Abstract
The ability of cells to collectively orient and align their behaviors is essential in multicellular organisms for unidirectional cilia beating, collective cell movements, oriented cell divisions, and asymmetric cell fate specification. The planar cell polarity pathway coordinates a vast and diverse array of collective cell behaviors by intersecting with downstream pathways that regulate cytoskeletal dynamics and intercellular signaling. How the planar polarity pathway translates directional cues to produce polarized cell behaviors is the focus of this review.
Keywords: planar polarity, cell polarity, cilia, asymmetric cell division, wing hairs, stereocilia, convergent extension, hair follicles, directional signaling
I. Introduction and the core PCP pathway
The coordinate polarization and alignment of cells over many cell distances is a phenomenon known as planar cell polarity (PCP). Most, if not all, epithelial tissues display some level of planar polarization, but it is perhaps most conspicuous in the epidermal appendages that cover the body surfaces like mammalian hairs, reptilian scales, and insect bristles. Internal tissues also display coordinately polarized patterns, which enable unidirectional beating of motile cilia, oriented cell divisions, and collective cell migrations that shape developing tissues. Despite the diversity of planar polarized structures in nature, they share certain characteristics, namely a global bias in orientation that is coordinated with the tissue axes, and precise local coordination with neighboring structures. The establishment of PCP requires cells to integrate directional information at the tissue, cellular, and subcellular scales, a process which is mediated by a set of conserved planar polarity pathways [1].
Molecularly, there are at least two partially independent but intersecting pathways known as the ‘core PCP’ and the ‘Fat/Dachsous PCP’ pathway. The core system was the first of the PCP pathways to be genetically defined in Drosophila. It is referred to as ‘core’ because its genes are required for each of the planar polarized structures in adult flies, and because it is highly conserved, having been shown to control planar polarized behaviors in cnidarians, nematodes, ascidians, amphibians, fish, and mammals [2, 3]. The major components of the core PCP pathway are the integral membrane proteins Frizzled (Fz), Van Gogh (Vang, Vangl in vertebrates), and Flamingo (Fmi, Celsr in vertebrates), and the cytoplasmic components Dishevelled (Dsh, Dvl in vertebrates), Prickle (Pk), and Diego (Dgo, Diversin in vertebrates) [1]. A key feature of the core PCP proteins is their planar asymmetric localization within individual cells. In the best-characterized example, the Drosophila wing blade, Fz, Dsh, and Dgo localize to intercellular junctions at the interface opposite Vang and Pk. Fmi, an atypical cadherin, localizes to both sides of the interface, and bridges opposite Fz and Vang complexes between neighboring cells [1,2]. This asymmetry is thought to arise from an initial global bias combined with feedback amplification to generate distinct non-overlapping membrane domains. Through intercellular interactions between Fz and Vang complexes, polarity is propagated from cell to cell. Current models for PCP establishment propose that the core PCP components interpret global directional cues, locally coordinate polarity between neighbors, and act on downstream tissue-specific effectors to organize polarized behaviors. Several excellent reviews cover the global inputs and interactions among core PCP components, as well as the Fat/Dachsous system, [1, 2, 4, 5] and therefore won’t be discussed in detail here. Instead, I review the literature on how the core PCP module interacts with diverse downstream cellular pathways to assemble collectively planar polarized behaviors.
Planar cell polarity is manifested both at the level of individual cells, through the asymmetric positioning or rotation subcellular structures, or at the level of cell clusters, which collectively orient as a unit. I categorize these polarized behaviors broadly as actin-based or microtubule-based. Furthermore, I also discuss how PCP orients collective cell motility during embryogenesis and how it directs asymmetric cell fates by biasing the direction of signaling and/or cell division. While this is not a comprehensive list of downstream outputs of the core PCP pathway, they are some of the best understood at a molecular level.
II. Planar polarization of actin-based structures
Some of the first planar polarized structures to be linked to the core PCP pathway were the actin-based trichomes and bristles covering the insect epidermis and the stereocilia bundles of the vertebrate inner ear. The prominent asymmetry of these cellular protrusions indicated that PCP acts on the cytoskeleton to organize its assembly in a directional manner.
Orienting actin-based cellular protrusions – Drosophila Wing Hairs
The cuticular hairs that decorate the insect body surface were of the first planar polarized structures to be genetically linked to the core PCP pathway [6, 7]. Indeed most of what is currently known about the PCP pathway has emerged from studies of Drosophila wing hairs - tiny protrusions comprised of actin bundles that emanate from the epidermal cell layer and connect to the outer cuticle. In the wing, a single actin-based hair emerges from the distal vertex of each epithelial cell and points in a distal orientation [8] (Fig. 1A). A conserved PCP pathway controls the global pattern of wing hairs by instructing the position of hair initiation [8, 9]. In the core class of PCP mutants (Fz, Vang, Dsh, Pk, Fmi, and Dgo), wing hairs emerge from aberrant positions on the cell surface, sometimes multiple in number, and point in disordered orientations [8]. Because the protein products of each of these genes asymmetrically localizes within each cell - with Fz, Fmi, Dsh, and Dgo at the distal cell cortex and Vang, Fmi, and Pk on proximal cortex - it is thought that the core PCP module instructs wing hair placement by restricting actin polymerization and bundling activities to distal cell edges.
Fig. 1. Planar polarization of actin-based structures.
(A) The Drosophila wing blade. The wing blade of Drosophila is decorated with actin-based hairs, or trichomes, the point in a distal direction. The core PCP components (red, blue) localize asymmetrically in the epithelial plane. The proximal core PCP components recruit the PCP effectors Inturned, Fuzzy and Fritz (green) to the proximal side of each cell. The PCP effectors then recruit Mwh, which represses actin polymerization activities, and restricts wing hair formation to the distal side. (B) Stereocilia bundles of the inner ear. Actin-rich stereoclila bundles form a V-shape whose vertex is positioned just below a microtubule-based kinocilium. The orientation of kinocilia is collectively aligned across the tissue by the core PCP pathway. PCP protein localization in inner ear cells is not strictly conserved with that of Drosophila, as Dvl and Fz are thought to be localized to opposite sides of the cell.
Genetic studies identified three classes of wing PCP mutants whose phenotypic and epistatic relationships suggest a functional hierarchy controlling actin polymerization [9]. At the top of this hierarchy is the core PCP module, which acts genetically upstream and is required for the subcellular localization of components of the other two classes [9–11]. The second class of mutants, Inturned (in), Fuzzy (Fy), and Fritz (Frtz), display multiple wing hairs at aberrant positions without affecting the asymmetric localization of the core components [9–12]. These so-called PCP effector genes function cell autonomously in wing epithelial cells and act genetically downstream of the core module [9, 12–14]. Like the core components, the protein products of the PCP effector genes also localize asymmetrically, but each is localized exclusively to the proximal side of each wing epithelial cell, opposite the position of the wing hair [10, 11] (Fig. 1A). While PCP effector localization requires core PCP components, it is yet not known if direct binding to Vang or Pk recruits them to the proximal side. Though their biochemical functions are largely unknown, all three PCP effectors physically interact and are mutually required for one another’s localization, suggesting they may function together as a protein complex [10, 15]. Moreover, their enrichment at the opposite side of the actin pre-hair suggests their activities oppose or limit actin polymerization, which is consistent with the ectopic sites of F-actin nucleation observed in In, Fy, and Frtz mutants. The best guess at present is that the PCP effector complex serves as a scaffold or adaptor for actin-regulatory proteins, which comprise the third and final class of wing polarity genes.
The multiple wing hairs (mwh) gene is the most downstream of the wing hair PCP genes [6, 9]. In mwh mutants, three or more ectopic hairs protrude from each wing cell due to excessive F-actin accumulation over the entire apical surface as well as a failure of actin pre-hairs to fully coalesce [9, 10, 16, 17]. The Mwh protein is distributed in a gradient within each wing cell with maximal enrichment on the proximal side. Both the core PCP module and the PCP effectors are necessary for Mwh proximal accumulation, which may be recruited by direct binding to Inturned [10, 16, 18]. Mwh encodes a novel protein containing G-protein binding (GBD) and FH3 homology domains with similarity to the diaphanous-related formin group of actin regulators. This homology suggested that Mwh is the protein that provides the link between PCP and the actin cytoskeleton. But unlike classical formins, which induce F-actin polymerization, Mwh acts an actin antagonist. Overexpression of Mwh delays pre-hair initiation and blocks the formation of F-actin structures in cultured S2 cells [10, 16]. Recent biochemical experiments found that Mwh can antagonize actin directly, as purified Mwh-GBD-FH3 fragments bind to actin in vitro and inhibit actin elongation into long filaments [17]. Mwh formin-like domains also have actin bundling activities in vitro, which explains why actin pre-hairs fail to fuse in mwh mutants [17]. Thus, there is a functional hierarchy controlling wing hair polarization where the core PCP module acts upstream to localize the PCP effector genes, which in turn localize and perhaps activate Mwh to restrict actin-polymerizing activities to the distal side of each cell. A distal-acting, positive regulator for actin pre-hair formation is probably also required but the nature of this pathway remains unknown.
The planar polarity of wing hairs requires several additional actin regulators including Rho1, the formin Diaphanous (Dia), cofilin, and non-muscle myosin [19–24]. Like the PCP effector genes, mutations in these other cytoskeletal regulators cause ectopic and/or misoriented hairs in the wing. However, placing these essential actin regulators into a functional hierarchy for wing polarity is complicated because of their numerous functions in cell shape, growth and division, apical basal polarization, and intercellular junctions, all of which can influence core PCP asymmetry and function in addition to morphogenesis of the actin pre-hair.
Whether this relatively simple pathway from the core PCP module to actin regulation is conserved in other actin-based planar polarity systems, is unclear. But given that the Mwh protein is specific to insects, it is likely that the core PCP module controls the spatial distribution of actin dynamics by distinct mechanisms in other systems. Intriguingly, the PCP effectors genes are highly conserved in vertebrates, where they unexpectedly function in ciliogenesis and convergent extension and seemingly not in the planar polarization of actin-based protrusions like stereocilia in the inner ear.
Orienting actin-based cellular protrusions - Stereocilia bundles of the vertebrate inner ear
Perhaps the most striking planar polarized actin-based structures are the mechanosensory stereocilia bundles of the vertebrate inner ear, which were the first actin-based structures to be genetically and molecularly linked to the core PCP module in mammals [25, 26]. The mechanosensory epithelium of the inner ear, which enables the sensation of hearing and balance, is composed of hair cells from which bundles of specialized microvilli called stereocilia emerge from the luminal surface. Stereocilia are arranged in closely packed rows in a U or V-shape configuration within each hair cell (Fig. 1B). The vertex of each stereocilia bundle aligns precisely with its neighbors along the epithelial plane, a pattern that is thought to be essential for coordinated and efficient mechanotransduction. Like insect wing hairs, inner ear hair cells require homologs of the core PCP module to globally orient. In Vangl2, Fz3/Fz6 double, Celsr1, and Dvl1/2 double, and Dvl3 mutants, stereocilia bundles assemble into proper V-shaped, staircase bundles but their vertices point with random orientations relative to the tissue axes [25, 27–30]. In addition, Vangl, Fz, Pk and Dvl proteins asymmetrically localize within hair cells and their surrounding support cells, demonstrating further conservation with invertebrate PCP [28, 29, 31, 32] (Fig. 1B). However, the relationship between different PCP protein asymmetries in the inner ear deviates from what has been shown invertebrates. For example Fz6 and Dvl2 appear to localize to opposite sides of the cell, rather than colocalize as would be predicted from Drosophila [28, 29] (Fig. 1B). Furthermore, whereas Fz6 localizes proximally in hair cells of the cochlea, it localizes distally in hair cells of another inner ear structure, the utricle [28]. Thus, the relationship between PCP protein asymmetry and the structural polarity of the hair bundle is not simple.
Whereas the positioning of actin pre-hairs in the insect wing involves core PCP-mediated inhibition of actin polymerization, stereocilia polarity is established by a distinct mechanism involving the migration of a primary cilium. During hair cell differentiation, and before actin microvilli have elaborated into mature stereocilia, a single, microtubule-based primary cilium elongates to become a non-sensory kinocilium [26]. The kinocilium migrates from an initially central position towards the cell cortex where it will associate with the vertex of the stereocilia bundle (Fig. 1B). Several pieces of evidence argue that the kinocilium acts as a landmark for stereocilia orientation and as an organizer of hair bundle organization. In the absence of functional kinocilium, as in Kif3a, Ift88, or Bardet-Biedl syndrome protein mutants, stereocilia bundles are malformed and point in random orientations [33–36]. Moreover, when the kinocilium is uncoupled from stereocilia, as in Rac1 or Lis1 mutants, they become misshaped and misoriented [37, 38]. These defects are not due to loss of core PCP function, which remains in tact as evidenced by normal subcellular localization of core PCP proteins in cilia mutants [34, 36]. Therefore, directional migration of the kinocilium is thought to be an instructive event for hair bundle orientation. But whereas in insect wing epithelial cells, the core PCP pathway is necessary for the off-center placement of actin protrusions, kinocilium displacement is independent of the core PCP system. What the core PCP module appears to do is coordinate the direction of kinocilium displacement with the tissue axes, a process requiring non-autonomous interactions between hair cells and their surrounding support cells. Kinocilium displacement, on the other hand, follows a cell-intrinsic polarity system that is largely separate from the core PCP system [39, 40], which will be discussed later in the review in the context of microtubule-based structures. Thus, PCP controls the polarity of stereocilia, not by regulating actin assembly directly but instead, by orienting the migration of a microtubule-based primary cilium.
Orienting collective cell motility by polarizing actin dynamics
The directed motility of collections of cells drives several essential morphogenetic processes during embryonic development, most notably gastrulation in early embryos. The term convergent extension refers to the process by which a sheet of cells simultaneously lengthens along one axis (extension) while shortening along the orthogonal axis (convergence) through directed cell movements [41]. This process transforms short and wide cell assemblies into long and narrow morphologies; and is widely employed during development for elongating the embryonic axis and shaping epithelial organs. Convergent extension during Xenopus gastrulation was the first vertebrate developmental process to be linked molecularly to the core PCP pathway [42–44]. Since then PCP has been shown to control convergent extension movements during gastrulation in several animal species including zebrafish, mouse, and ascidians, and in the elongation of other tissues during organogenesis including kidney tubules, the mouse limb, the neural plate and the cochlea (reviewed in [3]). The key underlying process driving tissue extension in each of these examples is directional cell intercalation. It should be noted that convergent extension is widely employed throughout the animal kingdom to elongate tissues and can be induced by upstream inputs that are independent of the core PCP system. Elongation of the Drosophila germband is a notable example [45]. So while the downstream mechanisms by which cell intercalate may be quite similar, more than one, and perhaps several upstream polarizing signals can coordinate the process. For the purposes of this review, we will focus on convergent extension driven by the core PCP system.
In the gastrulating vertebrate embryo, the body axis elongates along the anterior-posterior axis when cells of the mesoderm and neural plate undergo a process of mediolateral interacalation [46]. During mediolateral intercalation, cells that initially are laterally associated, interdigitate and line up along the anterior-posterior axis, thereby lengthening the embryo in the anterior-posterior direction while narrowing it along the medial-lateral axis [41] (Fig. 2A). Intercalation of mesodermal cells involves stereotypic cell behaviors that are controlled by the core PCP pathway. First, cells become elongated and extend actin-rich lamellipodia from their mediolateral, but not anterior posterior surfaces. Cells make contacts with their lateral neighbors via these lamellipodial protrusions, which exert traction forces and allow cells to crawl in between one another. Manipulation of core PCP proteins in Xenopus and zebrafish disrupts these coordinated cell behaviors [3, 47]. For example, interfering with Xenopus Dvl and Stbm (Vangl) causes cells to lose their elongated cell shapes and polarized lamellipodia protrusions. Lamellipodia become unstable and extend in all directions, leading to uncoordinated cellular movements and failure to intercalate [42, 48]. Thus, the core PCP module acts in convergent extension to coordinate the planar polarization of cell elongation and actin protrusions.
Fig. 2. Planar polarization of collective cell movements.
(A) Convergent extension during Xenopus gastrulation. Mesodermal cells undergo mediolateral directed intercalation to narrow and lengthen the body axis during gastrulation. Intercalation is driven by lamellipodia exerting traction on their neighbors and by shorting of anterior-posterior cell interfaces through actomyosin contractility. (B) Convergent extension of the neural plate in mouse and chick. In epithelia, mediolateral interaction is driven by junctional rearrangements. In this example, Celsr1 and Dvl organize actomyosin at anterior-posterior junctions, which undergo shrinking. When a new junction expand, the tissue narrows and lengthens as cells exchange neighbors.
What is the mechanism by which the PCP pathway drives mediolateral cell elongation, lamellipodia polarization, and mediolateral intercalation? The localizations of PCP proteins in cells undergoing convergent extension are less obviously polarized than in epithelia, perhaps due to the highly dynamic nature of intercalating cells. Nevertheless, PCP protein asymmetries have been observed for GFP-Pk in zebrafish, which preferentially accumulates to the anterior side of intercalating cells, and for Dvl, which localizes to the posterior [49] (Fig. 2A). Based on these localization patterns, PCP proteins could act to inhibit branched actin polymerization and protrusive activities along the A-P axis, thereby restricting lamellipodia formation to the mediolateral cell edges. Several lines of evidence connect Dvl molecularly to Rho activation, myosin contractility, and activation of Daam1 (a diaphanous-related formin), which would be predicted to inhibit lamellipodia formation wherever Dvl is localized. In Xenopus, Dvl has been shown to activate RhoA though its interaction with Daam1, and both Rho and Daam1 are required for convergent extension [50]. Dvl also binds to Xenopus WGEF, a Rho guanine nucleotide exchange factor required for convergent extension, which can activate RhoA in both cultured cells and Xenopus embryos [51]. While the specific downstream outputs of localized Rho activity in intercalating cells is unknown, based on the biochemical activities of RhoA and Daam1 [52, 53], one would predict these proteins induce acto-myosin contractility and polymerization of linear actin cables at sites of PCP localization.
Consistent with localized acto-myosin activities to PCP-enriched cell edges, convergent extension movements have also been shown to be coordinated by pulsed actomyosin contractions along anterior-posterior cell edges [54, 55] (Fig. 2A). With each pulse of actin, A-P edge length and cell area decrease as cells converge along the mediolateral axis. Interfering with Dvl function leads to a delocalization of actin pulses, a decreased rate of actin contractions [54], which correlates with convergent extension failure. The mechanism by which Dvl localizes acto-myosin dynamics is, in part, by helping to localize Septins to cell vertices. Septins are cytoskeletal elements that function as cortical diffusion barriers, and knockdown of Sept7 causes a loss of actin compartmentalization and myosin polarization [55]. These defects correlate with a loss of edge shrinking and cell intercalation in Sept7 morphants [55]. Thus, mesoderm cells appear to employ PCP-dependent cell crawling and edge shrinkage mechanisms to drive coordinated cell intercalation and axis elongation.
Epithelial tissues, like the neural epithelium, also elongate by convergent extension, but rather than cell crawling, these tightly adherent cells intercalate by rearranging their intercellular junctions. This mechanism for convergent extension was first described in Drosophila germ band extension, and is characterized by selective shrinkage and expansion of orthogonally oriented cell interfaces [56, 57] (Fig 2B). Repeated cycles of junctional shrinking and expansion transforms the germband from a short and wide epithelium into a long and narrow morphology (reviewed in [58]). Although Drosophila germband extension is regulated independently of PCP [45], epithelial intercalation in the mouse and chick neural plate and Xenopus kidney tubules occurs in a PCP-dependent manner [59–61]. Live imaging in the mouse neural plate revealed that epithelial cells converge into tetrads and multicellular rosettes as anterior-posterior oriented junctions contract and shrink. Rosettes then resolve through the formation of new mediolateral junctions causing cells to exchange neighbors and the tissue to elongate (Fig. 2B0. Disruption of Vangl2 inhibits directional rosette resolution in the neural plate, which correlates with a shortened body axis and complete failure to close the neural tube [60]. Like the shrinking cell interfaces in Xenopus mesoderm, localized myosin II contractility accompanies convergent extension in the neural plate, and is polarized toward anterior-posterior junctions [60, 61]. In chick, Celsr1 and Dvl promote anterior-posterior myosin enrichment by recruiting Rho kinase to cell interfaces via a Dvl-Daam1-PDZGEF dependent mechanism [61]. Thus, convergent extension movements across different cell types utilize PCP to localize actin polymerization and acto-myosin activities to drive coordinated cell intercalation.
III. Planar polarizing microtubule based structures
The core PCP module promotes the asymmetric positioning and rotation of microtubule based structures, most notably cilia and the mitotic spindle. The fact that the same core machinery can connect to diverse cytoskeletal structures suggests great flexibility in PCP binding partners. Here I discuss what is known about how the core PCP components link to and promote the asymmetric assembly of cilia and the spindle.
Orienting the Mitotic Spindle
During development, the process of asymmetric cell division generates cell fate diversity by unequally partitioning cell fate determinants to daughter cells. This process requires that both cell fate determinants and the mitotic spindle align with axis of polarity. In Drosophila sensory organ precursors (SOPs), PCP directs SOP cells to divide asymmetrically along the anterior-posterior axis, producing distinct cell types of the sensory bristle lineage. The fate of SOP daughter cells is determined by the differential inheritance of the Notch regulator, Numb, which is inherited only by the anterior-most daughter cell [62]. The core PCP system in this context plays two separate but crucial functions: 1) it breaks the planar symmetry of the PAR complex, which is responsible for asymmetric partitioning of Numb, and 2) it orients the mitotic spindle precisely with the anterior-posterior axis [62] (Fig. 3A). Together, PCP ensures the asymmetric inheritance of Numb in SOP daughter cells, and confers their distinct fates.
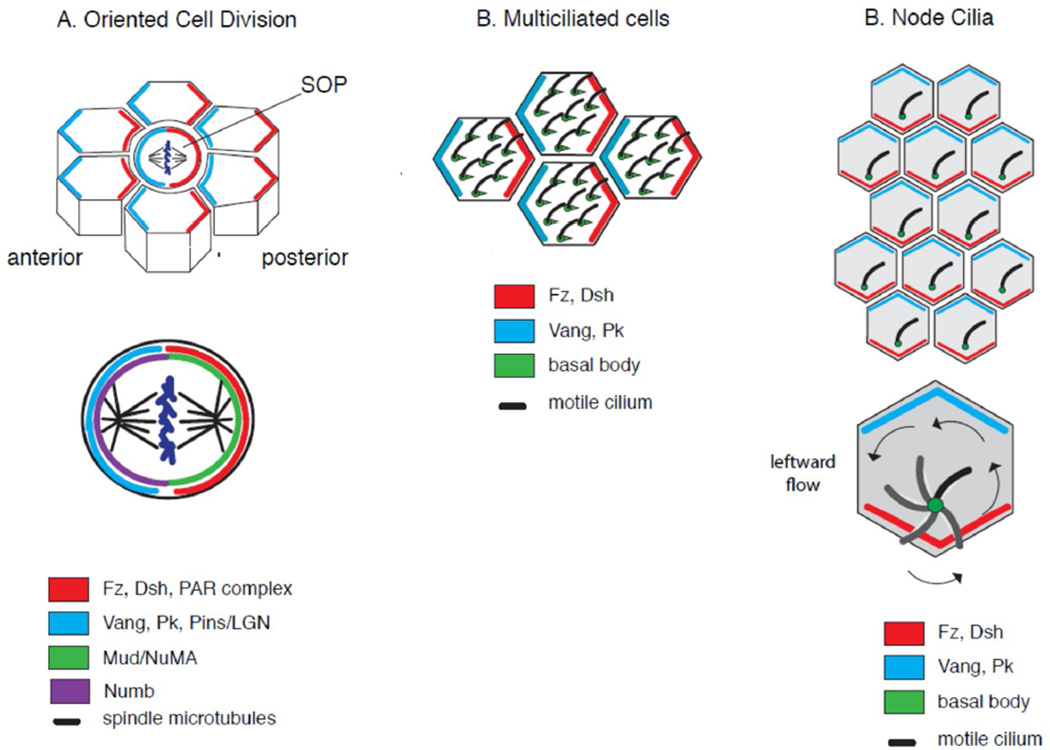
Fig. 3. Planar polarization of microtubule-based structures.
(A) Oriented cell division. The Drosophila sensory organ precursor (SOP) undergoes a planar, asymmetric division to produce two daughter cells with distinct cell fates. The core PCP pathway orients this division in part by interacting direction with the spindle orientation machinery (green) and pulling astral microtubules into a planar configuration. (B) Multiciliated cells. In this generalized example of a multiciliated epithelium, cilia motility and fluid flow are determined by the collective orientation of basal bodies (green). The direction of basal body polarity is referred to as rotational polarity. (C) Monociliated cells. In motile, monociliated cells, such as those of the mouse node, the direction of cilia motility and fluid flow are determined by the posterior displacement of cilia in each cell. The coordinated displacement of cellular structures along a tissue axis is referred to as translational polarity.gr3
Symmetry breaking in SOPs occurs during interphase, at least two hours prior to mitosis, as PAR complex proteins (Par3/Bazooka, aPKC, and Par6) become enriched on the Fz side of the cortex [63]. The posterior enrichment of the PAR complex leads to the phosphorylation of Numb by aPKC and its exclusion from the posterior, and thus, its restriction to the anterior side [62]. The initial bias of PAR proteins relies on PCP function, as Fz, Vang, and Dsh mutants show no bias in interphase PAR protein enrichment [63]. Later in mitosis, however, PAR proteins still polarize even in the absence of PCP, but their asymmetry is randomized with respect the anterior-posterior axis [64, 65]. Thus, PCP breaks the initial symmetry of SOPs cells, but a mitotic rescue mechanism restores PAR (and thus, Numb) polarity [63]. How PCP proteins cause a shift in the localization of apical PAR complexes towards the posterior in SOP cells, but not in the surrounding epithelium, is not known.
PCP plays a second role in SOP divisions by aligning the mitotic spindle with the anterior-posterior axis [62]. In this role, PCP appears to connect directly to the well-conserved spindle orientation machinery that tethers astral microtubules to the cell cortex and pulls the centrosome into register with the axis of polarity. At the posterior cortex of SOPs, Fz and Dsh interact with Mud/NuMA, which binds directly to the dynein complex, tethering one half of the spindle to the posterior [66] (Fig. 3A). On the anterior side, Pins/LGN also recruits Mud/NuMA-Dynein, bringing the spindle into register with the anterior-posterior axis [66] (Fig. 3A). An accessory pathway involving Dsh-mediated recruitment of Rho and Formin also promotes spindle alignment by modifying the cortical actin cytoskeleton to help anchor and position the mitotic spindle [67].
PCP utilizes similar actin and microtubule-based mechanisms to orient cell divisions in zebrafish embryos, where Vangl, and Dvl are required to bias early embryonic divisions along the animal-vegetal (A-V) axis [68]. As in Drosophila, zebrafish Dvl can bind to Mud/NuMA, and Mud/NuMA morphants display randomized embryonic divisions, suggesting that a conserved mechanism links PCP to astral microtubules in vertebrates [66]. In addition, PCP controls the positioning of polarized cortical actin caps in dividing cells, which align along the A-V axis and forecast the division plane [69]. These actin caps associate with a Anthrax receptor 2 – Rho - Formin complex, which is thought to exert torque on the spindle to align it with the actin cap [69]. It is not known how astral microtubules connect to and align with cortical actin.
Orienting motile cilia
Motile cilia direct fluid flow in several organ systems, notably, the mammalian oviduct, where flow propels the egg towards the uterus, and the vertebrate node, where leftward fluid flow establishes the left-right axis of the vertebrate body plan [3]. The successful generation of unidirectional flow relies on the collective and directed motility of cilia whose polarized motion is coordinated across the tissue. The direction of cilia motility is determined by the placement and orientation of its structural elements, which the core PCP module orients both within and between cells [70] (Fig. 3B–C).
Cilia are microtubule-based structures, nucleated from specialized centrioles called basal bodies. Basal bodies anchor at the apical cell surface of epithelial cells forming the base from which each cilium emanates. Monociliated cells, like those that decorate the vertebrate node, display rotational motility whose direction is determined by the asymmetric positioning and tilting of the basal body [71(Fig. 3C). On the other hand, muticiliated cells, or MCCs, like those lining the airways, oviduct, and brain ventricles, move with a front-to-back sweeping motion whose direction is determined by the orientation of the basal body{Brooks, 2014 #2] (Fig. 3B). Thus, ciliated cells can display three levels of polarity: the positional displacement of cilia is termed translational polarity, the direction of cilia motility is termed rotational polarity, and the coordination of these asymmetries between cells is tissue polarity. The core PCP module has been implicated in the coordination of all three levels of cilia polarity [70].
The requirements for the core PCP module in coordinated ciliary beating were first described in the MCCs of the Xenopus skin epidermis, a tissue with remarkable similarities to the mammalian airway. Expression of a dominant-negative version of Dvl (Xdd1) in the frog epidermis severely impaired planar polarization of basal bodies leading to disorganized cilia beating, and randomized fluid movements across the epidermis [72]. PCP has since been shown to control the polarization of MCCs in the trachea, ependymal cells of the brain and spinal cord, and of MCCs lining the oviduct [73–75]. Mice lacking coordinated cilia beating in these organ systems display lethal hydrocephalus and infertility, which are thought to be due to inefficient fluid flow [74, 75].
Tissue-level polarity
The tissue-level alignment of cilia polarity likely involves intercellular communication and cell-to-cell propagation of polarity. This was first demonstrated in a series of transplantation experiments in the frog epidermis, where normal cells were mixed with Vang, Fz or Dvl deficient cells. Cells lacking Vang and Fz, but not Dvl, induced polarity reorientation non-autonomously in neighboring control cells [76], a phenomenon that is well-described in Drosophila termed domineering non-autonomy. These results are consistent with current models of PCP where the transmembrane components (Fz, Vang, Fmi) coordinate tissue-level polarity through intercellular communication while the cytoplasmic factors (Dvl, Pk) function autonomously to reinforce asymmetry and build the structural polarity of the cell [77]. How cortical PCP complexes coordinate cilia polarity between neighboring cells is not entirely clear but a specialized pool of planar polarized microtubules may be involved. In both tracheal and ependymal MCCs, an enrichment of microtubule plus-ends is localized to apical junctions on the Fz-Dvl side of the cell [73, 78]. These junctional microtubules appear to connect to basal bodies, suggesting that Fz-Dvl complexes may coordinate tissue-level polarization by connecting basal body rotation to the cell cortex [73].
Tissue-level polarization of cilia motility also requires the hydrodynamic forces generated by fluid flow itself. While PCP imparts an initial bias in flow direction, fluid flow feeds back to reinforce directed cilia beating by refining the orientation of basal bodies into highly aligned arrays [79, 80]. Without cilia motility, basal bodies poorly align, but exogenously applied fluid flow can restore their collective polarity as long as cilia are motile [79]. Vangl2 is required for flow-induced cilia alignment, suggesting a function for core PCP in the mechanical response of cilia to flow [80]. Thus, motile cilia have a mechanical feedback mechanism to establish robust and coordinated tissue-level polarity.
Rotational polarity
In addition to tissue-level polarity, the core PCP system is responsible for the collective alignment of basal bodies within individual MCCs. The basal body itself is a polarized structure, characterized by a protrusion called the basal foot, whose orientation predicts the direction of cilia beating [70] (Fig. 3B). In MCCs, lattices of subapical actin and microtubule filaments connect adjacent basal bodies, ensuring their regular spacing and locally coordinating their rotational polarity [81]. The functions of these two cytoskeletal networks are distinct: disassembling subapical actin disrupts cilia spacing and coordination between cells, whereas microtubule depolymerization randomizes basal body orientations within cells [81]. In Celsr2 mutants or Dvl morphants, both subapical actin and cilia polarity are disrupted, suggesting organization of the subapical cytoskeletal networks relies on PCP [72, 78].
Because the direction of basal foot alignment strongly correlates with the axis of PCP asymmetry, it is tempting to speculate that asymmetric PCP protein localization directly controls basal body orientation. However, this model is challenged by the inconsistent relationship between PCP protein localization and the direction of fluid flow in different multiciliated tissues. For example, in the trachea, basal bodies are directed proximally (orally) towards the side of the cell where Fz6 and Dvl1/3 localize and opposite the Vang1/2 and Pk2 side of the cell [73]. This is similar to the reported localization of Vang2 in ependymal cells – opposite the direction of flow [78, 80]. However in the frog epidermis, the configuration is reversed, where Vangl1 and Pk2 orient toward the direction of flow [82]. This challenges the idea that asymmetric core PCP complexes couple directly to the cilia machinery to drive their polarized assembly, and suggests that different cell types can respond differently to PCP vectors. However one must interpret PCP localization data obtained by immunofluorescence cautiously, as conventional microscopy methods cannot resolve the two sides of an epithelial junction. Super resolution techniques or mosaic analyses, such as those performed in the frog epidermis [82], will allow for a more definitive localization of PCP asymmetry in multiciliated cell types.
Translational Polarity
In monociliated cells, such as those found in the node of vertebrate organs, flow is generated by the coordinated rotational motion of cilia, whose direction is determined by the asymmetric placement, or translational polarity, of ciliary structures [71]. In 2010, several papers reported functions for members of the core PCP module in the translational polarity of motile cilia in the vertebrate node. The mouse node is a transient fluid-filled cavity present early in embryonic development that is responsible for determination of the left-right body axis [71]. About 300 motile cilia produce a leftward fluid flow that is both necessary and sufficient for establishing left-right asymmetry, but the directional cues that bias leftward cilia rotation was not known. First, node cilia were shown to initially develop near the center of the cells’ apical surface, but then progressively acquire a posterior displacement [83]. Because of the node’s cupped shape, this posterior displacement tilts the cilium posteriorly, generating leftward rotation and flow (Fig. 3C). In Vangl and Dvl mutants, this posterior displacement is lost causing defective nodal fluid flow and randomization of left-right axial asymmetry [83–85]. At present, how Vangl and Dvl proteins control the asymmetric placement of cilia is not known. Translational polarity is also observed in the placement of the cilia patch in ependymal MCCs. But unlike the node, deletion of 5 out 6 Dvl alleles affects the tissue-level coordination of translational polarity but not the displacement itselfhata [86]. This is similar to the ependymal phenotype caused by deletion of Celsr1 [78].
Perhaps the best understood example of translational polarity is the non-motile kinocilium of the inner ear. Unlike the V-shaped sterocilia bundle, the kinocilium is a bonafide primary cilium comprised of a 9+2 microtubule architecture, and the position of the kinocilium predicts the orientation of stereocilia bundles (see Fig. 1B). During hair cell development the kinocilium migrates to a distal position within each cell, which is prefigured by an asymmetric crescent containing Galphai, mPins, and mInsc [39, 40]. Whereas the tissue-level alignment of Galphai/mPins crescents requires the core PCP protein Vangl2, Galphai and mPins are required cell-autonomously for the asymmetric displacement of the kinocilium [39, 40]. This mechanism is reminiscent of how PCP orients the mitotic spindle in Drosophila - by directly connecting astral microtubules to the cortex via the Galphai/Pins/Dynein machinery. Indeed, an enrichment of microtubule plus ends emanates towards the cortex from the basal body, suggesting a cortical Galphai/mPins/Dynein complex might capture the hair cell microtubule aster [39]. Again, the relationship between core PCP and Galphai crescent localization is inconsistent at different positions in the inner ear. For example, at the zone of reversal in the macula of inner ear, cilia polarity and Galphai crescents are reversed, whereas Fz6 and Pk2 localizations are maintained. This again suggests that different cell types can respond oppositely the to the same PCP vectors.
IV. Planar polarizing cell fate
The acquisition of new cell fates from equivalent progenitors is a driving force for the development and differentiation of multicellular organisms. We have already seen how PCP promotes cell fate asymmetry through oriented cell division. PCP also directs the orientation of cell fates decisions by biasing the direction of intercellular signaling events. By biasing the direction of juxtacrine signaling between neighboring cells within an epithelial sheet, PCP ensures cell fate specification occurs at the right position.
Directional Notch signaling in the specification of photoreceptors
The first example of directional cell fate specification by the PCP pathway was that of the R3/R4 photoreceptor pair in the Drosophila eye [87]. The photoreceptor clusters of Drosophila ommatidia are planar polarized as individual units, which collectively orient relative to the tissue equator (Fig. 4A). The polarity of the photoreceptor cluster arises when two cells, the R3 and R4 photoreceptors, become specified in a directional manner where equatorial-most cell of the cluster invariably gives rise to the R3 fate. The fates of the R3/R4 pair depend on their relative levels of Notch/Delta signaling such that the cell with higher Notch activity becomes R4 [88–90] (Fig. 4A). The directionality of this fate decision is determined by the core PCP system, which dampens Notch and/or enhances Delta in the cell located closer to the equator (where Fz ‘activity’ is proposed to be higher) (Fig. 4A). How PCP alters Notch and Delta in the R3 precursor is not entirely clear. In one model, Fz activity is graded from the equator to the margins and the cell with the highest Fz activity in each cluster activates Delta and Neuralized transcription. Consistently, when Fz is mosaically overexpressed or depleted, the cell with higher Fz levels in mosaic R3/R4 pairs displays increased Delta and Neuralized, expression, promoting Notch signaling, and R4 fate, in the neighboring cell [90, 91]. In this model, PCP leads to biased transcriptional activity in one of two cells, but the links between core PCP proteins and a downstream pathway leading to gene transcription is unknown. An alternative model proposes that PCP protein asymmetry could provide a localization or activity bias in Notch and/or Delta proteins at the interface between R3 and R4 precursor cells [92]. This model is supported by direct interactions between Dsh and Notch, and that mutations interfering with Notch and Dsh binding cause defects in the orientation of R3/R4 specification [92].
Fig. 4. Planar polarization of cell fates.
(A) R3/R4 photoreceptor fate in Drosophila ommatidia. R3/R4 photoreceptor fates are specified by directional signaling between R3 and R4 precursor cells. PCP biases the direction of Notch/Delta signaling such that the cell furthest from the equator activates Notch target genes an R4 fate. Later, the photoreceptor cluster rotates to form its mature, polarized configuration. (B) Bract cell fate in the Drosophila leg sensory organs. Bracts (green) are associated with each sensory organ in the Drosophila leg on the proximal side. Bract cell fate is specified by directional EGFR signaling. The socket precursor cell (blue), which produces the ligand Spitz, sends out proximal protrusions whose directionality are PCP dependent. The cell that comes into contact with these protrusions turns on EGFR target genes. (C) Asymmetric gene expression in the hair follicle. Mammalian hair follicles undergo PCP-depending anterior tilting and asymmetric gene expression. Core PCP proteins are asymmetrically localized in hair placodes and the surrounding epithelium. During tilting, anterior and posterior cells of the placode turn on distinct sets of genes. This asymmetry may be determined by the anterior displacement of the dermal condensate (grey).
Directional Notch signaling in joint specification
A striking phenotype observed in Drosophila core PCP mutants is the formation of ectopic leg joints [93, 94]. Joint specification is mediated by directional Notch signaling, where cells located distal, but not proximal, to ligand expression (the relevant ligand in this case is Serrate, not Delsta) activate Notch target genes and establish leg joint fate [94]. Although cells located proximal to Serrate ligand express the Notch receptor and are competent to signal, these cells do not normally activate Notch signaling and do not establish joint cell fates [93]. However, in the absence of core PCP function, as in dsh, pk, or fz mutants, cells proximal to Serrate expression ectopically activate Notch target genes and form excess joint fates, indicating that PCP acts to repress Notch signaling specifically in cells proximal to the ligand [94]. This function correlates with asymmetric PCP localization, where Fz and Dsh localize distally at the interface between non-responding and ligand-expressing cells [94]. Directional repression of Notch signaling appears to occur by Dsh-mediated endocytosis of Notch. Dsh overexpression causes Notch to accumulate in endosomes and blocks the expression of Notch target genes. Moreover, Dsh binds directly to Notch, possibly acting as a cytoplasmic adaptor for its endocytosis [94]. By selectively internalizing Notch receptors on the distal sides of each cell, PCP renders proximal cells irresponsive to the Serrate ligand, promoting unidirectional Notch signaling and fate specification.
Directional EGF signaling
Directional signaling through the EGFR pathway specifies another cell fate in the Drosophila leg, the bract cell, which produces a trichome associated with the sensory organs (bristles) that decorate the Drosophila leg [95] (Fig. 4B). But in contrast to leg joint specification where the signal reception is asymmetric, bract cell specification relies on asymmetric signal sending. EGFR signaling specifies the bract cell fate and it is the socket cell of the adjacent sensory organ that supplies the EGFR ligand, Spitz [96, 97] [95]. Although all socket cell neighbors are equivalent, only the most proximal cell signals and goes on to become a bract. The direction of EGFR signaling depends on the core PCP pathway; in a PCP-specific dsh mutant, bracts are specified at more variable orientations [95]. The asymmetry in signal transduction appears to arise, not from asymmetry in receptor localization or function, but rather in the asymmetric presentation of Spitz from ligand producing cells. Although socket cells are epithelial, closely packed and adherent with its neighbors, they send out elaborate protrusions that emanate in a proximal direction (Fig. 4B). These protrusions are dependent of the formin Dia for their extension and on Dsh for their proximal directionality and stability [95]. When Dia function is reduced, protrusions are diminished leading to a loss of bract cell fate [95]. These data suggest a model where PCP controls the direction of ligand presentation by orienting the direction of actin polymerization and cell protrusions. Neighboring cells that are contacted more extensively by ligand-presenting protrusions may reach a threshold of ligand binding that is sufficient to turn on EGFR target genes. Thus the core PCP pathway can influence the direction of signal transduction and cell fate specification by modulating the localization of ligands or of receptors in a planar polarized manner.
Asymmetry in hair follicles
The mammalian hair follicle is perhaps the most complex multicellular structure patterned and aligned by the core PCP pathway. Each hair follicle in its adult form is comprised of thousands of cells that are collectively oriented in a uniform orientation across the skin surface. Hair follicles display not only morphological asymmetry, tilting with an acute angle relative to the epithelial plane, but they also display molecular asymmetry, with distinct gene expression profiles on each side of the follicle [98] (Fig. 4C). Both asymmetries are acquired during the early stages of hair follicle development and rely on inputs from the core PCP pathway [98]. In the absence of PCP function, hair placodes emerge from the epidermis growing straight downward instead of at an angle, and as they elongate they attain random orientations across the skin surface [98–100]. Asymmetric gene expression is also lost in the follicles of PCP mutant mice, and the two sides of the follicle display mirror symmetry instead of asymmetric fates [98]. While asymmetric gene expression strongly correlates with the physical tilt of the hair follicle, it is not known how the follicles’ molecular and morphological asymmetries are connected. It is likely the dermal condensate, a cluster of fibroblasts that secrete crucial patterning and proliferative signals to overlying follicles, is involved in specifying anterior-posterior cell fates in each follicle. When hair placodes form an anterior tilt, the dermal condensate is carried with the base of the follicle, associating preferentially with the anterior-most cells [98] (Fig. 4C). Thus, anterior cells are positioned to receive higher concentrations of signals from the dermal condensate than posterior cells, which might be sufficient to induce distinct anterior-posterior cell fates. Consistent with this notion, embryos lacking Shh, which never form dermal condensates, fail to establish asymmetric cell fates in nascent hair placodes, despite establishing a relatively normal morphological tilt ([98], and Devenport, unpublished data). One possible model is that PCP primarily controls morphological asymmetry by modulating the cytoskeleton, which then indirectly establishes cell fate asymmetries through anterior displacement of the dermal condensate. Alternatively, an asymmetric signaling event similar to that in the Drosophila leg would not be difficult to envision in the developing hair follicles, but there is no evidence for such a mechanism at present. Whether these initial PCP-dependent asymmetries act as blueprint for later asymmetries in adult hair follicle structures, such as the posteriorly-placed arrector pili muscles and sebaceous glands, still remains to be determined [101].
V. Conclusions and perspectives
In summary, the core PCP pathway intersects with diverse cytoskeletal, signaling, and trafficking pathways to produce an amazing variety of planar polarized structures, of which this review highlights only the best studied thus far. Like other major developmental signaling pathways that are used over and over again in development yet produce distinct outcomes in new situations, the PCP pathway is used repeatedly in development to orient incredibly diverse cell behaviors. But while it is easy to envision how the developmental pathways that control gene transcription produce diverse outcomes depending on the history, memory, and combination of past patterning events through chromatin modifications, it is much more difficult to envision how the core PCP module produces distinct outcomes by regulating the cytoskeleton, which is comprised of proteins which are generally thought to be ubiquitous. Perhaps the answer lies in unidentified cell type-specific effectors, like Mwh, which appears to function exclusively in the insect wing. If such novel cell-type specific cytoskeletal regulators exist, they are likely only to be found through unbiased screens for new PCP genes and/or interactors.
Given the diversity of polarized outputs coordinated by PCP, can we draw any mechanistic links between different systems to gain a more generalized understanding of the downstream events governed by the core PCP pathway? While the differences between downstream mechanisms discussed here may far outweigh the similarities, a few common themes do emerge. First is the correlation between PCP protein asymmetry and the orientation of polarized outputs. Second is the involvement of Dsh/Dvl as a hub connecting the core PCP module to different downstream outputs. As a scaffold with just three distinct protein-protein interaction domains, it associates with a vast array of signaling, trafficking, and cytoskeletal proteins, not to mention its central role in the canonical Wnt/beta-catenin pathway. Perhaps the promiscuity of Dsh/Dvl and its ability to perhaps scaffold proteins into complexes enables PCP to impinge on a diverse set of outputs. A final repeating theme is the involvement of Rho family GTPases and their effectors that control the actin cytoskeleton. Even in cell types where they polarized output is microtubule based, PCP appears to have some effect of the actin cytoskeleton, which may be the primary and direct targets of the core PCP pathway.
As new functions and downstream targets of the PCP pathway are uncovered, it will be important to elucidate the precise downstream mechanisms connecting the transmembrane core PCP components to the most downstream effectors. Few, if any, PCP pathways are understood at this level of detail with the closest being the Drosophila wing blade. Moreover, deciphering the connection between PCP protein asymmetry and asymmetric assembly of polarized structures will also be a priority. The observation that, even within the same tissue, cells can reverse their structural polarity with respect to the PCP localization suggests that cells may express ‘reversal factors’ that lead to the opposite coupling of core PCP proteins with their downstream effectors. In Drosophila, Prickle appears to be such a reversal factor where, depending of which of two Prickle isoforms is expressed, cells can orient their structural polarity towards or away for Fz-Dsh localization. A similar mechanism may operate in vertebrate systems. Finally, as more PCP-dependent patterns continue to be discovered, particularly in mammals and lesser-studied model organisms where the tools to analyze PCP function continue to be developed, additional commonalities and differences are sure to be discovered.
Acknowledgments
D.D. is supported by NIH/NIAMS grant R01AR066070 as well as the Searle Scholars Program and a Vallee Foundation Young Investigator Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang Y, Mlodzik M. Wnt-Frizzled/Planar Cell Polarity Signaling: Cellular Orientation by Facing the Wind (Wnt) Annu Rev Cell Dev Biol. 2015;31:623–646. doi: 10.1146/annurev-cellbio-100814-125315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodrich LV, Strutt D. Principles of planar polarity in animal development. Development. 2011;138:1877–1892. doi: 10.1242/dev.054080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol. 2012;28:627–653. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- 4.Matis M, Axelrod JD. Regulation of PCP by the Fat signaling pathway. Genes & development. 2013;27:2207–2220. doi: 10.1101/gad.228098.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thomas C, Strutt D. The roles of the cadherins Fat and Dachsous in planar polarity specification in Drosophila. Developmental dynamics : an official publication of the American Association of Anatomists. 2012;241:27–39. doi: 10.1002/dvdy.22736. [DOI] [PubMed] [Google Scholar]
- 6.Gubb D, Garcia-Bellido A. A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. Journal of embryology and experimental morphology. 1982;68:37–57. [PubMed] [Google Scholar]
- 7.Vinson CR, Conover S, Adler PN. A Drosophila tissue polarity locus encodes a protein containing seven potential transmembrane domains. Nature. 1989;338:263–264. doi: 10.1038/338263a0. [DOI] [PubMed] [Google Scholar]
- 8.Adler PN. The frizzled/stan pathway and planar cell polarity in the Drosophila wing. Curr Top Dev Biol. 2012;101:1–31. doi: 10.1016/B978-0-12-394592-1.00001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong LL, Adler PN. Tissue polarity genes of Drosophila regulate the subcellular location for prehair initiation in pupal wing cells. The Journal of cell biology. 1993;123:209–221. doi: 10.1083/jcb.123.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strutt D, Warrington SJ. Planar polarity genes in the Drosophila wing regulate the localisation of the FH3-domain protein Multiple Wing Hairs to control the site of hair production. Development. 2008;135:3103–3111. doi: 10.1242/dev.025205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adler PN, Zhu C, Stone D. Inturned localizes to the proximal side of wing cells under the instruction of upstream planar polarity proteins. Current biology : CB. 2004;14:2046–2051. doi: 10.1016/j.cub.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Collier S, Lee H, Burgess R, Adler P. The WD40 repeat protein fritz links cytoskeletal planar polarity to frizzled subcellular localization in the Drosophila epidermis. Genetics. 2005;169:2035–2045. doi: 10.1534/genetics.104.033381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park WJ, Liu J, Sharp EJ, Adler PN. The Drosophila tissue polarity gene inturned acts cell autonomously and encodes a novel protein. Development. 1996;122:961–969. doi: 10.1242/dev.122.3.961. [DOI] [PubMed] [Google Scholar]
- 14.Collier S, Gubb D. Drosophila tissue polarity requires the cell-autonomous activity of the fuzzy gene, which encodes a novel transmembrane protein. Development. 1997;124:4029–4037. doi: 10.1242/dev.124.20.4029. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Yan J, Lee H, Lu Q, Adler PN. The proteins encoded by the Drosophila Planar Polarity Effector genes inturned, fuzzy and fritz interact physically and can re-pattern the accumulation of "upstream" Planar Cell Polarity proteins. Developmental biology. 2014;394:156–169. doi: 10.1016/j.ydbio.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan J, Huen D, Morely T, Johnson G, Gubb D, Roote J, Adler PN. The multiple-wing-hairs gene encodes a novel GBD-FH3 domain-containing protein that functions both prior to and after wing hair initiation. Genetics. 2008;180:219–228. doi: 10.1534/genetics.108.091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu Q, Schafer DA, Adler PN. The Drosophila planar polarity gene multiple wing hairs directly regulates the actin cytoskeleton. Development. 2015;142:2478–2486. doi: 10.1242/dev.122119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu Q, Yan J, Adler PN. The Drosophila planar polarity proteins inturned and multiple wing hairs interact physically and function together. Genetics. 2010;185:549–558. doi: 10.1534/genetics.110.114066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strutt DI, Weber U, Mlodzik M. The role of RhoA in tissue polarity and Frizzled signalling. Nature. 1997;387:292–295. doi: 10.1038/387292a0. [DOI] [PubMed] [Google Scholar]
- 20.Yan J, Lu Q, Fang X, Adler PN. Rho1 has multiple functions in Drosophila wing planar polarity. Developmental biology. 2009;333:186–199. doi: 10.1016/j.ydbio.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Q, Adler PN. The diaphanous gene of Drosophila interacts antagonistically with multiple wing hairs and plays a key role in wing hair morphogenesis. PloS one. 2015;10:e0115623. doi: 10.1371/journal.pone.0115623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blair A, Tomlinson A, Pham H, Gunsalus KC, Goldberg ML, Laski FA. Twinstar, the Drosophila homolog of cofilin/ADF, is required for planar cell polarity patterning. Development. 2006;133:1789–1797. doi: 10.1242/dev.02320. [DOI] [PubMed] [Google Scholar]
- 23.Winter CG, Wang B, Ballew A, Royou A, Karess R, Axelrod JD, Luo L. Drosophila Rho-associated kinase (Drok) links Frizzled-mediated planar cell polarity signaling to the actin cytoskeleton. Cell. 2001;105:81–91. doi: 10.1016/s0092-8674(01)00298-7. [DOI] [PubMed] [Google Scholar]
- 24.Franke JD, Montague RA, Kiehart DP. Nonmuscle myosin II is required for cell proliferation, cell sheet adhesion and wing hair morphology during wing morphogenesis. Developmental biology. 2010;345:117–132. doi: 10.1016/j.ydbio.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montcouquiol M, Rachel RA, Lanford PJ, Copeland NG, Jenkins NA, Kelley MW. Identification of Vangl2 and Scrb1 as planar polarity genes in mammals. Nature. 2003;423:173–177. doi: 10.1038/nature01618. [DOI] [PubMed] [Google Scholar]
- 26.May-Simera H, Kelley MW. Planar cell polarity in the inner ear. Curr Top Dev Biol. 2012;101:111–140. doi: 10.1016/B978-0-12-394592-1.00006-5. [DOI] [PubMed] [Google Scholar]
- 27.Curtin JA, Quint E, Tsipouri V, Arkell RM, Cattanach B, Copp AJ, Henderson DJ, Spurr N, Stanier P, Fisher EM, et al. Mutation of Celsr1 disrupts planar polarity of inner ear hair cells and causes severe neural tube defects in the mouse. Current biology : CB. 2003;13:1129–1133. doi: 10.1016/s0960-9822(03)00374-9. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Mark S, Zhang X, Qian D, Yoo SJ, Radde-Gallwitz K, Zhang Y, Lin X, Collazo A, Wynshaw-Boris A, et al. Regulation of polarized extension and planar cell polarity in the cochlea by the vertebrate PCP pathway. Nature genetics. 2005;37:980–985. doi: 10.1038/ng1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Etheridge SL, Ray S, Li S, Hamblet NS, Lijam N, Tsang M, Greer J, Kardos N, Wang J, Sussman DJ, et al. Murine dishevelled 3 functions in redundant pathways with dishevelled 1 and 2 in normal cardiac outflow tract, cochlea, and neural tube development. PLoS genetics. 2008;4:e1000259. doi: 10.1371/journal.pgen.1000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Montcouquiol M, Sans N, Huss D, Kach J, Dickman JD, Forge A, Rachel RA, Copeland NG, Jenkins NA, Bogani D, et al. Asymmetric localization of Vangl2 and Fz3 indicate novel mechanisms for planar cell polarity in mammals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:5265–5275. doi: 10.1523/JNEUROSCI.4680-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deans MR, Antic D, Suyama K, Scott MP, Axelrod JD, Goodrich LV. Asymmetric distribution of prickle-like 2 reveals an early underlying polarization of vestibular sensory epithelia in the inner ear. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:3139–3147. doi: 10.1523/JNEUROSCI.5151-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nature genetics. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 34.Sipe CW, Lu X. Kif3a regulates planar polarization of auditory hair cells through both ciliary and non-ciliary mechanisms. Development. 2011;138:3441–3449. doi: 10.1242/dev.065961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.May-Simera HL, Petralia RS, Montcouquiol M, Wang YX, Szarama KB, Liu Y, Lin W, Deans MR, Pazour GJ, Kelley MW. Ciliary proteins Bbs8 and Ift20 promote planar cell polarity in the cochlea. Development. 2015;142:555–566. doi: 10.1242/dev.113696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones C, Roper VC, Foucher I, Qian D, Banizs B, Petit C, Yoder BK, Chen P. Ciliary proteins link basal body polarization to planar cell polarity regulation. Nature genetics. 2008;40:69–77. doi: 10.1038/ng.2007.54. [DOI] [PubMed] [Google Scholar]
- 37.Grimsley-Myers CM, Sipe CW, Geleoc GS, Lu X. The small GTPase Rac1 regulates auditory hair cell morphogenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:15859–15869. doi: 10.1523/JNEUROSCI.3998-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sipe CW, Liu L, Lee J, Grimsley-Myers C, Lu X. Lis1 mediates planar polarity of auditory hair cells through regulation of microtubule organization. Development. 2013;140:1785–1795. doi: 10.1242/dev.089763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ezan J, Lasvaux L, Gezer A, Novakovic A, May-Simera H, Belotti E, Lhoumeau AC, Birnbaumer L, Beer-Hammer S, Borg JP, et al. Primary cilium migration depends on G-protein signalling control of subapical cytoskeleton. Nature cell biology. 2013;15:1107–1115. doi: 10.1038/ncb2819. [DOI] [PubMed] [Google Scholar]
- 40.Tarchini B, Jolicoeur C, Cayouette M. A molecular blueprint at the apical surface establishes planar asymmetry in cochlear hair cells. Developmental cell. 2013;27:88–102. doi: 10.1016/j.devcel.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 41.Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–1954. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- 42.Wallingford JB, Rowning BA, Vogeli KM, Rothbacher U, Fraser SE, Harland RM. Dishevelled controls cell polarity during Xenopus gastrulation. Nature. 2000;405:81–85. doi: 10.1038/35011077. [DOI] [PubMed] [Google Scholar]
- 43.Heisenberg CP, Tada M, Rauch GJ, Saude L, Concha ML, Geisler R, Stemple DL, Smith JC, Wilson SW. Silberblick/Wnt11 mediates convergent extension movements during zebrafish gastrulation. Nature. 2000;405:76–81. doi: 10.1038/35011068. [DOI] [PubMed] [Google Scholar]
- 44.Tada M, Smith JC. Xwnt11 is a target of Xenopus Brachyury: regulation of gastrulation movements via Dishevelled, but not through the canonical Wnt pathway. Development. 2000;127:2227–2238. doi: 10.1242/dev.127.10.2227. [DOI] [PubMed] [Google Scholar]
- 45.Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Developmental cell. 2004;6:343–355. doi: 10.1016/s1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- 46.Keller R, Shih J, Sater A. The cellular basis of the convergence and extension of the Xenopus neural plate. Developmental dynamics : an official publication of the American Association of Anatomists. 1992;193:199–217. doi: 10.1002/aja.1001930302. [DOI] [PubMed] [Google Scholar]
- 47.Skoglund P, Keller R. Integration of planar cell polarity and ECM signaling in elongation of the vertebrate body plan. Curr Opin Cell Biol. 2010;22:589–596. doi: 10.1016/j.ceb.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Developmental biology. 2002;247:165–181. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- 49.Yin C, Kiskowski M, Pouille PA, Farge E, Solnica-Krezel L. Cooperation of polarized cell intercalations drives convergence and extension of presomitic mesoderm during zebrafish gastrulation. The Journal of cell biology. 2008;180:221–232. doi: 10.1083/jcb.200704150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–854. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- 51.Tanegashima K, Zhao H, Dawid IB. WGEF activates Rho in the Wnt-PCP pathway and controls convergent extension in Xenopus gastrulation. The EMBO journal. 2008;27:606–617. doi: 10.1038/emboj.2008.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaiswal R, Breitsprecher D, Collins A, Correa IR, Jr, Xu MQ, Goode BL. The formin Daam1 and fascin directly collaborate to promote filopodia formation. Current biology : CB. 2013;23:1373–1379. doi: 10.1016/j.cub.2013.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Higashi T, Ikeda T, Shirakawa R, Kondo H, Kawato M, Horiguchi M, Okuda T, Okawa K, Fukai S, Nureki O, et al. Biochemical characterization of the Rho GTPase-regulated actin assembly by diaphanous-related formins, mDia1 and Daam1, in platelets. J Biol Chem. 2008;283:8746–8755. doi: 10.1074/jbc.M707839200. [DOI] [PubMed] [Google Scholar]
- 54.Kim HY, Davidson LA. Punctuated actin contractions during convergent extension and their permissive regulation by the non-canonical Wnt-signaling pathway. J Cell Sci. 2011;124:635–646. doi: 10.1242/jcs.067579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shindo A, Wallingford JB. PCP and septins compartmentalize cortical actomyosin to direct collective cell movement. Science. 2014;343:649–652. doi: 10.1126/science.1243126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–671. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 57.Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Developmental cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 58.Guillot C, Lecuit T. Mechanics of epithelial tissue homeostasis and morphogenesis. Science. 2013;340:1185–1189. doi: 10.1126/science.1235249. [DOI] [PubMed] [Google Scholar]
- 59.Lienkamp SS, Liu K, Karner CM, Carroll TJ, Ronneberger O, Wallingford JB, Walz G. Vertebrate kidney tubules elongate using a planar cell polarity-dependent, rosette-based mechanism of convergent extension. Nature genetics. 2012;44:1382–1387. doi: 10.1038/ng.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams M, Yen W, Lu X, Sutherland A. Distinct apical and basolateral mechanisms drive planar cell polarity-dependent convergent extension of the mouse neural plate. Developmental cell. 2014;29:34–46. doi: 10.1016/j.devcel.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nishimura T, Honda H, Takeichi M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell. 2012;149:1084–1097. doi: 10.1016/j.cell.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 62.Schweisguth F. Asymmetric cell division in the Drosophila bristle lineage: from the polarization of sensory organ precursor cells to Notch-mediated binary fate decision. Wiley Interdiscip Rev Dev Biol. 2015;4:299–309. doi: 10.1002/wdev.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Besson C, Bernard F, Corson F, Rouault H, Reynaud E, Keder A, Mazouni K, Schweisguth F. Planar Cell Polarity Breaks the Symmetry of PAR Protein Distribution prior to Mitosis in Drosophila Sensory Organ Precursor Cells. Current biology : CB. 2015;25:1104–1110. doi: 10.1016/j.cub.2015.02.073. [DOI] [PubMed] [Google Scholar]
- 64.Bellaiche Y, Beaudoin-Massiani O, Stuttem I, Schweisguth F. The planar cell polarity protein Strabismus promotes Pins anterior localization during asymmetric division of sensory organ precursor cells in Drosophila. Development. 2004;131:469–478. doi: 10.1242/dev.00928. [DOI] [PubMed] [Google Scholar]
- 65.Bellaiche Y, Gho M, Kaltschmidt JA, Brand AH, Schweisguth F. Frizzled regulates localization of cell-fate determinants and mitotic spindle rotation during asymmetric cell division. Nature cell biology. 2001;3:50–57. doi: 10.1038/35050558. [DOI] [PubMed] [Google Scholar]
- 66.Segalen M, Johnston CA, Martin CA, Dumortier JG, Prehoda KE, David NB, Doe CQ, Bellaiche Y. The Fz-Dsh planar cell polarity pathway induces oriented cell division via Mud/NuMA in Drosophila and zebrafish. Developmental cell. 2010;19:740–752. doi: 10.1016/j.devcel.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Johnston CA, Manning L, Lu MS, Golub O, Doe CQ, Prehoda KE. Formin-mediated actin polymerization cooperates with Mushroom body defect (Mud)-Dynein during Frizzled-Dishevelled spindle orientation. J Cell Sci. 2013;126:4436–4444. doi: 10.1242/jcs.129544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gong Y, Mo C, Fraser SE. Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature. 2004;430:689–693. doi: 10.1038/nature02796. [DOI] [PubMed] [Google Scholar]
- 69.Castanon I, Abrami L, Holtzer L, Heisenberg CP, van der Goot FG, Gonzalez-Gaitan M. Anthrax toxin receptor 2a controls mitotic spindle positioning. Nature cell biology. 2013;15:28–39. doi: 10.1038/ncb2632. [DOI] [PubMed] [Google Scholar]
- 70.Wallingford JB. Planar cell polarity signaling, cilia and polarized ciliary beating. Curr Opin Cell Biol. 2010;22:597–604. doi: 10.1016/j.ceb.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hashimoto M, Hamada H. Translation of anterior-posterior polarity into left-right polarity in the mouse embryo. Curr Opin Genet Dev. 2010;20:433–437. doi: 10.1016/j.gde.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 72.Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nature genetics. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vladar EK, Bayly RD, Sangoram AM, Scott MP, Axelrod JD. Microtubules enable the planar cell polarity of airway cilia. Current biology : CB. 2012;22:2203–2212. doi: 10.1016/j.cub.2012.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tissir F, Qu Y, Montcouquiol M, Zhou L, Komatsu K, Shi D, Fujimori T, Labeau J, Tyteca D, Courtoy P, et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nature neuroscience. 2010;13:700–707. doi: 10.1038/nn.2555. [DOI] [PubMed] [Google Scholar]
- 75.Shi D, Komatsu K, Hirao M, Toyooka Y, Koyama H, Tissir F, Goffinet AM, Uemura T, Fujimori T. Celsr1 is required for the generation of polarity at multiple levels of the mouse oviduct. Development. 2014;141:4558–4568. doi: 10.1242/dev.115659. [DOI] [PubMed] [Google Scholar]
- 76.Mitchell B, Stubbs JL, Huisman F, Taborek P, Yu C, Kintner C. The PCP pathway instructs the planar orientation of ciliated cells in the Xenopus larval skin. Current biology : CB. 2009;19:924–929. doi: 10.1016/j.cub.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Devenport D. The cell biology of planar cell polarity. The Journal of cell biology. 2014;207:171–179. doi: 10.1083/jcb.201408039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Boutin C, Labedan P, Dimidschstein J, Richard F, Cremer H, Andre P, Yang Y, Montcouquiol M, Goffinet AM, Tissir F. A dual role for planar cell polarity genes in ciliated cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E3129–E3138. doi: 10.1073/pnas.1404988111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mitchell B, Jacobs R, Li J, Chien S, Kintner C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- 80.Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi JM, Strehl L, Hirota Y, Desoeuvre A, Boutin C, Han YG, et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nature cell biology. 2010;12:341–350. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- 81.Werner ME, Hwang P, Huisman F, Taborek P, Yu CC, Mitchell BJ. Actin and microtubules drive differential aspects of planar cell polarity in multiciliated cells. The Journal of cell biology. 2011;195:19–26. doi: 10.1083/jcb.201106110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Butler MT, Wallingford JB. Control of vertebrate core PCP protein localization and dynamics by Prickle2. Development. 2015 doi: 10.1242/dev.121384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, Nonaka S, Takada S, Hatta K, Wynshaw-Boris A, et al. Planar polarization of node cells determines the rotational axis of node cilia. Nature cell biology. 2010;12:170–176. doi: 10.1038/ncb2020. [DOI] [PubMed] [Google Scholar]
- 84.Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–382. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, Axelrod JD. Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PloS one. 2010;5:e8999. doi: 10.1371/journal.pone.0008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ohata S, Nakatani J, Herranz-Perez V, Cheng J, Belinson H, Inubushi T, Snider WD, Garcia-Verdugo JM, Wynshaw-Boris A, Alvarez-Buylla A. Loss of Dishevelleds disrupts planar polarity in ependymal motile cilia and results in hydrocephalus. Neuron. 2014;83:558–571. doi: 10.1016/j.neuron.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zheng L, Zhang J, Carthew RW. frizzled regulates mirror-symmetric pattern formation in the Drosophila eye. Development. 1995;121:3045–3055. doi: 10.1242/dev.121.9.3045. [DOI] [PubMed] [Google Scholar]
- 88.Cooper MT, Bray SJ. Frizzled regulation of Notch signalling polarizes cell fate in the Drosophila eye. Nature. 1999;397:526–530. doi: 10.1038/17395. [DOI] [PubMed] [Google Scholar]
- 89.Tomlinson A, Struhl G. Decoding vectorial information from a gradient: sequential roles of the receptors Frizzled and Notch in establishing planar polarity in the Drosophila eye. Development. 1999;126:5725–5738. doi: 10.1242/dev.126.24.5725. [DOI] [PubMed] [Google Scholar]
- 90.Fanto M, Mlodzik M. Asymmetric Notch activation specifies photoreceptors R3 and R4 and planar polarity in the Drosophila eye. Nature. 1999;397:523–526. doi: 10.1038/17389. [DOI] [PubMed] [Google Scholar]
- 91.del Alamo D, Mlodzik M. Frizzled/PCP-dependent asymmetric neuralized expression determines R3/R4 fates in the Drosophila eye. Developmental cell. 2006;11:887–894. doi: 10.1016/j.devcel.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 92.Strutt D, Johnson R, Cooper K, Bray S. Asymmetric localization of frizzled and the determination of notch-dependent cell fate in the Drosophila eye. Current biology : CB. 2002;12:813–824. doi: 10.1016/s0960-9822(02)00841-2. [DOI] [PubMed] [Google Scholar]
- 93.Bishop SA, Klein T, Arias AM, Couso JP. Composite signalling from Serrate and Delta establishes leg segments in Drosophila through Notch. Development. 1999;126:2993–3003. doi: 10.1242/dev.126.13.2993. [DOI] [PubMed] [Google Scholar]
- 94.Capilla A, Johnson R, Daniels M, Benavente M, Bray SJ, Galindo MI. Planar cell polarity controls directional Notch signaling in the Drosophila leg. Development. 2012;139:2584–2593. doi: 10.1242/dev.077446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Peng Y, Han C, Axelrod JD. Planar polarized protrusions break the symmetry of EGFR signaling during Drosophila bract cell fate induction. Developmental cell. 2012;23:507–518. doi: 10.1016/j.devcel.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.del Alamo D, Terriente J, Diaz-Benjumea FJ. Spitz/EGFr signalling via the Ras/MAPK pathway mediates the induction of bract cells in Drosophila legs. Development. 2002;129:1975–1982. doi: 10.1242/dev.129.8.1975. [DOI] [PubMed] [Google Scholar]
- 97.Held LI., Jr Bristles induce bracts via the EGFR pathway on Drosophila legs. Mechanisms of development. 2002;117:225–234. doi: 10.1016/s0925-4773(02)00212-5. [DOI] [PubMed] [Google Scholar]
- 98.Devenport D, Fuchs E. Planar polarization in embryonic epidermis orchestrates global asymmetric morphogenesis of hair follicles. Nature cell biology. 2008;10:1257–1268. doi: 10.1038/ncb1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guo N, Hawkins C, Nathans J. Frizzled6 controls hair patterning in mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9277–9281. doi: 10.1073/pnas.0402802101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y, Chang H, Nathans J. When whorls collide: the development of hair patterns in frizzled 6 mutant mice. Development. 2010;137:4091–4099. doi: 10.1242/dev.057455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chang H, Nathans J. Responses of hair follicle-associated structures to loss of planar cell polarity signaling. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E908–E917. doi: 10.1073/pnas.1301430110. [DOI] [PMC free article] [PubMed] [Google Scholar]