Abstract
Aging is a major risk factor for carotid artery disease that may lead to stroke and dementia. Vascular effects associated with aging include increased vasomotor tone, as well as enhanced contractility to endothelial vasoconstrictor prostanoids and reduced nitric oxide (NO) bioactivity partly due to increased oxidative stress. We hypothesized that vascular NADPH oxidase (Nox)-derived superoxide may be involved in prostanoid- and NO-related functional aging. NO-mediated relaxations and prostanoid-mediated contractions to acetylcholine as well as phenylephrine-dependent contractions were investigated in the carotid artery from young (4 months) and aged mice (24 months). Gene expression of Nox subunits and endothelial NO synthase (eNOS) was determined in the carotid artery and aorta. In young mice, the thromboxane-prostanoid receptor antagonist SQ 29,548 fully blocked acetylcholine-induced contractions while reducing responses to phenylephrine by 75 %. The Nox2-targeted inhibitor Nox2ds-tat and the superoxide scavenger tempol reduced acetylcholine-stimulated, prostanoid-mediated contractions by 85 and 75 %, respectively, and phenylephrine-dependent contractions by 45 %. Unexpectedly, in aged mice, the substantial Nox2-dependent component of acetylcholine- and phenylephrine-induced, prostanoid-mediated contractions was abolished. In addition, endothelium-dependent, NO-mediated relaxations were impaired with aging. The expression of Nox subunits was greater in the aorta compared with the carotid artery, in which Nox1 was undetectable. eNOS gene expression was reduced in the aorta of aged compared to young mice. In conclusion, aging decreases prostanoid-mediated contractility in the carotid artery involving a loss of Nox2 activity and is associated with impaired endothelium-dependent, NO-mediated relaxation. These findings may contribute to a better understanding of the pathophysiology of carotid artery disease and the aging process.
Keywords: Aging, Carotid artery, NADPH oxidase, Nox2, Prostanoid, Superoxide
Introduction
Aging is a major non-modifiable risk factor for atherosclerosis in carotid arteries (Gorelick et al. 2011). In view of recent increases in human life expectancy, the clinical consequences of carotid artery disease such as stroke and dementia and the associated social and economical burden of disability are becoming increasingly important (Gerland et al. 2014; Gorelick et al. 2011). Carotid artery disease has been associated with age-dependent stiffening and increased vasomotor tone (Gorelick et al. 2011; Zieman et al. 2005), which involve abnormal release of vasodilators such as nitric oxide (NO) and contracting factors such as vasoconstrictor prostanoids (Barton 2014; Feletou and Vanhoutte 2006; Seals et al. 2011). Vascular aging is commonly associated with reduced bioavailability of NO and impairment of endothelium-dependent, NO-mediated vasodilation (Barton 2014; Seals et al. 2011). At the same time, various arterial beds display increased vasoconstrictor responses to prostanoids (Heymes et al. 2000; Novella et al. 2013; Shi et al. 2008; Wong et al. 2009), which activate thromboxane-prostanoid (TP) receptors on underlying smooth muscle cells to induce rapid changes in vascular tone (Feletou and Vanhoutte 2006). Aging also results in increased vascular production of reactive oxygen species (ROS) such as superoxide and hydrogen peroxide (Barton 2014; Donato et al. 2007; Feletou and Vanhoutte 2006; Mukai et al. 2002; Oudot et al. 2006; Seals et al. 2011; Shi et al. 2008). ROS may directly activate TP receptors (Cosentino et al. 1994; Feletou and Vanhoutte 2006; Gao and Lee 2005; Katusic et al. 1993; Shi et al. 2008; Tang et al. 2007; Yang et al. 2002) or inactivate NO through formation of peroxynitrite (Feletou and Vanhoutte 2006). In addition, prostanoid-mediated activation of TP receptors can increase vascular ROS production while further reducing the bioavailability of NO (Zhang et al. 2011). These findings suggest a complex interplay between prostanoid-, ROS-, and NO-dependent signaling processes that remain incompletely understood in vascular aging, particularly in the carotid artery.
The formation of ROS is tightly regulated as it modulates multiple signaling pathways to control normal vascular homeostasis, including vascular tone (Brandes et al. 2010; Lassegue et al. 2012). Under healthy conditions, ROS are generated mainly by vascular NADPH oxidase (Nox) proteins, the only enzymes whose primary function is to produce ROS (Brandes et al. 2010; Lassegue et al. 2012). Of the seven known mammalian Nox catalytic isoforms, Nox1 and Nox2 contribute to stimulus-dependent acute superoxide production in rodents (Brandes et al. 2010; Lassegue et al. 2012), and increased expression of Nox2 has been reported in the aorta of aged rats (Oudot et al. 2006). Although Nox2 has been shown to contribute to TP receptor-dependent superoxide production in endothelial cells (Zhang et al. 2011), it remains unknown whether activation of Nox2 is directly involved in prostanoid-mediated vascular contractions.
Despite the important clinical implications, functional aging in carotid arteries is poorly understood. Since we have previously shown that contractions to endothelin-1, a mediator causally involved in age-dependent vascular changes (Barton 2014), are in part mediated by Nox2 in the carotid artery (Meyer et al. 2014b), we hypothesized that Nox2 would also be involved in prostanoid- and NO-dependent regulation of vascular tone with aging. Using carotid arteries from young and aged mice, we therefore set out to study responses to acetylcholine, a muscarinic agonist widely used to elicit endothelium-dependent, NO-mediated relaxations at low concentrations, followed by endothelium-dependent contractions mediated by vasoconstrictor prostanoids at higher concentrations, with both responses being particularly potent in this vascular bed (Crauwels et al. 2000; Traupe et al. 2002; Zhou et al. 2005). We also examined contractions to the α1-adrenergic agonist phenylephrine, a known activator of Nox2 (Hahn et al. 2014) that mediates prostanoid and superoxide formation in rat carotid arteries (Pereira et al. 2010), as well as gene expression levels of Nox subunits and endothelial NO synthase (eNOS).
Materials and methods
Materials
The thromboxane-prostanoid (TP) receptor antagonist [1S-[1α,2α(Z),3α,4α]]-7-[3-[[2- [(phenylamino)carbonyl]hydrazino] methyl]-7-oxabicyclo[2.2.1]hept- 2-yl]-5-heptenoic acid (SQ 29,548) was from Cayman Chemical (Ann Arbor, MI, USA), the superoxide dismutase mimetic 1-oxyl-2,2,6,6-tetramethyl-4-hydroxypiperidine (tempol) was from Tocris Bioscience (Bristol, United Kingdom), and the Nox2-targeted inhibitor Nox2ds-tat (Csanyi et al. 2011; Rey et al. 2001) was from Anaspec (Fremont, CA, USA). All other drugs were from Sigma-Aldrich (St. Louis, MO, USA). Tempol was dissolved in ethanol (final ethanol concentration 0.1 % v/v in the organ chamber, which lacks superoxide scavenging capacity (Huisman et al. 2004)). All other drugs were dissolved in water. Stock solutions were diluted in physiological saline solution (PSS; composition in mmol/L, 129.8 NaCl; 5.4 KCl; 0.83 MgSO4; 0.43 NaH2PO4; 19 NaHCO3; 1.8 CaCl2; and 5.5 glucose; pH 7.4) to the required concentrations before use.
Animals
Male C57BL/6J mice (Harlan Laboratories, Indianapolis, IN, USA) were kept at the University of New Mexico Animal Resources Facility on a 12-h light-dark cycle with unlimited access to standard rodent chow and water. Young (4 months of age) and aged animals (24 months of age) were sacrificed by intraperitoneal injection of sodium pentobarbital (2.2 mg/g body weight). All procedures were approved by the University of New Mexico Institutional Animal Care and Use Committee and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Blood pressure measurements
Systolic and diastolic blood pressure levels were measured in trained conscious mice using a non-invasive tail cuff volume-pressure recording system (CODA-6, Kent Scientific, Torrington, CT) as described (Meyer et al. 2013).
Vascular ring preparation and myography set-up
Immediately after sacrifice, the aorta and both common carotid arteries were carefully excised and cleaned of perivascular adipose and connective tissue. The suprarenal segment of the abdominal aorta and a portion of carotid arteries were snap-frozen in liquid nitrogen and stored at −80 °C until subsequent molecular analyses. Remaining carotid arteries were cut into 2 mm long rings in cold (4 °C) PSS. Vessels were mounted in organ chambers of a Mulvany-Halpern myograph (620 M Multi Wire Myograph System, Danish Myo Technology, Aarhus, Denmark) for recording of isometric tension using a PowerLab 8/35 data acquisition system and LabChart Pro software (AD Instruments, Colorado Springs, CO, USA) as described (Meyer et al. 2014b).
Vascular reactivity experiments
Experiments to study vascular reactivity of carotid arteries were performed as described (Meyer et al. 2014b). Briefly, vessels were equilibrated in PSS (37 °C, bubbled with 21 % O2, 5 % CO2, and balanced N2, pH 7.4) and stretched stepwise to the optimal level of passive tension for force generation (8.8 mN as determined in preceding experiments). Functional integrity of the vascular smooth muscle was confirmed by repeated exposures to KCl (PSS with equimolar substitution of 60 mmol/L potassium for sodium), which yielded equal generation of active force in preparations from young and old mice (7.7 ± 0.4 and 8.3 ± 0.4 mN, respectively). Where indicated, arteries were treated with the small-molecule cell-permeable superoxide dismutase mimetic tempol (100 μmol/L) or the Nox2-targeted inhibitor Nox2ds-tat (3 μmol/L) (Csanyi et al. 2011; Meyer et al. 2014a, b; Rey et al. 2001) for 30 min. Thereafter, concentration-responses to acetylcholine (0.1 nmol/L–10 μmol/L) were recorded in arteries equally precontracted with phenylephrine to a stable plateau between 46 ± 5 and 57 ± 8 % of KCl (60 mmol/L)-induced contractions across treatment groups. Identical experiments were carried out in the presence of the TP receptor antagonist SQ 29,548 (1 μmol/L) to assess the mechanism underlying prostanoid-mediated contractions in response to acetylcholine (level of precontraction, 33 ± 5 %). In the presence and absence of each inhibitor, concentration-response curves to phenylephrine (1 nmol/L–1 μmol/L) were also obtained. Functional integrity of endothelial cells was confirmed if rings displayed relaxant responses to acetylcholine >85 % of precontraction; all other preparations were excluded from analyses.
Quantification of steady-state mRNA expression levels
Total RNA from the aorta and carotid artery was extracted and reverse transcribed as described (Meyer et al. 2014b). SYBR Green-based detection of amplified gene-specific complementary DNA (cDNA) fragments was performed on a 7500 FAST real-time PCR system (Applied Biosystems, Carlsbad, CA, USA). The following sets of primers were used: 5′-CAT CCA GTC TCC AAA CAT GAC A-3′ (forward) and 5′-GCT ACA GTG GCA ATC ACT CCA G-3′ (reverse) for amplification of a specific cDNA fragment encoding for murine Nox1 (GenBank ID: NM_172203.1); 5′-ACT CCT TGG GTC AGC ACT GG-3′ (forward) and 5′-GTT CCT GTC CAG TTG TCT TCG-3′ (reverse) for amplification of a specific cDNA fragment encoding for murine Nox2 (GenBank ID: NM_007807.4); 5'-CAT CGT GGC TAC TGC TGG AC-3' (forward) and 5'-TGG ACC CCT TTT TCC TCT TT-3' (reverse) for amplification of a specific cDNA fragment encoding for murine p22phox (GenBank ID: NM_007806.3); 5′-CCA GTG AAT CTG GGC CTA TG-3′ (forward) and 5′-ACA GTG GTG TCG CAC TTC AG-3′ (reverse) for amplification of a specific cDNA fragment encoding for murine Rac1 (GenBank ID: NM_009007.2); 5′-AGA TAC GGG ACT GGC ACC G-3′ (forward) and 5′-CAT CCT AGC CAG CGG CTC TC-3′ (reverse) for amplification of a specific cDNA fragment encoding for murine Noxa1 (GenBank ID NM_172204.4); 5′-AGA GCC TGC AAT TAC TAC CA-3′ (forward) and 5′-GTG GAT TTG CTG CTC TGT AG-3′ (reverse) for amplification of a specific cDNA fragment encoding for murine eNOS (GenBank ID: NM_008713); 5′-TTC ACC ACC ATG GAG AAG GC-3′ (forward) and 5′-GGC ATG GAC TGT GGT CAT GA-3′ (reverse) for amplification of a specific cDNA fragment encoding for murine GAPDH (GenBank NM_008084), which served as housekeeping control.
Calculations and statistical analyses
Responses to acetylcholine are expressed relative to precontraction and contractions to phenylephrine relative to KCl. Area under the curve, EC50 values (as negative logarithm, pD2), and maximal effects were calculated by fitting of concentration-response curves as described (DeLean et al. 1978). Area under the concentration-response curve of acetylcholine- and phenylephrine-mediated contractions was determined using the trapezoidal rule approximation. Relative gene expression was calculated based on the 2−ΔCT method. Data were analyzed using two-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test or the unpaired Student’s t-test as appropriate (Prism version 5.0 for Macintosh, GraphPad Software, San Diego, CA, USA). Values are expressed as mean ± sem; n equals the number of animals used. Statistical significance was accepted at a p value <0.05.
Results
Reduced acetylcholine-induced, prostanoid-mediated contractions with aging
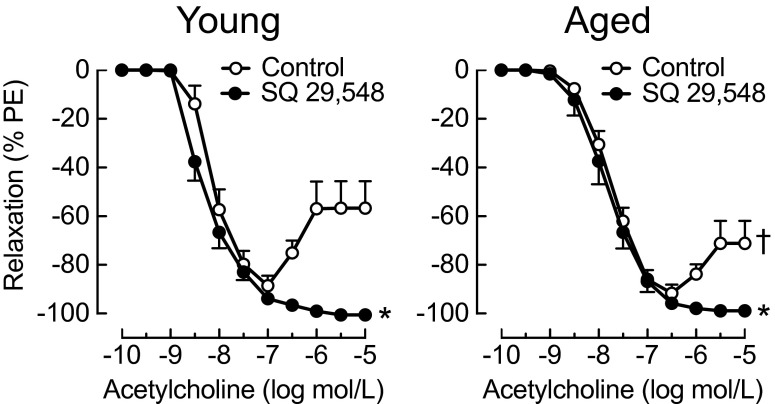
We studied vascular reactivity in the carotid artery of young and aged animals in response to the muscarinic agonist acetylcholine, which displays endothelium-dependent, NO-mediated relaxation at low concentrations, followed by rapid, transient, prostanoid-mediated contraction at concentrations ≥100 nmol/L (Fig. 1, see Fig. 3 for representative tracings) (Crauwels et al. 2000; Traupe et al. 2002; Zhou et al. 2005). Unexpectedly, acetylcholine-induced contractions in arteries from aged mice were less potent and the onset of contractions began at higher concentrations compared to young mice (1 vs. 0.3 μmol/L, respectively, Fig. 1). Consistent with the response being mediated by vasoconstrictor prostanoids (Feletou and Vanhoutte 2006), the TP receptor antagonist SQ 29,548 completely blocked contractions to acetylcholine in arteries from both young and aged mice (n = 4–5, p < 0.001 vs. control, Fig. 1). Furthermore, in the presence of SQ 29,548, relaxant responses to acetylcholine were less potent in carotid arteries from aged compared with young mice as indicated by reduced sensitivity (pD2 value) and smaller area under the curve values, although maximal responses were preserved (Fig. 1 and Table 1). These findings indicate reduced receptor-stimulated NO bioactivity associated with aging in the carotid artery.
Fig. 1.
Effect of aging on acetylcholine-induced relaxations and contractions in the carotid artery. Vessels were isolated from young and aged mice 4 and 24 months of age, respectively, and concentration-dependent responses to acetylcholine were recorded in the absence and presence of the TP receptor antagonist SQ 29,548 (1 μmol/L). *p < 0.001 vs. control; † p < 0.05 vs. young. All data (n = 4–5 per group) represent mean ± sem. PE phenylephrine
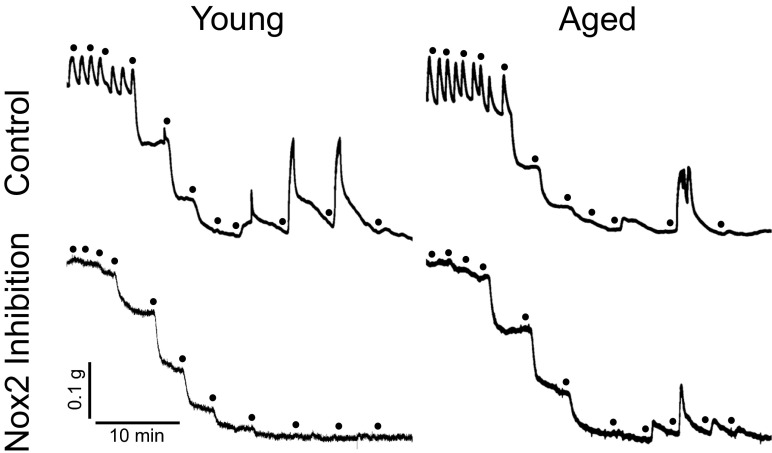
Fig. 3.
Original recordings of acetylcholine-induced, nitric oxide-mediated relaxations and contractions to prostanoids in the carotid artery. Vessels were obtained from young and aged mice, 4 and 24 months of age, respectively. Half-log increases of cumulative acetylcholine concentrations (0.1 nmol/L–10 μmol/L) are indicated by dots. Acetylcholine at concentrations ≥100 nmol/L stimulates prostanoid-dependent contractions (top) that are potently inhibited by the Nox2-targeted inhibitor Nox2ds-tat (3 μmol/L) only in young mice (bottom). Note that the rapid oscillatory behavior present in the upper two recordings before the onset of relaxation is not representative of any particular study group
Table 1.
Maximal responses, pD2 values, and area under the curve of endothelium-dependent relaxations to acetylcholine. Responses were determined in the presence of the TP receptor antagonist SQ 29,548 (1 μmol/L). Values were calculated by fitting of concentration-response curves (DeLean et al. 1978). Area under the curve is expressed as arbitrary units. All data (n = 4–5 per group) are mean ± sem
| Young | Aged | p value | |
|---|---|---|---|
| Maximal responses (% PE) | −98 ± 1 | −98 ± 1 | 1.0 |
| pD2 values (−log mol/L) | 8.3 ± 0.1 | 7.8 ± 0.1 | 0.01 |
| Area under the curve (AU) | 318 ± 8 | 275 ± 14 | 0.04 |
PE phenylephrine, AU arbitrary units
Prostanoid-mediated contractions become Nox2-independent with aging
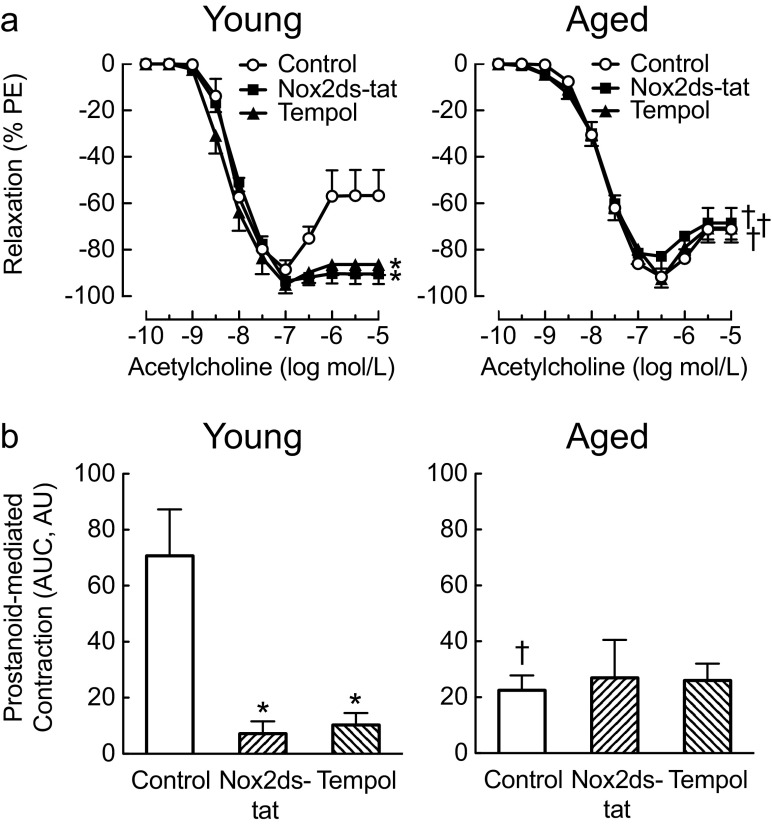
To study the role of vascular ROS bioactivity in prostanoid-mediated contractions, the superoxide dismutase mimetic tempol was employed. Scavenging of superoxide reduced contractions to acetylcholine by ~75 % in arteries from young mice (from 35 ± 9 to 9 ± 3 %, n = 4–5, p < 0.05 vs. control, Fig. 2a). Since vascular Nox2 is a key source of superoxide (Brandes et al. 2010; Lassegue et al. 2012) and has been implicated in TP receptor signaling (Zhang et al. 2011), we next treated carotid arteries of young mice with the Nox2-targeted inhibitor Nox2ds-tat (Csanyi et al. 2011; Rey et al. 2001). This inhibitor consists of a 9-amino acid peptide that prevents activation of the catalytic Nox2 subunit by cytosolic proteins, coupled to the 11-amino acid HIV-tat peptide sequence that facilitates cellular entry (Csanyi et al. 2011; Rey et al. 2001). Nox2ds-tat greatly reduced contractions to acetylcholine in carotid arteries of young mice (85 % reduction, from 35 ± 9 to 5 ± 3 %, n = 4–5, p < 0.01 vs. control, Figs. 2a and 3). By contrast and unexpectedly, neither tempol nor Nox2ds-tat had any effect on prostanoid-mediated contractions in carotid arteries of aged mice (Figs. 2a and 3). When analyzed by area under the curve, both aging and treatment with tempol or Nox2ds-tat reduced prostanoid-dependent contractions by ~70 and ~85 %, respectively (n = 4–6, p < 0.05 vs. control in young animals, Fig. 2b), with no effect of the inhibitors of superoxide bioavailability in arteries of aged mice. Furthermore, neither tempol nor Nox2ds-tat had any effect on relaxations to acetylcholine irrespective of the age of the animals (Fig. 2a). Taken together, these findings indicate that a substantial portion of prostanoid-mediated contractions in the carotid artery of young mice requires functional Nox2. Furthermore, Nox2 as an essential mediator of these contractions becomes inactive with aging.
Fig. 2.
Age-dependent differential effect of functional Nox2 on the responses to vasoconstrictor prostanoids. Carotid arteries obtained from 4-month-old (young) and 24-month-old mice (aged) were pretreated with the Nox2-targeted inhibitor Nox2ds-tat (3 μmol/L) or the superoxide dismutase mimetic tempol (100 μmol/L). Concentration-dependent responses to acetylcholine were recorded (a) and area under the curve (AUC) calculated from prostanoid-mediated contractions stimulated by acetylcholine (b). AUC is expressed as arbitrary units (AU). *p < 0.05 vs. control; † p < 0.05 vs. young. All data (n = 4–6 per group) represent mean ± sem. PE phenylephrine
Nox2 partially mediates contractions to phenylephrine: loss with aging
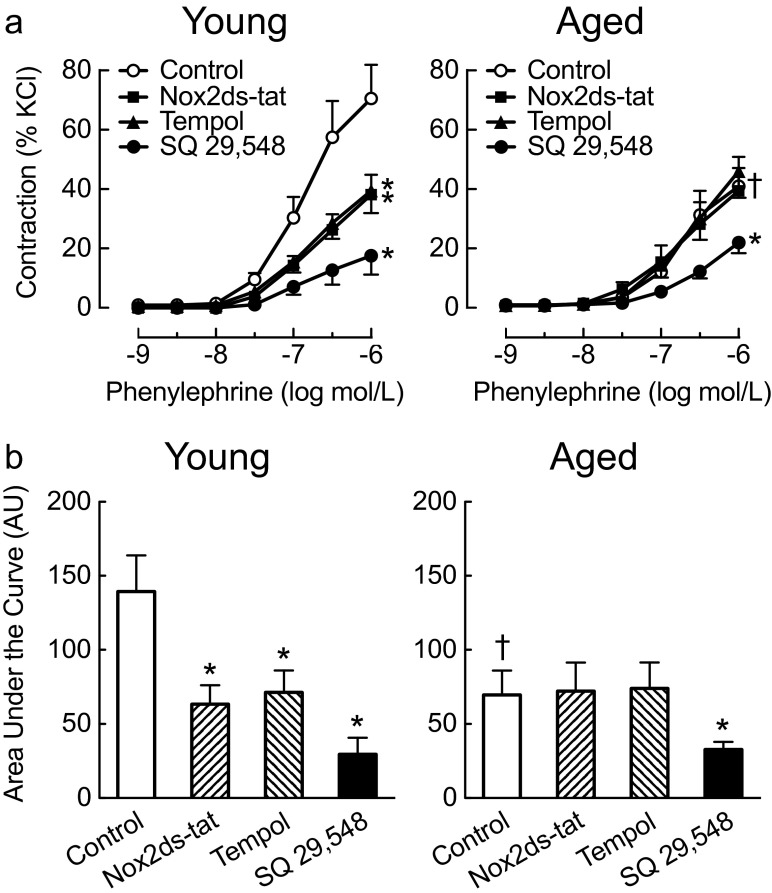
Given that vascular activity of Nox2 determines age-dependent contractility to prostanoids, we sought to extend these findings by studying contractions to the α1-adrenergic agonist phenylephrine, a known activator of Nox2 (Hahn et al. 2014). Importantly, phenylephrine-dependent contractions involve formation of prostanoids and superoxide in carotid arteries (Pereira et al. 2010). In young mice, we indeed found that both Nox2ds-tat and tempol reduced carotid artery contractions to phenylephrine by ~45 % (n = 4–6, each p < 0.01 vs. control, Fig. 4), while TP receptor inhibition by SQ 29,548 reduced contractions by 75 % (from 71 ± 11 to 18 ± 6 %, n = 4–5, p < 0.001 vs. control, Fig. 4). In contrast, phenylephrine-induced contractions in aged mice remained partially sensitive to inhibition by SQ 29,548, but were unaffected by Nox2ds-tat or tempol (n = 5–7, Fig. 4). The maximum contractility (at 1 μmol/L phenylephrine) in carotid arteries of aged mice matched that of Nox2ds-tat-treated arteries from young mice (representing a 42 % reduction from 71 ± 11 % in young controls to 41 ± 10 % in aged controls, n = 5, p < 0.01, Fig. 4), which further supports the conclusion that the loss of Nox2 activity is a contributing factor in the age-dependent reduced contractility of the carotid artery, similar to that observed for acetylcholine-stimulated, prostanoid-mediated contractions (Figs. 2 and 3).
Fig. 4.
Effect of aging on Nox2-dependent contractions to phenylephrine. Concentration-dependent contractions to phenylephrine (a) in carotid arteries from young (4-month-old) and aged mice (24-month-old) were determined in the absence and presence of the Nox2-targeted inhibitor Nox2ds-tat (3 μmol/L), the superoxide dismutase mimetic tempol (100 μmol/L), and the TP receptor antagonist SQ 29,548 (1 μmol/L). Area under the curve (b) is given as arbitrary units (AU). *p < 0.05 vs. control; † p < 0.05 vs. young. All data (n = 4–7 per group) represent mean ± sem
Heterogeneity in vascular gene expression of Nox subunits and eNOS: role of aging
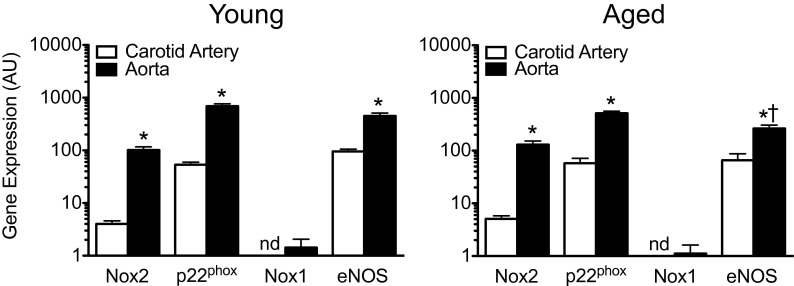
Since the assessment of vascular reactivity suggested age-dependent reductions in the activity of Nox2 and eNOS, we next determined their gene expression levels in the carotid artery and compared it with the aorta of young and aged mice. We found that expression of the Nox2 catalytic subunit, its transmembrane scaffolding protein p22phox (Brandes et al. 2010; Lassegue et al. 2012) and eNOS was markedly lower in the carotid artery than in the aorta of young mice (n = 4, each p < 0.01, Fig. 5). Aging had no effect on expression of the Nox subunits in either vessel and had no effect on eNOS messenger RNA (mRNA) levels in the carotid artery. In the aorta, a downregulation of eNOS mRNA by 42 % was observed with aging (n = 4, p < 0.05, Fig. 5). Carotid artery gene expression of the activating proteins Rac1 and Noxa1 (Brandes et al. 2010; Lassegue et al. 2012) remained unaffected by aging (not shown). By contrast, Nox1 mRNA was expressed only in the aorta (at low levels), with Nox1 mRNA being undetectable in the carotid artery regardless of the age of the animals (Fig. 5).
Fig. 5.
Age-dependent vascular gene expression levels of Nox2, p22phox, Nox1, and endothelial NO synthase (eNOS). Steady-state mRNA levels in the carotid artery and in the aorta from young and aged mice (4 and 24 months of age) were determined by quantitative PCR. *p < 0.01 vs. carotid artery; † p < 0.05 vs. young. All data (n = 4 per group) represent mean ± sem. nd not detectable
Blood pressure and body weight in young and aged animals
Changes in blood pressure or body weight alter responses to vasoconstrictor prostanoids in carotid arteries and other vascular beds (Feletou and Vanhoutte 2006; Traupe et al. 2002). However, in line with previous reports in mice (Durrant et al. 2009), we found no change in systolic and diastolic blood pressure levels in aged compared to young animals (Table 2). Similarly, body weight of 4-month-old mice was not different when compared with 24-month-old mice (Table 2).
Table 2.
Blood pressure and body weight in young and aged mice. Systolic and diastolic blood pressure values were determined in conscious animals using a non-invasive tail cuff volume-pressure recording system. All data (n = 5–8 per group) are mean ± sem
| Young | Aged | p value | |
|---|---|---|---|
| Systolic blood pressure (mmHg) | 118 ± 2 | 115 ± 2 | 0.32 |
| Diastolic blood pressure (mmHg) | 88 ± 3 | 87 ± 2 | 0.79 |
| Body weight (g) | 31.1 ± 1.0 | 30.8 ± 1.1 | 0.87 |
Discussion
In the present study, we report for the first time that Nox2 is largely responsible for prostanoid-mediated contractions in the carotid artery of healthy mice. Contrary to our hypothesis, we found that this ROS-dependent mechanism does not increase but instead becomes inactivated with aging. Thus, TP receptor-mediated contractions to acetylcholine in the murine carotid artery involve (i) a substantial superoxide/Nox2-dependent component that becomes inactive with aging and (ii) a superoxide/Nox2-independent component that remains functional irrespective of age. These findings suggest that Nox2 is centrally involved in the generation of TP receptor-mediated contractions in adult healthy mice and provide evidence that NADPH oxidase-dependent contractile mechanisms may not uniformly increase with aging in all vascular beds.
Increased ROS production has been causally implicated in endothelial cell dysfunction with vascular aging in animals and humans (Barton 2014; Donato et al. 2007; Feletou and Vanhoutte 2006; Mukai et al. 2002; Oudot et al. 2006; Seals et al. 2011; Shi et al. 2008), and increased expression of Nox2 was found in the aorta of aged rats (Oudot et al. 2006). In addition, enhanced acetylcholine-stimulated contractions with aging have been observed in the aorta of rats and hamsters as well as rat femoral arteries (Heymes et al. 2000; Shi et al. 2008; Wong et al. 2009). In contrast, we unexpectedly found that with aging, the potency of responses to vasoconstrictor prostanoids in the carotid artery decreases due to a loss of Nox2 and superoxide bioactivity. Vascular reactivity in murine common carotid arteries may be unique since this vascular bed, compared with other arteries, exhibits very high basal NO bioactivity (Crauwels et al. 2000), which, in turn, may be a consequence of the loss of agonist-induced Nox2 activity with aging reported in the present study (Barton 2014; Feletou and Vanhoutte 2006). Therefore and in parallel with other vascular beds such as the aorta (Barton et al. 1997; Mukai et al. 2002; Shi et al. 2008), the observed age-dependent shift to the right of the concentration-response curve to acetylcholine likely represents a significant impairment of endothelial cell function that may be of relevance for the development of carotid artery disease (Feletou and Vanhoutte 2006). However, maximal relaxant responses were preserved possibly due to the exceptionally high NO producing capacity of this artery (Crauwels et al. 2000). Similarly, eNOS expression remained unaffected by aging in the carotid artery, whereas it was reduced in the aorta of aged compared to young mice. Our observations are in line with previous reports demonstrating that maximal NO-mediated relaxations to acetylcholine as well as eNOS expression in the carotid artery are preserved in old mice at 24 months of age (Modrick et al. 2012) and only begin to decline as the animals age further (Durrant et al. 2009; Fleenor et al. 2012). Together, these findings indicate that functional aging in the common carotid artery may substantially differ compared to previous findings in other vascular beds (Barton et al. 1997; Meyer et al. 2014a).
ROS play an integral role as amplifiers of prostanoid-mediated contractions (Cosentino et al. 1994; Feletou and Vanhoutte 2006; Gao and Lee 2005; Katusic et al. 1993; Shi et al. 2008; Vanhoutte 2013; Yang et al. 2002), and stimulation of Nox2 was shown in response to TP receptor activation in endothelial cells (Zhang et al. 2011). In the present study, we show that in healthy young mice, Nox2 mediates a large portion of acetylcholine-induced carotid artery contractions by utilizing Nox2ds-tat, a peptide directed against the Nox2 catalytic subunit (Csanyi et al. 2011; Rey et al. 2001). To the best of our knowledge, this is the first demonstration of a direct involvement and requirement of NADPH oxidase for prostanoid-mediated contractions. Diphenylene iodonium and apocynin have previously been used to inhibit contractile responses to acetylcholine (Gao and Lee 2005; Ling et al. 2005), yet these substances do not selectively target NADPH oxidase and have off-target antioxidant effects by interfering with several flavoenzyme oxidoreductases, including NO synthase (Brandes et al. 2010; Drummond et al. 2011; Vanhoutte 2013). In line with the fact that Nox2 primarily produces superoxide (Brandes et al. 2010; Lassegue et al. 2012), the superoxide dismutase mimetic tempol largely inhibited prostanoid-mediated contractions in the present study. These findings are consistent with previous observations in the canine basilar artery demonstrating that superoxide directly contributes to contractions induced by acetylcholine (Cosentino et al. 1994; Katusic et al. 1993). Interestingly, Nox2-dependent ROS production has been reported following stimulation by cyclooxygenase (COX)-derived prostaglandin E2 (Wang et al. 2013), although direct generation of ROS by COX has also been proposed (Tang et al. 2007; Vanhoutte 2013).
It has been noted that Nox2ds-tat may also inhibit the activation of Nox1 due to high sequence homology with the Nox2 isoform (Brandes et al. 2010; Lassegue et al. 2012). However, we were unable to detect mRNA transcripts of Nox1 in carotid arteries from both young and aged animals. The Nox1 gene was detectable at substantially lower levels than Nox2 or p22phox in the aorta of these animals, a vessel that generally displays higher gene expression levels of Nox subunits than the carotid artery. We therefore conclude that the Nox2 isoform is predominantly involved in the contractile responses mediated by vasoconstrictor prostanoids. Lack of Nox1 expression in murine carotid arteries may also explain why contractions to angiotensin II in this vascular bed are weak and unaffected by Nox inhibition (Kretz et al. 2006; Meyer et al. 2014b), since angiotensin II-mediated contractions to a substantial part depend on Nox1 activity (Mehta and Griendling 2007). In contrast, angiotensin II-induced contractions are slightly more potent and partially Nox-dependent in the suprarenal abdominal aorta (M. R. Meyer and E. R. Prossnitz, unpublished observation), where low levels of Nox1 mRNA are detectable.
Consistent with previous observations in mice (Modrick et al. 2012), we observed no effect of aging on vascular gene expression levels of Nox2. Similarly, mRNA levels of the scaffolding subunit p22phox or the activating proteins Rac1 and Noxa1 (Brandes et al. 2010; Lassegue et al. 2012) remained unaffected by aging. The observed age-dependent loss of Nox2-mediated, prostanoid-dependent contractions therefore reinforces the notion that levels of Nox mRNA expression do not necessarily translate into Nox activity (Brandes et al. 2010). Furthermore, agonists other than acetylcholine or phenylephrine may still be capable of stimulating NADPH oxidases or alternative sources of ROS, such as uncoupled eNOS, mitochondria, or xanthine oxidase (Barton 2014; Feletou and Vanhoutte 2006; Seals et al. 2011), which may become active in the carotid artery with aging.
To further strengthen our findings regarding the role of Nox2 as an age-dependent factor contributing to vascular contractility, we studied responses to the α1-adrenergic agonist phenylephrine, which, in the carotid artery, involve prostanoid and superoxide formation (Pereira et al. 2010), particularly through activation of Nox2 (Hahn et al. 2014). In arteries from young mice, contractions to phenylephrine were largely TP receptor-dependent and reduced by inhibition of Nox2 and scavenging of superoxide, effects consistent with the observed reduction of acetylcholine-induced, TP receptor-mediated contractions. This Nox2-dependent component was completely abolished with aging, resulting in an overall reduced contractile response that remained partially sensitive to TP receptor blockade. In support of the preserved prostanoid-dependent component of contractions to phenylephrine and acetylcholine in carotid arteries of aged mice observed in the present study, we have previously reported that angiotensin II-mediated contractions, a prototypical NADPH oxidase-dependent event (Mehta and Griendling 2007), become fully COX-dependent in murine carotid arteries during early aging, i.e., adulthood at 7 months of age (Kretz et al. 2006).
In summary, the present study provides evidence that the functional aging process in the common carotid artery likely involves Nox2- and eNOS-dependent pathways. While impairment in NO-mediated dilation has been associated with aging of other vascular beds such as the aorta (Barton 2014; Barton et al. 1997; Seals et al. 2011), an age-dependent increase in vasoconstrictor responses to prostanoids has commonly been found (Heymes et al. 2000; Novella et al. 2013; Shi et al. 2008; Wong et al. 2009). The present study is the first to demonstrate that aging reduces prostanoid-mediated contractions due to a lack of functional Nox2 activity in the murine carotid artery, which displays generally lower expression levels of Nox subunits than the aorta. Given that Nox2 activity reduces NO bioavailability and increases atherogenesis (Judkins et al. 2010), the findings of the present study may serve as an explanation for the differential susceptibility of the common carotid artery to atherosclerotic vascular disease compared to coronary and other arterial beds in humans (Dalager et al. 2007; Solberg and Eggen 1971). Instead, alternative age-dependent factors such as deregulated production of extracellular matrix proteins, inflammatory responses, or changes in mechanical vascular stimulation (Zieman et al. 2005) may play a more prominent role in the carotid artery. Furthermore, since mechanisms implicated in vascular aging may not be uniformly active in different vascular beds and since the usefulness of existing pharmacological and lifestyle interventions to control vascular risk factors such as diabetes or hyperlipidemia is uncertain in the carotid artery (Gorelick et al. 2011), additional studies to identify specific mechanisms that determine the aging process in the common carotid artery should be pursued, particularly given the increasing prevalence of clinical consequences such as stroke and cognitive impairment in our aging population (Gerland et al. 2014; Gorelick et al. 2011).
Acknowledgments
We thank Dr. Chelin Hu and Daniel F. Cimino for expert technical assistance. This study was supported by the National Institutes of Health (R01 CA127731 and CA163890 to E.R.P.), Dedicated Health Research Funds from the University of New Mexico School of Medicine allocated to the Signature Program in Cardiovascular and Metabolic Diseases (to E.R.P.), and the Swiss National Science Foundation (grants 135874 and 141501 to M.R.M. and grants 108258 and 122504 to M.B.). N.C.F. was supported by NIH training grant HL07736.
References
- Barton M. Aging and endothelin: determinants of disease. Life Sci. 2014;118:97–109. doi: 10.1016/j.lfs.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Barton M, Cosentino F, Brandes RP, Moreau P, Shaw S, Luscher TF. Anatomic heterogeneity of vascular aging: role of nitric oxide and endothelin. Hypertension. 1997;30:817–824. doi: 10.1161/01.HYP.30.4.817. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Weissmann N, Schroder K. NADPH oxidases in cardiovascular disease. Free Radic Biol Med. 2010;49:687–706. doi: 10.1016/j.freeradbiomed.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Sill JC, Katusic ZS. Role of superoxide anions in the mediation of endothelium-dependent contractions. Hypertension. 1994;23:229–235. doi: 10.1161/01.HYP.23.2.229. [DOI] [PubMed] [Google Scholar]
- Crauwels HM, Van Hove CE, Herman AG, Bult H. Heterogeneity in relaxation mechanisms in the carotid and the femoral artery of the mouse. Eur J Pharmacol. 2000;404:341–351. doi: 10.1016/S0014-2999(00)00619-1. [DOI] [PubMed] [Google Scholar]
- Csanyi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egana L, Lopes LR, Jackson HM, Kelley EE, Pagano PJ. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med. 2011;51:1116–1125. doi: 10.1016/j.freeradbiomed.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalager S, Paaske WP, Kristensen IB, Laurberg JM, Falk E. Artery-related differences in atherosclerosis expression: implications for atherogenesis and dynamics in intima-media thickness. Stroke. 2007;38:2698–2705. doi: 10.1161/STROKEAHA.107.486480. [DOI] [PubMed] [Google Scholar]
- DeLean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978;235:E97–E102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, Seals DR. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- Drummond GR, Selemidis S, Griendling KK, Sobey CG. Combating oxidative stress in vascular disease: NADPH oxidases as therapeutic targets. Nat Rev Drug Discov. 2011;10:453–471. doi: 10.1038/nrd3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant JR, Seals DR, Connell ML, Russell MJ, Lawson BR, Folian BJ, Donato AJ, Lesniewski LA. Voluntary wheel running restores endothelial function in conduit arteries of old mice: direct evidence for reduced oxidative stress, increased superoxide dismutase activity and down-regulation of NADPH oxidase. J Physiol. 2009;587:3271–3285. doi: 10.1113/jphysiol.2009.169771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (The Wiggers Award Lecture) Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. doi: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- Fleenor BS, Seals DR, Zigler ML, Sindler AL. Superoxide-lowering therapy with TEMPOL reverses arterial dysfunction with aging in mice. Aging Cell. 2012;11:269–276. doi: 10.1111/j.1474-9726.2011.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao YJ, Lee RM. Hydrogen peroxide is an endothelium-dependent contracting factor in rat renal artery. Br J Pharmacol. 2005;146:1061–1068. doi: 10.1038/sj.bjp.0706423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerland P, Raftery AE, Sevcikova H, Li N, Gu D, Spoorenberg T, Alkema L, Fosdick BK, Chunn J, Lalic N, Bay G, Buettner T, Heilig GK, Wilmoth J. World population stabilization unlikely this century. Science. 2014;346:234–237. doi: 10.1126/science.1257469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn NE, Musters RJ, Fritz JM, Pagano PJ, Vonk AB, Paulus WJ, van Rossum AC, Meischl C, Niessen HW, Krijnen PA. Early NADPH oxidase-2 activation is crucial in phenylephrine-induced hypertrophy of H9c2 cells. Cell Signal. 2014;26:1818–1824. doi: 10.1016/j.cellsig.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymes C, Habib A, Yang D, Mathieu E, Marotte F, Samuel J, Boulanger CM. Cyclo-oxygenase-1 and -2 contribution to endothelial dysfunction in ageing. Br J Pharmacol. 2000;131:804–810. doi: 10.1038/sj.bjp.0703632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman A, Van De Wiel A, Rabelink TJ, Van Faassen EE. Wine polyphenols and ethanol do not significantly scavenge superoxide nor affect endothelial nitric oxide production. J Nutr Biochem. 2004;15:426–432. doi: 10.1016/j.jnutbio.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Judkins CP, Diep H, Broughton BR, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR. Direct evidence of a role for Nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in ApoE-/- mice. Am J Physiol Heart Circ Physiol. 2010;298:H24–H32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- Katusic ZS, Schugel J, Cosentino F, Vanhoutte PM. Endothelium-dependent contractions to oxygen-derived free radicals in the canine basilar artery. Am J Physiol. 1993;264:H859–H864. doi: 10.1152/ajpheart.1993.264.3.H859. [DOI] [PubMed] [Google Scholar]
- Kretz M, Mundy AL, Widmer CC, Barton M. Early aging and anatomic heterogeneity determine cyclooxygenase-mediated vasoconstriction to angiotensin II in mice. J Cardiovasc Pharmacol. 2006;48:30–33. doi: 10.1097/01.fjc.0000242061.18981.d3. [DOI] [PubMed] [Google Scholar]
- Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling X, Cota-Gomez A, Flores NC, Hernandez-Saavedra D, McCord JM, Marecki JC, Haskins K, McDuffie M, Powers K, Kench J, Oka M, McMurtry I, Flores SC. Alterations in redox homeostasis and prostaglandins impair endothelial-dependent vasodilation in euglycemic autoimmune nonobese diabetic mice. Free Radic Biol Med. 2005;39:1089–1098. doi: 10.1016/j.freeradbiomed.2005.05.027. [DOI] [PubMed] [Google Scholar]
- Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Fredette NC, Barton M, Prossnitz ER. Regulation of vascular smooth muscle tone by adipose-derived contracting factor. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Barton M, Prossnitz ER. Functional heterogeneity of NADPH oxidase-mediated contractions to endothelin with vascular aging. Life Sci. 2014;118:226–231. doi: 10.1016/j.lfs.2013.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Fredette N, Barton M, Prossnitz ER. Endothelin-1 but not angiotensin II contributes to functional aging in murine carotid arteries. Life Sci. 2014;118:213–218. doi: 10.1016/j.lfs.2014.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrick ML, Kinzenbaw DA, Chu Y, Sigmund CD, Faraci FM. Peroxisome proliferator-activated receptor-gamma protects against vascular aging. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1184–R1190. doi: 10.1152/ajpregu.00557.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai Y, Shimokawa H, Higashi M, Morikawa K, Matoba T, Hiroki J, Kunihiro I, Talukder HM, Takeshita A. Inhibition of renin-angiotensin system ameliorates endothelial dysfunction associated with aging in rats. Arterioscler Thromb Vasc Biol. 2002;22:1445–1450. doi: 10.1161/01.ATV.0000029121.63691.CE. [DOI] [PubMed] [Google Scholar]
- Novella S, Dantas AP, Segarra G, Novensa L, Heras M, Hermenegildo C, Medina P. Aging enhances contraction to thromboxane A2 in aorta from female senescence-accelerated mice. Age (Dordr) 2013;35:117–128. doi: 10.1007/s11357-011-9337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudot A, Martin C, Busseuil D, Vergely C, Demaison L, Rochette L. NADPH oxidases are in part responsible for increased cardiovascular superoxide production during aging. Free Radic Biol Med. 2006;40:2214–2222. doi: 10.1016/j.freeradbiomed.2006.02.020. [DOI] [PubMed] [Google Scholar]
- Pereira AC, Olivon VC, de Oliveira AM. An apparent paradox: attenuation of phenylephrine-mediated calcium mobilization and hyperreactivity to phenylephrine in contralateral carotid after balloon injury. J Cardiovasc Pharmacol. 2010;56:162–170. doi: 10.1097/FJC.0b013e3181e571cd. [DOI] [PubMed] [Google Scholar]
- Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ Res. 2001;89:408–414. doi: 10.1161/hh1701.096037. [DOI] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Man RY, Vanhoutte PM. Two isoforms of cyclooxygenase contribute to augmented endothelium-dependent contractions in femoral arteries of 1-year-old rats. Acta Pharmacol Sin. 2008;29:185–192. doi: 10.1111/j.1745-7254.2008.00749.x. [DOI] [PubMed] [Google Scholar]
- Solberg LA, Eggen DA. Localization and sequence of development of atherosclerotic lesions in the carotid and vertebral arteries. Circulation. 1971;43:711–724. doi: 10.1161/01.CIR.43.5.711. [DOI] [PubMed] [Google Scholar]
- Tang EH, Leung FP, Huang Y, Feletou M, So KF, Man RY, Vanhoutte PM. Calcium and reactive oxygen species increase in endothelial cells in response to releasers of endothelium-derived contracting factor. Br J Pharmacol. 2007;151:15–23. doi: 10.1038/sj.bjp.0707190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traupe T, Lang M, Goettsch W, Munter K, Morawietz H, Vetter W, Barton M. Obesity increases prostanoid-mediated vasoconstriction and vascular thromboxane receptor gene expression. J Hypertens. 2002;20:2239–2245. doi: 10.1097/00004872-200211000-00024. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. One or two, does it matter as long as the arterial wall is coxygenated? Hypertension. 2013;62:244–246. doi: 10.1161/HYPERTENSIONAHA.113.01566. [DOI] [PubMed] [Google Scholar]
- Wang G, Sarkar P, Peterson JR, Anrather J, Pierce JP, Moore JM, Feng J, Zhou P, Milner TA, Pickel VM, Iadecola C, Davisson RL. COX-1-derived PGE2 and PGE2 type 1 receptors are vital for angiotensin II-induced formation of reactive oxygen species and Ca(2+) influx in the subfornical organ. Am J Physiol Heart Circ Physiol. 2013;305:H1451–H1461. doi: 10.1152/ajpheart.00238.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong SL, Leung FP, Lau CW, Au CL, Yung LM, Yao X, Chen ZY, Vanhoutte PM, Gollasch M, Huang Y. Cyclooxygenase-2-derived prostaglandin F2alpha mediates endothelium-dependent contractions in the aortae of hamsters with increased impact during aging. Circ Res. 2009;104:228–235. doi: 10.1161/CIRCRESAHA.108.179770. [DOI] [PubMed] [Google Scholar]
- Yang D, Feletou M, Boulanger CM, Wu HF, Levens N, Zhang JN, Vanhoutte PM. Oxygen-derived free radicals mediate endothelium-dependent contractions to acetylcholine in aortas from spontaneously hypertensive rats. Br J Pharmacol. 2002;136:104–110. doi: 10.1038/sj.bjp.0704669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Song P, Xu J, Zou MH. Activation of NAD(P)H oxidases by thromboxane A2 receptor uncouples endothelial nitric oxide synthase. Arterioscler Thromb Vasc Biol. 2011;31:125–132. doi: 10.1161/ATVBAHA.110.207712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Varadharaj S, Zhao X, Parinandi N, Flavahan NA, Zweier JL. Acetylcholine causes endothelium-dependent contraction of mouse arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1027–H1032. doi: 10.1152/ajpheart.00226.2005. [DOI] [PubMed] [Google Scholar]
- Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]