Abstract
We describe the performance of 292 4 – 17-year-olds with Williams syndrome (WS) on the Kaufman Brief Intelligence Test-2 (KBIT-2). Mean IQ Composite, Verbal standard score (SS), and Nonverbal SS were in the borderline range relative to the general population, with variability similar to the general population. Correlations between SSs and CA were close to 0, with no significant sex differences. There was a significant effect of maternal education on Verbal SS. The KBIT-2 appropriately captures the full range of performance of 8 – 17-year-olds with WS for the abilities measured and of all but the very lowest-functioning 5 – 7-year-olds. However, the KBIT-2 does not contain easy enough items to assess adequately the abilities of the lowest quartile of 4-year-olds.
Keywords: intellectual abilities, Williams syndrome, maternal education, Kaufman Brief Intelligence Test-2
Introduction
Williams syndrome (WS) is a neurogenetic disorder caused by a microdeletion of approximately 26 genes on 7q11.23 (Hillier et al., 2003). It occurs in 1:7,500 live births and affects males and females equally (Strømme, Bjørnstad, & Ramstad, 2002). WS is characterized by unique cognitive, behavioral, and medical profiles (see Mervis & Morris, 2007 for review). Findings from early studies involving very small samples (e.g. Bellugi, Marks, Bihrle, & Sabo, 1988) suggested that individuals with WS exhibit significant cognitive deficits but spared expressive language. More recent large-sample studies have challenged this position (e.g. Martens, Wilson, & Reutens, 2008; Mervis & John, 2010a, 2010b; Mervis & Morris, 2007). On measures of intellectual abilities, mean verbal and nonverbal reasoning abilities are typically in the borderline to moderate intellectual disability range with standard scores (SSs) ranging from the severe intellectual disability level to average relative to the general population (see Martens et al., 2008; Mervis & John, 2010a for review). In contrast, mean spatial abilities are typically in the mild to moderate intellectual disability range, with SSs ranging from the severe intellectual disability level to low average relative to the general population. Performance is best on measures of concrete, single-word vocabulary such as the Peabody Picture Vocabulary Test – Fourth Edition (PPVT-4; Dunn & Dunn, 2007) and Expressive Vocabulary Test – Second Edition (Williams, 2007). Mean SSs on these measures are typically in the low average to average range, with SSs ranging from the severe intellectual disability level to above average relative to the general population.
American researchers studying individuals with WS most commonly report the Kaufman Brief Intelligence Test—Second Edition (KBIT-2; Kaufman & Kaufman, 2004) to characterize participants’ intellectual abilities. The KBIT-2 assesses verbal and nonverbal intelligence independently, providing a Verbal SS, Nonverbal SS, and an IQ Composite. Unlike full-scale IQ tests, the KBIT-2 does not assess visuospatial construction. The KBIT-2 is the intellectual ability test of choice for many researchers studying WS for two primary reasons. First, the KBIT-2 takes less than half the time to administer than a full-scale IQ test (e.g., DAS-II, WISC, WAIS). Second, administration requires considerably less training; the authors note that the KBIT-2 can be administered by properly trained paraprofessionals or technicians (Kaufman & Kaufman, 2004). In addition, the KBIT-2 is sometimes used in order to provide a measure of intellectual ability that does not include visuospatial construction, the area of greatest weakness for most individuals with WS.
We have identified 23 published articles that have used the KBIT-2 to characterize the intellectual abilities of individuals with WS participating in cross-sectional studies. Descriptive statistics for KBIT-2 IQ Composite, Verbal SS, and Nonverbal SS are presented in Table 1, for each study for which these statistics were reported. The grand mean and standard deviation (SD) also are provided based on the studies for which sample mean and SD were reported. The grand mean of the studies was in the borderline range for all three measures, with SSs ranging from 24 – 30 points lower than for the general population. For the 20 articles that reported the age range of the participants, the median of the youngest age was 7.08 years and the median of the oldest age was 34.00 years. Across the 23 studies, median sample size was 23.50 individuals. Due to the rarity of WS, it often is difficult to obtain a large number of participants across a restricted range. Thus, most studies report small samples, often across a wide age range.
Table 1.
Prior Cross-sectional Studies Reporting KBIT-2 Standard Scores (SS) for Individuals with Williams Syndrome
| Authors (year) | N | Sample | CA (in years) | IQ Composite SS | Verbal SS | Nonverbal SS | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |||
| Klein-Tasman et al. (2011) | 84 | C | 9.4 (3.9) | 4 –16 | 70.63 (13.86) | NR | NR | NR | NR | NR |
| Leyfer et al. (2012) | 183 | C | 7.3(1.8) | 5.0 – 10.9 | 75.59 (15.32) | 40 – 114 | NR | NR | NR | NR |
| van der Fluit et al. (2012) | 24 | C | 12.4 (2.7) | 8.1 – 15.8 | 65.71 (11.99) | NR | 73.08 (11.96) | NR | 66.29 (13.56) | NR |
| Hoffmann et al. (2013) | 18 | C | 11.7 (3.7) | 6 – 16.7 | 74 (16) | 53 – 100 | 74 (12) | 56 – 92 | 80 (18) | 55 – 109 |
| Plesa Skwerer et al. (2013) | 21 | C | 8.5 (2.3) | 5.2 – 12.8 | 75 (14.1) | 52 – 96 | NR | NR | NR | NR |
| Plesa Skwerer et al. (2006) | ||||||||||
| Experiment 1a | 43 | C+A | 20.7 (7.4) | 12.1 – 36.1 | 70.2 (11.8) | 52 – 100 | NR | NR | NR | NR |
| Palomares et al. (2008) | 12 | C+A | 18 | 11.3 – 24.4 | 68 | 40 – 88 | NR | NR | NR | NR |
| Goldman et al. (2009) | 23 | C+A | 25.5 (8.0) | 17 – 35 | 66.1 (14.5) | NR | 79.3 (15.8) | NR | 60.6 (17.7) | NR |
| Palomares et al. (2009) | 11 | C+A | 18.6 | 11.8 – 24.4 | 68 | 40 – 88 | NR | NR | NR | NR |
| Lakusta et al. (2010) | ||||||||||
| Experiment 1b | 19 | C+A | 16.8 | 9.8 – 27.6 | 67.05 (12.35) | 40 – 88 | NR | NR | NR | NR |
| Martens et al. (2011) | ||||||||||
| Experiment 1 | 33 | C+A | 20.4 | 6 – 59 | 68.62 (13.01) | NR | 76.11 (11.85) | NR | 67.97 (14.97) | NR |
| Experiment 2 | 36 | C+A | 22.2 | 7 – 50 | 71.06 (14.02) | NR | 75.78 (8.13) | NR | 72.39 (19.56) | NR |
| Palomares et al. (2011) | 11 | C+A | 21.3 | 9 – 33 | 82 | 63 – 94 | NR | NR | NR | NR |
| Plesa Skwerer et al. (2011) | 42 | C+A | 22.2 (5.7) | 12.5 – 34.5 | 68.8 (12.4) | 41 – 91 | NR | NR | NR | NR |
| Martens et al. (2012) | 30 | C+A | 20.8 (10.1) | 8 – 42 | 73.9 (14.2) | NR | NR | NR | NR | NR |
| Opfer & Martens (2012) | 30 | C+A | C: 11.8; A: 33.7 | C: 6 – 17; A: 18 – 56 | 67.28 | 45 – 93 | 75.31 | 56 – 101 | 66.25 | 44 – 87 |
| Pryweller et al. (2012)c | 29 | C+A | 25.2 (8.4) | 16 – 59 | 67.03 (17.17) | 40 – 102 | 72.48 (17.64) | 40 – 107 | 68.52 (18.15) | 40 – 98 |
| Lense & Dykens (2013a) | 46 | C+A | 23.1 (9.6) | 7 – 49 | 69.84 (14.31) | 41 – 100 | 74.81 (11.68) | 43 – 102 | 71.93 (17.07) | 40 – 109 |
| Lense et al. (2013) | 73 | C+A | 26.2 (9.4) | 10 – 51 | 70 (14.5) | 43 – 97 | 76.8 (12.0) | 54 – 108 | 69.8 (17.1) | 40 – 97 |
| Palomares & Shannon (2013) | 17 | C+A | NR | 8.3 – 35.8 | 73 | 49 – 90 | NR | NR | NR | NR |
| Yoshioka et al. (2013) | 19 | C+A | 17.4 | 7 – 32 | 82 | 54 – 94 | NR | NR | NR | NR |
| Key & Dykens (2011) | 21 | A | 26.2 (8.3) | NR | 74.25 (17.19) | NR | 79.55 (13.75) | NR | 69.20 (24.78) | NR |
| Lense et al. (2011) | 14 | A | 28.4 (10.6) | NR | 77.64 (16.31) | NR | 83.00 (14.21) | NR | 77.07 (16.38) | NR |
| Lense & Dykens (2013b) | 19 | A | 27.8 (6.4) | NR | 67.4 (13.8) | NR | NR | NR | NR | NR |
| Grand mean (SD)d | 71.38 (14.27) | 76.38 (12.90) | 70.00 (17.73) | |||||||
Note. CA = chronological age; SD = standard deviation; SS = standard score; NR = Not Reported; C = children; A = adults.
This article included a second experiment. The authors stated that the majority of the individuals in Experiment 2 also participated in Experiment 1.
This article included a second experiment. The authors indicated that 11 of the 12 individuals in Experiment 2 also participated in Experiment 1.
Calculated from supplemental data.
Based on all studies for which the relevant statistic was reported.
One longitudinal study of performance on the KBIT-2 by 40 children with WS has been reported (Mervis, Kistler, John, & Morris, 2012). Children were tested 4 – 7 times (mean: 5.55) over 3 – 7 years, with at least 11.75 months between assessments. Results indicated that Composite IQ, Verbal SS, and Nonverbal SS remained stable for children with WS. No sex differences were found. This suggests that intellectual abilities as measured by the KBIT-2 are generally independent of age, at least in the 4 – 17 year age range. However, individual differences were detected in the rate of change of the IQ Composite and Nonverbal SS. Furthermore, individual differences were detected in the IQ Composite, Verbal SS, and Nonverbal SS intercepts. This indicates there is considerable variability in performance on the KBIT-2 across ages 4 – 17 years, confirming the importance of large samples to best characterize performance on the KBIT-2 by children with WS.
An important variable found to affect intellectual abilities both in typically developing children and in children at risk due to low birth weight or preterm birth is maternal education (see Mervis et al., 2012 for review). Maternal education has been shown to have the strongest and most consistent effect on verbal abilities. In some studies with large sample sizes, a smaller but significant effect has been found for overall intellectual abilities and much less consistently for nonverbal abilities (Performance IQ). In the only study to directly evaluate this relation for children with WS, Mervis et al. (2012) found that maternal education was significantly related to KBIT-2 Verbal SS intercept but not to Nonverbal SS or IQ Composite intercepts or to any of the slopes. For Verbal SS, children whose mothers had a bachelor degree scored an average of 8 points higher than those whose mothers did not.
Given the widespread use of the KBIT-2 to characterize the intellectual abilities of individuals with WS, it is important to determine the characteristics of this measure for this population by evaluating the adequacy of the KBIT-2 for capturing the full range of abilities of individuals with WS, including low-functioning young children. To do so, in the current study we focused on a very large cross-sectional sample of children with WS aged 4 – 17 years. Our first aim was to provide a descriptive report of the full range of performance on the KBIT-2 by children with WS. For this purpose, we considered overall performance, variability, differences between Verbal and Nonverbal SSs, differences between the scaled scores for the two Verbal subtests, and correlations between performance on scales or subtests. Possible differences as a function of sex, chronological age (CA), or maternal education also were evaluated. Our second aim was to determine the appropriateness of the KBIT-2 for capturing the performance of lower functioning children aged 4 – 17 years with WS on the types of verbal and nonverbal abilities measured by this assessment. For this purpose, we first determined the proportion of children who were unable to answer any item correctly on either the Verbal or Nonverbal scale (raw score of 0). (If a child earned a raw score of 0 on a scale, then his/her performance would not be adequately captured by that scale.) Second, we determined the proportion of children who responded correctly to at least one item on the Verbal or Nonverbal scale but who earned the lowest possible IQ Composite, Verbal SS, or Nonverbal SS. We further evaluated these children’s performance to determine if it was adequately captured by the relevant scale(s). (For example, if the child’s raw score corresponded to the highest raw score assigned to the lowest possible SS, then his/her performance would be adequately captured by that scale. If the raw score was lower, then his/her performance would not be adequately assessed by that scale.)
Methods
Participants
Participants were 292 children (150 girls, 142 boys) with WS ranging in age from 4.01 to 17.98 years (M = 9.59, Mdn = 8.72, SD = 4.07). Children were included in the sample if they had a genetically-confirmed classic-length deletion of the WS region, had English as a native language or had been in an English-speaking school for at least 3 years, and did not have any additional diagnoses associated with intellectual disability. The children came from 40 different states in the US and from Canada, England, and South Africa. English was the native language of 96% of the children (280); the native language of the remaining children was Spanish (7), Chinese (2), or one of the languages of India (3). The age composition of the participants was: 96 (47 girls, 49 boys) 4 – 6-year-olds, 131 (60 girls, 71 boys) 7 – 12 year-olds, and 65 (43 girls, 22 boys) 13 – 17 year-olds. Based on the exclusionary criteria, 21 additional children were not included in the sample. Six children were excluded because their deletions were either longer (2 girls, 1 boy) or shorter (2 girls, 1 boy) than the classic deletion. Fifteen children with classic deletions were excluded because they had a comorbid diagnosis of fetal alcohol syndrome (1 girl, 1 boy) or a gold-standard (based on ADOS, ADI-R, and clinical judgment) co-morbid diagnosis of an autism spectrum disorder (3 girls, 10 boys).
Some participants completed the KBIT-2 as part of a longitudinal study of cognitive and language development of individuals with WS. For these individuals, data from the most recent KBIT-2 administration were used. The longitudinal participants included the 40 children comprising the Mervis et al. (2012) longitudinal sample. For almost all of these children the data point included in the present study was more recent than the latest data point included for that child in Mervis et al. (2012). Level of maternal education varied; 187 mothers had a bachelor degree or higher and 105 did not. The racial/ethnic background of the participants was: 229 White non-Hispanic (78.42%), 29 White Hispanic (9.93%), 8 African American non-Hispanic (2.74%), 7 Asian non-Hispanic (2.40%), 12 biracial non-Hispanic (4.11%; 4 African American/White, 2 Asian/White, 1 Asian/African American, 1 Hawaiian/Pacific Islander/White, 4 American Indian/White), 3 biracial Hispanic (1.03%; 2 American Indian/White, 1 Asian/White), 3 tri-racial non-Hispanic (1.03%; 1 American Indian/African American/White, 1 African American/Pacific Islander/White, 1 Asian/Hawaiian/White), and 1 tri-racial Hispanic (0.34%; American Indian/African American/White).
Materials
The KBIT-2 (Kaufman & Kaufman, 2004) is a brief assessment of intellectual ability normed for ages 4 – 90 years. The KBIT-2 assesses verbal and nonverbal intelligence independently, providing a Verbal SS, Nonverbal SS, and an IQ Composite SS. All SSs have a mean of 100 (SD = 15) for the general population, with a range from 40 to 160. The KBIT-2 has minor differences in the lowest possible SS that can be obtained at the younger age ranges. Raw scores of 0 yield SSs higher than 40 until 5 years 4 months for the Verbal SS, 7 years 4 months for the Nonverbal SS, and 5 years 8 months for the IQ Composite.
The IQ Composite measures general intelligence and is determined from an individual’s performance on the Verbal and Nonverbal scales. The Verbal scale measures crystallized intelligence (breadth and depth of acquired knowledge, including vocabulary, and the ability to use that knowledge to solve problems). Two subtests are included: Verbal Knowledge (measuring receptive language and general information) and Riddles (measuring verbal reasoning and comprehension). Scaled scores (M = 10, SD = 3, range = 1 – 19 for the general population) are provided, allowing a comparison of performance across these subtests. The Nonverbal scale measures fluid intelligence (ability to think logically and solve novel problems; language is not required for solution). The Nonverbal scale includes one subtest, Matrices This subtest measures understanding of relations among either concrete stimuli (pictures of objects or people) or abstract stimuli (designs or symbols). Thus, the KBIT-2 Nonverbal scale measures nonverbal reasoning but does not measure visuospatial construction. (In contrast, Performance IQ on the Wechsler IQ tests and the DAS-II Special Nonverbal Composite are based on assessments of both nonverbal reasoning and visuospatial construction.)
As reported by the KBIT-2 test authors, intercorrelations between Verbal SS and Nonverbal SS were .49 for ages 4 – 6 years, .48 for ages 7 – 12 years, and .53 for ages 13–18 years for the norming sample. Comparisons of the SSs obtained by females and males for each of the age groups also were reported. For ages 7 – 12 years, a small but significant difference was found for Nonverbal SS with females scoring 2.4 points higher than males. The authors indicate this difference has no practical consequence. No other significant sex differences were detected.
Procedure
The KBIT-2 was administered to each child following the standardized procedures, as part of a larger assessment of intellectual and language abilities. Children were tested individually in a quiet room in either the authors’ laboratory, a collaborator’s laboratory, or at meeting sites for national or regional Williams Syndrome Association conferences. Test administrators were carefully-trained staff members who had bachelor degrees, doctoral students in Psychology, or individuals who had completed their Ph.D. in Psychology. All tests were scored separately by two staff members; disagreements were very rare and were resolved by discussion with a third staff member.
Results
Due to non-normality of the data, nonparametric statistics were used.
Overall Performance
Descriptive statistics for SSs and scaled scores are reported in Table 2. According to the test authors’ descriptive categories, mean scores for each SS fell in the below average range, with a range from the lower extreme to average relative to the general population. Scaled scores followed the same trend; mean Verbal Knowledge and Riddles scaled scores were in the below average range, with a range from the lower extreme to average. SDs for both SSs and scaled scores were similar to those for the general population.
Table 2.
Descriptive Statistics for KBIT-2 Standard Scores (SSs) and Scaled Scores and Correlations with Chronological Age (CA) for 4- to 17-year-olds with Williams Syndrome
| KBIT-2 Component | Descriptive Statistics | Correlations with CA | |||
|---|---|---|---|---|---|
|
| |||||
| Mean | Median | SD | Range | Spearman rho (p-value) | |
| IQ Composite SS | 73.50 | 74.00 | 15.44 | 40 – 111 | −.06 (.35) |
| Verbal SS | 76.57 | 77.00 | 14.81 | 40 – 112 | −.08 (.18) |
| Nonverbal SS | 76.78 | 78.50 | 15.82 | 40 – 112 | −.01 (.85) |
| Verbal Knowledge scaled score | 6.22 | 6.00 | 2.86 | 1 – 13 | −.13 (.02) |
| Riddles scaled score | 5.17 | 5.00 | 2.73 | 1 – 13 | −.06 (.30) |
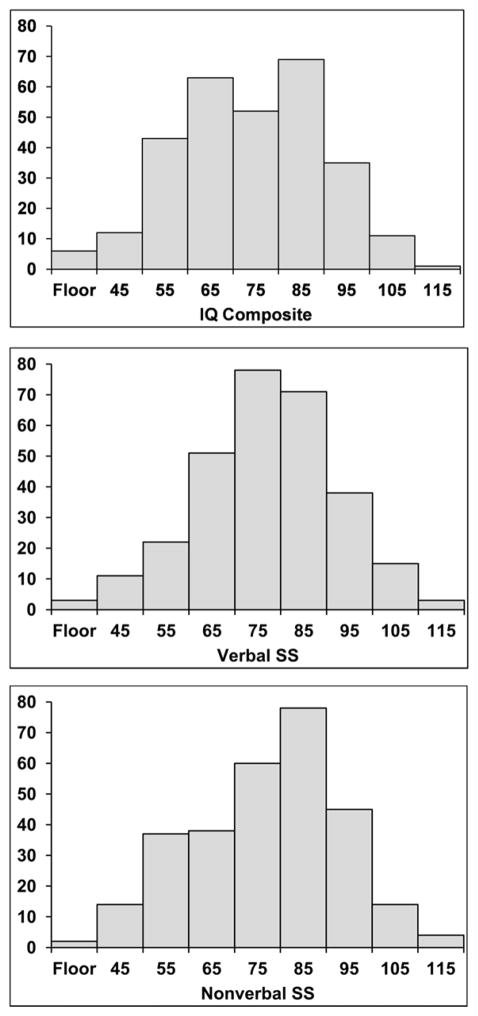
Histograms of the SS distribution are depicted in Figure 1, separately for IQ Composite, Verbal SS, and Nonverbal SS. SSs were in the average range (85 – 115) for 25.7% of children for IQ Composite, 29.5% for Verbal, and 33.2% for Nonverbal, with SSs of 100 or higher earned by 4.1% of children for IQ Composite, 6.2% for Verbal, and 6.2% for Nonverbal. SSs were in the below average range (70 – 84) for 31.8% of children for IQ Composite, 40.8% for Verbal, and 35.6% for Nonverbal. SSs were in the lower extreme range (40 – 69) for 42.5% of children for IQ Composite, 29.8% for Verbal, and 31.2% for Nonverbal.
Figure 1.
Histograms for KBIT-2 Standard Scores (SS) for 4- to 17-year-olds with WS
Comparison of Scales and Subtests
To compare overall performance on the Verbal and Nonverbal scales, a Wilcoxon signed rank test was conducted. The median Verbal SS (77.00) and Nonverbal SS (78.50) were highly similar (Z = 0.04, p = .97, r = .002). At the individual level, the test authors’ criterion for a significant difference between Verbal SS and Nonverbal SS (difference of 17 points for age 4 years, 15 for ages 5 – 10 years, and 13 for ages 11 – 17; p < .05) was met by 27.4% of the children. Nonverbal SS was significantly higher than Verbal SS for 14.0% of the children, and 13.4% had significantly higher Verbal SS than Nonverbal SS. The test authors set their criterion for an unusually large difference between an individual’s Verbal SS and Nonverbal SS as the difference shown by the most extreme 10% of the normative sample. The criterion difference ranged from ≥ 20 – 26 points depending on the participant’s age. In the current sample of children with WS, the test authors’ criterion was met by 5.9% (2 out of 34) of the 4-year-olds, 10.0% (3 out of 30) of the 5-year-olds, 10.2% (6 out of 59) of the children aged 6 – 7 years, 8.5% (6 out of 71) of those aged 8 – 10 years, 7.0% (3 out of 43) of those aged 11 – 13 years, and 10.9% (6 out of 55) of those aged 14 – 17 years. Of the 26 children who met this criterion (8.9% of the full sample), Verbal SS was significantly higher than Nonverbal SS for 11 (3.8% of the full sample) and Nonverbal SS was significantly higher than Verbal SS for 15 (5.1%).
Median Verbal Knowledge scaled score was significantly higher than median Riddles scaled score as indicated by a Wilcoxon signed rank test, with a medium effect size (Z = −7.52, p < .001, r = −.44). At the individual level, the test authors’ criterion for a significant difference between the Verbal Knowledge and Riddles scaled scores (difference of at least 3 scaled-score points) was met by 27.7% of children. The Verbal Knowledge scaled score was significantly higher than the Riddles scaled score for 22.6% of children; 5.1% performed significantly higher on Riddles than Verbal Knowledge.
To confirm that the components of the KBIT-2 are related yet distinctive enough to contribute uniquely to IQ Composite, Spearman correlations were conducted between the scale SSs and between the Verbal subtest scaled scores. Verbal SS and Nonverbal SS were significantly correlated (rs = .66, p < .001). Spearman correlations between Verbal SS and Nonverbal SS were significant for each of the child age groups provided in the KBIT-2 manual: rs = .72 for ages 4 – 6 years, .65 for ages 7 – 12 years, and .56 for ages 13 – 17 years (all ps ≤ .001). Verbal Knowledge scaled score was highly correlated with Riddles scaled score (rs = .71, p < .001). This relation was also significant for each of the child age groups: rs = .68 for ages 4 – 6 years, .77 for ages 7 – 12 years, and .65 for ages 13 – 17 years (all ps ≤ .001).
Chronological Age and Sex Differences
Possible differences as a function of CA or sex were considered. To evaluate the relation between CA and scale SSs or subtest scaled scores, Spearman correlations were conducted (Table 2). CA was very weakly negatively correlated with Verbal Knowledge scaled score (rs = −.13, p = .024). None of the remaining correlations with CA was significant; all were close to 0 (all ps ≥ .18).
No significant sex differences in the distributions of the IQ Composite, Verbal SS, and Nonverbal SS (ps ≥ .101) or the Verbal scaled scores (ps ≥ .067) were found as indicated by Mann-Whitney U tests. Further analyses comparing SSs obtained by females and males were conducted for the age groups provided in the KBIT-2 manual. No significant sex differences were found for any of the SSs across ages 4 – 6 years (ps ≥ .11), 7 – 12 years (ps ≥ .46), or 13 – 17 years (ps ≥ .48). A similar pattern was observed for the Verbal Knowledge and Riddles scaled scores with no significant sex differences detected across ages 4 – 6 years (ps ≥ .12), 7 – 12 years (ps ≥ .39), or 13 – 17 years (ps ≥ .85).
Maternal Education
Descriptive statistics for SSs and scaled scores are reported separately in Table 3 for children whose mothers had a bachelor degree or higher and children whose mothers did not have a bachelor degree. To evaluate possible differences in the distributions of the SSs or scaled scores across the two levels of maternal education, Mann-Whitney U tests were conducted. Verbal SS differed significantly as a function of maternal education, with a moderate effect size (Z = 3.63, p < .001, r = .21). Median Verbal SS was 8 points higher for children whose mothers had at least a bachelor degree than for children whose mothers did not. The same pattern and moderate effect size was observed for the two Verbal subtests (ps = .001, rs = .19). Children of mothers with at least a bachelor degree had median Verbal Knowledge scaled score and Riddles scaled score that were 1 scaled score point higher than those of children whose mothers did not.
Table 3.
Descriptive statistics for KBIT-2 Standard Scores (SSs) and Scaled Scores as a Function of Maternal Education
| No Bachelor Degree | Bachelor Degree or Higher | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| KBIT-2 Component | Mean | Median | SD | Range | Mean | Median | SD | Range |
| IQ Composite SS | 70.91 | 69.00 | 14.41 | 40 – 111 | 74.95 | 77.00 | 15.84 | 40 – 107 |
| Verbal SS | 72.99 | 72.00 | 13.29 | 43 – 110 | 78.57 | 80.00 | 15.27 | 40 – 112 |
| Nonverbal SS | 75.82 | 76.00 | 15.40 | 40 – 112 | 77.32 | 81.00 | 16.06 | 40 – 110 |
| Verbal Knowledge scaled score | 5.55 | 6.00 | 2.70 | 1 – 13 | 6.59 | 7.00 | 2.94 | 1 – 13 |
| Riddles scaled score | 4.54 | 5.00 | 2.34 | 1 – 12 | 5.53 | 6.00 | 2.82 | 1 – 13 |
The distribution of Nonverbal SS did not differ significantly as a function of maternal education level (p = .28). A small but significant difference across levels of maternal education was detected for IQ Composite (Z = 2.34, p = .020, r = .14). The median IQ Composite differed by 8 points, with children whose mothers had at least a bachelor degree scoring higher.
Evaluation of Appropriateness
Our second aim was to evaluate the appropriateness of the KBIT-2 for capturing the performance of lower functioning children aged 4 – 17 years with WS on the types of verbal and nonverbal abilities measured by this assessment. For this purpose, we first determined the proportion of children who were unable to answer any item correctly on either the Verbal or Nonverbal scale. Second, we determined the proportion of children who were able to correctly respond to at least one item on the Verbal or Nonverbal scale but who earned the lowest possible IQ Composite, Verbal SS, and/or Nonverbal SS.
Raw scores of 0
In the KBIT-2 manual, the authors indicate that the test cannot adequately capture the abilities of individuals who earn raw scores of 0 on the Verbal or Nonverbal scales. However, they state that it is appropriate to interpret the Verbal SS for individuals who obtain a raw score of 0 on only one of the two Verbal subtests (Verbal Knowledge or Riddles). In the present sample, 14 children earned a raw score of 0 on the Nonverbal scale. Of these children, 9 were 4 years old (26.5% of 4-year-olds), 2 were 5 years old, and 2 were 7 years old (4.5% of 5 – 7-year-olds). Three children of these children, all aged 4 years, also earned a raw score of 0 on the Verbal scale (8.8% of 4-year-olds). No child aged 8 – 17 years earned a raw score of 0 on the Nonverbal scale, and no child aged 5 – 17 years earned a raw score of 0 on the Verbal scale.
Standard scores of 40
The lowest possible SS that can be obtained on the KBIT-2 is 40. Therefore, the abilities of individuals with relatively severe intellectual disabilities may not be captured adequately by the KBIT-2 even if they are able to respond correctly to at least one item. To determine if these children’s abilities were adequately assessed, we further examined the performance of children who earned a raw score of at least 1 on the Verbal and/or the Nonverbal scale but who nonetheless earned a SS of 40 on that scale and/or on IQ Composite. If a Verbal SS and/or Nonverbal SS of 40 was earned, the raw score(s) for the relevant scale(s) was evaluated. If the obtained raw score was the highest possible raw score corresponding to a SS of 40, the child’s abilities were considered to be adequately captured. If the raw score obtained was less than the highest possible raw score corresponding to a SS of 40, then the child performed below floor on that scale and the KBIT-2 did not capture the type of ability measured by that scale for that child. If the child’s IQ Composite was 40, we determined if the Verbal SS and Nonverbal SS adequately captured the child’s performance. If they did, then the IQ Composite also accurately captured the child’s abilities.
The Nonverbal SSs of all of the children who responded correctly to at least one item on that scale were above 40. Of the 279 children who responded correctly to at least one item on the Verbal scale, SSs of 40 were obtained by 6: three (1.1%) for Verbal SS and four (1.4%) for IQ Composite (one of whom also earned a SS of 40 on the Verbal scale). For four of the six children, both the Verbal SS and the IQ Composite adequately captured their abilities. One of these children (aged 7 years) earned a Verbal SS of 40, a Nonverbal SS above 40, and an IQ Composite of 40. His Verbal raw score was the highest possible raw score corresponding to a Verbal SS of 40. Thus, he truly earned the Verbal SS of 40, indicating his abilities were adequately captured by the KBIT-2. The other three children (aged 7 years, 8 years, and 12 years) also earned an IQ Composite of 40 but their Verbal SS and Nonverbal SS were both above 40. Thus, they did not perform at floor on either of the scales included in the IQ Composite and the KBIT-2 was able to capture their abilities adequately.
For the two remaining children, the KBIT-2 did not adequately capture their verbal abilities and/or nonverbal reasoning abilities. One 7-year-old obtained a raw score that was 1 point below the highest possible raw score corresponding to a Verbal SS of 40. He also earned a raw score of 0 on the Nonverbal scale. Therefore, the KBIT-2 could not adequately capture his verbal or nonverbal reasoning abilities. One 10-year-old earned a Verbal raw score 2 points below the highest possible raw score corresponding to a Verbal SS of 40. Her Nonverbal SS was above 40 (as was her IQ Composite). The KBIT-2 adequately captured this child’s nonverbal reasoning abilities but did not adequately capture her verbal abilities.
Discussion
The present study reports the performance of a large sample of children with WS on the KBIT-2. Mean SSs were 73.50 for IQ Composite, 76.57 for the Verbal scale, and 76.78 for the Nonverbal scale. A wide range of abilities was detected, with SSs and Verbal subtest scaled scores ranging from the severe intellectual disability level to average relative to the general population. The SDs of the SSs and Verbal subtest scaled scores were similar to the general population. Correlations with CA were close to 0, and no sex differences were found. These results suggest that children with WS, as a group, develop at a consistent rate relative to the KBIT-2 normative sample with similar amounts of variability. However, the average SSs and Verbal subtest scaled scores of children with WS are lower than the general population. Mean KBIT-2 Verbal and Nonverbal SSs in the present study were similar to the mean Verbal cluster SS (74.06) and Nonverbal Reasoning cluster SS (78.89) on the Differential Ability Scales-II (Elliott, 2007) previously reported for 120 children with WS aged 4 – 17 years (Mervis & John, 2010a).
Comparisons to KBIT-2 Performance Previously Reported for Individuals with WS
The mean IQ Composite for the current sample is 2 points higher than the grand mean of the reported IQ Composite means for the 23 previous articles that have used the KBIT-2 to characterize the intellectual abilities of individuals with WS participating in cross-sectional studies; the SD for IQ Composite is 1 point higher than the mean SD for the 22 previous articles that reported the IQ Composite SD. The mean Verbal SS for the current sample is the same as the grand mean Verbal SS for the 10 previous articles that reported Verbal SS, with a SD that was almost 2 points higher. In contrast, the mean Nonverbal SS for the current sample was almost 7 points higher than the grand mean Nonverbal SS for the 10 previous articles for which mean Nonverbal SS had been reported, with a SD that was 2 points lower. All of the SDs in the current study are closer to the SDs for the KBIT-2 normative sample than are the means of the previously reported SDs. Given that the sample size in the present study is more than 12 times that of the median sample size for previous studies, it is likely that the mean Nonverbal SS for the present sample is a more accurate estimate of the mean nonverbal reasoning abilities of children with WS than is the mean of the reported mean Nonverbal SSs for previous studies and that the SDs in the present study are a more accurate indication of the variability among children with WS.
Three of the previous articles reporting KBIT-2 results for individuals with WS included a statistical comparison of Verbal SS and Nonverbal SS at the group level. For two studies, no significant difference was found (Pryweller et al., 2012; van der Fluit et al., 2012); in the third study, Verbal SS was significantly higher than Nonverbal SS (Key & Dykens, 2011). None of these studies evaluated differences between Verbal SS and Nonverbal SS at the individual level. At the group level, our findings were consistent with those of Pryweller et al. (2012) and van der Fluit et al. (2012); we found no significant group-level difference. Nevertheless, at the individual level, 27.4% of the participants in our study evidenced a significant difference between Verbal SS and Nonverbal SS, with differences as large as 37 points. Approximately half of the children who evidenced a significant difference had a significantly higher Verbal SS and approximately half had a significantly higher Nonverbal SS.
None of the previous studies of individuals with WS that included the KBIT-2 compared performance on the two Verbal subtests. In the current study, we found that at the group level, performance on the Verbal Knowledge subtest (measuring primarily receptive single-word vocabulary) was significantly better than performance on the Riddles subtest (measuring verbal reasoning and comprehension). At the individual level, 27.7% of the children met the test authors’ criterion for a significant difference between the Verbal Knowledge and Riddles scaled scores, with 22.6% scored significantly higher on the Verbal Knowledge subtest and only 5.1% scoring significantly higher on the Riddles subtest. Better performance on the Verbal Knowledge subtest than the Riddles subtest both at the group level and at the individual level is consistent with prior findings: When children with WS complete a comprehensive neuropsychological evaluation, they typically earn their highest SS on the measure of single-word receptive vocabulary (e.g. PPVT-4; see Mervis & John, 2010a).
Comparisons with KBIT-2 Normative Sample
Relative to the KBIT-2 normative sample, the mean SSs of the current sample of children with WS were almost 2 SDs below the general population mean. At the same time, the SDs for the current sample were similar to those for the normative sample. Consistent with the findings for the KBIT-2 normative sample, no meaningful sex differences or CA differences in SSs were detected for the current sample. An unusually large difference between Verbal SS and Nonverbal SS was evidenced 8.9% of the current sample in comparison to 10% of the normative sample. Thus, extreme individual differences between verbal abilities and nonverbal reasoning abilities in the present sample of children with WS occurred at a rate similar to that of the normative sample.
Overall, correlations between Verbal SS and Nonverbal SS were slightly higher for the current sample of children with WS than for the normative sample. This finding is consistent with those of Detterman and Daniel (1989), who divided the WISC-R and WAIS-R normative samples into separate groups based on IQ-equivalent scores and then computed the correlations among subtests. These authors found that these correlations were highest for the lowest IQ-equivalent band (< 78) and next highest for the second-lowest IQ-equivalent band (78 – 92). For the current sample, the magnitudes of the correlation between KBIT-2 Verbal SS and Nonverbal SS both for the full sample and for each age band indicate that the two scales are related yet distinctive enough to contribute uniquely to IQ Composite for children aged 4 – 17 years with WS. Furthermore, correlations between Verbal Knowledge and Riddles were moderately high within the current sample indicating that the verbal subtests are related yet distinctive enough to contribute uniquely to Verbal SS for children with WS.
Maternal Education
Possible differences as a function of maternal education level also were evaluated. Maternal education was dichotomized as mothers who had at least a bachelor degree and those who did not. The distribution of Verbal SSs for the high maternal-education group was significantly higher than that for the low maternal-education group, with a median difference of 8 points. This finding is consistent with that of the previous longitudinal KBIT-2 study (Mervis et al., 2012), which included a much smaller sample. The finding also fits with those of previous studies of typically developing children or children at risk due to low birth weight or preterm birth. For the current sample of children with WS, the Nonverbal SS distributions did not differ significantly for the two maternal-education groups. The distribution of IQ Composite was significantly higher for children in the high maternal-education group than for children in the low maternal-education group. However, this effect was due primarily to the difference observed between the distributions of Verbal SSs. Overall, these results suggest that factors associated with higher maternal education have a significant impact on crystallized intelligence for children with WS, as has been previously reported for children in the general population.
Appropriateness of KBIT-2 for Assessing Lower-functioning Children with WS
The second aim of the current study was to determine the appropriateness of the KBIT-2 for capturing the performance of lower functioning children with WS aged 4 – 17 years on the types of verbal and nonverbal abilities measured. The KBIT-2 adequately assessed the nonverbal reasoning abilities of all 168 children aged 8 – 17 years and the verbal abilities of all but one child in this age range (99.4%). Of the 89 children aged 5 – 7 years, the KBIT-2 adequately assessed the nonverbal reasoning abilities of 85 (95.5%) and the verbal abilities of 88 (98.9%). The KBIT-2 was less effective at adequately assessing the abilities of 4-year-olds with WS. Of the 34 4-year-olds with WS, the KBIT-2 adequately captured the Nonverbal abilities of 25 (73.5%) and the Verbal abilities of 30 (85.7%). Thus the KBIT-2 can adequately capture the full range of nonverbal reasoning and verbal performance of children with WS aged 8 – 17 years and can adequately measure these abilities for all but the very lowest functioning children with WS aged 5 – 7 years (in our sample, the lowest 4.5%). However, the KBIT-2 does not include items that are simple enough to assess these abilities in the lowest quartile of 4-year-olds with WS.
Inappropriateness of the KBIT-2 for Assessing the Cognitive Profile Associated with WS
The cognitive profile of individuals with WS is sometimes simplistically described as relatively preserved verbal abilities in the context of severely impaired nonverbal abilities. This corresponds to a common view of WS in the popular media (see Mervis & John, 2010a), as well as the view of some academicians who have not studied children with WS directly (e.g., Piattelli-Palmarini, 2001). In this context, the KBIT-2, which is composed of a Verbal scale and a Nonverbal scale, might on the surface appear to be a good measure to demonstrate this profile.
It is important to note, however, that “nonverbal” abilities are typically taken to include both nonverbal reasoning abilities and visuospatial construction abilities (e.g., Performance IQ on the Wechsler tests, Special Nonverbal Composite on the DAS-II). In contrast, the KBIT-2 Nonverbal scale measures nonverbal reasoning but does not measure visuospatial construction. Thus, if the “nonverbal” weakness relative to verbal abilities for individuals with WS involves visuospatial construction rather than nonverbal reasoning, the KBIT-2 will not be able to detect this profile. Findings from studies of children with WS using the DAS-II (Elliott, 2007), which measures both nonverbal reasoning abilities and visuospatial abilities but in two separate composites (Nonverbal Reasoning cluster and Spatial cluster) address this possibility directly. On the DAS-II, mean Verbal cluster SS is similar to mean Nonverbal Reasoning cluster SS and both are significantly higher than mean Spatial cluster SS, with a difference of about 20 points (Mervis & John, 2010a, 2010b). At the individual level, 75% of a sample of 83 4 – 17-year-olds with WS scored significantly higher on the DAS-II Nonverbal Reasoning cluster than on the Spatial cluster, 72% scored significantly higher on the Verbal cluster than on the Spatial cluster, and 2% scored significantly higher on the Spatial cluster than on the Verbal cluster. Only 27% evidenced a significant difference in SSs between the Nonverbal Reasoning cluster and the Verbal cluster (Mervis & John, 2010b), with half of the children evidencing a significant difference earning a significantly higher Verbal cluster SS and half earning a significantly higher Nonverbal Reasoning cluster SS (unpublished data). Thus, the major cognitive weakness relative to verbal abilities for individuals with WS appears to be visuospatial construction rather than nonverbal abilities in general.
In this context, it is not surprising that the patterns of performance on the KBIT-2 Verbal and Nonverbal scales in the present study strongly parallel the findings just described for the DAS-II Verbal and Nonverbal Reasoning clusters. In particular, mean KBIT-2 Verbal and Nonverbal SSs are very similar, and 27.4% of children evidence a significant difference between the two SSs, with about half earning a significantly higher Verbal SS and half earning a significantly higher Nonverbal SS. Thus, despite the names of its two scales, the KBIT-2 is not an appropriate measure for assessing the cognitive profile associated with WS. In order to provide an appropriate assessment for the WS cognitive profile, in addition to measuring verbal abilities, both nonverbal reasoning and visuospatial construction abilities need to be measured, and they must be assessed separately.
Limitations and Future Directions
Although this study contains a much larger sample of individuals with WS who have completed the KBIT-2 than any of the previous studies, the current sample was restricted to ages 4 – 17 years. Many of the cross-sectional studies that commonly report the KBIT-2 to characterize the intellectual abilities of participants with WS either include both children and adults or are restricted to adults. Thus, replication of these findings across the lifespan is needed. The finding that children whose mothers have a high level of education (relative to the levels of maternal education included in the sample) have higher verbal abilities has been replicated repeatedly for a variety of types of children (see Mervis et al., 2012 for review), including the present sample of children with WS. Higher maternal education is correlated with other characteristics such as amount of time spent reading and talking with one’s child (e.g. Fletcher, Cross, Tanney, Schneider, & Finch, 2008; Hart & Risley, 1995) a supportive interaction style (e.g. Fewell & Deutscher, 2002; Hart, & Risley, 1995), and higher family income (e.g. Gershoff, Aber, Raver, & Lennon, 2007). In order to better understand which factors associated with a higher maternal education level are most relevant for child verbal abilities, it will be important to examine the inter-relations of these factors with each other and with child verbal abilities, using samples larger than the present one. Furthermore, as congenital heart disease is very common among children with WS (e.g. Collins, 2013) and surgery for heart disease and other medical complications such as repeated ear infections or inguinal hernia also is common (e.g. Collins, 2013; Mervis & Morris, 2007; Morris, 2006), it would be of interest to evaluate the impact of congenital heart disease, multiple ear infections, and/or early exposure to anesthesia on the verbal and nonverbal reasoning abilities of children with WS. Negative effects of congenital heart disease on language and/or intellectual abilities have been reported both for children without syndromes associated with intellectual disability (e.g. Miatton, de Wolf, François, Thiery, & Vingerhoets, 2007; Schaefer et al., 2013) and children with Down syndrome (Visootsak, Hess, Bakeman, & Adamson, 2013). Additionally, in a population-based study, exposure to anesthesia prior to age 3 years was related to significantly lower scores on both language and nonverbal reasoning measures at age 10 years even after adjusting for demographic factors and gender (e.g. Ing et al., 2012). Finally, the appropriateness of the KBIT-2 for assessing the verbal and nonverbal reasoning abilities of children with other genetic syndromes should be addressed.
Conclusion
The results of the present cross-sectional study of the performance of 4 – 17-year-old children with WS on the KBIT-2 indicated that mean SSs are in the borderline range relative to the general population, with variability similar to the general population. The range of intellectual ability levels was from severe intellectual disability to average. Correlations of CA with SSs were close to 0, and no significant sex differences were found. Children whose mothers had a bachelor degree scored on average 8 points higher on Verbal SS, and, in turn, on IQ Composite, than children whose mothers did not have a bachelor degree. The KBIT-2 can effectively capture the full range of performance by children aged 8 – 17 years with WS on the abilities measured by this assessment and can adequately assess these abilities for all but the very lowest functioning children with WS aged 5 – 7 years (4.5% of the children in this age range). However, the KBIT-2 does not include easy enough items to assess adequately the verbal and nonverbal reasoning abilities of lower-functioning 4-year-olds with WS. In line with the conclusion of the longitudinal KBIT-2 study (Mervis, et al., 2012), the KBIT-2 is a reasonable measure to consider when evaluating possible genotype/phenotype relations involving intellectual, verbal, and/or nonverbal reasoning abilities. This is especially the case for studies in which the youngest children are at least 5 years old and that either include an age range too broad to be captured by a single full-scale assessment of intellectual abilities with an adequate floor for children with WS and/or which do not include enough time or appropriate staff expertise to administer an appropriate full-scale intellectual assessment. Under similar circumstances, the KBIT-2 also would be useful for characterizing the verbal and nonverbal reasoning abilities of individuals with WS included in clinical trials or intervention studies.
Acknowledgments
An earlier version of this study was presented at the 2013 Gatlinburg Conference on Research and Theory in Intellectual Developmental Disabilities in San Antonio, TX. This research was supported by grants R37 HD29957 from the National Institute of Child Health and Human Development and R01 NS35102 from the National Institute of Neurological Disorders and Stroke. We thank the many children and their families who have participated in our research. We also thank the members of our laboratory who administered the Kaufman Brief-Intelligence Test-2 to the participants in this study. The Williams Syndrome Association (WSA) facilitated the conduct of this study by providing space to test research participants at WSA national and regional family conventions.
References
- Bellugi U, Marks S, Bihrle A, Sabo H. Dissociation between language and cognitive functions in Williams syndrome. In: Bishop D, Mogford K, editors. Language development in exceptional circumstances. London: Churchill Livingstone; 1988. pp. 177–189. [Google Scholar]
- Collins RT. Cardiovascular disease in Williams syndrome. Circulation. 2013;127:2125–2134. doi: 10.1161/CIRCULATIONAHA.112.000064. [DOI] [PubMed] [Google Scholar]
- Detterman DK, Daniel MH. Correlations of mental tests with each other and with cognitive variables are highest for low IQ groups. Intelligence. 1989;13:349–359. doi: 10.1016/S0160-2896(89)80007-8. [DOI] [Google Scholar]
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test—Fourth edition. Minneapolis, MN: Pearson Assessments; 2007. [Google Scholar]
- Elliott CD. Differential Ability Scales—Second edition. San Antonio, TX: Psychological Corporation; 2007. [Google Scholar]
- Fewell RR, Deutscher B. Contributions of receptive vocabulary and maternal style variables to later verbal ability and reading in low-birthweight children. Topics in Early Childhood Special Education. 2002;22:181–190. doi: 10.1177/027112140202200401. [DOI] [Google Scholar]
- Fletcher KL, Cross JR, Tanney AL, Schneider M, Finch WH. Predicting language development in children at risk: The effects of quality and frequency of caregiver reading. Early Education and Development. 2008;19:89–111. doi: 10.1080/10409280701839106. [DOI] [Google Scholar]
- Gray JC, III, Krazinski AW, Schoepf UJ, Meinel FG, Pietris NP, Suranyi P, Hlavacek AM. Cardiovascular manifestations of Williams syndrome: Imaging findings. Journal of Cardiovascular Computed Tomography. 2013;7:400–407. doi: 10.1016/j.jcct.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Gershoff ET, Aber JL, Raver CC, Lennon MC. Income is not enough: Incorporating material hardship into models of income associations with parenting and child development. Child Development. 2007;78:70–95. doi: 10.1111/j.1467-8624.2007.00986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SE, Malow BA, Newman KD, Roof EE, Dykens EM. Sleep patterns and daytime sleepiness in adolescents and young adults with Williams syndrome. Journal of Intellectual Disability Research. 2009;53:182–188. doi: 10.1111/j.1365-2788.2008.01140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B, Risley TR. Meaningful differences in the everyday experience of young American children. Baltimore, MD: Paul H Brookes Publishing; 1995. [Google Scholar]
- Hillier LW, Fulton RS, Fulton LA, Graves TA, Pepin KH, Wagner-McPherson C, … Wilson RK. The DNA sequence of chromosome 7. Nature. 2003;424:157–164. doi: 10.1038/nature01782. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Martens MA, Fox R, Rabidoux P, Andridge R. Pragmatic language assessment in Williams syndrome: A comparison of the Test of Pragmatic Language-2 and the Children’s Communication Checklist-2. American Journal of Speech-Language Pathology. 2013;22:198–204. doi: 10.1044/1058-0360(2012/11-0131). [DOI] [PubMed] [Google Scholar]
- Ing C, DiMaggio C, Whitehouse A, Hegarty MK, Brady J, … Sun LS. Long-term differences in language and cognitive function after childhood exposure to anesthesia. Pediatrics. 2012;130:e476–485. doi: 10.1542/peds.2011-3822. [DOI] [PubMed] [Google Scholar]
- Kaufman AS, Kaufman NL. Kaufman Brief Intelligence Test, second edition. Circle Pines, MN: American Guidance Services; 2004. [Google Scholar]
- Key AF, Dykens EM. Electrophysiological study of local/global processing in Williams Syndrome. Journal of Neurodevelopmental Disorders. 2011;3:28–38. doi: 10.1007/s11689-010-9064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein-Tasman BP, Li-Barber KT, Magargee ET. Honing in on the social phenotype in Williams syndrome using multiple measures and multiple raters. Journal of Autism and Developmental Disorders. 2011;41:341–351. doi: 10.1007/s10803-010-1060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakusta L, Dessalegn B, Landau B. Impaired geometric reorientation caused by genetic defect. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2010;107:2813–2817. doi: 10.1073/pnas.0909155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lense MD, Dykens EM. Cortisol reactivity and performance abilities in social situations in adults with Williams syndrome. American Journal on Intellectual and Developmental Disabilities. 2013;118:381–393. doi: 10.1352/1944-7558-118.5.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lense MD, Key AP, Dykens EM. Attentional disengagement in adults with Williams syndrome. Brain and Cognition. 2011;77:201–207. doi: 10.1016/j.bandc.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lense MD, Shivers CM, Dykens EM. (A)musicality in Williams syndrome: Examining relationships among auditory perception, musical skill, and emotional responsiveness to music. Frontiers in Psychology. 2013;4 doi: 10.3389/fpsyg.2013.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lense MM, Dykens EE. Musical learning in children and adults with Williams syndrome. Journal of Intellectual Disability Research. 2013;57:850–860. doi: 10.1111/j.1365-2788.2012.01611.x. [DOI] [PubMed] [Google Scholar]
- Leyfer O, John AE, Woodruff-Borden J, Mervis CB. Factor structure of the Children’s Behavior Questionnaire in children with Williams syndrome. Journal of Autism and Developmental Disorders. 2012;42:2346–2353. doi: 10.1007/s10803-012-1482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens MA, Wilson SJ, Reutens DC. Research review: Williams syndrome: a critical review of the cognitive, behavioral, and neuroanatomical phenotype. Journal of Child Psychology and Psychiatry. 2008;49:576–608. doi: 10.1111/j.1469-7610.2008.01887.x. [DOI] [PubMed] [Google Scholar]
- Martens MA, Hasinski AE, Andridge RR, Cunningham WA. Continuous cognitive dynamics of the evaluation of trust worthiness in Williams syndrome. Frontiers in Psychology. 2012;3 doi: 10.3389/fpsyg.2012.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens MA, Jungers MK, Steele AL. Effect of musical experience on verbal memory in Williams syndrome: Evidence from a novel word learning task. Neuropsychologia. 2011;49:3093–3102. doi: 10.1016/j.neuropsychologia.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Mervis CB, John AE. Vocabulary abilities of children with Williams syndrome: Strengths, weaknesses, and relation to visuospatial construction ability. Journal of Speech, Language, and Hearing Research. 2008;51:967–982. doi: 10.1044/1092-4388(2008/071). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, John AE. Cognitive and behavioral characteristics of children with Williams syndrome: Implications for intervention approaches. American Journal of Medical Genetics Part C. 2010a;154C:229–248. doi: 10.1002/ajmg.c.30263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mervis CB, John AE. Williams syndrome: Psychological characteristics. In: Shapiro B, Accardo P, editors. Neurogenetic syndromes: Behavioral issues and their treatment. Baltimore: MD: Brookes; 2010b. pp. 81–98. [Google Scholar]
- Mervis CB, Morris CA. Williams syndrome. In: Mazzocco MMM, Ross JL, editors. Neurogenetic developmental disorders: Variation of manifestation in childhood. Cambridge, MA: MIT Press; 2007. pp. 199–262. [Google Scholar]
- Mervis CB, Kistler DJ, John AE, Morris CA. Longitudinal assessment of intellectual abilities of children with Williams syndrome: Multilevel modeling of performance on the Kaufman Brief Intelligence Test – Second edition. American Journal on Intellectual and Developmental Disabilities. 2012;117:134–155. doi: 10.1352/1944-7558-117.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miatton M, De Wolf D, François K, Thiery E, Vingerhoets G. Neuropsychological performance in school-aged children with surgically corrected congenital heart disease. The Journal of Pediatrics. 2007;151:73–78. doi: 10.1016/j.jpeds.2007.02.020. [DOI] [PubMed] [Google Scholar]
- Morris CA. The dysmorphology, genetics, and natural history of Williams-Beuren syndrome. In: Morris CA, Lenhoff HM, Wang PP, editors. Williams-Beuren syndrome: Research, evaluation, and treatment. Baltimore, MD: Johns Hopkins University Press; 2006. pp. 3–17. [Google Scholar]
- Opfer JE, Martens MA. Learning without representational change: Development of numerical estimation in individuals with Williams syndrome. Developmental Science. 2012;15:863–875. doi: 10.1111/j.1467-7687.2012.01187.x. [DOI] [PubMed] [Google Scholar]
- Palomares M, Shannon MT. Global dot integration in typically developing children and in Williams Syndrome. Brain and Cognition. 2013;83:262–270. doi: 10.1016/j.bandc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Palomares M, Englund JA, Ahlers S. Patterns and trajectories in Williams Syndrome: The case of visual orientation discrimination. Research in Developmental Disabilities. 2011;32:1021–1029. doi: 10.1016/j.ridd.2011.01.038. [DOI] [PubMed] [Google Scholar]
- Palomares M, Landau B, Egeth H. Orientation perception in Williams syndrome: Discrimination and integration. Brain and Cognition. 2009;70:21–30. doi: 10.1016/j.bandc.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piattelli-Palmarini M. Speaking of learning: how do we acquire our marvellous facility for expressing ourselves in words? Nature. 2001;411:887–888. [Google Scholar]
- Plesa Skwerer D, Ammerman E, André M, Ciciolla L, Fine AB, Tager-Flusberg H. A multimeasure approach to investigating affective appraisal of social information in Williams syndrome. Journal of Neurodevelopmental Disorders. 2011;3:325–334. doi: 10.1007/s11689-011-9100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesa Skwerer D, Ammerman E, Tager-Flusberg H. Do you have a question for me? How children with Williams syndrome respond to ambiguous referential communication during a joint activity. Journal of Child Language. 2013;40:266–289. doi: 10.1017/S0305000912000360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesa Skwerer D, Verbalis A, Schofield C, Faja S, Tager-Flusberg H. Social-perceptual abilities in adolescents and adults with Williams syndrome. Cognitive Neuropsychology. 2006;23:338–349. doi: 10.1080/02643290542000076. [DOI] [PubMed] [Google Scholar]
- Pryweller JR, Avery SN, Blackford JU, Dykens EM, Thornton-Wells TA. The effect of intellectual ability on functional activation in a neurodevelopmental disorder: preliminary evidence from multiple fMRI studies in Williams syndrome. Journal of Neurodevelopmental Disorders. 2012;4:1–8. doi: 10.1186/1866-1955-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer C, Rhein M, Knirsch W, Huber R, Natalucci G, Caflisch J, … Latal B. Neurodevelopmental outcome, psychological adjustment, and quality of life in adolescents with congenital heart disease. Developmental Medicine & Child Neurology. 2013;55:1143–1149. doi: 10.1111/dmcn.12242. [DOI] [PubMed] [Google Scholar]
- Searcy YM, Lincoln A, Rose F, Klima E, Bavar N, Korenberg JR. The relationship between age and IQ in adults with Williams syndrome. American Journal on Mental Retardation. 2004;109:231–236. doi: 10.1352/0895-8017(2004)109<231:TRBAAI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Strømme P, Bjørnstad PG, Ramstad K. Prevalence estimation of Williams syndrome. Journal of Child Neurology. 2002;17:269–271. doi: 10.1177/088307380201700406. [DOI] [PubMed] [Google Scholar]
- van der Fluit F, Gaffrey MS, Klein-Tasman BP. Social cognition in Williams syndrome: Relations between performance on the social attribution task and cognitive and behavioral characteristics. Frontiers in Psychology. 2012;3 doi: 10.3389/fpsyg.2012.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visootsak J, Hess B, Bakeman R, Adamson LB. Effect of congenital heart defects on language development in toddlers with Down syndrome. Journal of Intellectual Disability Research. 2013;57:887–892. doi: 10.1111/j.1365-2788.2012.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KT. Expressive Vocabulary Test. 2. Minneapolis, MN: Pearson Assessments; 2007. [Google Scholar]
- Yoshioka T, Dillon MR, Beck GC, Rapp B, Landau B. Tactile localization on digits and hand: Structure and development. Psychological Science. 2013;24:1653–1663. doi: 10.1177/0956797613478617. [DOI] [PMC free article] [PubMed] [Google Scholar]