Abstract
Bone loss occurs insidiously and initially asymptomatically; therefore, osteoporosis is frequently diagnosed only after the first clinical fracture. The aim of this study was to test the hypothesis is that by simply observing the behavior of cultured peripheral monocytes, it might be possible to diagnose altered bone remodeling and, therefore, limit the complications associated with osteoporosis, especially fractures. Monocytes isolated as mononuclear precursors from healthy and ovariectomized rats were cultured both in basal and differentiation medium for up to 3 weeks. Viability and differentiation capability towards the osteoclastic phenotype was checked by light microscopy at early times, whereas differentiation state and synthetic activity (tartrate-resistant acid phosphatase (TRAP) staining; phalloidin, fluorescin isothiocynate (FITC) staining, cathepsin K, metalloproteinase 7 and 9, MMP-7 and MMP-9) were measured at 1, 2, and 3 weeks. Compared to their controls, monocytes isolated from ovariectomized rats proliferate and lean toward the osteoclastic phenotype in the absence of differentiating factors. In both culture conditions, osteoclasts from ovariectomized rats showed significantly higher productions of cathepsin K, MMP-7, and MMP-9 than those of cells isolated from healthy rats, steadily over time. These results obtained in an animal osteoporotic model, if confirmed by clinical studies, open up the possibility to assess the presence of an alteration in bone remodeling with a simple in vitro diagnostic test requiring a small blood sample and less than 48 h. This might allow to early select patients with a spontaneous viability and differentiation of monocytes to osteoclasts for further diagnostic techniques.
Keywords: In vitro method, Screening, Monitoring, Monocytes, Osteoclasts, Bone loss
Introduction
Osteoporosis is a major health problem considering that by 2025, more than 3 million osteoporosis-related fractures per year are expected, with an annual cost of more than $25 billion (Lewiecki 2013). Osteoporosis is defined as a systemic skeletal disease characterized by reduced bone mass and microarchitectural deterioration of bone tissue, resulting in increased bone fragility and susceptibility to fracture and uncoupling of osteoblast-mediated bone formation and osteoclast-mediated bone resorption. This definition is based on four essential aspects: (1) osteoporosis is a systemic condition, affecting the whole skeleton; (2) the health risk associated with osteoporosis is the propension to fracture, in the absence of which the disease is “silent,” with patients unaware of underlying poor bone quality; (3) low bone mass or bone mineral density (BMD) are important diagnostic factors, but not the only ones; and (4) the loss of structural integrity, recognized as microarchitectural deterioration, is of critical importance considering that about 50 % of patients with fragility fractures do not have osteoporosis based on BMD (by using the World Health Organization T-score criteria of −2.5) (Schuit 2004).
Screening by bone densitometry is important in 65-year-old women and younger postmenopausal women with additional risk factors for osteoporosis/fracture (US Preventive Task Force 2011). There is insufficient evidence to make recommendations about the screening and appropriate age to stop it; moreover, the harm versus benefit of screening for osteoporosis in men is unknown (US Preventive Task Force 2011).
Over the years, the diagnosis and monitoring of osteoporosis has been performed through imaging methods, mainly by exploiting the sensitivity of X-ray absorption to the calcium content of the tissue. Dual-energy X-ray absorptiometry (DXA) is still the gold standard (Kanis 2013) because of its high precision and low radiation dose (1-50 μSv) and the possibility to evaluate both the whole skeleton and peripheral sites. However, the technique has important drawbacks, deriving the result from 2D measurements and therefore not able to provide information on the volumetric density and bone microstructural characteristics. Moreover, no less important, the results can be altered by the presence of comorbidities (obesity, osteomalacia, etc.) (Link 2012).
With regard to this, quantitative computed tomography (qCT) provides more detailed information about the structure of trabecular bone and can distinguish between mild fractures and deformities. Nevertheless, it is an expensive procedure and like DXA exposes the patient to radiation and requires well-trained personnel. Conversely, ultrasound methods can provide quick and low-cost information about bone mineralization without radiation exposure, but there is still room for improvement (Pisani 2013).
Finally, research into the biomarkers of bone metabolism and their alterations in bone diseases have also helped to identify patients at high risk of fracture and monitor the efficacy of antiresorptive therapies and bone-forming agents (McCormick 2007). However, the current biochemical markers of bone metabolism have some limitations. These include (1) a lack of tissue specificity for bone, (2) inability to distinguish the metabolic activity of the different skeletal compartments, although they can be differently affected by diseases and treatments, and (3) they are all protein-based markers, although circulating mRNA might also be of value as early biomarkers (Garnero 2004).
The search is ongoing for a method that is simple, inexpensive, predictable, easy to perform, minimally invasive, feasible for every laboratory and every country, and determined with precision in a short time to detect and monitor pathological conditions associated to bone resorption.
Osteoclasts, derived from the fusion of marrow-derived mononuclear phagocytes, play a key role in bone loss in osteoporosis (Shalhoub 2000; Shih 1996; Faust 1999; Massey 1999). These cells differentiate under the influence of several cytokines, namely, macrophage colony stimulating factor (M-CSF), receptor activator of nuclear factor kappa-B ligand (RANKL), and parathyroid hormone (PTH). M-CSF is a potent stimulator of proliferation and differentiation of monocyte-macrophage lineage cells. In addition to M-CSF, RANKL promotes differentiation and fusion of osteoclast precursors and activates mature osteoclasts to resorb bone, by binding its specific receptor RANK (Boyle 2003). PTH also increases the resorptive activity of pre-existing osteoclasts by primary interaction with cells of the osteoblastic lineage. Mature multinucleated bone-resorbing osteoclasts are recognized by the expression of key markers, including tartrate-resistant acid phosphatase (TRAP) (Helfrich 1987), cathepsin K (Inaoka 1995), matrix metalloproteinase 9 (MMP-9) (Hentunen 1999), and the alpha V beta 3 integrin chains (Clover 1992).
The aim of this study was to develop an in vitro diagnostic method to identify a bone resorption state leading to osteoporosis by taking a blood sample. The hypothesis is that by simply observing the behavior of cultured peripheral monocytes, it might be possible to diagnose altered bone remodeling and, therefore, limit the complications associated with osteoporosis, especially fractures, in patients suspected to be at risk or suffering from pathologically increased bone resorption (due to age, lifestyle, acquired and genetic pathologies, or treatments with drugs with a known effect on bone remodeling).
Osteoclasts isolated as mononuclear precursors in peripheral blood mononuclear cells (PBMCs) from healthy and ovariectomized (OVX) rats were cultured for 1, 2, and 3 weeks with and without osteoclast-stimulating factors (M-CFS, RANKL, PTH) to test in vitro spontaneous osteoclast formation. Cell viability, at early times, differentiation state, and synthetic activity (i.e., TRAP and fluorescein isothiocyanate (FITC)-conjugate phalloidin staining, cathepsin K, metalloproteinase-7 (MMP)-7, and MMP-9) were also measured.
Materials and methods
Study design
The study was performed in accordance with the European and Italian Laws on animal experimentation. Blood was obtained immediately after the euthanasia of rats from an uncorrelated in vivo study and previously approved by the Ethical Committee of the Rizzoli Orthopedic Institute and by the appropriate public authorities.
Twelve 2-month-old Sprague-Dawley female rats (Charles River Italia SpA, Lecco), 200 ± 25 g b.w., were housed under controlled conditions (room temperature 20 ± 0.5 °C; relative humidity 55 ± 5 %; 12 h light and 12 h darkness) and supplied with 250 g/rat/week standard diet (Laboratorio Dottori Piccioni SRL, Gessate, Milano) and water ad libitum. The animals were divided in two groups of six: group 1 underwent bilateral ovariectomy (OVX) and group 2 underwent a simulated ovariectomy and served as a sham-operated group (SHAM). Twelve weeks after ovariectomy, all the animals were euthanized by an i.v. injection of 1 ml Tanax (Hoechst AG, Frankfurt-am-Main, Germany), and the osteoporotic condition was evaluated by bone ultrasound measurements (quantitative ultrasound (QUS)), microtomography (micro-CT), and histology on iliac crest bone biopsies.
QUS, micro-CT, and histology on iliac crest bone biopsies
Immediately after ovariectomy and 3 months later, the animals underwent QUS measurements, by DBM Sonic Bone Profiler, (IGEA SRL, Carpi, Italy) under general anesthesia as previously described (Giavaresi 2000). Microtomographic 3D analysis was performed by using CTAN software (Skyscan). The volume of interest selected in each sample was a cylinder with a 1.5-mm diameter and 3-mm height, completely included in the trabecular bone tissue, and the bone volume fraction parameter (BV/TV, %) was measured.
The iliac crest bone biopsies used for histological investigations were fixed in buffered 4 % paraformaldehyde, embedded in methyl methacrylate (Merck, Shuchardt, Ottobrunn, Germany), sectioned (EXAKT Cutting Systems, GmbH Apparatus GmbH Co., Norderstedt, Germany), and finally superficially de-plasticized and stained with toluidine blue, Fuchsin Acid, and Fast Green.
Isolation of mononuclear cells and differentiation of osteoclasts
Osteoclasts (OC) were obtained from the peripheral blood mononuclear cells (PBMCs) of OVX and SHAM rats using heparin as an anticoagulant. PBMCs were separated on a Ficoll-Histopaque gradient (Sigma-Aldrich, MO, USA), according to the following protocol: a volume of peripheral blood was diluted 1:1 with pre-warmed PBS and carefully layered on Histopaque 1077 (ratio 2:1). Density gradient centrifugation (700g at RT for 30 min) was used to separate the mononuclear cells from the other cell fractions of blood. After centrifugation, the PBMCs accumulated at the interface between PBS, and Ficoll were collected, washed twice with PBS, resuspended in an appropriate volume of basal medium (Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich, MO, USA) + 10 % fetal calf serum (FCS; Lonza, Verviers, Belgium)), and counted in a Neubauer chamber after a brief incubation with Turk solution.
The PBMCs derived from SHAM (PBMCSHAM) and OVX (PBMCOVX) rats were both seeded at a density of 1.5 × 106/cm2 in DMEM with 10 % FCS. One culture day later, the non-adherent cells were removed and the adherent cells were reefed with different media, representing alternative cell culture conditions:
Basal medium only (CTR−): PBMCSHAM or PBMCOVX cultured with DMEM + 10 % FCS in the absence of differentiating factors towards the OC phenotype
Differentiation medium (CTR+): PBMCSHAM or PBMCOVX cultured with basal medium implemented with differentiating factors towards the OC phenotype (30 ng/ml of RANKL, 25 ng/ml of M-CSF, and 10-7 M PTH) (Peprotech, Rocky Hill, NJ)
Media were changed, in all the tested conditions, twice a week.
PBMC viability
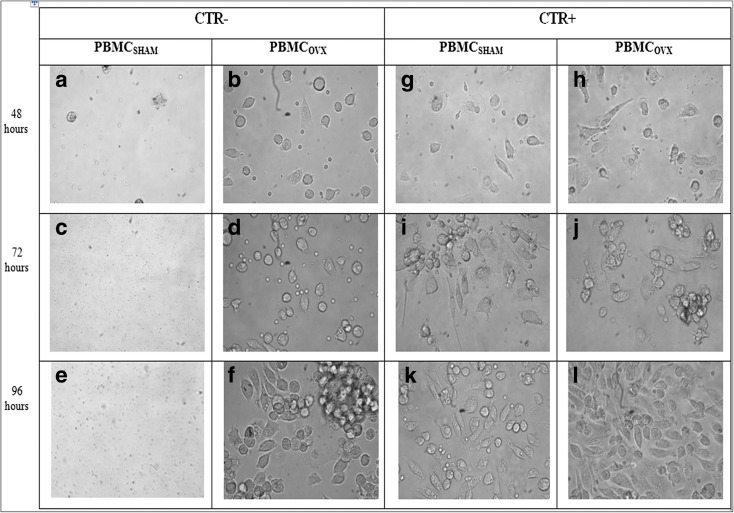
After removing the non-adherent cells (one culture days later), PBMCSHAM or PBMCOVX were examined by light microscope (Olympus IX 71) at 48, 72, and 96 h, and the images acquired by a digital image capture system (×40 objective and an Olympus XC camera), considering 10 regions of interest (ROI) (resolution, 1280 × 960 pixel; area, 7.2 × 103 μm2) for each examined sample.
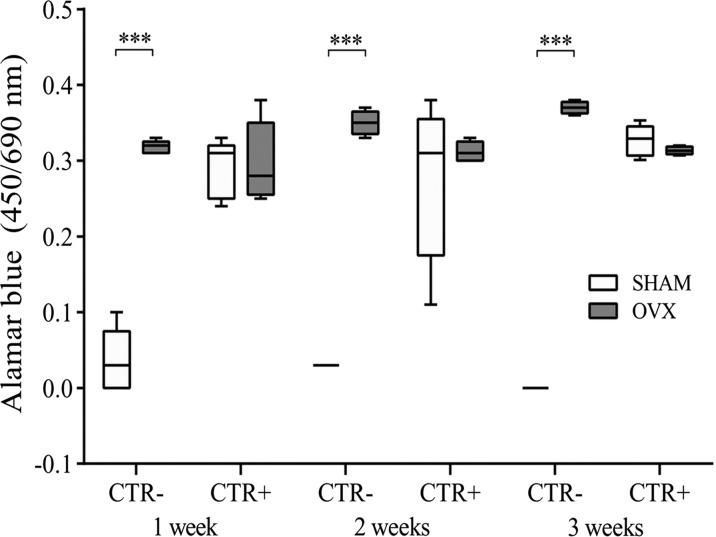
In addition, at 1, 2, and 3 weeks of cell culture, the Alamar blue dye test (Serotec, Oxford, UK) was used to evaluate cell viability. The reagent is a dye, which incorporates an oxidation-reduction indicator that changes color in response to the chemical reduction of growth medium, resulting from cell growth. It was added to each culture well (1:10 v/v) for 4 h at 37 °C. After transferring the supernatants to 96-well plates, the absorbance was read spectrophotometrically at 570- and 600-nm wavelengths (for the fully oxidized and reduced forms of reagent) by MicroPlate reader (BioRad, CA, USA). The results, obtained as optical density (OD), were processed following the manufacturer’s instructions and expressed as reduction percentage.
PBMC differentiation
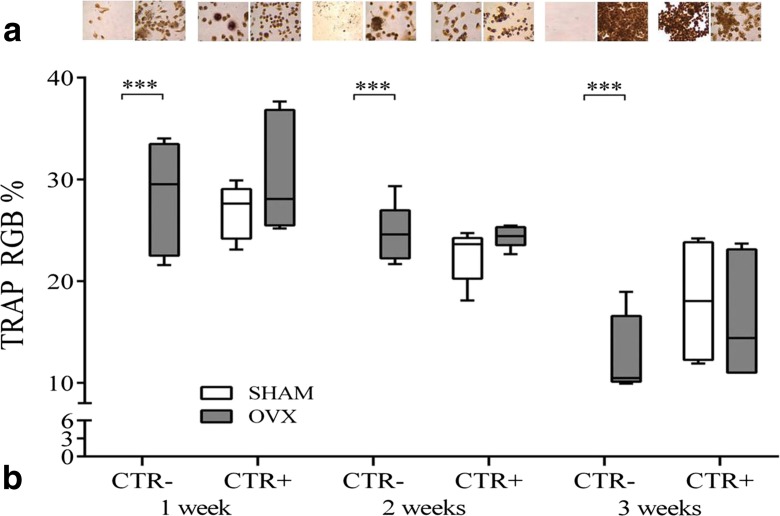
At 1, 2, and 3 weeks of cell culture, PBMC differentiation was evaluated by tartrate-resistant acid phosphatase (TRAP) histochemical staining, according to the manufacturer’s instructions (387A-KT, SIGMA, St. Louis, MO, USA). The large, multinucleated cells (three or more nuclei), which developed a brown color, were scored as positive cells. The ratio between the brown-colored region and total image area was measured using an image analysis system (Leica QWIN, Leica Microsystems Ltd., United Kingdom). The brown color was defined on the red-green-blue (RGB) scale as [R = 36 ÷ 86; G = 23 ÷ 40; B = 14 ÷ 24]. Images were taken using a standard light microscope (Olympus IX71, Olympus Italia Srl, Italy) equipped with a digital camera (XCell, Olympus Italia Srl, Italy) at ×40 magnification. In addition, the number of osteoclasts, in 10 ROI (resolution, 1280 × 960 pixel; area, 7.2 × 103 μm2), were also counted at 1, 2, and 3 weeks of cell culture. To avoid biases due to subjectivity, the evaluation and measurements were performed by two experienced, blinded investigators.
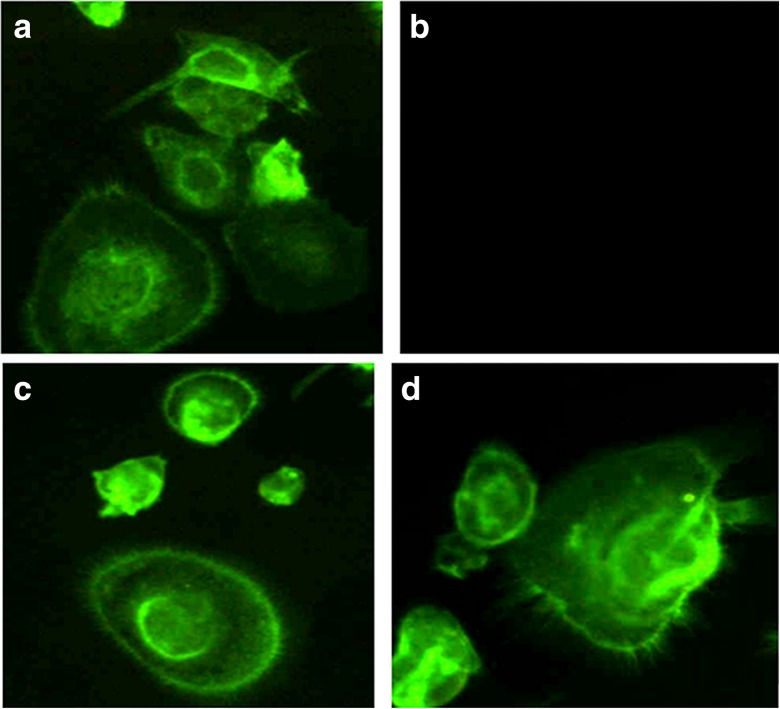
Three weeks after seeding the cells, each culture was pre-washed with PBS and fixed in a solution of 4 % formaldehyde in PBS for 10 min at 37 °C. Then, the samples were permeabilized with 0.1 % Triton X-100 for 15 min and washed in PBS and a FITC-conjugate phalloidin solution (1:100 in PBS) was added for 30 min at 37 °C. After washing with PBS, the samples were examined by fluorescence microscope (Olympus IX 71) and the images acquired by a digital image capture system (×40 objective and an Olympus XC camera).
PBMC activity
At 1, 2, and 3 weeks of cell culture, the supernatants from each culture condition were collected from all wells and centrifuged to remove any apoptotic cells. Aliquots were dispensed in Eppendorf tubes for storage at −80 °C and assayed for cathepsin K (CTSK; Enzyme-linked Immunosorbent Assay Kit, Uscn Life Science Inc., Wuhan, China), the lysosomal collagenase responsible for the degradation of the organic bone matrix during bone remodeling; matrix metalloproteinase-7 (MMP-7; Enzyme-linked Immunosorbent Assay Kit Uscn, Life Science Inc., Wuhan, China); and matrix metalloproteinase-9 (MMP-9; Enzyme-linked Immunosorbent Assay Kit Uscn, Life Science Inc., Wuhan, China), belonging to the group of the stromelysin and gelatinase matrix metalloproteinase, respectively.
Statistical analyses
Statistical analysis was performed using SPSS v.21.0 software (SPSS Inc., Chicago, Illinois, USA). Data are reported as mean ± SD at a significance level of p < 0.05. After verifying the normal distribution and homogeneity of variance, a one-way ANOVA was performed for comparison between groups. Finally, Scheffé’s post hoc multiple comparison tests were performed to detect significant differences between groups.
Results
QUS, micro-CT, and histology on iliac crest bone biopsies
The reliability of the animal model used and the development of osteoporosis were confirmed by the tests performed. QUS showed that the value of SoS (m/s) was 4 % lower in the OVX group (1802 ± 35 m/s) in comparison to the SHAM group (1881 ± 61 m/s) (p < 0.005). Microtomographic analysis highlighted a significant difference (p < 0.005) in bone density (BV/TV, %) between the SHAM (27.3 ± 4.5 %) and OVX (16.9 ± 3.9 %) groups 3 months after ovariectomy. The qualitative histological evaluation provided further confirmation of the development of an osteoporotic condition.
PBMC viability
After 48, 72, and 96 h of culture with different media (basal medium, CTR−; differentiation medium, CTR+), PBMCSHAM and PBMCOVX cultures were observed by light microscope (Fig. 1). At each experimental time, PBMCSHAM in CTR− showed a lower number (≤5 for ROI) of cells compared to that of PBMCOVX (≥15 for ROI) (Fig. 1a-f). Conversely, both PBMCSHAM and PBMCOVX highlighted the presence of a high number of cells (≥15 for ROI) in CTR+ at each experimental time (Fig. 1g-n). These evidences showed that, unlike what was observed in PBMCSHAM, PBMCOVX are able to survive even without stimulation with osteoclast-differentiated medium (Fig. 1b, d, f). These results were also confirmed by the Alamar blue test, which highlighted an increased viability in PBMCOVX cultured in CTR− in comparison to PBMCSHAM in the same condition, at each experimental time (p < 0.0005) (Fig. 2). Concerning the cultures in differentiating medium (CTR+), no significant differences were found among the OVX and SHAM groups (Fig. 2).
Fig. 1.
Characteristics of PBMC isolated as mononuclear precursors from healthy and ovariectomized rats. Morphology of PBMCSHAM and PBMCOVX after 48, 72, and 96 h exposed to a–f CTR− medium or to g–n CT+R+ medium
Fig. 2.
Cell viability after 1, 2, and 3 weeks of culture (mean ± SD, n = 4 triplicates). CTR−: basal culture medium (without RANKL, M-CSF, PTH); CTR+: differentiated medium (with RANKL, M-CSF, PTH). Scheffé post hoc multiple comparison test between PBMCOVX and PBMCSHAM for each culture condition: 1st week, CTR− PBMCOVX versus PBMCSHAM ***p < 0.0005; 2nd week, CTR− PBMCOVX versus PBMCSHAM ***p < 0.0005; 3rd week, CTR− PBMCOVX versus PBMCSHAM ***p < 0.0005
PBMC differentiation
TRAP assay was used for osteoclast differentiation. Foreseeable and as widely described, the absence of differentiating factors in culture medium prevents PBMCSHAM from viability and obviously maturation towards the OC lineage; PBMCOVX, instead, showed significant values of occurred differentiation at 1, 2, and 3 weeks when compared to PBMCSHAM (p < 0.0005) (Fig. 3). These results were also confirmed by the osteoclast count where PBMCOVX showed a significantly higher number of osteoclasts at 1, 2, and 3 weeks when compared to those of PBMCSHAM (p < 0.0005) (Table 1). With regard to CTR+ cultures, TRAP staining was positive for PBMCSHAM, which showed signs of maturation at the first, second, and third weeks (Fig. 3).
Fig. 3.
a A multinucleated TRAP-positive osteoclast was observed by TRAP staining. Cells containing tartrate-sensitive acid phosphatase are devoid of activity, and only the cells containing tartrate acid-resistant phosphatase show maroon dye deposits at the sites of activity. Staining confirmed that the presence of TRAP-positive multinucleated osteoclast-like cells was limited to CTR− PBMCOVX, CTR+ PBMCOVX, and PBMCSHAM. b RGB evaluation of TRAP staining after 1, 2, and 3 weeks of culture (mean ± SD, n = 4 triplicates). CTR−: basal culture medium (without RANKL, M-CSF, PTH); CTR+: differentiated medium (with RANKL, M-CSF, PTH). Scheffé post hoc multiple comparison test between PBMCOVX and PBMCSHAM for each culture condition: 1st week, CTR− PBMCOVX versus PBMCSHAM ***p < 0.0005; 2nd week, CTR− PBMCOVX versus PBMCSHAM ***p < 0.0005; 3rd week, CTR− PBMCOVX versus PBMCSHAM ***p < 0.0005
Table 1.
Number of osteoclasts (three or more nuclei) evaluated in 10 regions of interest (ROI) at 1, 2, and 3 weeks of cell culture at ×40 magnification (mean ± SD)
| Condition | SHAM | OVX | ||
|---|---|---|---|---|
| Time | CTR− | CTR+ | CTR− | CTR+ |
| 1 week | 0*** | 113 ± 12 | 109 ± 11 | 126 ± 28 |
| 2 weeks | 0*** | 130 ± 24 | 140 ± 22 | 152 ± 37 |
| 3 weeks | 0*** | 167 ± 37 | 169 ± 33 | 189 ± 26 |
Scheffé post hoc multiple comparison test between PBMCOVX and PBMCSHAM for each culture condition:
***p < 0.005; 1st week, CTR− PBMCOVX versus PBMCSHAM;
2nd week, CTR− PBMCOVX versus PBMCSHAM;
3rd week, CTR− PBMCOVX versus PBMCSHAM
Three weeks after cell seeding, PBMC cultures were also analyzed by labeling the F-actin cytoskeleton with phalloidin (Fig. 4). PBMCOVX in CTR− showed the typical organization of the actin network in mature and functional multinucleated osteoclasts (Fig. 4a), described as an actin ring, whereas PBMCSHAM did not show the presence of any actin ring (Fig. 4b). With regard to CTR+ cultures, phalloidin staining highlighted mature and functional osteoclasts in both PBMCOVX and PBMCSHAM groups (Fig. 4c, d).
Fig. 4.
Osteoclasts stained with phalloidin to reveal cell shape through definition of the F-actin cytoskeleton. a PBMCOVX–CTR−; b PBMCSHAM–CTR−; c PBMCOVX–CTR+; d PBMCSHAM–CTR+
PBMC activity
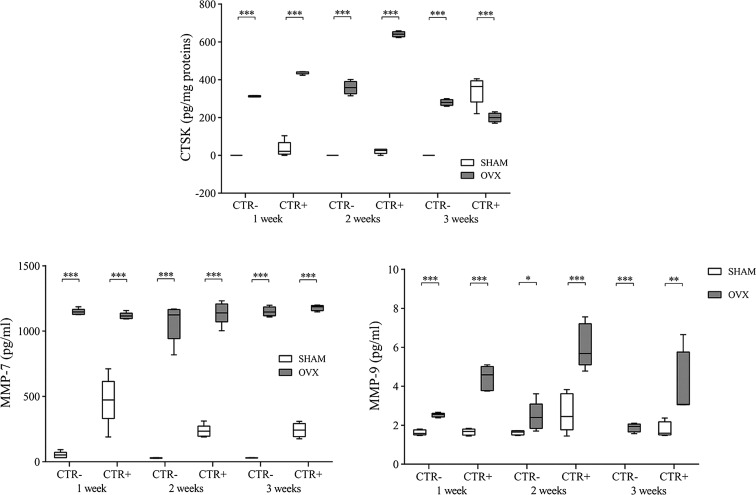
In CTR− culture, low levels of cathepsin K were found in the PBMCSHAM group, whereas the PBMCOVX group showed significantly higher values at all evaluated time points (p < 0.0005) (Fig. 5). On the other hand, with regard to the CTR+ culture, cathepsin K values were significantly greater in the PBMCOVX group than in the PBMCSHAM group at the first 2 weeks (p < 0.0005) (Fig. 5a). In the third week, however, there was a reversal of the trend, with significantly higher values in the PBMCSHAM group compared to those of the PBMCOVX group (p < 0.0005) (Fig. 5a).
Fig. 5.
Cathepsin K, MMP-7, and MMP-9 after 1, 2, and 3 weeks of culture (mean ± SD, n = 4 triplicates). CTR−: basal culture medium (without RANKL, M-CSF, PTH); CTR+: differentiated medium (with RANKL, M-CSF, PTH). Scheffé post hoc multiple comparison test between PBMCOVX and PBMCSHAM for each culture condition: cathepsin K: 1st week, CTR− PBMCOVX versus PBMCSHAM ***p < 0.0005, CTR+ PBMCOVX versus PBMCSHAM ***p < 0.0005; 2nd week, CTR− PBMCOVX versus PBMCSHAM ***p < 0.0005, CTR+ PBMCOVX versus PBMCSHAM ***p < 0.0005; 3rd week, CTR− PBMCOVX versus PBMCSHAM ***p < 0.0005, CTR+ PBMCOVX versus PBMCSHAM ***p < 0.0005. MMP-7: 1st week, CTR− PBMCOVX versus PBMCSHAM ***p < 0.0005, CTR+ PBMCOVX versus PBMCSHAM ***p < 0.0005; 2nd week, CTR− PBMCOVX versus PBMCSHAM ***p < 0.0005, CTR+ PBMCOVX versus PBMCSHAM ***p < 0.0005; 3rd week, CTR− PBMCOVX versus PBMCSHAM ***p < 0.0005, CTR+ PBMCOVX versus PBMCSHAM ***p < 0.0005. MMP-9: 1st week, CTR− PBMCOVX versus PBMCSHAM ***p < 0.0005, CTR+ PBMCOVX versus PBMCSHAM ***p < 0.0005; 2nd week, CTR− PBMCOVX versus PBMCSHAM p < 0.05, CTR+ PBMCOVX versus PBMCSHAM ***p < 0.0005; 3rd week, CTR− PBMCOVX versus PBMCSHAM ***p < 0.0005, CTR+ PBMCOVX versus PBMCSHAM p < 0.005
Concerning MMP-7 production, both in CTR− and CTR+ cultures, levels were significantly higher in the PBMCOVX group than in the PBMCSHAM group (p < 0.0005) (Fig. 5b).
Interestingly, the production of MMP-9 was generally greater in the OVX group (Fig. 5c). In particular, in the CTR− culture, values were statistically higher in the PBMCOVX group compared to those of the PBMCSHAM group, where levels were very low if not null, at 1 (p < 0.0005), 2 (p < 0.05), and 3 (p < 0.0005) weeks (Fig. 5c). Also in the CTR+ culture, values indicate an overall increased secretory activity in the OVX group with respect to those of the other groups (Fig. 5c).
Discussion
The aim of the study was to investigate the possibility of identifying an unbalanced bone turnover by the observation of monocyte viability, isolated from peripheral blood, cultured in the absence of specific factors, normally required for their survival and differentiation towards the osteoclastic phenotype. A standardized model of osteoporosis in OVX rats was used, and the osteoporosis development 12 weeks after OVX was confirmed by QUS, micro-CT, and histology on iliac crest bone biopsies.
PBMCs were cultured with and without differentiating factors as RANKL, M-CSF, and PTH, which represent the essential factors for osteoclast differentiation. As expected, PBMCs collected from animals in healthy bone status (PBMCSHAM) showed viability and osteoclastogenesis only if differentiating factors were added to culture medium; otherwise, PBMCs did not differentiate into osteoclasts and died within 48 h of culture. Conversely, in the absence of differentiating factors, PBMCs from estrogen-deficient osteoporotic animals (PBMCOVX) not only maintained viability at 48, 72, and 96 h but also differentiated into osteoclasts after only a week. Moreover, in CTR− culture conditions, the cell viability of the PBMCOVX group was significantly higher compared to that of the PBMCSHAM group at each experimental time. This result, together with the evaluation of TRAP, osteoclast number, and phalloidin staining, showed that PBMCOVX cells in contrast to PBMCSHAM cells are able to differentiate into osteoclasts even without the presence of the factors necessary and mandatory for their differentiation.
It is known that estrogen withdrawal following menopause leads to an increase in the production of hematopoietic GFs, such as M-CSF, and proinflammatory cytokines, including interleukin 1, interleukin 6, and tumor necrosis factor from the stroma, monocytes, and lymphoid cells (Chen 2009). Increased levels of these factors are believed to stimulate the differentiation of myeloid precursor cells into osteoclasts (Roodman 1999). However, to our knowledge, there are no data in the literature that link spontaneous monocyte viability to in vitro diagnosis of osteoporosis or altered bone remodeling.
Normal bone resorption and remodeling depend critically upon the synthesis and secretion, into the resorption cavity, of cathepsin K, mediated by active osteoclasts through the “ruffled border” (Fuller 2008; Le Gall 2007; Littlewood-Evans 1997; Motyckova 2002; Saftig 1998; Troen 2004; Yasuda 2005). In addition, cathepsin K breaks down type-I collagen protein, an important constituent in bones. In accordance with a previous study (Kiviranta 2001), our results showed that the osteoporotic condition leads to an increase in cathepsin K expression. Whereas cathepsin-K is critical for bone resorption (Saftig 2002), the role of MMPs is less clear. MMPs, by removing the collagenous layer from the bone surface before the demineralization process, trigger osteoclastic resorption (Delaissè 2000). MMPs are also implicated in the cleaning of resorption pits from remaining collagen fibrils by bone lining cells, prior to refilling the pit with new bone matrix components produced by the osteoblasts (Everts 2002). Nevertheless, to date, neither in vitro nor in vivo studies have shown evidence that the osteoporotic microenvironment may provide a favorable condition for MMP-7 production. In the current study, MMP-7 showed a significant increase in osteoclast activity when derived from OVX rats than from healthy ones in all tested culture conditions and experimental times. Regarding the role of MMP-9 in osteoporotic conditions, Zhao et al. suggested that MMP-9 might play a key role in the development of bone loss in osteoporosis (Wang 2005; Zhao 1997). Supporting this assumption, in our study, CTR− and CTR+ culture conditions also showed higher values of MMP-9 production in osteoporotic conditions compared to those of healthy ones. However, as for osteoclast formation and differentiation, no data correlate MMP synthesis with in vitro diagnosis of osteoporosis.
The spontaneous osteoclast formation of PBMCOVX observed in our study is in agreement with a previous study (D’Amelio 2004), which explained spontaneous osteoclastogenesis with a higher production of tumor necrosis factor-alpha (TNF-α) and RANKL. However, it is important to highlight that mature multinucleated bone-resorbing osteoclasts are also recognized by the expression of other key osteoclast markers that include TRAP (Helfrich 1987), cathepsin K (Inaoka 1995), and MMP-9 (Hentunen 1999). Jevon et al. observed that the proportion of circulating precursors in the peripheral blood of primary osteoporosis patients did not increase relatively to controls (Jevon 2003). These results are not in disagreement with ours because PBMCs were cultured in medium implemented with RANKL and M-CSF that, as observed in our study, mask the endogenous differences between PBMCs from osteoporotic and from healthy subjects. Conversely, Jevon et al. support the utmost importance of a differentiation medium in osteoclastogenesis in healthy conditions (Jevon 2003).
As Europe’s population ages and the burden of chronic diseases, such as osteoporosis, rises, in vitro diagnostics, and biomarkers will become more and more important and healthcare systems, striving for greater efficiency and sustainability, will increasingly focus on disease prevention, early intervention, and monitoring. Diagnostics also plays an important role in public health programs, which have always involved sophisticated and expensive diagnostic procedures, but whose clinical relevance requires more effective screening means. Besides intrinsic issues in the use of imaging techniques, in fact, serious drawbacks for patients are the availability of health facilities performing these procedures and waiting times, which may both vary considerably even within the same country. Efforts to adopt substitutive laboratory tests, at least in the early phases of screening/diagnosis, have so far been unsatisfactory. The literature includes studies aimed at showing a correlation between serum markers or hormone levels and bone fractures (Garnero 2014; Eastell 2008). However, the results are not often predictive, because they are sometimes distorted by the full clinical picture of the analyzed subjects. Furthermore, a common uncoupled bone formation/resorption evaluation of bone turnover may be ineffective and provide altered results.
To our knowledge, there are no diagnostic methods that relate osteoporosis with in vitro spontaneous monocyte viability rate. In this study, we suggest the use of a new in vitro tool for the assessment of osteoporosis to diagnose and monitor the disease process and susceptibility and determine a course of treatment. The present method involves the spontaneous ability of PBMC to differentiate into multinucleated osteoclasts. Unlike more complex medical technologies, the in vitro method of the present study allows an easier and quicker diagnosis, because it only requires a biological sample to isolate monocyte-macrophagic cells and analyze their spontaneous differentiation into osteoclasts.
At this stage of the research, the study has some limitations, for example, the use of animal cells. However, the ovariectomized model used as a source of PBMCs is a well-recognized model for estrogen-deficiency osteoporosis (Li 2012); it is reported that bone loss in these models shares many similarities with bone loss in early postmenopausal women, including an increase in bone turnover with bone resorption in excess of formation (Davidge 2001; Sakakura 2001). A clinical study has been designed and approved by the local Ethical Committee of the Rizzoli Orthopedic Institute (Protocol MET-3D; approved May 22, 2015), and informed consent was obtained from all subjects. Enrolled osteoporosis (OP) patients had a BMD T-score value less or equal to −2.5 SD, measured by DXA. Till now, preliminary and partial data confirmed the same results already observed in rats: when using monocytes from PBMC of nine OP patients (six women and three men; submitted to surgery because of fragility fractures) and six not OP patients (three women and three men;age, sex, and BMI were matched for each group) (unpublished data). Other studies are also needed to evaluate cell behavior at different stages of OP development, to correlate exactly quantitative data on PBMC viability and bone densitometry and fracture risk.
This in vitro diagnostic method might complement existing protocols, and a comprehensive approach would also involve other approaches especially to assess anatomical fracture risks and identify underlying disease mechanisms, but could offer the possibility to screen a large number of patients through a common blood collection, eventually feasible simultaneously with routinely serum evaluations. On the basis of its outcome, patients may be referred or not to additional diagnostic investigations for the determination of altered bone metabolism, with important benefits for both health facilities and users.
Acknowledgments
This work was supported by Grants from Rizzoli Orthopedic Institute (Ricerca Corrente), “Cinque x mille 2010,” and to the Operational Programme ERDF 2007-2013 in the region Emilia-Romagna: Activity The 1.1 “Creation of technology centers for Industrial research and technological transfer.”
Conflict of interest
Melania Maglio, Gianluca Giavaresi, and Stefania Pagani declare that they have no competing interests. Francesca Salamanna, Roberto Giardino, and Milena Fini hold a patent on the described method.
References
- Boyle WJ, Simonet WS, Lacey DL (2003) Osteoclast differentiation and activation. Nature. 423(6937):337-42. doi:10.1038/nature01658 [DOI] [PubMed]
- Chen FP, Wang KC, Huang JD (2009) Effect of estrogen on the activity and growth of human osteoclasts in vitro. Taiwan J Obstet Gynecol. 48(4):350-5. doi:10.1359/jbmr.2002.17.1.77 [DOI] [PubMed]
- Clover J, Dodds RA, Gowen M (1992) Integrin subunit expression by human osteoblasts and osteoclasts in situ and in culture. J Cell Sci. 103 (Pt 1):267-71. [DOI] [PubMed]
- D’Amelio P, Grimaldi A, Pescarmona GP, Tamone C, Roato I, Isaia G (2005) Spontaneous osteoclast formation from peripheral blood mononuclear cells in postmenopausal osteoporosis. FASEB J. 19(3):410-2. doi:10.1096/fj.04-2214fje. Epub 2004 Dec. 20. [DOI] [PubMed]
- Davidge ST, Zhang Y, Stewart KG (2001) A comparison of ovariectomy models for estrogen studies. Am J Physiol Regul Integr Comp Physiol. 280(3):R904-7. [DOI] [PubMed]
- Delaissé JM, Engsig MT, Everts V, del Carmen Ovejero M, Ferreras M, Lund L, Vu TH, Werb Z, Winding B, Lochter A, Karsdal MA, Troen T, Kirkegaard T, Lenhard T, Heegaard AM, Neff L, Baron R, Foged NT (2000) Proteinases in bone resorption: obvious and less obvious roles. Clin Chim Acta. 291(2):223-34. doi:10.1359/jbmr.2002.17.1.77 [DOI] [PubMed]
- Eastell RHannon RA (2008) Biomarkers of bone health and osteoporosis risk. Proc Nutr Soc. 67(2):157-62. doi:10.1017/S002966510800699X [DOI] [PubMed]
- Everts V, Delaissé JM, Korper W, Jansen DC, Tigchelaar-Gutter W, Saftig P, Beertsen W (2002) The bone lining cell: its role in cleaning Howship’s lacunae and initiating bone formation. J Bone Miner Res. 17(1):77-90. doi:10.1359/jbmr.2002.17.1.77 [DOI] [PubMed]
- Faust J, Lacey DL, Hunt P, Burgess TL, Scully S, Van G, Eli A, Qian Y, Shalhoub V (1999) Osteoclast markers accumulate on cells developing from human peripheral blood mononuclear precursors. J Cell Biochem. 72(1):67-80. doi:10.1002/(SICI)1097-4644(19,990,101)72:1%3C67::AID-JCB8%3E3.0.CO;2-A [DOI] [PubMed]
- Fuller K, Lawrence KM, Ross JL, Grabowska UB, Shiroo M, Samuelsson B, Chambers TJ (2008) Cathepsin K inhibitors prevent matrix-derived growth factor degradation by human osteoclasts. Bone. 42(1):200-11. doi:10.1016/j.bone.2007.09.044 [DOI] [PubMed]
- Garnero P, Delmas PD (2004) Contribution of bone mineral density and bone turnover markers to the estimation of risk of osteoporotic fracture in postmenopausal women. J Musculoskelet Neuronal Interact. 4(1):50-63. [PubMed]
- Garnero P (2014) New developments in biological markers of bone metabolism in osteoporosis. Bone. 66 46-55. doi:10.1359/jbmr.2002.17.1.77 [DOI] [PubMed]
- Giavaresi G, De Terlizzi F, Gnudi S, Cadossi R, Aldini NN, Fini M, Rocca M, Ripamonti C, Brandi ML, Giardino R (2000) Discriminant capacity of quantitative ultrasound versus dual X-ray absorptiometry to determine cancellous bone loss in ovariectomized rats. Bone. 26(3):297-303. 10.1016/S8756-3282(99)00267-7. [DOI] [PubMed]
- Helfrich MH, Thesingh CW, Mieremet RH, van Iperen-van Gent AS (1987) Osteoclast generation from human fetal bone marrow in cocultures with murine fetal long bones. A model for in vitro study of human osteoclast formation and function. Cell Tissue Res. 249(1):125-36. [DOI] [PubMed]
- Hentunen TA, Jackson SH, Chung H, Reddy SV, Lorenzo J, Choi SJ, Roodman GD (1999) Characterization of immortalized osteoclast precursors developed from mice transgenic for both bcl-X(L) and simian virus 40 large T antigen. Endocrinology. 140(7):2954-61. http://dx.doi.org/10.1210/endo.140.7.6867 [DOI] [PubMed]
- Inaoka T, Bilbe G, Ishibashi O, Tezuka K, Kumegawa M, Kokubo T (1995) Molecular cloning of human cDNA for cathepsin K: novel cysteine proteinase predominantly expressed in bone. Biochem Biophys Res Commun. 206(1):89-96. doi:10.1006/bbrc.1995.1013 [DOI] [PubMed]
- Jevon M, Hirayama T, Brown MA, Wass JA, Sabokbar A, Ostelere S, Athenasou NA (2003) Osteoclast formation from circulating precursors in osteoporosis. Scand J Rheumatol. 32(2):95-100. [DOI] [PubMed]
- Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster J-Y (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 24: 23-57. doi:10.1007/s00198-012-2074 [DOI] [PMC free article] [PubMed]
- Kiviranta R1, Morko J, Uusitalo H, Aro HT, Vuorio E, Rantakokko J (2001) Accelerated turnover of metaphyseal trabecular bone in mice overexpressing cathepsin K. J Bone Miner Res. 16(8):1444-52. doi:10.1359/jbmr.2001.16.8.1444 [DOI] [PubMed]
- Le Gall C, Bellahcène A, Bonnelye E, Gasser JA, Castronovo V, Green J, Zimmermann J, Clézardin P (2007) A cathepsin K inhibitor reduces breast cancer induced osteolysis and skeletal tumor burden. Cancer Res. 67(20):9894-902. doi:10.1158/0008-5472.CAN-06-3940 [DOI] [PubMed]
- Lewiecki EM, Cummings SR, Cosman F (2013) Treat-to-target for osteoporosis: is now the time? J Clin Endocrinol Metab. 98(3):946-53. doi:10.1210/jc.2012-3680 Epub 2013 Jan. 21. [DOI] [PubMed]
- Li YF, Zhou CC, Li JH, Luo E, Zhu SS, Feng G, Hu J (2012) The effects of combined human parathyroid hormone (1-34) and zoledronic acid treatment on fracture healing in osteoporotic rats. Osteoporos Int. 23(4):1463-74. doi:10.1007/s00198-011-1751-6. Epub 2011 Sep. 3. [DOI] [PubMed]
- Link TM (2012) Osteoporosis imaging: state of the art and advanced imaging. Radiology. 263(1):3-17 doi:10.1148/radiol.12110462 [DOI] [PMC free article] [PubMed]
- Littlewood-Evans AJ, Bilbe G, Bowler WB, Farley D, Wlodarski B, Kokubo T, Inaoka T, Sloane J, Evans DB, Gallagher JA (1997) The osteoclast-associated protease cathepsin K is expressed in human breast carcinoma. Cancer Res. 57(23):5386-90. [PubMed]
- Massey HM, Flanagan AM (1999) Human osteoclasts derive from CD14-positive monocytes. Br J Haematol. 106(1):167-70. doi:10.1046/j.1365-2141.1999.01491.x [DOI] [PubMed]
- McCormick RK (2007) Osteoporosis: integrating biomarkers and other diagnostic correlates into the management of bone fragility. Altern Med Rev. 12(2):113-45. [PubMed]
- Motyckova G, Fisher DE (2002) Pycnodysostosis: role and regulation of cathepsin K in osteoclast function and human disease. Curr Mol Med. 2(5):407-21. doi:10.2174/1,566,524,023,362,401 [DOI] [PubMed]
- Pisani P, Renna MD, Conversano F, Casciaro E, Muratore M, Quarta E, Di Paola M, Casciaro S (2013) Screening and early diagnosis of osteoporosis through X-ray and ultrasound based techniques. World J Radiol. 5 (11): 398-410. doi:10.4329/wjr.v5.i11.398 [DOI] [PMC free article] [PubMed]
- Roodman GD (1999) Cell biology of the osteoclast. Exp Hematol. 27(8):1229-41. doi:10.1016/S0301-472X(99)00061-2 [DOI] [PubMed]
- Saftig P, Hunziker E, Wehmeyer O, Jones S, Boyde A, Rommerskirch W, Moritz JD, Schu P, von Figura K (1998) Impaired osteoclastic bone resorption leads to osteopetrosis in cathepsin-K-deficient mice. Proc Natl Acad Sci U S A. 95(23):13,453-8. [DOI] [PMC free article] [PubMed]
- Sakakura Y, Shide N, Tsuruga E, Irie K, Yajima T (2001) Effects of running exercise on the mandible and tibia of ovariectomized rats. J Bone Miner Metab. 19(3):159-67. [DOI] [PubMed]
- Schuit SC, van Meurs JB, Bergink AP, van der Klift M, Fang Y, Leusink G, Hofman A, van Leeuwen JP, Uitterlinden AG, Pols HA (2004) Height in pre- and postmenopausal women is influenced by estrogen receptor alpha gene polymorphisms. J Clin Endocrinol Metab. 89(1):303-9. http://dx.doi.org/10.1210/jc.2003-031095 [DOI] [PubMed]
- Shalhoub V, Elliott G, Chiu L, Manoukian R, Kelley M, Hawkins N, Davy E, Shimamoto G, Beck J, Kaufman SA, Van G, Scully S, Qi M, Grisanti M, Dunstan C, Boyle WJ, Lacey DL (2000) Characterization of osteoclast precursors in human blood. Br J Haematol. 111(2):501-12. doi:10.1111/j.1365-2141.2000.02379.x. [DOI] [PubMed]
- Shih C, Bernard GW (1996) Peripheral blood mononuclear cells develop into multinucleated osteoclasts in tissue culture. Anat Rec. 245(1):41-5. [DOI] [PubMed]
- Troen BR (2004) The role of cathepsin K in normal bone resorption. Drug News Perspect. 17(1):19-28. [DOI] [PubMed]
- U.S. Preventive Services Task Force (2011) Screening for osteoporosis: U.S. preventive services task force recommendation statement. Ann Intern Med. 154(5):356-64. doi:10.7326/0003-4819-154-5-201,103,010-00,307. Epub 2011 Jan. 17. [DOI] [PubMed]
- Wang FQ, So J, Reierstad S, Fishman DA (2005) Matrilysin (MMP-7) promotes invasion of ovarian cancer cells by activation of progelatinase. Int J Cancer. 114(1):19-31. doi:10.1002/ijc.20697 [DOI] [PubMed]
- Yasuda Y, Kaleta J, Brömme D (2005) The role of cathepsins in osteoporosis and arthritis: rationale for the design of new therapeutics. Adv Drug Deliv Rev. 57(7):973-93. doi:10.1016/j.addr.2004.12.013 [DOI] [PubMed]
- Zhao H, Cai G, Du J, Xia Z, Wang L, Zhu T (1997) Expression of matrix metalloproteinase-9 mRNA in osteoporotic bone tissues. J Tongji Med Univ. 17(1):28-31. [DOI] [PubMed]