Abstract
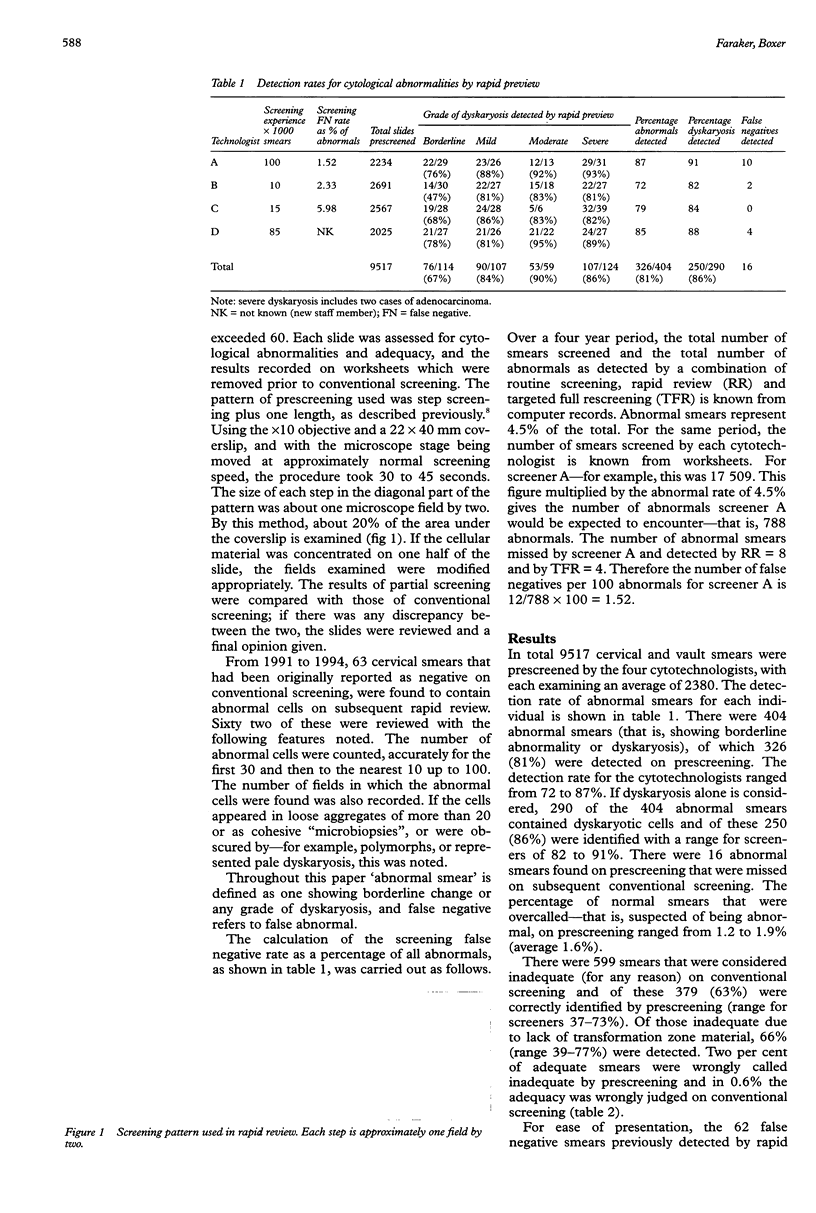
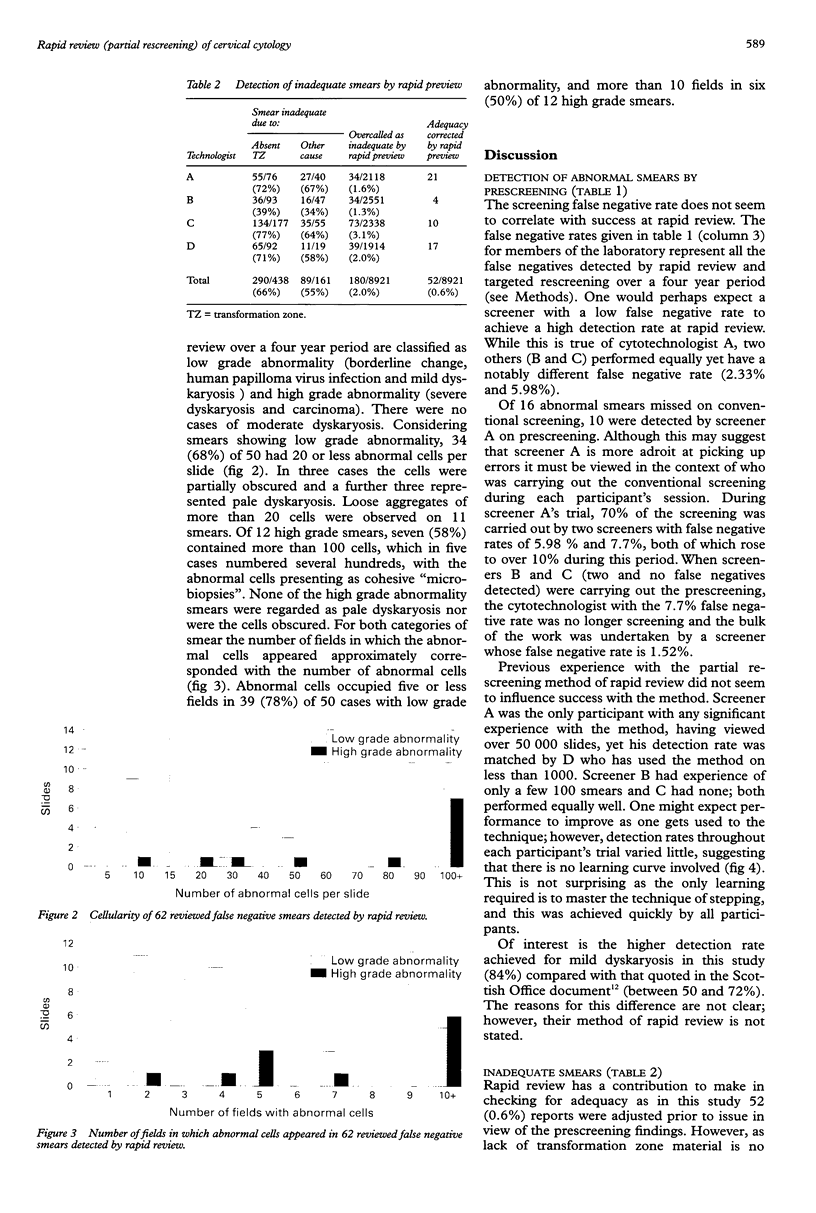
AIMS: To determine the sensitivity of the partial rescreening method of rapid review for internal quality control of cervical cytology; to determine which staff members are most suited to undertake it; and to investigate the cell patterns of false negative smears previously detected by the method. METHODS: As a prospective study 9517 cervical smears were partially screened by four cytotechnologists using the 'step' method prior to conventional screening and the results compared with the final report. As a retrospective study 62 false negative smears that had been identified by the method over four years were reviewed. RESULTS: A detection rate for dyskaryosis of 86% (range 82-91%) was achieved. Sixteen abnormal smears were missed on conventional screening that had been detected by prescreening. Review of the 62 false negatives revealed three patterns: (1) scanty abnormal cells; (2) abundant dyskaryotic cells presenting as "microbiopsies"; and (3) abundant, readily recognisable abnormal cells. CONCLUSIONS: Partial rescreening enables the detection of errors due to both fatigue and misinterpretation. In this laboratory the method has, together with targeted full rescreening, reduced the false negative report rate from 5.0% to 0.4%. For laboratories using a rapid review method to reduce false negative reports, a prescreening trial is recommended in order (1) to select the most effective review method and the staff most suited to undertake it; and (2) to determine the laboratory's sensitivity with the method, as this is required for accurate estimation of the false negative rate.
Full text
PDF
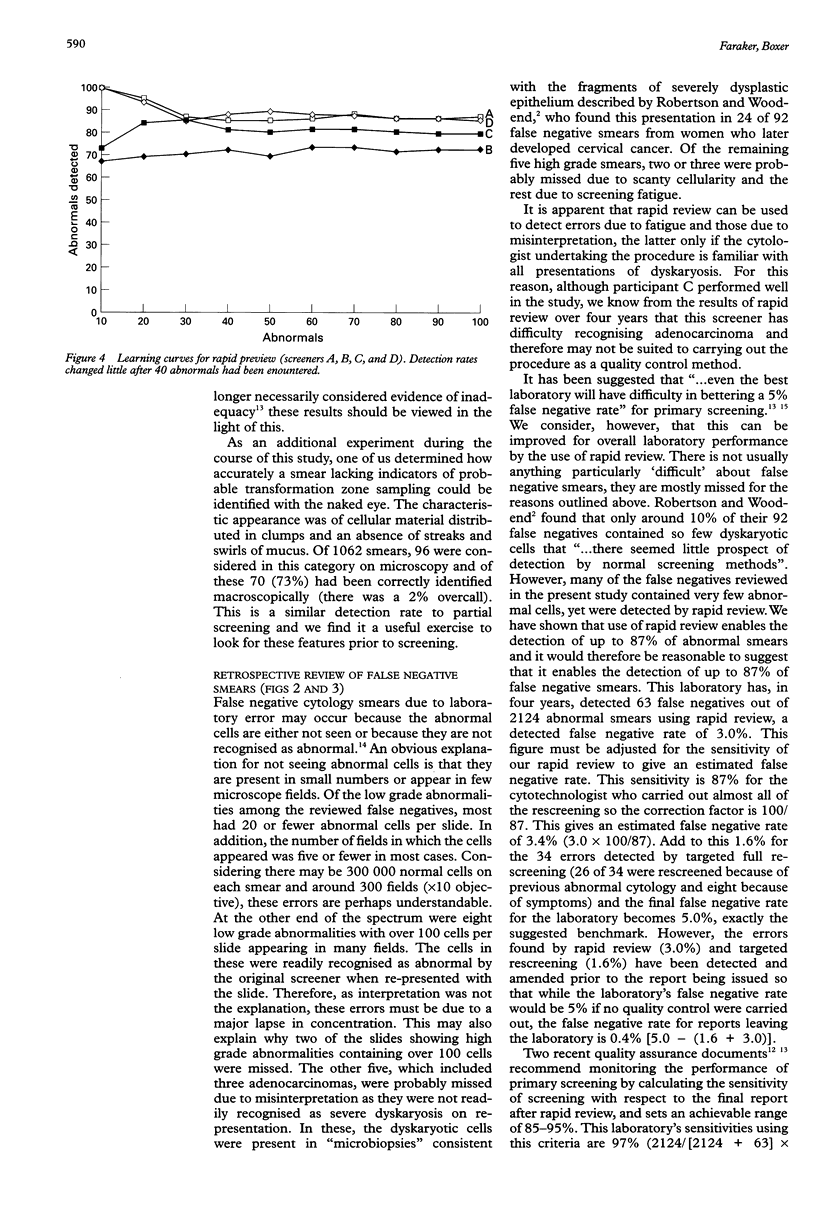

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker A., Melcher D., Smith R. Role of re-screening of cervical smears in internal quality control. J Clin Pathol. 1995 Nov;48(11):1002–1004. doi: 10.1136/jcp.48.11.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrossian C. W., Gupta P. K. Cytology in the headlines. Diagn Cytopathol. 1994;11(1):1–3. doi: 10.1002/dc.2840110102. [DOI] [PubMed] [Google Scholar]
- Dudding N. Rapid rescreening of cervical smears: an improved method of quality control. Cytopathology. 1995 Apr;6(2):95–99. doi: 10.1111/j.1365-2303.1995.tb00453.x. [DOI] [PubMed] [Google Scholar]
- Faraker C. A. Partial rescreening of all negative smears: an improved method of quality assurance in laboratories undertaking cervical screening. Cytopathology. 1993;4(1):47–50. doi: 10.1111/j.1365-2303.1993.tb00072.x. [DOI] [PubMed] [Google Scholar]
- Faraker C. A. Partial rescreening. Cytopathology. 1995 Feb;6(1):59–61. doi: 10.1111/j.1365-2303.1995.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Koss L. G. Cervical (Pap) smear. New directions. Cancer. 1993 Feb 15;71(4 Suppl):1406–1412. doi: 10.1002/cncr.2820710405. [DOI] [PubMed] [Google Scholar]
- Koss L. G., Lin E., Schreiber K., Elgert P., Mango L. Evaluation of the PAPNET cytologic screening system for quality control of cervical smears. Am J Clin Pathol. 1994 Feb;101(2):220–229. doi: 10.1093/ajcp/101.2.220. [DOI] [PubMed] [Google Scholar]
- Koss L. G. The Papanicolaou test for cervical cancer detection. A triumph and a tragedy. JAMA. 1989 Feb 3;261(5):737–743. [PubMed] [Google Scholar]
- Robertson J. H., Woodend B. Negative cytology preceding cervical cancer: causes and prevention. J Clin Pathol. 1993 Aug;46(8):700–702. doi: 10.1136/jcp.46.8.700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater D. Cervical cytology internal quality assurance--what are the national standards? Cytopathology. 1994 Aug;5(4):207–210. doi: 10.1111/j.1365-2303.1994.tb00421.x. [DOI] [PubMed] [Google Scholar]
- van der Graaf Y., Vooijs G. P., Gaillard H. L., Go D. M. Screening errors in cervical cytologic screening. Acta Cytol. 1987 Jul-Aug;31(4):434–438. [PubMed] [Google Scholar]