Abstract
A 23-year-old human immunodeficiency virus (HIV)-infected Indian woman was admitted to a tertiary care hospital with generalized erythematosus rash all over her body with difficulty in swallowing for the previous 3 days. She also presented with swelling of the lips and redness of both eyes along with nausea, anorexia, slight headache, and fever, which appeared immediately after the initiation of a new regime of antiretroviral treatment with tenofovir (300 mg once daily), lamivudine (300 mg once daily), and efavirenz (600 mg once daily). Presumptive diagnosis of efavirenz-induced Stevens–Johnson syndrome was made after excluding other causes. Efavirenz was withdrawn, followed by tenofovir and lamivudine. Supportive care was provided to the patient during her hospital stay. She recovered after 2 weeks. Thus, strict vigilance of adverse drug reaction is required in patients on a highly active antiretroviral therapy regimen.
Key Points
| Efavirenz is a non-nucleoside reverse transcriptase inhibitor that can be added to tenofovir and lamivudine as first-line antiretroviral therapy. |
| In patients taking efavirenz, 0.14 % may develop Stevens–Johnson syndrome. |
| Here, a patient with human immunodeficiency virus developed Stevens–Johnson syndrome 2 weeks after the introduction of efavirenz (600 mg once daily). |
Introduction
Stevens–Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are severe cutaneous adverse reactions frequently caused by exposure to drugs and can result in significant morbidity and mortality. SJS and TEN are rare variants of severe, adverse cutaneous drug reactions characterized by extensive painful erythematous macules, ultimately evolving to epidermal detachment and mucous membrane erosions resulting from massive apoptosis of epithelial cells [1]. The most common drugs responsible for SJS are sulfonamides [2], sulfones [3], thiacetazone [4], and antiretrovirals [5, 6] in human immunodeficiency virus (HIV)-infected individuals. Nevirapine has the highest incidence (32–48 %) [5, 6] associated with this syndrome and efavirenz was associated with less than 0.14 % of cases among the antiretroviral drugs [7]. Efavirenz, a non-nucleoside reverse transcriptase inhibitor (NNRTI), is widely prescribed as a part of the combination therapy of HIV infection because of its convenience, effectiveness, and long-term tolerability. The combination of efavirenz plus two nucleoside reverse transcriptase inhibitors is a preferred regimen for treatment-naive patients. More importantly, it can be safely combined with rifampicin and is useful in treating patients with associated tuberculosis. Rash with efavirenz is a frequent occurrence (27 %), developing usually in the first weeks of treatment, resolving spontaneously, and rarely requiring drug discontinuation [8]. SJS is characterized by the development of macules that spread quickly to form epidermal blisters, necrosis, and sloughing. SJS caused by efavirenz is very rare and infrequently reported in the literature [7, 8]. Diagnosis of SJS is usually performed by the characterization of lesions and distinctive clinical features. The mainstay of treatment is supportive. Cyclosporine, plasma exchange, intravenous immunoglobulin, and early corticosteroid pulse therapy are used in mitigating the morbidity and mortality. Mortality can be lowered with early initiation of treatment. Here, we present the case of a 23-year-old woman who developed generalised erythematosus rash following treatment with efavirenz-based highly active antiretroviral therapy.
Case Report
A female patient, aged 23 years, was admitted to a tertiary care hospital with complaints of generalised erythematosus rash all over her body with difficulty in swallowing for the previous 3 days. She had swelling of the lips and redness of eyes along with nausea, anorexia, slight headache, and fever. The rash first appeared on the face and then spread throughout her body. The rashes were erythematosus, macular, and presented symmetrically on the face and upper part of torso along with a burning sensation (Fig. 1). On examination, it was revealed that she had diffuse exfoliating exanthema with generalized bullous eruption that involved the trunk and face (Fig. 2). The rashes appeared as macules that subsequently developed into papules and vesicles. Some lesions had the appearance of a target lesion with two zones of color. Some of these lesions eventually became bullous and later ruptured, leaving denuded skin. In addition, she had facial and lip swelling (Fig. 3), oral ulceration, and difficulty opening her mouth and in swallowing. There was presence of edema, sloughing, blistering, and ulceration in mucosal examinations of the eye and mouth along with conjunctivitis.
Fig. 1.
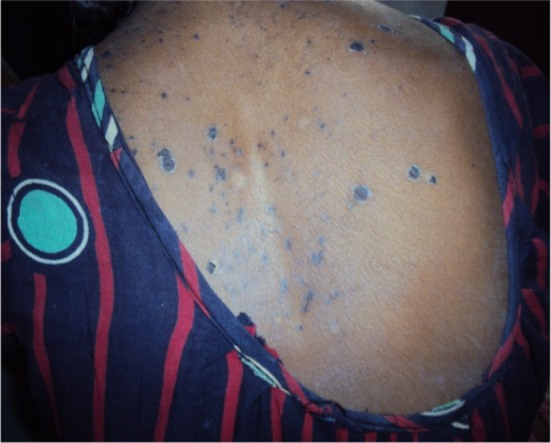
Skin rashes in Stevens–Johnson syndrome with efavirenz treatment in the upper part of the torso
Fig. 2.
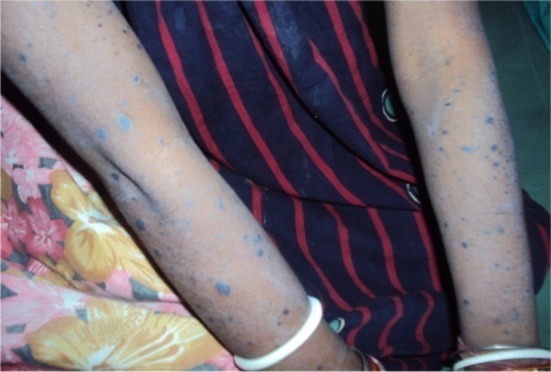
Eruption in the extremity in Stevens–Johnson syndrome with efavirenz treatment
Fig. 3.
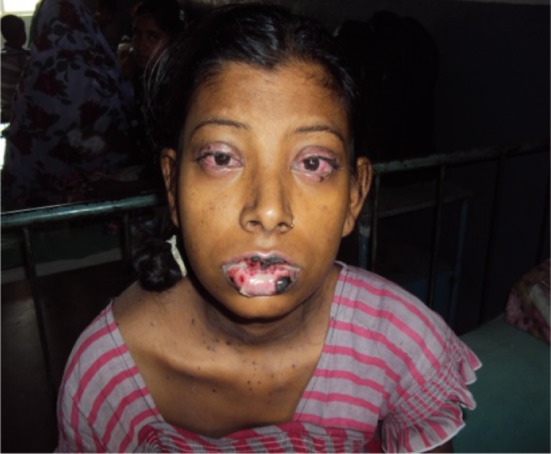
Facial and lip swelling along with conjunctivitis of the eyes in Stevens–Johnson syndrome with efavirenz treatment
On history elicitation, it was revealed that the patient had been immune compromised for the previous 6 months. She had been diagnosed with HIV infection 6 months prior with a CD4 count of 243 cells/mm3. Serologic tests for hepatitis B virus and hepatitis C virus were negative. A serologic evaluation of the patient was also performed for herpes simplex virus and the titers were not significantly elevated, thus ruling out the possibility of herpes simplex virus infection. Treatment was initiated with the antiretroviral regime, ziduvudine (300 mg twice daily), lamivudine (150 mg twice daily), and nevirapine (200 mg once daily) initially. Then, 2 months before admission, she developed severe anemia and was simultaneously diagnosed with pulmonary tuberculosis, for which anti-tuberculosis drugs were prescribed according to the CAT 1 (Category 1) regime under the directly observed treatment-short course (DOTS) strategy. The drugs were isoniazid (300 mg), rifampicin (450 mg), pyrazinamide (1500 mg), and ethambutol (1200 mg) administered three times weekly as per DOTS. The CAT 1 regimen is given to patients who are new sputum smear-positive seriously ill or new sputum smear-negative seriously ill or with new extra-pulmonary tuberculosis. Seriously ill also includes any patient, pulmonary or extra-pulmonary, who is HIV positive and declares his/her sero-status to the categorizing/treating medical officer [9]. The treatment in Category 1 consists of an intensive phase of isoniazid, rifampicin, pyrazinamide, and ethambutol administered under direct supervision three times weekly on alternate days for 2 months (24 dosages), followed by a continuation phase of isoniazid and rifampicin three times weekly on alternate days for 4 months [9]. A liver function test revealed a serum bilirubin level of 2.0 mg/dL, alanine aminotransferase of 148 U/L (normal 30–65 U/L), aspartate aminotransferase of 156 U/L (normal 15–37 U/L), and alkaline phosphatase of 188 U/L (normal 50–136). The antiretroviral regime was then changed to a combination of tenofovir (300 mg once daily), lamivudine (300 mg once daily), and efavirenz (600 mg once daily) about 2 weeks before admission. Therefore, for the last 2 weeks, the patient was taking anti-tuberculosis drugs under the CAT 1 regimen and antiretroviral drugs of a tenofovir-lamivudine-efavirenz regimen simultaneously at the time of admission with rashes. She was not taking any other drugs or antibiotics such as co-trimoxazole for the previous 2 weeks. Thus, immediately after starting with a new antiretroviral regime with tenofovir-lamivudine-efavirenz, she started developing the previously mentioned symptoms and was admitted to hospital after 2 weeks. Based on the presumptive diagnosis of SJS, efavirenz was discontinued immediately, and tenofovir and lamivudine 2 days later.
The body surface area involvement of the patient at the time of presentation was 8 %. She was treated with intravenous fluid, corticosteroids, and oral antihistamines. After treatment of the skin lesions by the topical application of mupirocin, 0.9 % NaCl, and 0.5 % AgNO3 three times a day for 14 days, the skin condition gradually improved. An ophthalmology consultation was sought for the management of ocular lesions and the mucosal lesions were managed symptomatically. Rashes disappeared after 2 weeks. The causality assessment as per the Naranjo algorithm [10] and World Health Organization-Uppsala Monitoring Centre criteria [11] revealed the adverse drug reaction to be Probable (Naranjo score 7) with efavirenz.
Discussion
SJS is an immune-complex-mediated severe hypersensitivity reaction of skin and mucous membranes. It was first recognized in 1922 by Stevens and Johnson [12]. It usually presents with flu-like symptoms, followed by the appearance of a painful red or purplish rash that spreads and may become bullous and rupture later. The typical pathognomonic lesion of SJS appears as that of a target lesion, whereas typical lesions of erythema multiforme have only two zones of color. The most commonly affected parts of the body are the palms, soles, dorsum of the hands, and extensor surfaces. It is a milder form of TEN [13].
Multiple factors may play a role in the development of SJS. Although it may be presumed to be caused by viral infections (commonly herpes simplex virus) and neoplasias (carcinomas and lymphomas), the most common cause are drugs, such as allopurinol, antibiotics, anticonvulsants, and non-steroid anti-inflammatory drugs [14]. SJS has been categorized to three forms, SJS, TEN, and SJS/TEN overlap by a consensus classification published first in 1993 [15]. All three are part of a spectrum of severe cutaneous reactions that affect skin and mucous membranes [15].
The underlying mechanism of SJS is still not clear, an idiosyncratic delayed hypersensitivity reaction (type IV) has been attributed to its pathophysiology [16]. NNRTIs such as delavirdine, efavirenz, nevirapine, and etravirine can all cause skin rash. The rash associated with NNRTIs is usually erythematous, maculopapular, and widespread [7, 8].
Early recognition of the drug reaction and discontinuation of the presumptive drug is the cornerstone of therapy in SJS. Treatment of SJS is usually to provide supportive care. There is a lack of universally accepted and specific treatments. Furthermore, the use of corticosteroids is controversial [17]; though they are effective in the initial phase, they can result in an increased risk of infection and a delay in wound healing, particularly during bullous eruption or mucosal erosion. According to some retrospective studies, intravenous immunoglobulin may be effective in stopping the progression of SJS but other studies showed limited benefit on the mortality rate or progression of the disease [14].
In our case, the patient developed SJS immediately after administration of efavirenz. According to the National AIDS Control Organization guideline [18], in HIV patients receiving the concurrent rifampicin-containing anti-tuberculosis regimen, efavirenz should be instituted for the duration of the anti-tuberculosis treatment. Perhaps, for this, efavirenz might be substituted for nevirapine. Though other antiretroviral and anti-tuberculosis drugs were also present, it was only after starting efavirenz that the symptoms of SJS developed. Tenofovir was also introduced along with efavirenz, but there was no previous report or evidence of SJS with tenofovir in the indexed literature. Thus, the causality assessment showed a stronger correlation between efavirenz and SJS rather than tenofovir and SJS. Furthermore, as anti-tuberculosis drugs were present for the previous 2 months, they could not be implicated in developing SJS in this patient. However, there is an incidence of association of SJS with efavirenz, albeit only in less than 0.14 % of cases [13, 14]. Thus, efavirenz was suspected to be implicated in developing SJS in our case.
The SCORTEN score used to prognosticate risk factors for death from SJS [19] was zero in this patient. Efavirenz was not re-challenged here but as per the World Health Organization-Uppsala Monitoring Centre (WHO-UMC) classification this case was categorized as probable. Conjunctival and vaginal swabs were tested for viral, bacterial, and fungal cultures and found to be negative. Efavirenz was withdrawn immediately. Two days later, the other antiretroviral drugs were also discontinued. The patients took 2 weeks to recover.
Conclusion
The sudden-onset positive drug history, temporal relationship, stinging eyes, difficulty in swallowing, erythematosus rash over the face and trunk, as well as cracking and fissuring of the lips with bloody crusting indicated a diagnosis of SJS. In summary, this report of efavirenz-induced SJS underscores the importance that physicians should be cautious about the fact that SJS can be developed within only a few days after ingestion of an efavirenz-based highly active antiretroviral therapy regimen. This case suggests that physicians treating individuals with HIV infection should consider a diagnosis of SJS in patients not only on a nevirapine-containing treatment regimen but in patients on an efavirenz-containing treatment presenting only with mucositis.
Acknowledgments
Sabyasachi Paik, Agnik Pal, Sukanta Sen, Netai Pramanick and Santanu K. Tripathi declare that they have no conflict of interest. No financial support was received for the conduct of this study or preparation of this manuscript.
Contributions
NP: identified the case and followed up; SP and AP: drafted the article; AP and SS: collected and assembled clinical data and the causality assessment; SKT: critically reviewed the scientific writing. All the authors finalized the manuscript.
Compliance with Ethical Standards
Informed consent
Written informed consent was obtained from the patient for the publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor of this journal.
References
- 1.Bastuji-Garin S, Razny B, Stern RS, Shear NH, Naldi L, Roujeau JC. Clinical classification of cases of toxic epidermal necrolysis, Steven–Johnson syndrome and erythema multiforme. Arch Dermatol. 1993;129:92–96. doi: 10.1001/archderm.1993.01680220104023. [DOI] [PubMed] [Google Scholar]
- 2.Carr A, Swanson C, Penny R, Cooper DA. Clinical and laboratory markers of hypersensitivity to trimethoprim-sulfamethoxazole in patients with Pneumocystis carinii pneumonia and AIDS. J Infect Dis. 1993;167(1):180–185. doi: 10.1093/infdis/167.1.180. [DOI] [PubMed] [Google Scholar]
- 3.Pertel P, Hischtick R. Adverse reactions to dapsone in persons infected with human immunodeficiency virus. Clin Infect Dis. 1994;18:630–632. doi: 10.1093/clinids/18.4.630. [DOI] [PubMed] [Google Scholar]
- 4.Nunn P, Kibuga D, Gathua S, Brindle R, Imalingat A, Wasunna K, Lucas S, Gilks C, Omwega M, Were J, et al. Cutaneous hypersensitivity reactions due to thiacetazone in HIV-1 seropositive patients treated for tuberculosis. Lancet. 1991;337(8742):627–630. doi: 10.1016/0140-6736(91)92447-A. [DOI] [PubMed] [Google Scholar]
- 5.Fagot JP, Mockenhaupt M, Bouwes-Bavinck JN, Naldi L, Viboud C, Roujeau JC, EuroSCAR Study Group Nevirapine and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. AIDS. 2001;15(14):1843–1848. doi: 10.1097/00002030-200109280-00014. [DOI] [PubMed] [Google Scholar]
- 6.Dodi F, Alessandrini A, Camera M, Gaffuri L, Morandi N, Pagano G. Stevens–Johnson syndrome in HIV patients treated with nevirapine: two case reports. AIDS. 2002;16(8):1197–1198. doi: 10.1097/00002030-200205240-00022. [DOI] [PubMed] [Google Scholar]
- 7.Zangerle R. Cutaneous drug reactions in HIV-infected patients. Dermatol Ther. 1999;12:115–130. [Google Scholar]
- 8.Hilal-Dandan R, Brunton LL. Antiretroviral agents and treatment of HIV infection. In: Goodman & Gilman’s manual of pharmacology and therapeutics. 2nd ed. New York: McGraw Hill Education; 2014. p. 993–1014.
- 9.Technical and Operational Guidelines for Tuberculosis Control. Revised National Tuberculosis Control Programme. Central TB Division, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; 2005. Available from: http://tbcindia.nic.in/pdfs/Technical%20&%20Operational%20guidelines%20for%20TB%20Control.pdf. Accessed 11 Aug 2015.
- 10.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–245. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization-Uppsala Monitoring Centre. The use of the WHO-UMC system for standardised case causality assessment. Available from: http://www.WHO-UMC.org/graphics/4409.pdf. Accessed 30 Sept 2014.
- 12.Ward KE, Archambault R, Mersfelder TL. Severe adverse skin reactions to non-steroidal antiinflammatory drugs: a review of the literature. Am J Health Syst Pharm. 2010;67(3):206–213. doi: 10.2146/ajhp080603. [DOI] [PubMed] [Google Scholar]
- 13.Rehmus WE. Steven-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) In: Porter RS, editor. The Merck manual (online version) 19. Whitehouse Station, NJ: Merck & Co; 2013. [Google Scholar]
- 14.Letko E, Papaliodis DN, Papaliodis GN, Daoud YJ, Ahmed AR, Foster CS. Stevens–Johnson syndrome and toxic epidermal necrolysis: a review of the literature. Ann Allergy Asthma Immunol. 2005;94:419–436. doi: 10.1016/S1081-1206(10)61112-X. [DOI] [PubMed] [Google Scholar]
- 15.Mockenhaupt M. The current understanding of Stevens-Johnson syndrome and toxic epidermal necrolysis. Exp Rev Clin Immunol. 2011;7(6):803–815. doi: 10.1586/eci.11.66. [DOI] [PubMed] [Google Scholar]
- 16.Leeder JS. Mechanisms of idiosyncratic hypersensitivity reactions to antiepileptic drugs. Epilepsia. 1998;39(Suppl. 7):S8–S16. doi: 10.1111/j.1528-1157.1998.tb01679.x. [DOI] [PubMed] [Google Scholar]
- 17.Azfar NA, Zia MA, Malik LM, Khan AR, Jahangir M. Role of systemic steroids in the outcome of Stevens–Johnson syndrome and toxic epidermal necrolysis. J Pakistan Assoc Derma. 2010;20:158–162. [Google Scholar]
- 18.National AIDS Control Organization (NACO), Government of India. Antiretroviral therapy guidelines for HIV-infected adults and adolescents. May 2013. Available from: http://www.naco.gov.in. Accessed 21 Sept 2015.
- 19.Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149–153. doi: 10.1046/j.1523-1747.2000.00061.x. [DOI] [PubMed] [Google Scholar]


