (0.3) (BL) to 5.5 (0.2) mm (12 months, subject level) | |||||||
| No significant differences between groups at 12 months | |||||||
| Renvert et al. [31] | RCT, parallel | 31 patients | PD ≥4 mm, BOP + and/or pus | 6 months | OHI + ultrasonic device with hydroxyapatite fluid polish | OHI + mechanical debridement (titanium curettes) | Test |
| 31 implants machined and medium-rough surfaces | Bone loss <2.5 mm | BI: 1.7 (0.6) (BL) to 1.2 (0.7) (6 months, subject level) | |||||
| PD: 4.3 (0.6) (BL) to 3.9 (0.8) mm (6 months, subject level) | |||||||
| Control | |||||||
| BI: 1.7 (0.9) (BL) to 1.4 (1.0) (6 months, subject level) | |||||||
| PD: 6.2 (1.6) (BL) to 6.3 (2.2) mm (6 months, subject level) | |||||||
| No significant differences between groups | |||||||
| Renvert et al. [30] | RCT, parallel | 42 patients | PD ≥5 mm, BOP + and/or pus | 6 months | OHI + air abrasive device, glycine powder | OHI + Er:YAG laser device (cone-shaped glass fibre tip, 12.7 J/cm2) | Test |
| 90 implants machined and medium-rough surfaces | Bone loss >3 mm | PD changes: 0.9 (0.8) mm (6 months, implant level) | |||||
| Radiographic bone level change: −0.3(0.9) mm (6 months, subject level) | |||||||
| Positive treatment outcome: 47 % | |||||||
| Control | |||||||
| PD changes: 0.8 (0.5) mm (6 months, implant level) | |||||||
| Radiographic bone level change: −0.1(0.8) mm (6 months, subject level) | |||||||
| Positive treatment outcome: 44 % | |||||||
| No significant differences between groups | |||||||
| Sahm et al.; John et al. [27, 33] | RCT, parallel | 32 patients (BL) | PD ≥4 mm, BOP + with suppuration | 12 months | OHI + air abrasive device, glycine powder | OHI + mechanical debridement (carbon curettes + 0.1 % CHX) | Test |
| 25 patients (12 months) | Bone loss ≤33 % | BOP: 99.0 (4.1) (BL) to 57.8 (30.7) % (12 months, subject level) | |||||
| 36 implants | PD: 3.7 (1.0) (BL) to 3.2 (1.1) mm (12 months, subject level) | ||||||
| 8 implant systems | Control | ||||||
| BOP: 94.7 (13.7) (BL) to 78.1 (30.0) % (12 months, subject level) | |||||||
| PD: 3.9 (1.1) (BL) to 3.5 (1.2) mm (12 months, subject level) | |||||||
| BOP: significant difference between groups at 3, 6 and 12 months |
BI bleeding index, BL baseline, BOP bleeding on probing, CHX chlorhexidine digluconate, OHI oral hygiene instructions, PD probing pocket depth, RCT randomized controlled clinical study
Table 7.
Included studies—nonsurgical treatment of peri-implantitis: adjunctive antiseptic therapy
| Publication | Design | Population | Case definition | Period | Test | Control | Mean (SD) outcome |
|---|---|---|---|---|---|---|---|
| Machtei et al. [23] | Multicentre RCT, parallel | 60 patients | PD = 6–10 mm and BOP + radiographic bone loss | 6 months | OHI + ultrasonic debridement + matrix containing 2.5-mg CHX chips (i.e. up to 4 per implant site) | OHI + ultrasonic debridement + biodegradable crosslinked gelatin matrix chip | Test |
| 77 implants | Repeated application at sites with PD ≥6 mm at 2, 4, 6, 8, 12 and 18 weeks | Repeated application at sites with PD ≥6 mm at 2, 4, 6, 8, 12 and 18 weeks | BOP: 100 (0.0) (BL) to 42.5 (50.0) % (6 months, subject level) | ||||
| PD: 7.6 (1.1) (0.0) to 5.47 (1.86) mm (6 months, subject level) | |||||||
| Control | |||||||
| BOP: 100 (0.0) (BL) to 54.5 (50.5) % (6 months, subject level) | |||||||
| PD: 7.21 (1.08) (BL) to 5.48 (1.25) mm (6 months, subject level) | |||||||
| BOP and PD reductions at 6 months not sign. different between groups |
BL baseline, BOP bleeding on probing, CHX chlorhexidine digluconate, OHI oral hygiene instructions, PD probing pocket depth, RCT randomized controlled clinical study
Table 8.
Included studies—nonsurgical treatment of peri-implantitis: adjunctive antibiotic therapy
| Publication | Design | Population | Case definition | Period | Test | Control | Mean (SD) outcome |
|---|---|---|---|---|---|---|---|
| Büchter et al. [26] | RCT, parallel | 28 patients | PD >5 mm | 18 weeks | OHI + mechanical debridement (plastic curettes) + 0.2 % CHX pocket irrigation + 8 % doxycycline hyclate | OHI + mechanical debridement (plastic curettes) + 0.2 % CHX pocket irrigation | Test |
| 48 implants medium-rough surfaces | Bone loss >50 % | BOP: 0.54 (0.07) (BL) to 0.27 (0.06) % (18 weeks, subject level) | |||||
| PD: 5.64 (0.32) (BL) to 4.49 (0.29) mm (18 weeks, subject level) | |||||||
| Control | |||||||
| BOP: 0.63 (0.06) (BL) to 0.50 (0.07) % (18 weeks, subject level) | |||||||
| PD: 5.68 (0.28) (BL) to 5.4 (0.34) mm (18 weeks, subject level) | |||||||
| BOP and PD reductions sign. higher in the test group | |||||||
| Renvert et al. [32] | RCT, parallel | 32 patients | PD ≥4 mm, BOP + with suppuration | 12 months | OHI + mechanical debridement (scalers + rubber cup + polishing) + 1 mg minoycycline microspheres | OHI + mechanical debridement (scalers + rubber cup + polishing) + 1.0 % chlorhexidine gel | Test |
| 1–5 (test)/1–6 (control) implants per patient machined surfaces | Bone loss ≤3 threads | BOP: 88 (12) (BL) to 71 (22) % (12 months, subject level) | |||||
| Presence of anaerobic bacteria | PD: 3.9 (0.7) (BL) to 3.6 (0.6) mm (12 months, subject level) | ||||||
| Control | |||||||
| BOP: 86 (14) (BL) to 78 (13) % (12 months, subject level) | |||||||
| PD: 3.9 (0.3) (BL) to 3.9 (0.4) mm (12 months, subject level) | |||||||
| PD reductions at 12 months sign. higher in the test group | |||||||
| Comparable microbiological improvements in both groups | |||||||
| Renvert et al. [29] | RCT, parallel | 32 patients | PD ≥4 mm, BOP + with suppuration | 12 months | OHI + mechanical debridement + 1 mg minoycycline microspheres | OHI + mechanical debridement + 0.5 ml of 1.0 % CHXgel | Test |
| 95 implants machined surfaces | Bone loss ≤3 threads | Treatment was repeated at days 30 and 90 | Treatment was repeated at days 30 and 90 | BOP: 86.5 (20.1) (BL) to 48.1 (20.7) % (12 months, implant level) | |||
| Presence of anaerobic bacteria | PD: 3.85 (1.04) (BL) to 3.55 (0.98) mm (12 months, implant level) | ||||||
| Radiographic bone levels: 0.77 (0.85) (BL) to 0.7 (0.85) mm (12 months, implant level) | |||||||
| Control | |||||||
| BOP: 89.2 (17.2) (BL) to 63.5 (19.2) % (12 months, implant level) | |||||||
| PD: 3.87 (1.16) (BL) to 3.72 (1.02) mm (12 months, implant level) | |||||||
| Radiographic bone levels: 0.41 (0.7) (BL) to 0.46 (0.76) mm (12 months, implant level) | |||||||
| BOP reductions at 12 months sign. higher in the test group | |||||||
| Comparable microbiological improvements in both groups | |||||||
| Schär et al.; Bassetti et al. [34, 37] | RCT, parallel | 40 patients | PD = 4–6 mm, BOP + bone loss = 0.5–2 mm | 12 months | OHI + mechanical debridement (titanium curettes + glycin powder air polishing, pocket irrigation using 3 % hydrogen peroxide) + aPDT (660 nm, phenothiazine chloride dye) | OHI + mechanical debridement (titanium curettes + glycin powder air polishing, pocket irrigation using 3 % hydrogen peroxide) + minocycline microspheres | Test |
| 40 implants medium-rough surfaces | BOP change: 57 % (12 months, subject level) | ||||||
| PD changes: 0.56 mm (12 months, subject level) | |||||||
| Complete resolution of mucosal inflammation: 31.6 % | |||||||
| Control | |||||||
| BOP change: 65 % (12 months, subject level) | |||||||
| PD changes: 0.11 mm (12 months, subject level) | |||||||
| Complete resolution of mucosal inflammation: 35.0 % | |||||||
| No significant differences in clinical, microbiological and immunological parameters between groups |
aPDT antimicrobial photodynamic therapy, BL baseline, BOP bleeding on probing, CHX chlorhexidine digluconate, OHI oral hygiene instructions, PD probing pocket depth, RCT randomized controlled clinical study
Table 9.
Included studies—surgical treatment of peri-implantitis: alternative measures for surface decontamination
| Publication | Design | Population | Case definition | Period | Test | Control | Mean (SD) outcome |
|---|---|---|---|---|---|---|---|
| Deppe et al. [40] | CCT, parallel | 32 patients | PD ≥5 mm, BOP + progressive vertical bone loss | 5 years | OHI + open flapa surgery + air polishing + carbon dioxide laser (cw mode, 2.5 W, 12 × 5 s) decontamination + soft tissue resection | OHI + open flapb surgery + air polishing + soft tissue resection | Test |
| 73 implants machined, rough- and medium-rough surfaces | SBI: 0.6 (0.3) (BL) to 1.8 (1.1) (48 months, implant level) | ||||||
| PD: 6.1 (1.6) (BL) to 3.4 (1.5) mm (48 months, implant level) | |||||||
| Control | |||||||
| SBI: 0.7 (0.8) (BL) to 1.1 (1.2) (48 months, implant level) | |||||||
| PD: 5.1 (1.3) (BL) to 4.3 (1.2) mm (48 months, implant level) | |||||||
| Comparable outcomes in both groups | |||||||
| De Waal et al. [38] | RCT, parallel | 30 patients | PD ≥5 mm, BOP + and/or suppuration | 12 months | OHI/mechanical debridement + resective therapy (apical re-positioned flap + bone re-contouring) + surface debridement using surgical gauzes soaked in saline + decontamination using 0.12 % CHX + 0.05 % cetylpyridinium chloride CPC | OHI/mechanical debridement + resective therapy (apical re-positioned flap + bone re-contouring) + surface debridement using surgical gauzes soaked in saline + decontamination using placebo solution | Test |
| 79 implants machined, rough- and medium-rough surfaces | Bone loss ≥2 mm | BOP: 80.4 (26.5) (BL) to 60.5 (30.1) % (12 months, implant level) | |||||
| PD: 6.6 (1.6) (BL) to 4.3 (2.1) mm (12 months, implant level) | |||||||
| MBL: 4.3 (2.1) (BL) to 5.0 (2.5) mm (12 months, implant level) | |||||||
| Control | |||||||
| BOP: 79.7 (28.1) (BL) to 57.2 (29.0) % (12 months, implant level) | |||||||
| PD: 5.5 (1.4) (BL) to 3.7 (0.8) mm (12 months, implant level) | |||||||
| MBL: 3.6 (1.9) (BL) to 3.9 (2.0) mm (12 months, implant level) | |||||||
| No sign. differences in BOP and PD reductions between groups | |||||||
| De Waal et al. [39] | RCT, parallel | 44 patients | PD ≥5 mm, BOP + and/or suppuration | 12 months | OHI/mechanical debridement + resective therapy (apical re-positioned flap + bone re-contouring) + surface debridement using surgical gauzes soaked in saline + decontamination using 0.12 % CHX + 0.05 % cetylpyridinium chloride | OHI/mechanical debridement + resective therapy (apical re-positioned flap + bone re-contouring) + surface debridement using surgical gauzes soaked in saline + decontamination using 2.0 % CHX | Test |
| 108 implants machined, rough- and medium-rough surfaces | Bone loss ≥2 mm | BOP: 82.1 (23.9) (BL) to 42.7 (34.2) % (12 months, implant level) | |||||
| PD: 4.7 (1.0) (BL) to 3.0 (0.7) mm (12 months, implant level) | |||||||
| MBL: 4.0 (1.5) (BL) to 4.3 (1.7) mm (12 months, implant level) | |||||||
| Control | |||||||
| BOP: 74.2 (27.8) (BL) to 37.0 (35.5) % (12 months, implant level) | |||||||
| PD: 5.0 (1.2) (BL) to 2.9 (0.7) mm (12 months, implant level) | |||||||
| MBL: 4.1 (1.6) (BL) to 4.1 (1.7) mm (12 months, implant level) | |||||||
| No sign. differences in BOP and PD reductions between groups | |||||||
| Papadopoulos et al. [41] | RCT, parallel | 16 patients | PD ≥6 mm, BOP + and/or suppuration | 6 months | Mechanical debridement + open flap surgery + mechanical debridement (plastic curettes) and cotton swabs soaked in saline | Mechanical debridement + open flap surgery + mechanical debridement (plastic curettes) and cotton swabs soaked in saline + 980 nm diode laser application | Test |
| 16 implants | Radiographic bone loss ≥2 mm | BOP: 81.2 (BL) to 23.8 % (6 months, implant level) | |||||
| PD: 5.92 (BL) to 4.44 mm (6 months, implant level) | |||||||
| Control | |||||||
| BOP: 81.2 (BL) to 23.8 % (6 months, implant level) | |||||||
| PD: 5.52 (BL) to 4.31 mm (6 months, implant level) | |||||||
| Sign. BOP and PD reductions in both groups at 6 months |
BL baseline, BOP bleeding on probing, CCT non-randomized controlled clinical study, CHX chlorhexidine digluconate, MBL marginal bone level, OHI oral hygiene instructions, PD probing pocket depth, RCT randomized controlled clinical study, SBI sulcus bleeding index
aSubgroup analysis of n = 17 implants
bSubgroup analysis of n = 16 implants
Table 10.
Included studies—surgical treatment of peri-implantitis: adjunctive resective therapy
| Publication | Design | Population | Case definition | Period | Test | Control | Mean (SD) outcome |
|---|---|---|---|---|---|---|---|
| Romeo et al. [42, 57] | RCT, parallel | 17 patients | Suppuration or sulcus bleeding, PD >4 mm horizontal peri-implant translucency | 36 months systemic antibiotic medication (Amoxicillin for 8 days) | Full mouth disinfection/mechanical debridement + resective therapy (apical re-positioned flap + bone re-contouring) + decontamination using metronidazole + tetracycline hydrochloride (3 min) + implantoplasty using diamond and arkansas burs/silicone polishers | Full mouth disinfection/mechanical debridement + resective therapy (apical re-positioned flap + bone re-contouring) + decontamination using metronidazole + tetracycline hydrochloride (3 min) | Test |
| 22 implants rough surfaces | BOP: 2.83 (0.47) (BL) to 0.5 (0.69) (24 monthsa, implant level) PD: 5.79 (1.69) (BL) to 3.58 (1.06) mm (24 monthsa, implant level) |
||||||
| MBL: 0.0–0.01 mm (36 months, implant level) | |||||||
| Control | |||||||
| BOP: 2.86 (0.35) (BL) to 2.33 (0.74) (24 monthsa, implant level) | |||||||
| PD: 6.52 (1.62) (BL) to 5.5 (1.47) mm (24 monthsa, implant level) | |||||||
| MBL: 1.44–1.54 mm (36 months, implant level) |
BL baseline, BOP bleeding on probing, MBL marginal bone loss, PD probing pocket depth, RCT randomized controlled clinical study
aAll patients of the control group were discontinued from the study due to persistent clinical signs of inflammation
Table 11.
Included studies—surgical treatment of peri-implantitis: adjunctive augmentative therapy
| Publication | Design | Population | Case definition | Period | Test | Control | Mean (SD) outcome |
|---|---|---|---|---|---|---|---|
| Schwarz et al. [48, 51, 54] | RCT, parallel | 20 patients | PD >6 mm, BOP + and/or pus | 4 years nonsubmerged healing | OHI + initial nonsurgical therapy | OHI + initial nonsurgical therapy | Test |
| 21 implants machined and medium-rough surfaces | Intrabony defect >3 mm | Open flap surgery + mechanical debridement (plastic curettes) + nanocrystalline hydroxyapatite paste | Open flap surgery + mechanical debridement (plastic curettes) + bovine-derived xenograft + native collagen barrier membrane | BOP reduction: 32 % (4 years, subject level) | |||
| PD reduction: 2.5 (0.9) mm (4 years, subject level) | |||||||
| Control | |||||||
| BOP reduction: 51 % (4 years, subject level) | |||||||
| PD reduction: 1.1 (0.3) mm (4 years, subject level) | |||||||
| BOP and PD reductions sign. higher at control sites | |||||||
| Deppe et al. [40] | CCT, parallel | 32 patients | PD ≥5 mm, BOP + progressive vertical bone loss | 5 years | Group 2 OHI + open flapa surgery + air polishing + carbon dioxide laser (cw mode, 2.5 W, 12 × 5 s) decontamination + beta tricalcium phosphate + cortical bone chips harvested from the retromoar area (50:50) + nonresorbable synthetic barrier membrane | Group 4 OHI + open flapb surgery + air polishing + beta tricalcium phosphate + cortical bone chips harvested from the retromoar area (50:50) + nonresorbable synthetic barrier membrane | Test |
| 73 implants machined, rough- and medium-rough surfaces | SBI: 0.5 (0.8) (BL) to 2.1 (1.4) (48 months, implant level) | ||||||
| PD: 4.8 (1.4) (BL) to 2.5 (1.1) mm (48 months, implant level) | |||||||
| Control | |||||||
| SBI: 1.2 (0.6) (BL) to 1.9 (1.0) (48 months, implant level) | |||||||
| PD: 5.7 (1.4) (BL) to 2.5 (1.4) mm (48 months, implant level) | |||||||
| Comparable outcomes in both groups | |||||||
| Roos-Jansaker et al. [45–47] | CCT, parallel | 25 patients | BOP + and/or pus | 5 years transmucosal healing systemic antibiotic medication (amoxicillin + metronidazole for 10 days) | Removal of the suprastructure | Removal of the suprastructure | Test |
| 45 implants machined and medium-rough surfaces | Bone loss ≥3 threads one- to four-wall defects | Open flap surgery + debridement + decontamination using hydrogen peroxide 3 % algae derived xenograft + resorbable synthetic barrier membrane | Open flap surgery + debridement + decontamination using hydrogen peroxide 3 % algae derived xenograft + | PD reduction: 3.0 (2.4) mm (5 years, implant level) | |||
| Radiographic defect fill: 0.1 (0.5) mm (5 years, implant level) | |||||||
| Control | |||||||
| PD reduction: 3.3 (2.0) mm (5 years, implant level) | |||||||
| Radiographic defect fill: 0.1 (0.5) mm (5 years, implant level) | |||||||
| Comparable defect fill and BOP reductions in both groups | |||||||
| Schwarz et al. [53] | CCT, parallel | 27 patients | PD >6 mm, BOP + and/or pus | 12 months nonsubmerged healing | Circumferential-type (Ie) defects OHI + initial nonsurgical therapy | Buccal dehiscence-type defects with a semicircular (Ib) or circular component (Ic) | Test Ib |
| 27 implants machined and medium-rough surfaces | Intrabony defect >3 mm | Open flap surgery + mechanical debridement (carbon curettes) + decontamination (cotton pellets soaked in saline) | OHI + initial nonsurgical therapy Open flap surgery + Mechanical debridement (carbon curettes) + decontamination (cotton pellets soaked in saline) | BOP reduction: 38.9 (16.6) % (12 months, subject level) | |||
| Supracrestal component ≤1 mm | Bovine-derived xenograft + native collagen barrier membrane | Bovine-derived xenograft + native collagen barrier membrane | PD reduction: 1.6 (0.9) mm (12 months, subject level) | ||||
| Test Ic | |||||||
| BOP reduction: 25.9 (14.7) % (12 months, subject level) | |||||||
| PD reduction: 1.6 (0.7) mm (12 months, subject level) | |||||||
| Control Ie | |||||||
| BOP reduction: 61.1 (16.7) % (12 months, subject level) | |||||||
| PD reduction: 2.7 (0.7) mm (12 months, subject level) | |||||||
| Sign. difference in BOP reductions between Ic and Ie | |||||||
| Rocuzzo et al. [44] | CCT, parallel | 26 patients | PD ≥6 mm | 12 months nonsubmerged healing simultaneous connective tissue graft at sites lacking keratinized mucosa systemic antibiotic medication (amoxicillin + clavulanic acid for 6 days) | SLA surfaced implants | TPS surfaced implants | Test |
| 26 implants rough and medium-rough surfaces | Crater-like (intrabony) defects | OHI | OHI | BOP reduction: 60.4 % (12 months, subject level) | |||
| Open flap surgery + mechanical debridement (plastic curettes) + decontamination (24 % EDTA and 1 % CHX gel) + bovine-derived xenograft | Open flap surgery + mechanical debridement (plastic curettes) + decontamination (24 % EDTA and 1 % CHX gel) + bovine-derived xenograft | PD reduction: 3.4 (1.7) mm (12 months, subject level) | |||||
| Radiographic defect fill: 1.9 (1.3) mm (12 months, subject level) | |||||||
| Control | |||||||
| BOP reduction: 33.9 % (12 months, subject level) | |||||||
| PD reduction: 2.1 (1.2) mm (12 months, subject level) | |||||||
| Radiographic defect fill: 1.6 (0.7) mm (12 months, subject level) | |||||||
| BOP and PD reductions sign. higher in the test group | |||||||
| Schwarz et al. [49, 50, 52] | RCT, parallel | 17 patients | PD >6 mm, BOP + and/or pus | 4 years nonsubmerged healing | OHI + initial nonsurgical therapy | OHI + initial nonsurgical therapy | Test |
| 17 implants machined and medium-rough surfaces | Intrabony defect >3 mm | Open flap surgery + debridement + decontamination using an Er:YAG laser device (cone-shaped glass fibre tip) at 11.4 J/cm2 implantoplasty at bucally and supracrestally exposed implant parts | Open flap surgery + Mechanical debridement (plastic curettes) + decontamination (cotton pellets soaked in saline) implantoplasty at bucally and supracrestally exposed implant parts | BOP reduction: 71.6 (24.9) % (4 years, subject level) | |||
| Supracrestal component >1 mm | Bovine-derived xenograft + native collagen barrier membrane at intrabony components | Bovine-derived xenograft + native collagen barrier membrane at intrabony components | PD reduction: 1.3 (1.8) mm (4 years, subject level) | ||||
| Control | |||||||
| BOP reduction: 85.2 (16.4) % (4 years, subject level) | |||||||
| PD reduction: 1.2 (1.9) mm (4 years, subject level) | |||||||
| BOP and PD reductions comparable in both groups | |||||||
| Aghanzadeh et al. [43] | RCT, parallel | 45 patients | PD ≥2 mm, BOP + and pus | 12 months nonsubmerged healing systemic antibiotic medication (Azithromycin for 4 days) | Open flap surgery + mechanical debridement (titanium instruments) + decontamination using hydrogen peroxide 3 % cortical bone chips harvested from the mandibular ramus + resorbable synthetic barrier membrane | Open flap surgery + mechanical debridement (titanium instruments) + decontamination using hydrogen peroxide 3 % bovine-derived xenograft + resorbable synthetic barrier membrane | Test |
| 75 implants medium-rough surfaces | Bone loss ≥2 mm | BOP reduction: 44.8 (6.3) % (12 months, implant level) | |||||
| Angular defects ≥3 mm in depth | PD reduction: 2.0 (0.3) mm (12 months, implant level) | ||||||
| Radiographic bone level gain: 0.2 (0.3) mm (12 months, implant level) | |||||||
| Control | |||||||
| BOP reduction: 50.4 (5.3 %) (12 months, implant level) | |||||||
| PD reduction: 3.1 (0.2)mm (12 months, implant level) | |||||||
| Radiographic bone level gain: 0.8 (0.4) mm (12 months, implant level) | |||||||
| PD reductions and bone level gains were significantly higher at control sites | |||||||
| Wohlfahrt et al. [55, 58] | RCT, parallel | 33 patients | PD ≥5 mm, BOP + intrabony defects ≥4 mm | 12 months submerged healing for 6 months | Open flap surgery + mechanical debridement (titanium curettes) + conditioning using 24 % ethylenediaminetetraacetic acid gel (2 min) + augmentation of intrabony defect components using porous titanium granules | Open flap surgery + mechanical debridement (titanium currettes) + conditioning using 24 % ethylenediaminetetraacetic acid gel (2 min) | Test |
| 33 implants medium-rough surfaces | BOP reduction: 0.38 (2.1) % (12 months, implant level) | ||||||
| PD reduction: 1.7 (1.7) mm (12 months, implant level) | |||||||
| Radiographic defect fill: 57.0 (45.1) % (12 months, implant level) | |||||||
| Control | |||||||
| BOP reduction: 0.56 (2.9) % (12 months, implant level) | |||||||
| PD reduction: 2.0 (2.3) mm (12 months, implant level) | |||||||
| Radiographic defect fill: −14.8 (83.4) % (12 months, implant level) no sign. reductions in BOP scores in both groups comparable reductions in MMP-8 and bone level markers |
BL baseline, BOP bleeding on probing, CCT non-randomized controlled clinical study, CHX chlorhexidine dugluconate, MMP-8 matrixmetalloproteinase-8, PD probing pocket depth, RCT randomized controlled clinical study, SBI sulcus bleeding index, SLA sand blasted and acid etched, TPS titanium plasma flamed
aSubgroup analysis of n = 11 implants
bSubgroup analysis of n = 13 implants
Nonsurgical treatment of peri-implant mucositis
The case definitions markedly differed among the studies investigated. While three studies considered mucosal inflammation in the absence of radiographic bone loss [15–17], four studies also accepted a bone resorption of up to 3 mm for defining peri-implant mucositis [18–21]. Moreover, these studies used several clinical parameters to assess mucosal inflammation, employed various oral hygiene instructions (OHI) and defined different intervals for maintenance care. Despite significant improvements in all of the clinical parameters investigated, test (i.e. alternative or adjunctive methods for biofilm removal, adjunctive antiseptic therapy, or adjunctive antibiotic therapy) and control treatments were commonly associated with residual gingival index (GI), BI, and/or BOP scores at 3 to 12 months after therapy (Tables 3, 4 and 5).
Alternative or adjunctive measures for biofilm removal
Three studies reported on alternative or adjunctive measures for biofilm removal (Table 3).
In particular, one RCT and one CCT compared the clinical efficacy of adjunctive air polishing (glycine powder) to OHI and mechanical debridement using either ultrasonic scalers or hand instruments [15, 18]. Both test and control groups were associated with significant improvements in mean BI and PD scores after therapy. When evaluating absolute values at 6 months, mean BI and PD scores were significantly lower following adjunctive air polishing to teflon curettes [18]. One RCT compared a repeated (3 and 6 months) monotherapy using an air abrasive device to ultrasonic scaling. After a healing period of 12 months, both groups revealed comparable BOP reductions and frequencies of diseased sites [21] (Table 3).
Adjunctive antiseptic therapy
Three RCTs reported on the adjunctive antispectic therapy to OHI and mechanical debridement [17, 20, 22] (Table 4).
In particular, one RCT assessed the adjunctive application of phosphoric acid gel to carbon curettes and rubber cup polishing, which was provided once every month in both groups. At 5 months, test sites revealed a significantly higher reduction in mean gingival index (GI) (modified, but not specified) and colony-forming units when compared with control sites, respectively [22]. Porras et al. [20] compared OHI + mechanical debridement with and without local pocket irrigation using chlorhexidine digluconate (CHX) + topical CHX gel application + CHX mouthwash (twice for 10 days). At 3 months, mean mucosal bleeding (mBI), BOP scores and microbiological parameters did not significantly differ between test and control groups. However, the test group revealed a significantly higher change in mean PD scores [20]. In another RCT, topical CHX gel application + full mouth disinfection + CHX mouthrinse (2×/day) and tonsil spraying (1×/day) for 14 days was compared with OHI + mechanical debridement (plastic scaler + polyetheretherketone-coated ultrasonic instruments) + full mouth scaling alone. While both treatment procedures were associated with significant PD reductions at 8 months, the BOP scores did not significantly differ to baseline in both groups [17] (Table 4).
Adjunctive antibiotic therapy
Two studies reported on the adjunctive antibiotic (i.e. local or systemic) therapy to OHI and mechanical debridement [16, 19] (Table 5).
In particular, one RCT compared the adjunctive local delivery of tetracycline HCl (25 %) fibres for 10 days (test) to mechanical debridement alone (control). A complete or partial fibre loss was noted at three implants after 7 to 10 following application. While test sites were associated with marked BOP improvements, control sites revealed a further increase of BOP scores at 3 months [16]. In another RCT, a total of 45 patients were randomly allocated to either OHI + mechanical debridement (titanium curettes + rubber polishing) + systemic antibiotic medication (Azithromycin® 500 mg day 1 and 250 mg days 2–4) (test), or OHI + mechanical debridement alone (control). The subject-based, per-protocol analysis at 6 months did not reveal any significant differences between test and control groups for all clinical and microbiological parameters investigated [19] (Table 5).
Nonsurgical treatment of peri-implantitis
In all studies investigated, peri-implantitis was commonly defined by BOP and a radiographic bone loss. However, the reference points (i.e. baseline radiographs) and thresholds used to identify bone level changes were either not specified [23–25] or exhibited large variations [26–34]. Radiographic bone level changes as treatment outcome were merely assessed in three studies [28–30].
Despite significant improvements in all of the clinical and microbiological parameters investigated, test (i.e. alternative methods for biofilm removal, adjunctive antiseptic therapy, or adjunctive antibiotic therapy) and control treatments were commonly associated with residual BI and BOP scores at 3 to 12 months after therapy (Tables 6, 7 and 8).
Alternative measures for biofilm removal
Six RCTs (corresponding to 7 publications) reported on the efficacy of alternative measures for biofilm removal (Table 6). In particular, two studies employed the same type of an ultrasonic device used with a hydroxyapatite fluid polish [28, 31], while two studies reported on erbium-doped yttrium aluminum garnet (Er:YAG) laser monotherapy [24, 25] and two publications on glycine powder air polishing [27, 33]. One study compared Er:YAG laser monotherapy versus air polishing [30].
At 3 months after therapy, nonsurgical ultrasonic debridement was associated with a reduction in mean BOP scores, whereas these values further increased at control sites (i.e. carbon fibre curettes). However, these differences, as well as those noted for mean PD and radiographic bone level changes, did not reach statistical significance between groups [28]. Similarly, when comparing ultrasonic scaling with mechanical debridement using titanium curettes, Renvert et al. [31] also failed to identify any significant between group differences in mean BI and PD reductions at 6 months. Furthermore, both procedures did not reduce bacterial load [35] (Table 6).
In two RCTs, the efficacy of Er:YAG laser monotherapy was compared to that of mechanical debridement using carbon fibre curettes + adjunctive local antiseptic CHX irrigation/application [24, 25]. After 6 months of healing, Er:YAG laser application was associated with significantly lower mean BOP scores than the control treatment. However, these improvements failed to reach statistical significance at 12 months, particularly at advanced sites [24, 25] (Table 6). In one RCT, glycine powder air polishing resulted in a significantly higher reduction of mean BOP scores at 3, 6, and 12 months when compared with mechanical debridement + local antiseptic therapy using CHX. The application of this specific air abrasive device was not associated with any emphysema formation or complications in peri-implant wound healing [27, 33]. At more advanced sites, Er:YAG laser monotherapy and glycine powder air polishing resulted in comparable BOP/PD reductions and crestal bone level changes, but failed to reduce bacterial load [36] (Table 6).
Adjunctive antiseptic therapy
One multicenter RCT reported on the adjunctive antispective therapy to ultrasonic debridement (Table 7). In particular, a CHX containing matrix was repeatedly applied at 2, 4, 6, 8, 12 and 18 weeks until PD was reduced to ≤5 mm. At 6 months, CHX chips resulted in a significantly higher PD reduction than the placebo chips [23].
Adjunctive antibiotic therapy
Three RCTs reported on the adjunctive local antibiotic therapy to mechanical debridement (Table 8). In particular, minocycline microspheres were either applied once [32] at baseline or repeatedly [29] at 30 and 90 days and compared with local antispectic therapy using CHX gel (1.0 %). At 12 months, minocycline was associated with significantly higher BOP (refers to a repeated application) and PD (refers to a single application) reductions when compared with the control group. The radiographic (refers to a repeated application) and microbiological analyses failed to reveal any significant differences between both groups. Similar clinical outcomes were also noted when doxycycline hyclate was used as an adjunct to mechanical debridement [26].
In one RCT, adjunctive local antibiotic therapy (minocycline microspheres) was compared to adjunctive antimicrobial photodynamic therapy. At 12 months, both test and control groups were associated with significant but comparable clinical, microbiological and immunological improvements [34, 37] (Table 8).
The weighted mean (WM) BOP and PD reductions following conventional nonsurgical treatment (referring to the control groups in respective studies) [23–27] amounted to 31.12 % [SE = 9.14; 95 % CI (12.20, 49.05)] and 0.71 mm [SE = 0.32; 95 % CI (0.07, 1.35)], respectively. The weighted mean (WM) BOP and PD reductions for alternative/adjunctive measures (i.e. air polishing, aPDT, CHX chip, doxycycline, Er:YAG laser) [23–27] amounted to 42.85 % [SE = 9.24; 95 % CI (24.70, 60.97)] and 0.87 mm [SE = 0.29; 95 % CI (0.29, 1.44)], respectively.
Surgical treatment of peri-implantitis
Twelve studies (18 publications) reported on the surgical treatment of peri-implantitis, employing either alternative measures for surface decontamination (3 RCTs and 1 CCT) [38–41], adjunctive resective (1 RCT) [42] or augmentative (4 RCTs and 4 CCTs) [40, 43–55] therapy. In these studies, peri-implantitis was commonly defined by BOP and a radiographic bone level changes. However, the thresholds used to assess bone loss revealed large variations and defect configurations (i.e. supra-/intrabony defects) [56] were rarely reported (Tables 9, 10 and 11).
Alternative measures for surface decontamination
In one CCT, Deppe et al. [40] assessed the clinical efficacy of carbon dioxide laser decontamination used as an adjunct to resective flap surgery + air polishing (control). While the test treatment improved the clinical outcomes over the control measure at 4 months, mean SBI and PD values were comparable in both groups at about 5 years (Table 9).
In further two studies, De Waal et al. [38, 39] employed an open flap debridement using gauzes soaked in sterile saline + bone re-contouring + apical flap re-positioning and compared one test (0.12 % CHX + 0.05 % cetylpyridinium chloride) and two control (placebo solution or 2.0 % CHX) measures for surface decontamination. At 12 months, the test and both control procedures were associated with marked but comparable reductions in mean BOP and PD scores, respectively. Furthermore, between group comparisons failed to reveal any significant differences in mean marginal bone loss after therapy [38, 39]. Similarly, Papadopoulos et al. [41] also failed to reveal any significant clinical improvements in mean BOP and PD scores at 6 months, when a 980-nm diode laser was used as an adjunct to mechanical open flap debridement (Table 9).
Adjunctive resective therapy
One study assessed the clinical efficacy of an implantoplasty (diamond/arkansas burs + silicone polishers) when used as an adjunct to open flap debridement + bone re-contouring + apical flap re-positioning [42]. At 24 months, all patients from the control group had to be discontinued from the study due to persistent active signs of peri-implant inflammation. This was associated with elevated mBI and PD scores when compared with the test group. On the contrary, resective therapy resulted in significantly higher mean mucosal recessions (1.64 ± 1.29 vs. 2.3 ± 1.45 mm) but no pseudopocket formation [42], while test sites were associated with stable radiographic bone levels at 3 years, the interproximal bone loss at control sites amounted to 1.45–1.54 mm [57] (Table 10).
The calculated WM BOP [38, 39, 55] and PD [38–40, 55] reductions following surgical treatment (i.e. open flap with and without soft tissue resection) amounted to 34.81 % [SE = 8.95; 95 % CI (17.25, 52.37)] and 1.75 mm [SE = 0.34; 95 % CI (1.08, 2.42)].
Adjunctive augmentative therapy
The clinical efficacy of adjunctive augmentative therapy to open flap debridement (titanium curettes + conditioning using 24 % ethylenediaminetetraacetic acid gel + submerged healing for 6 months) was merely assessed in one study [55]. Notably, 12/16 control and 13/16 test sites revealed a premature exposure during the submerged healing phase of 6 months. At 12 months after therapy, the application of porous titanium granules to the intrabony defect components resulted in a significantly higher percentage of radiographic defect fill when compared with open flap surgery alone. Moreover, the test group was associated with an increase in implant stability quotient, whereas these values further decreased at control sites. However, both groups revealed comparable PD reductions and only minor improvements in mean BOP scores [55]. The immunological analysis did not reveal any significant between differences in the reduction of MMP-8 levels or bone level markers [58] (Table 11). Four RCTs and four CCTs compared different augmentation protocols employing various methods for surface decontamination, bone fillers (i.e. alloplastic, xenogenic, autogenous) and barrier membranes (synthetic, native collagen) over a period of up to 5 years [40, 43–55]. The majority of these studies considered PD and BOP reductions as primary outcomes but also reported on radiographic defect fill (Table 11).
WM BOP [43, 49, 51, 53, 55] and PD [40, 43–55] reductions following adjunctive augmentative therapy amounted to 50.73 % [SE = 3.5; 95 % CI (43.87, 57.59)] and 2.20 mm [SE = 0.22; 95 % CI (1.76, 2.64)], respectively. The outcomes of therapy was mainly influenced by the type of bone filler (i.e. a slowly resorbing bovine-derived mineral was superior to autogenous bone and an alloplastic material), defect characteristics (i.e. circumferential-type defects were superior to dehiscence-type defects) and implant surface characteristics (i.e. moderately rough surfaces were superior to rough surfaces) (Table 11).
Meta-analysis
Meta-analysis to estimate the weighted mean difference (WMD) between test and control treatments was conducted on RCTs reporting on similar assessments of either absolute BOP or PD scores.
Nonsurgical treatment of peri-implant mucositis—adjunctive antiseptics/antibiotics
Based on four and four studies, WMD in BOP [16, 17, 19, 22] and PD [17, 19, 20, 22] scores amounted to −8.16 % [SE = 4.61; p > 0.05; 95 % CI (−17.20, 0.88)] and −0.15 mm [SE = 0.13; p > 0.05; 95 % CI (−0.42, 0.11)], not favouring local antiseptic or antibiotic (i.e. local and systemic) therapy as an adjunct to mechanical debridement (p value for heterogeneity: 0.42, I2 = 0.0 % = low heterogeneity; p value for heterogeneity: 0.45, I2 = 0.0 % = low heterogeneity, respectively) (Fig. 2a, b).
Fig. 2.
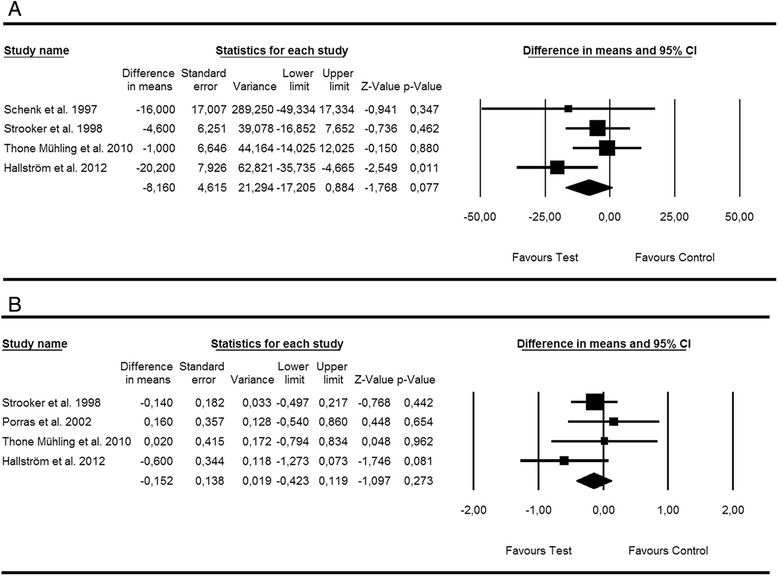
Forest plot indicating weighted mean difference (95 % CI) in the reduction of primary outcomes following nonsurgical treatment of peri-implant mucositis. a Adjunctive antiseptic/antibiotic therapy—BOP. b Adjunctive antiseptic/antibiotic therapy—PD
Egger’s linear regression method revealed symmetrical plots for changes in BOP (p = 0.51) and PD (p = 0.69) thus suggesting the absence of publication bias.
Nonsurgical treatment of peri-implantitis—alternative methods for biofilm removal
Based on three studies [24, 25, 27], WMD in BOP scores between test and control groups amounted to −23.12 % [SE = 4.81; p < 0.001; 95 % CI (−32.56, −13.69)] favouring alternative methods (i.e. Er:YAG laser, glycine air polishing,) for biofilm removal over mechanical debridement (p value for heterogeneity: 0.55, I2 = 0.0 % = low heterogeneity; 0.39) (Fig. 3a). Based on five studies [24, 25, 27, 28, 31], WMD in PD scores between test and control groups amounted to −0.49 mm [SE = 0.21; p < 0.05; 95 % CI (−0.91, −0.08)] not favouring alternative methods (i.e. Er:YAG laser, glycine air polishing, ultrasonic system) for biofilm removal over mechanical debridement (p value for heterogeneity: 0.029, I2 = 62.8 % = substantial heterogeneity; 0.39) (Fig. 3b).
Fig. 3.
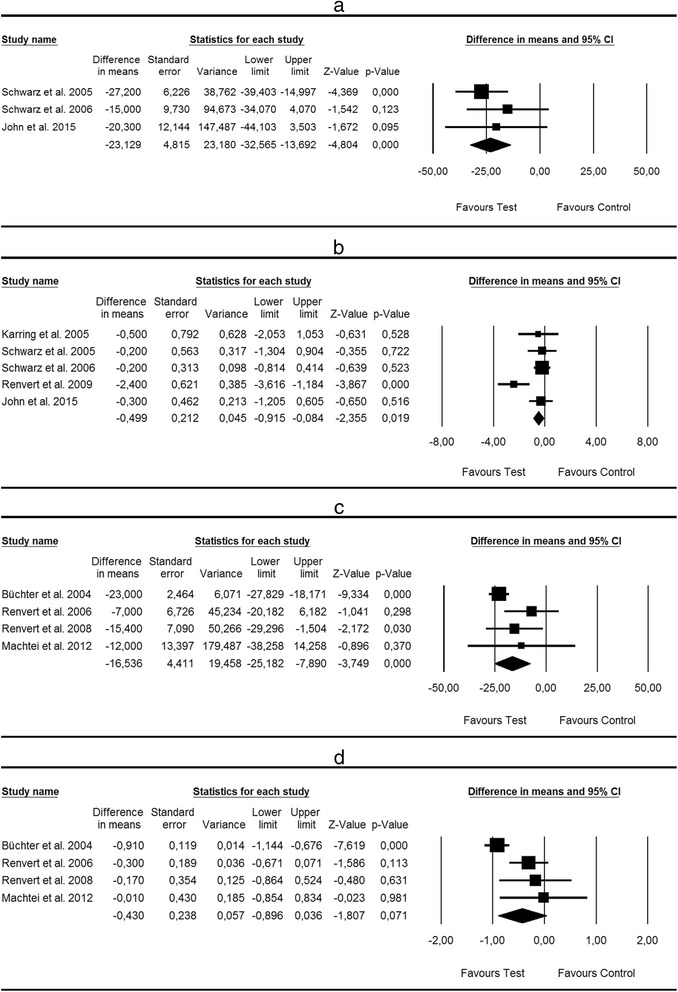
Forest plot indicating weighted mean difference (95 % CI) in the reduction of primary outcomes following nonsurgical treatment of peri-implantitis. a Alternative measures for biofilm removal—BOP. b Alternative measures for biofilm removal—PD. c Adjunctive antibiotic therapy—BOP. d Adjunctive antiseptic/antibiotic therapy—PD
Egger’s linear regression method revealed symmetrical plots for changes in BOP (p = 0.41) and PD (p = 0.39) thus suggesting the absence of any publication bias.
Nonsurgical treatment of peri-implantitis—adjunctive antiseptic/antibiotic therapy
Based on four studies [23, 26, 29, 32], WMD in BOP scores between test and control groups amounted to −16.53 % [SE = 4.41; p < 0.001; 95 % CI (−25.18, −7.89)] favouring local antibiotic therapy as an adjunct to mechanical debridement (p value for heterogeneity: 0.113, I2 = 49.77 % = moderate heterogeneity) (Fig. 3c). WMD in PD scores between test and control groups amounted to −0.829 mm [SE = 0.51; p > 0.05; 95 % CI (−1.84, 0.18)] not favouring antiseptic/antibiotic therapy as an adjunct to mechanical debridement (p value for heterogeneity: 0.000, I2 = 87.37 % = considerable heterogeneity) (Fig. 3d).
Egger’s linear regression method revealed symmetrical plots for changes in BOP (p = 0.17) and PD (p = 0.07) thus suggesting the absence of any publication bias.
Surgical treatment of peri-implantitis - alternative measures for surface decontamination
Based on two studies [38, 39], WMD in BOP and PD scores between test and control groups amounted to 5.61 % [SE = 7.68; p > 0.05; 95 % CI (−9.44, 20.68)] and 0.22 mm [SE = 0.22; p > 0.05; 95 % CI (−0.20, 0.65)] not favouring alternative (i.e. CHX + CPC) over conventional (i.e. CHX) measures for surface decontamination (p value for heterogeneity: 0.76, I2 = 0.0 % = low heterogeneity; 0.60, I2 = 0.0 % = low heterogeneity, respectively) (Fig. 4a, b).
Fig. 4.
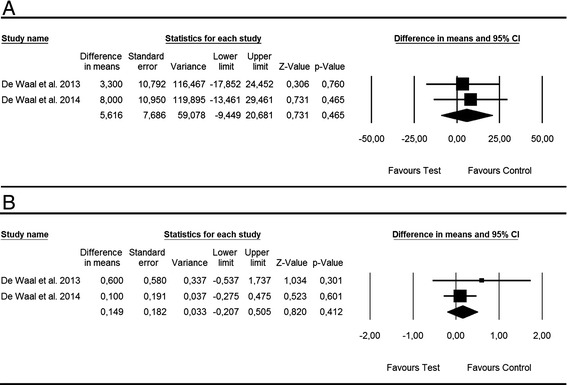
Forest plot indicating weighted mean difference (95 % CI) in the reduction of primary outcomes following surgical treatment of peri-implantitis. a Alternative measures for surface decontamination—BOP. b Alternative measures for surface decontamination—PD
Discussion
The present systematic review and meta-analysis was conducted to address the following focused question: “In patients with peri-implant mucositis and peri-implantitis, what is the efficacy of nonsurgical (i.e. referring to peri-implant mucositis and peri-implantitis) and surgical (i.e. referring to peri-implantitis) treatments with alternative or adjunctive measures on changing signs of inflammation compared with conventional nonsurgical and surgical treatments alone?”.
Basically, the literature search revealed that only a few studies considered appropriate test and control treatments needed to address the aforementioned focused question. In particular, this was true for 8 (7 RCTs and 1 CCT) [15–22] and 9 (9 RCTs) [23–26, 28, 29, 31–33] studies reporting on the nonsurgical treatment of peri-implant mucositis and peri-implantitis, as well as 5 RCTs [38, 39, 41, 42, 55] reporting on the surgical treatment of peri-implantitis. In addition, 5 RCTs and 4 CCTs not implementing appropriate control measures (i.e. mechanical/ultrasonic debridement or open flap debridement alone) but reporting on changes in primary outcomes were included for the estimation of the overall efficacy (referring to WM changes in BOP and PD scores) of nonsurgical [30, 34] and surgical [40, 43, 44, 46, 48, 52, 53] treatments of peri-implantitis. Moreover, it must be emphasized that the percentage across all included studies for high risk of bias items was 34.1 %, thus pointing to a need to improve the quality of reporting in future studies.
Within these limitations, the current data synthesis revealed that for the nonsurgical treatment of peri-implant mucositis, WMD in BOP [16, 17, 19, 22] and PD [15, 17, 19, 20, 22] scores amounted to −8.16 % and −0.15 mm, not favouring local antiseptic or antibiotic (i.e. local and systemic) therapy as an adjunct to mechanical debridement alone. Basically, these data corroborate the findings of a recent systematic review and meta-analysis, also indicating that adjunctive therapy may not improve the efficacy of professionally administered plaque removal in reducing BOP (i.e. local antiseptic or local/systemic antibiotics), GI and PD (i.e. local antiseptics, systemic antibiotics, air abrasive device) scores at mucositis sites [9]. When considering the present narrative data synthesis on the adjunctive [15] or alternative use of glycine powder air polishing [21], it was also noted that this device did not reveal any major improvements in BI/BOP scores or disease resolution over the respective control measures. In this context, it must be emphasized that BOP is the key parameter for the diagnosis of peri-implant mucositis [1], and the “resolution of peri-implant mucosal inflammation as evidenced by the absence of BOP” was the suggested endpoint following nonsurgical treatment of mucositis lesions [13]. All these data, taken together with the present findings support the view that OHI and mechanical debridement with or without polishing tools may be defined as a current standard of care for the management of peri-implant mucositis [6].
In contrast, for the nonsurgical treatment of peri-implantitis, WMD in BOP scores amounted to −16.53 % [23, 26, 29, 32] and 23.12 % [24, 25, 27], thus favouring either adjunctive local antibiotic therapy or alternative measures for plaque removal (i.e. Er:YAG laser or glycine powder air polishing) over respective control treatments. However, these improvements were not observed when analysing WMD in PD scores between test and control groups. Basically, these observations were also supported by the differences in the estimated WM BOP (31.12 vs. 42.85 %) and PD (0.71 vs. 0.87 mm) reductions noted following nonsurgical treatment of peri-implantitis using either conventional [23–27] or alternative/adjunctive measures (i.e. air polishing, aPDT, CHX chip, doxycycline, Er:YAG laser) [23–27], respectively.
Since the suggested endpoint following nonsurgical treatment of peri-implantitis is a “composite outcome of disease resolution including the absence of deep PD with bleeding and suppuration” [13], one has to critically emphasize the limited efficacy at “deep sites”. In particular, several studies reported on increasing BOP scores between 3 and 12 months following nonsurgical treatment of “severe” peri-implantitis sites using either mechanical debridement, adjunctive aPDT, Er:YAG laser monotherapy or glycine powder air polishing. The efficacy of all treatment procedures investigated was higher at “moderately” deep sites [24, 27, 33, 59]. In this context, however, one also has to realize that PD scores at implant sites may be influenced by a variety of different local factors, including the soft tissue thickness, vertical implant positioning, or a specific design of the implant-abutment connection (e.g. platform-switching). Accordingly, the classification of a “deep” pocket needs to be accomplished on an individual basis and disease severity should also consider “proportions of affected implants per patient in the presence of multiple implants” [6].
When further analysing the present results, it was also noted that nonsurgical treatment of peri-implantitis commonly failed to result in major microbiological improvements [32, 34–37], thus potentially explaining the frequency of residual BOP scores at respective sites.
At the time being, there is a lack of clinical studies aimed at comparing the efficacy of nonsurgical and surgical treatments of peri-implantitis. However, a preclinical study employing the ligature model has indicated that open flap debridement was associated with significant histological improvements in osseous defect fill and establishment of a new bone-to-implant when compared with nonsurgical treatments. The latter outcome was mainly influenced by the method of surface decontamination [60]. Accordingly, a “proven method of decontaminating the implant surface” has been defined as a critical component in surgical therapy [13]. However, the present qualitative and quantitative analysis has indicated that the clinical, radiographical and microbiological outcomes following either open flap debridement or surgical augmentative therapy were not influenced by the decontamination protocol, including chemical or photothermal (i.e. carbon dioxide, diode- or Er:YAG laser radiation) approaches [38–41, 49, 50, 52]. Moreover, two RCTs [38, 39] reported on an additional bone loss at 6 and 12 months after open flap debridement, thus indicating that disease resolution (i.e. “absence of deep probing pocket depths with bleeding and suppuration and no additional bone loss”) [13] was commonly not achieved.
The present data synthesis also revealed a lack of RCTs/CCTs implementing appropriate test and control groups to assess the efficacy of adjunctive resective or augmentative measures over open flap debridement alone. The available studies have indicated that resective surgery (i.e. apical re-positioned flap + bone contouring) + implantoplasty was more effective in obtaining and maintaining disease resolution over resective surgery alone [42, 57]. In contrast, surgical augmentative therapy of the intrabony defect component using porous titanium granules was associated with a significantly higher radiographic defect fill, but failed to improve a reduction in mean BOP and PD scores over the control treatment [55]. When considering the estimated WM BOP (50.73 vs. 34.81 %) [38, 39, 43, 49, 51, 53, 55] and PD (2.20 vs. 1.75 mm) [38–40, 43–55] reductions, the clinical outcomes obtained following adjunctive augmentative therapy tended to be improved when compared with surgical measures alone. However, it has to be realized that for the data synthesis in the latter group, surgical procedures with and without soft tissue resection were combined, and therefore, the interpretation of the overall performance of surgical measures without augmentative measures on PD reductions is difficult. Moreover, obvious variations in the surgical procedures, including different decontamination protocols, administration of prophylactic systemic antibiotics, and modes of healing (i.e. open vs. submerged) may not allow for a direct comparison of these estimated outcomes. Basically, the estimated WM BOP and PD reductions corroborate those calculations reported in a recent systematic review on reconstructive procedures for the management of peri-implantitis. When also case series were included in the meta-analysis, these values amounted to 45.8 and 2.97 mm [61]. Furthermore, the present qualitative analysis of the available data on surgical augmentative therapy have indicated, that the outcomes of therapy may be influenced by several local factors, mainly including the physicochemical properties of the bone filler [43, 48, 51, 54], the defect configuration [53], as well as implant surface characteristics [44]. Any beneficial effect of a resorbable synthetic barrier membrane could not be identified [45–47]. Nevertheless, the available evidence did not allow for any conclusive statements on the potential superiority of any particular augmentation protocol.
Finally, it must be emphasized that laser therapy, the application of bone grafts and barrier membranes were reported to be associated with the highest cost-effectiveness ratio (i.e. costs and proportions of lost implants) among 11 treatment procedures investigated [62].
Conclusions
While OHI + mechanical debridement alone was found to be effective for the management of peri-implant mucositis, alternative/adjunctive measures may improve the efficacy over/of conventional treatments at peri-implantitis sites. Adjunctive resective and/or augmentative measures are promising; however, their beneficial effect on the clinical outcome of surgical treatments needs to be further investigated.
Acknowledgements
The authors appreciate the additional information provided during the process of “contact” with the following authors:
Herbert Deppe, Department of Oral and Maxillofacial Surgery at the Klinikum Rechts Der Isar, Technische Universität München, Munich, Germany; Eli Machtei, Department of Periodontology, School of Graduate Dentistry, Rambam HCC, Technion - Israeli Institute of Technology, Haifa, Israel; Giovanni Salvi, Department of Periodontology, School of Dental Medicine, University of Bern, Bern, Switzerland; Yvonne de Waal, Center for Dentistry and Oral Hygiene, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands.
Abbreviations
- aPDT
antimicrobial photodynamic therapy
- BOP
bleeding on probing
- CAL
clinical attachment level
- CCD
controlled clinical study
- CHX
chlorhexidine digluconate
- Er:YAG laser
erbium-doped yttrium aluminum garnet laser
- GI
gingival index
- mBI
(modified) bleeding index
- MMP
matrixmetalloproteinase
- MR
mucosal recession
- OHI
oral hygiene instructions
- PD
probing pocket depth
- RCT
randomized controlled clinical study
- WM
weighted mean changes
- WMD
weighted mean differences
Footnotes
Competing interests
Frank Schwarz and Jürgen Becker received lecture fees and/or unrestricted research grants from Geistlich Biomaterials, Rooth, Switzerland; Osteology Foundation, Lucerne, Switzerland; EMS, Nyon, Switzerland. Frank Schwarz, Andrea Schmucker and Jürgen Becker declare that they have no competing interests related to this systematic review.
Authors’ contributions
FS and JB have made substantial contributions to study conception and interpretation of data as well as manuscript preparation. AS and FS were involved in the literature search and data management as well as the statistical analysis. All authors read and approved the final manuscript.
References
- 1.Lang NP, Berglundh T. Periimplant diseases: where are we now? Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38(Suppl 11):178–81. doi: 10.1111/j.1600-051X.2010.01674.x. [DOI] [PubMed] [Google Scholar]
- 2.Derks J, Tomasi C. Peri-implant health and disease. A systematic review of current epidemiology. J Clin Periodontol. 2015;42 Suppl 16:158–71. doi: 10.1111/jcpe.12334. [DOI] [PubMed] [Google Scholar]
- 3.Renvert S, Polyzois I. Risk indicators for peri-implant mucositis: a systematic literature review. J Clin Periodontol. 2015;42(Suppl 16):172–86. doi: 10.1111/jcpe.12346. [DOI] [PubMed] [Google Scholar]
- 4.Schwarz F, Mihatovic I, Golubovich V, Eick S, Iglhaut T, Becker J. Experimental peri-implant mucositis at different implant surfaces. J Clin Periodontol. 2014;41:513–20. doi: 10.1111/jcpe.12240. [DOI] [PubMed] [Google Scholar]
- 5.Costa FO, Takenaka-Martinez S, Cota LO, Ferreira SD, Silva GL, Costa JE. Peri-implant disease in subjects with and without preventive maintenance: a 5-year follow-up. J Clin Periodontol. 2012;39:173–81. doi: 10.1111/j.1600-051X.2011.01819.x. [DOI] [PubMed] [Google Scholar]
- 6.Jepsen S, Berglundh T, Genco R, Aass AM, Demirel K, Derks J, et al. Primary prevention of peri-implantitis: managing peri-implant mucositis. J Clin Periodontol. 2015;42(Suppl 16):152–7. doi: 10.1111/jcpe.12369. [DOI] [PubMed] [Google Scholar]
- 7.Heitz-Mayfield LJ, Mombelli A. The therapy of peri-implantitis: a systematic review. Int J Oral Maxillofac Implants. 2014;29(Suppl):325–45. doi: 10.11607/jomi.2014suppl.g5.3. [DOI] [PubMed] [Google Scholar]
- 8.Klinge B, Meyle J. Working G. Peri-implant tissue destruction. The Third EAO Consensus Conference 2012. Clin Oral Implants Res. 2012;23 Suppl 6:108–10. doi: 10.1111/j.1600-0501.2012.02555.x. [DOI] [PubMed] [Google Scholar]
- 9.Schwarz F, Becker K, Sager M. Efficacy of professionally administered plaque removal with or without adjunctive measures for the treatment of peri-implant mucositis. A systematic review and meta-analysis. J Clin Periodontol. 2015;42 Suppl 16:202–13. doi: 10.1111/jcpe.12349. [DOI] [PubMed] [Google Scholar]
- 10.Claffey N, Clarke E, Polyzois I, Renvert S. Surgical treatment of peri-implantitis. J Clin Periodontol. 2008;35:316–32. doi: 10.1111/j.1600-051X.2008.01277.x. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller SA, Forrest JL. Enhancing your practice through evidence-based decision making: PICO, learning how to ask good questions. J Evid Base Dent Pract. 2001;1:136–41. doi: 10.1016/S1532-3382(01)70024-3. [DOI] [Google Scholar]
- 13.Sanz M, Chapple IL, Working Group 4 of the VEWoP Clinical research on peri-implant diseases: consensus report of Working Group 4. J Clin Periodontol. 2012;39 Suppl 12:202–6. doi: 10.1111/j.1600-051X.2011.01837.x. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://www.cochrane-handbook.org. 2011.
- 15.Ji YJ, Tang ZH, Wang R, Cao J, Cao CF, Jin LJ. Effect of glycine powder air-polishing as an adjunct in the treatment of peri-implant mucositis: a pilot clinical trial. Clin Oral Implants Res. 2014;25:683–9. doi: 10.1111/clr.12123. [DOI] [PubMed] [Google Scholar]
- 16.Schenk G, Flemmig TF, Betz T, Reuther J, Klaiber B. Controlled local delivery of tetracycline HCl in the treatment of periimplant mucosal hyperplasia and mucositis. A controlled case series. Clin Oral Implants Res. 1997;8:427–33. doi: 10.1034/j.1600-0501.1997.080510.x. [DOI] [PubMed] [Google Scholar]
- 17.Thone-Mühling M, Swierkot K, Nonnenmacher C, Mutters R, Flores-de-Jacoby L, Mengel R. Comparison of two full-mouth approaches in the treatment of peri-implant mucositis: a pilot study. Clin Oral Implants Res. 2010;21:504–12. doi: 10.1111/j.1600-0501.2009.01861.x. [DOI] [PubMed] [Google Scholar]
- 18.De Siena F, Corbella S, Taschieri S, Del Fabbro M, Francetti L. Adjunctive glycine powder air-polishing for the treatment of peri-implant mucositis: an observational clinical trial. Int J Dent Hyg. 2014;14. doi:10.1111/idh.12114. [DOI] [PubMed]
- 19.Hallström H, Persson GR, Lindgren S, Olofsson M, Renvert S. Systemic antibiotics and debridement of peri-implant mucositis. A randomized clinical trial. J Clin Periodontol. 2012;39:574–81. doi: 10.1111/j.1600-051X.2012.01884.x. [DOI] [PubMed] [Google Scholar]
- 20.Porras R, Anderson GB, Caffesse R, Narendran S, Trejo PM. Clinical response to 2 different therapeutic regimens to treat peri-implant mucositis. J Periodontol. 2002;73:1118–25. doi: 10.1902/jop.2002.73.10.1118. [DOI] [PubMed] [Google Scholar]
- 21.Riben Grundström C, Norderyd O, Andre U, Renvert S. Treatment of peri-implant mucositis using a glycine powder air-polishing or ultrasonic device. A randomized clinical trial. J Clin Periodontol. 2015;42:462–9. doi: 10.1111/jcpe.12395. [DOI] [PubMed] [Google Scholar]
- 22.Strooker H, Rohn S, Van Winkelhoff AJ. Clinical and microbiologic effects of chemical versus mechanical cleansing in professional supportive implant therapy. Int J Oral Maxillofac Implants. 1998;13:845–50. [PubMed] [Google Scholar]
- 23.Machtei EE, Frankenthal S, Levi G, Elimelech R, Shoshani E, Rosenfeld O, et al. Treatment of peri-implantitis using multiple applications of chlorhexidine chips: a double-blind, randomized multi-centre clinical trial. J Clin Periodontol. 2012;39:1198–205. doi: 10.1111/jcpe.12006. [DOI] [PubMed] [Google Scholar]
- 24.Schwarz F, Bieling K, Bonsmann M, Latz T, Becker J. Nonsurgical treatment of moderate and advanced periimplantitis lesions: a controlled clinical study. Clin Oral Investig. 2006;10:279–88. doi: 10.1007/s00784-006-0070-3. [DOI] [PubMed] [Google Scholar]
- 25.Schwarz F, Sculean A, Rothamel D, Schwenzer K, Georg T, Becker J. Clinical evaluation of an Er : YAG laser for nonsurgical treatment of peri-implantitis: a pilot study. Clin Oral Implants Res. 2005;16:44–52. doi: 10.1111/j.1600-0501.2004.01051.x. [DOI] [PubMed] [Google Scholar]
- 26.Büchter A, Meyer U, Kruse-Losler B, Joos U, Kleinheinz J. Sustained release of doxycycline for the treatment of peri-implantitis: randomised controlled trial. Br J Oral Maxillofac Surg. 2004;42:439–44. doi: 10.1016/j.bjoms.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 27.John G, Sahm N, Becker J, Schwarz F. Nonsurgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine. Twelve-month follow-up of a prospective, randomized, controlled clinical study. Clin Oral Investig. 2015. doi:10.1007/s00784-015-1406-7. [DOI] [PubMed]
- 28.Karring ES, Stavropoulos A, Ellegaard B, Karring T. Treatment of peri-implantitis by the VectorR system. A pilot study. Clin Oral Implants Res. 2005;16:288–93. doi: 10.1111/j.1600-0501.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 29.Renvert S, Lessem J, Dahlen G, Renvert H, Lindahl C. Mechanical and repeated antimicrobial therapy using a local drug delivery system in the treatment of peri-implantitis: a randomized clinical trial. J Periodontol. 2008;79:836–44. doi: 10.1902/jop.2008.070347. [DOI] [PubMed] [Google Scholar]
- 30.Renvert S, Lindahl C, Roos Jansker A-M, Persson GR. Treatment of peri-implantitis using an Er:YAG laser or an air-abrasive device: a randomized clinical trial. J Clin Periodontol. 2011;38:65–73. doi: 10.1111/j.1600-051X.2010.01646.x. [DOI] [PubMed] [Google Scholar]
- 31.Renvert S, Samuelsson E, Lindahl CPersson GR. Mechanical non-surgical treatment of peri-implantitis: a double-blind randomized longitudinal clinical study. I: clinical results. J Clin Periodontol. 2009;36:604–9. doi: 10.1111/j.1600-051X.2009.01421.x. [DOI] [PubMed] [Google Scholar]
- 32.Renvert S, Lessem J, Dahlen G, Lindahl C, Svensson M. Topical minocycline microspheres versus topical chlorhexidine gel as an adjunct to mechanical debridement of incipient peri-implant infections: a randomized clinical trial. J Clin Periodontol. 2006;33:362–9. doi: 10.1111/j.1600-051X.2006.00919.x. [DOI] [PubMed] [Google Scholar]
- 33.Sahm N, Becker J, Santel T, Schwarz F. Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: a prospective, randomized, controlled clinical study. J Clin Periodontol. 2011;38:872–8. doi: 10.1111/j.1600-051X.2011.01762.x. [DOI] [PubMed] [Google Scholar]
- 34.Schär D, Ramseier CA, Eick S, Arweiler NB, Sculean A, Salvi GE. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: six-month outcomes of a prospective randomized clinical trial. Clin Oral Implants Res. 2013;24:104–10. doi: 10.1111/j.1600-0501.2012.02494.x. [DOI] [PubMed] [Google Scholar]
- 35.Persson GR, Samuelsson E, Lindahl C, Renvert S. Mechanical non-surgical treatment of peri-implantitis: a single-blinded randomized longitudinal clinical study. II Microbiological results. J Clin Periodontol. 2010;37:563–73. doi: 10.1111/j.1600-051X.2010.01561.x. [DOI] [PubMed] [Google Scholar]
- 36.Persson GR, Roos-Jansker AM, Lindahl C, Renvert S. Microbiologic results after non-surgical erbium-doped:yttrium, aluminum, and garnet laser or air-abrasive treatment of peri-implantitis: a randomized clinical trial. J Periodontol. 2011;82:1267–78. doi: 10.1902/jop.2011.100660. [DOI] [PubMed] [Google Scholar]
- 37.Bassetti M, Schär D, Wicki B, Eick S, Ramseier CA, Arweiler NB, et al. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin Oral Implants Res. 2013;25:279–87. doi: 10.1111/clr.12155. [DOI] [PubMed] [Google Scholar]
- 38.de Waal YC, Raghoebar GM, Huddleston Slater JJ, Meijer HJ, Winkel EG, van Winkelhoff AJ. Implant decontamination during surgical peri-implantitis treatment: a randomized, double-blind, placebo-controlled trial. J Clin Periodontol. 2013;40:186–95. doi: 10.1111/jcpe.12034. [DOI] [PubMed] [Google Scholar]
- 39.de Waal YC, Raghoebar GM, Meijer HJ, Winkel EG, van Winkelhoff AJ. Implant decontamination with 2% chlorhexidine during surgical peri-implantitis treatment: a randomized, double-blind, controlled trial. Clin Oral Implants Res. 2014. doi:10.1111/clr.12419. [DOI] [PubMed]
- 40.Deppe H, Horch HH, Neff A. Conventional versus CO2 laser-assisted treatment of peri-implant defects with the concomitant use of pure-phase beta-tricalcium phosphate: a 5-year clinical report. Int J Oral Maxillofac Implants. 2007;22:79–86. [PubMed] [Google Scholar]
- 41.Papadopoulos CA, Vouros I, Menexes G, Konstantinidis A. The utilization of a diode laser in the surgical treatment of peri-implantitis. A randomized clinical trial. Clin Oral Investig. 2015. doi:10.1007/s00784-014-1397-9. [DOI] [PubMed]
- 42.Romeo E, Ghisolfi M, Murgolo N, Chiapasco M, Lops D, Vogel G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part I: clinical outcome. Clin Oral Implants Res. 2005;16:9–18. doi: 10.1111/j.1600-0501.2004.01084.x. [DOI] [PubMed] [Google Scholar]
- 43.Aghazadeh A, Persson GR, Renvert S. A single-centre randomized controlled clinical trial on the adjunct treatment of intra-bony defects with autogenous bone or a xenograft: results after 12 months. J Clin Periodontol. 2012;39(7):666–73. [DOI] [PubMed]
- 44.Roccuzzo M, Bonino F, Bonino L, Dalmasso P. Surgical therapy of peri-implantitis lesions by means of a bovine-derived xenograft: comparative results of a prospective study on two different implant surfaces. Surgical treatment of peri-implantitis. J Clin Periodontol. 2011;38(8):738–45. [DOI] [PubMed]
- 45.Roos-Jansaker AM, Persson GR, Lindahl C, Renvert S. Surgical treatment of peri-implantitis using a bone substitute with or without a resorbable membrane: a 5-year follow-up. J Clin Periodontol. 2014;41:1108–14. doi: 10.1111/jcpe.12308. [DOI] [PubMed] [Google Scholar]
- 46.Roos-Jansaker AM, Renvert H, Lindahl C, Renvert S. Surgical treatment of peri-implantitis using a bone substitute with or without a resorbable membrane: a prospective cohort study. J Clin Periodontol. 2007;34:625–32. doi: 10.1111/j.1600-051X.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- 47.Roos-Jansker A-M, Lindahl C, Pesson GR, Renvert S. Long-term stability of surgical bone regenerative procedures of peri-implantitis lesions in a prospective case–control study over 3 years. J Clin Periodontol. 2011;38:590–7. doi: 10.1111/j.1600-051X.2011.01729.x. [DOI] [PubMed] [Google Scholar]
- 48.Schwarz F, Bieling K, Latz T, Nuesry E, Becker J. Healing of intrabony peri-implantitis defects following application of a nanocrystalline hydroxyapatite (Ostim) or a bovine-derived xenograft (Bio-Oss) in combination with a collagen membrane (Bio-Gide). A case series. J Clin Periodontol. 2006;33:491–9. doi: 10.1111/j.1600-051X.2006.00936.x. [DOI] [PubMed] [Google Scholar]
- 49.Schwarz F, Hegewald A, John G, Sahm N, Becker J. Four-year follow-up of combined surgical therapy of advanced peri-implantitis evaluating two methods of surface decontamination. J Clin Periodontol. 2013;40:962–7. doi: 10.1111/jcpe.12143. [DOI] [PubMed] [Google Scholar]
- 50.Schwarz F, John G, Mainusch S, Sahm N, Becker J. Combined surgical therapy of peri-implantitis evaluating two methods of surface debridement and decontamination. A two-year clinical follow up report. J Clin Periodontol. 2012;39:789–97. doi: 10.1111/j.1600-051X.2012.01867.x. [DOI] [PubMed] [Google Scholar]
- 51.Schwarz F, Sahm N, Bieling K, Becker J. Surgical regenerative treatment of peri-implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane: a four-year clinical follow-up report. J Clin Periodontol. 2009;36:807–14. doi: 10.1111/j.1600-051X.2009.01443.x. [DOI] [PubMed] [Google Scholar]
- 52.Schwarz F, Sahm N, Iglhaut G, Becker J. Impact of the method of surface debridement and decontamination on the clinical outcome following combined surgical therapy of peri-implantitis: a randomized controlled clinical study. J Clin Periodontol. 2011;38:276–84. doi: 10.1111/j.1600-051X.2010.01690.x. [DOI] [PubMed] [Google Scholar]
- 53.Schwarz F, Sahm N, Schwarz K, Becker J. Impact of defect configuration on the clinical outcome following surgical regenerative therapy of peri-implantitis. J Clin Periodontol. 2010;37:449–55. doi: 10.1111/j.1600-051X.2010.01540.x. [DOI] [PubMed] [Google Scholar]
- 54.Schwarz F, Sculean A, Bieling K, Ferrari D, Rothamel D, Becker J. Two-year clinical results following treatment of peri-implantitis lesions using a nanocrystalline hydroxyapatite or a natural bone mineral in combination with a collagen membrane. J Clin Periodontol. 2008;35:80–7. doi: 10.1111/j.1600-051X.2007.01168.x. [DOI] [PubMed] [Google Scholar]
- 55.Wohlfahrt JC, Lyngstadaas SP, Ronold HJ, Saxegaard E, Ellingsen JE, Karlsson S, et al. Porous titanium granules in the surgical treatment of peri-implant osseous defects: a randomized clinical trial. Int J Oral Maxillofac Implants. 2012;27:401–10. [PubMed] [Google Scholar]
- 56.Schwarz F, Herten M, Sager M, Bieling K, Sculean A, Becker J. Comparison of naturally occurring and ligature-induced peri-implantitis bone defects in humans and dogs. Clin Oral Implants Res. 2007;18:161–70. doi: 10.1111/j.1600-0501.2006.01320.x. [DOI] [PubMed] [Google Scholar]
- 57.Romeo E, Lops D, Chiapasco M, Ghisolfi M, Vogel G. Therapy of peri-implantitis with resective surgery. A 3-year clinical trial on rough screw-shaped oral implants. Part II: radiographic outcome. Clin Oral Implants Res. 2007;18:179–87. doi: 10.1111/j.1600-0501.2006.01318.x. [DOI] [PubMed] [Google Scholar]
- 58.Wohlfahrt JC, Aass AM, Granfeldt F, Lyngstadaas SP, Reseland JE. Sulcus fluid bone marker levels and the outcome of surgical treatment of peri-implantitis. J Clin Periodontol. 2014;41:424–31. doi: 10.1111/jcpe.12229. [DOI] [PubMed] [Google Scholar]
- 59.Deppe H, Mucke T, Wagenpfeil S, Kesting M, Sculean A. Nonsurgical antimicrobial photodynamic therapy in moderate vs severe peri-implant defects: a clinical pilot study. Quintessence Int. 2013;44:609–18. doi: 10.3290/j.qi.a29505. [DOI] [PubMed] [Google Scholar]
- 60.Schwarz F, Jepsen S, Herten M, Sager M, Rothamel D, Becker J. Influence of different treatment approaches on non-submerged and submerged healing of ligature induced peri-implantitis lesions: an experimental study in dogs. J Clin Periodontol. 2006;33:584–95. doi: 10.1111/j.1600-051X.2006.00956.x. [DOI] [PubMed] [Google Scholar]
- 61.Khoshkam V, Chan HL, Lin GH, Mac Eachern MP, Monje A, Suarez F, et al. Reconstructive procedures for treating peri-implantitis: a systematic review. J Dent Res. 2013;92:131S–8. doi: 10.1177/0022034513509279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwendicke F, Tu YK, Stolpe M. Preventing and treating peri-implantitis: a cost-effectiveness analysis. J Periodontol. 2015;9:1–15. doi: 10.1902/jop.2015.150071. [DOI] [PubMed] [Google Scholar]
- 63.Lavigne SE, Krust-Bray KS, Williams KB, Killoy WJ, Theisen F. Effects of subgingival irrigation with chlorhexidine on the periodontal status of patients with HA-coated integral dental implants. Int J Oral Maxillofac Implants. 1994;9:156–62. [PubMed] [Google Scholar]
- 64.Ciancio SG, Lauciello F, Shibly O, Vitello M, Mather M. The effect of an antiseptic mouthrinse on implant maintenance: plaque and peri-implant gingival tissues. J Periodontol. 1995;66:962–5. doi: 10.1902/jop.1995.66.11.962. [DOI] [PubMed] [Google Scholar]
- 65.Felo A, Shibly O, Ciancio SG, Lauciello FR, Ho A. Effects of subgingival chlorhexidine irrigation on peri-implant maintenance. Am J Dent. 1997;10:107–10. [PubMed] [Google Scholar]
- 66.Bach G, Neckel C, Mall C, Krekeler G. Conventional versus laser-assisted therapy of periimplantitis: a five-year comparative study. Implant Dent. 2000;9:247–51. doi: 10.1097/00008505-200009030-00010. [DOI] [PubMed] [Google Scholar]
- 67.Dörtbudak O, Haas R, Bernhart T, Mailath-Pokorny G. Lethal photosensitization for decontamination of implant surfaces in the treatment of peri-implantitis. Clin Oral Implants Res. 2001;12:104–8. doi: 10.1034/j.1600-0501.2001.012002104.x. [DOI] [PubMed] [Google Scholar]
- 68.Khoury F, Buchmann R. Surgical therapy of peri-implant disease: a 3-year follow-up study of cases treated with 3 different techniques of bone regeneration. J Periodontol. 2001;72:1498–508. doi: 10.1902/jop.2001.72.11.1498. [DOI] [PubMed] [Google Scholar]
- 69.Roos-Jansaker AM, Renvert H, Lindahl C, Renvert S. Submerged healing following surgical treatment of peri- implantitis: a case series. J Periodontol. 2001;72:1498–508. doi: 10.1902/jop.2001.72.11.1498. [DOI] [PubMed] [Google Scholar]
- 70.Duarte PM, de Mendonca AC, Maximo MB, Santos VR, Bastos MF, Nociti FH. Effect of anti-infective mechanical therapy on clinical parameters and cytokine levels in human peri-implant diseases. J Periodontol. 2009;80:234–43. doi: 10.1902/jop.2009.070672. [DOI] [PubMed] [Google Scholar]
- 71.Maximo MB, de Mendonca AC, Renata Santos V, Figueiredo LC, Feres M, Duarte PM. Short-term clinical and microbiological evaluations of peri-implant diseases before and after mechanical anti-infective therapies. Clin Oral Implants Res. 2009;20:99–108. doi: 10.1111/j.1600-0501.2008.01618.x. [DOI] [PubMed] [Google Scholar]
- 72.Ramberg P, Lindhe J, Botticelli D, Botticelli A. The effect of a triclosan dentifrice on mucositis in subjects with dental implants: a six-month clinical study. J Clin Dent. 2009;20:103–7. [PubMed] [Google Scholar]
- 73.Corbella S, Del Fabbro M, Taschieri S, De Siena F, Francetti L. Clinical evaluation of an implant maintenance protocol for the prevention of peri-implant diseases in patients treated with immediately loaded full-arch rehabilitations. Int J Dent Hyg. 2011;9:216–22. doi: 10.1111/j.1601-5037.2010.00489.x. [DOI] [PubMed] [Google Scholar]
- 74.Heitz-Mayfield LJ, Salvi GE, Botticelli D, Mombelli A, Faddy M, Lang NP, et al. Anti-infective treatment of peri-implant mucositis: a randomised controlled clinical trial. Clin Oral Implants Res. 2011;22:237–41. doi: 10.1111/j.1600-0501.2010.02078.x. [DOI] [PubMed] [Google Scholar]
- 75.De Angelis N, Felice P, Grusovin MG, Camurati A, Esposito M. The effectiveness of adjunctive light-activated disinfection (LAD) in the treatment of peri-implantitis: 4-month results from a multicentre pragmatic randomised controlled trial. Eur J Oral Implantol. 2012;5:321–31. [PubMed] [Google Scholar]
- 76.Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, Ramseier CA. Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res. 2012;23:182–90. doi: 10.1111/j.1600-0501.2011.02220.x. [DOI] [PubMed] [Google Scholar]
- 77.De Siena F, Francetti L, Corbella S, Taschieri S, Del Fabbro M. Topical application of 1% chlorhexidine gel versus 0.2% mouthwash in the treatment of peri-implant mucositis. An observational study. Int J Dent Hyg. 2013;11:41–7. doi: 10.1111/idh.12002. [DOI] [PubMed] [Google Scholar]
- 78.McKenna DF, Borzabadi-Farahani A, Lynch E. The effect of subgingival ozone and/or hydrogen peroxide on the development of peri-implant mucositis: a double-blind randomized controlled trial. Int J Oral Maxillofac Implants. 2013;28:1483–9. doi: 10.11607/jomi.3168. [DOI] [PubMed] [Google Scholar]
- 79.Flichy-Fernandez AJ, Ata-Ali J, Alegre-Domingo T, Candel-Marti E, Ata-Ali F, Palacio JR, et al. The effect of orally administered probiotic lactobacillus reuteri-containing tablets in peri-implant mucositis: a double-blind randomized controlled trial. J Periodontal Res. 2015. doi:10.1111/jre.12264. [DOI] [PubMed]


