Abstract
Testosterone replacement improves metabolic parameters and cognitive function in hypogonadism. However, the effects of testosterone therapy on cognition in obese condition with testosterone deprivation have not been investigated. We hypothesized that testosterone replacement improves cognitive function in testosterone-deprived obese rats by restoring brain insulin sensitivity, brain mitochondrial function, and hippocampal synaptic plasticity. Thirty male Wistar rats had either a bilateral orchiectomy (ORX: O, n = 24) or a sham operation (S, n = 6). ORX rats were further divided into two groups fed with either a normal diet (NDO) or a high-fat diet (HFO) for 12 weeks. Then, ORX rats in each dietary group were divided into two subgroups (n = 6/subgroup) and were given either castor oil or testosterone (2 mg/kg/day, s.c.) for 4 weeks. At the end of this protocol, cognitive function, metabolic parameters, brain insulin sensitivity, hippocampal synaptic plasticity, and brain mitochondrial function were determined. We found that testosterone replacement increased peripheral insulin sensitivity, decreased circulation and brain oxidative stress levels, and attenuated brain mitochondrial ROS production in HFO rats. However, testosterone failed to restore hippocampal synaptic plasticity and cognitive function in HFO rats. In contrast, in NDO rats, testosterone decreased circulation and brain oxidative stress levels, attenuated brain mitochondrial ROS production, and restored hippocampal synaptic plasticity as well as cognitive function. These findings suggest that testosterone replacement improved peripheral insulin sensitivity and decreased oxidative stress levels, but failed to restore hippocampal synaptic plasticity and cognitive function in testosterone-deprived obese rats. However, it provided beneficial effects in reversing cognitive impairment in testosterone-deprived non-obese rats.
Keywords: Testosterone replacement, Obese insulin resistance, Testosterone deprivation, Brain mitochondrial function, Hippocampal synaptic plasticity, Cognitive function
Introduction
Testosterone plays a major role in reproductive function by promoting secondary sexual characteristics and emotions (Janowsky 2006). Previous studies demonstrated that testosterone plays an important role in metabolic activities, including the regulation of glucose and lipid metabolism (Aydilek and Aksakal 2005; Christoffersen et al. 2010). Growing evidence has demonstrated that testosterone deficiency is associated with an increased incidence of obesity (Shi et al. 2013; Stellato et al. 2000). In addition, a positive correlation between obese-insulin resistance and testosterone deficiency has been reported (Grossmann et al. 2008; Haffner et al. 1994), but contradictory findings have been demonstrated in some animal models (Borst and Conover 2006; Erben et al. 2000; Kakolewski et al. 1968). In addition, our previous studies demonstrated that 15 weeks of a high-fat diet (HFD) consumption in male rats induced not only obese-related insulin resistance, but also impaired brain insulin sensitivity and brain mitochondrial dysfunction, leading to cognitive decline (Pintana et al. 2013; Pintana et al. 2012; Pipatpiboon et al. 2013). However, the effects of testosterone deprivation under obese insulin-resistant conditions on cognitive function have not been thoroughly investigated.
Although testosterone replacement has been prescribed to patients with hypogonadism to improve the quality of life and overall health, including the improvement of cognitive function in men (Donatucci et al. 2014) and animal models (Bassil and Morley 2010 ; Cunningham and Toma 2010; Margo and Winn 2006), several studies found that testosterone replacement in the hypogonadal condition has either no effect or adverse effects on cognition in animal models (Emamian et al. 2010; Harooni et al. 2008; Khorshidahmad et al. 2012; Naghdi and Asadollahi 2004) and humans (Kenny et al. 2004; Lu et al. 2006; Maki et al. 2007; Vaughan et al. 2007; Wolf et al. 2000). In addition, previous studies reported that a high dosage of testosterone could lead to increased cell apoptosis (Cunningham et al. 2009), neuronal toxicity (Buletko et al. 2012), and impaired learning and decreased memory capability in animal models (Emamian et al. 2010; Harooni et al. 2008; Naghdi et al. 2005; Spritzer et al. 2011).
At present, the effects of testosterone replacement on brain insulin receptor function, brain mitochondrial function, and cognitive function in either testosterone deprivation with or without obesity have not been investigated. The hypotheses of the present study are as follows:
Testosterone-deprived rats with or without obesity have impaired brain insulin receptor function, decreased brain insulin signaling, impaired brain mitochondrial function, and impaired hippocampal synaptic plasticity, thus leading to cognitive decline
Testosterone replacement restores these impairments in conditions of testosterone deprivation with or without obesity.
Materials and methods
Animal models and experimental protocols
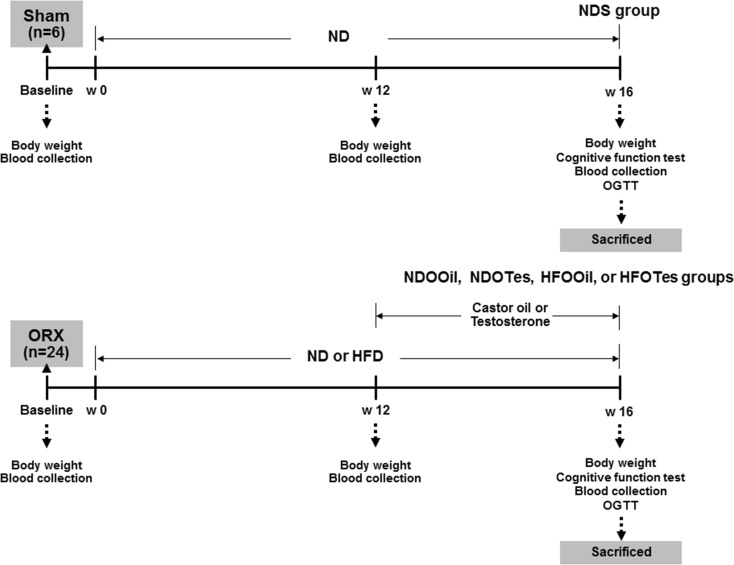
All experiments were conducted in accordance with the approved protocol of the Faculty of Medicine, Chiang Mai University Institutional Animal Care and Use Committee, in compliance with NIH guidelines. Thirty male Wistar rats, weighing 180–200 g (aged ~5–6 weeks old) obtained from the National Animal Center, Salaya Campus, Mahidol University, Bangkok, Thailand, were used. All animals were housed in environmentally controlled conditions (25 ± 0.5 °C and a 12-h light/dark cycle) and allowed to acclimate for 1 week. Rats were then divided into two groups, either control sham-operated rats (S) (n = 6) or bilateral orchiectomized (O) rats (n = 24), as described previously (Pongkan et al. 2015). Rats in the sham-operated group were fed with a normal diet (ND) for 16 weeks, and this group was the control group (NDS). Rats in the orchiectomized group were further divided into two dietary groups, where each group was fed on either a normal diet (ND 19.77 % E fat, NDO) or a high-fat diet (HFD 59.28 % E fat, HFO) for 12 weeks (Pratchayasakul et al. 2011). At the end of week 12, the rats in each dietary group were subdivided into two subgroups (n = 6/subgroup). Each subgroup were given castor oil (NDOOil and HFOOil) or testosterone replacement 2 mg/kg/days (NDOTes and HFOTes), via subcutaneous injection for 4 weeks (Pongkan et al. 2015). The cognitive function was subsequently determined by the Morris water maze (MWM) test at the end of week 16 of the experimental protocol. Blood samples were collected from a tail vein at week 12, and week 16 of the experimental protocol for further plasma analysis. At the end of the experimental protocol, rats were deeply anesthetized with isoflurane and killed by decapitation. The brain of each rat was quickly removed and carefully sliced in preparation for investigation, including extracellular recording (insulin-induced long-term depression (LTD) and hippocampal synaptic long-term potentiation (LTP)), immunoblot analysis, brain mitochondrial function and brain malondialdehyde (MDA) measurement. The experimental protocol is summarized in Fig. 1.
Fig. 1.
The experimental protocol of the study. ND normal diet, HFD high-fat diet, ORX orchiectomy, NDS sham-operated rats with normal diet feeding, NDOOil orchiectomized-operated rats with normal diet feeding and treated with castor oil, NDOTes orchiectomized-operated rats with normal diet feeding and treated with testosterone, HFOOil orchiectomized-operated rats with high-fat diet feeding and treated with castor oil, HFOTes orchiectomized-operated rats fed with a high-fat diet and treated with testosterone, w week, OGTT oral glucose tolerance test
Blood sample assays
Plasma glucose and cholesterol levels were determined by colorimetric assay (Biotech, Bangkok, Thailand). Plasma HDL and LDL levels were determined using a commercial colorimetric assay kit (Biovision, CA, USA). Plasma insulin levels were determined using the sandwich enzyme-linked immunosorbent assay (ELISA) kit (Millipore, MI, USA). Peripheral insulin resistance was assessed using the homeostasis model assessment (HOMA) as described in previous studies (Pipatpiboon et al. 2013; Pratchayasakul et al. 2011). An oral glucose tolerance test (OGTT) was performed as described previously (Pipatpiboon et al. 2013). Areas under the curves (AUCs) were calculated to evaluate glucose tolerance. Plasma testosterone levels were determined as described previously (Pongkan et al. 2015).
Serum and brain MDA levels
The high-performance liquid chromatography (HPLC) method was used to evaluate concentrations of serum and brain MDA as described previously (Candan and Tuzmen 2008; Mateos et al. 2005; Pintana et al. 2013; Pintana et al. 2014).
Brain slice preparation
At the end of the experimental protocol, animals were anesthetized with isoflurane and decapitated. The hippocampal slices were prepared as described previously for extracellular recording to determine insulin-induced LTD and hippocampal synaptic LTP (Pipatpiboon et al. 2013; Pipatpiboon et al. 2012; Pratchayasakul et al. 2011).
Extracellular recordings from the hippocampal slices for insulin-induced LTD
An extracellular recording of hippocampal slices for insulin-induced LTD was performed as described in the previous studies (Pipatpiboon et al. 2013; Pipatpiboon et al. 2012; Pratchayasakul et al. 2011). Field excitatory postsynaptic potentials (fEPSPs) were evoked by stimulating the Schaffer collateral-commissural pathway with a bipolar tungsten electrode, while recordings were taken from the stratum radiatum of the hippocampal CA1 region with micropipettes (3 MΩ) filled with 2 M NaCl. Stimulus frequency was 0.033 Hz. The stimulus intensity of fEPSPs was adjusted to 0.8–1.0 mV in amplitude. Hippocampal slices were perfused with aCSF (as a baseline condition) for 10 min, and then perfused with aCSF plus 500 nM insulin (as insulin-induced LTD) for an additional 10 min. After this, the slices were perfused with aCSF for an additional 50 min and readings recorded. Data was filtered at 3 kHz, digitized at 10 kHz, and stored in a computer using pClamp 9.2 software (Axon Instruments, CA, USA). The initial slope of the fEPSP was measured and plotted against time.
Extracellular recordings from hippocampal slices for synaptic LTP
The examination of electrical-induced hippocampal synaptic LTP was performed as described in the previous study (Sripetchwandee et al. 2014). fEPSPs were evoked by stimulating the Schaffer collateral-commissural pathway with a bipolar tungsten electrode, while the fEPSP recordings were taken from the stratum radiatum of the hippocampal CA1 region with micropipettes (3 MΩ) filled with 2 M NaCl. LTP was induced by delivering high-frequency stimulation (HFS; four trains at 100 Hz; 0.5-s duration; 20-s interval). Experiments were performed for at least 40 min after HFS. The amount of potentiation was calculated at 40 min after tetanus. All data were filtered at 3 kHz, digitized at 10 kHz, and stored in a computer using pClamp9.2 software (Axon Instruments, CA, USA). The initial slopes of the fEPSPs were measured and plotted against time.
Immunoblotting for brain insulin signaling
To investigate the expression of insulin receptor phosphorylation (p-IR) and insulin receptors (IRs), homogenate brain slices from each subgroup were used as described in the previous study (Pipatpiboon et al. 2013; Pipatpiboon et al. 2012; Pratchayasakul et al. 2011). Electrophoresis and immunoblotting were carried out on IR tyrosine phosphorylation using rabbit antibodies for IR at tyrosine. The level of IR protein expression was assessed with homogenates prepared from another set of four whole brain slices. These proteins were resolved by an immunoblot assay conducted with rabbit anti-IR at tyrosine phosphorylation. These proteins were resolved by an immunoblot assay conducted with rabbit anti-IR at tyrosine phosphorylation (p-IRtyr1162/1163) (1:1000; sc-25103-R; Santa Cruz Biotechnology, CA, USA), IR (1:1000; sc-711; Santa Cruz Biotechnology, CA, USA). For a loading control, an immunoblot for each membrane was incubated with anti-β-actin (1:4000; #4967; Cell Signaling Technology, MA, USA). All membranes for visualizing the phosphorylation and the protein levels of IR expression were incubated with secondary goat anti-rabbit antibody conjugated with horseradish peroxidase (1:2000; #7074; Cell Signaling Technology, MA, USA). The protein bands were visualized on Amersham hyperfilm ECL (GE Healthcare, Buckinghamshire, UK) using Amersham ECL Western blot detection reagents (GE, Healthcare). Band densities of phosphorylated IR were represented as a ratio of insulin stimulation (+) to no insulin stimulation (−) and were normalized to total IR. In addition, band intensities were quantified by Scion Imaging and the results were shown as average signal intensity (arbitrary) units.
Brain mitochondrial function study
Brain mitochondria were isolated using the method described previously (Pipatpiboon et al. 2013). Mitochondrial protein concentration was measured using BCA assay (Pipatpiboon et al. 2013), and brain mitochondrial function including brain mitochondrial reactive oxygen species (ROS), mitochondrial membrane potential change (ΔΨm), and mitochondrial swelling were determined. Brain mitochondrial reactive oxygen species (ROS) were measured using dichloro-hydrofluoresceindiacetate (DCFHDA) fluorescent dye. The change in ΔΨm was measured using the fluorescent dye 5,5′,6, 6′-tetrachloro-1,1′,3,3′-tetraethyl benzimidazolcarbocyanine iodide (JC-1), and brain mitochondrial swelling was determined by measuring the change in the absorbance of brain mitochondrial suspension at 540 nm. All were determined by following the methods described previously (Pintana et al. 2013; Pipatpiboon et al. 2013).
Cognitive function test
The open-field test (OFT) was used to screen locomotor activity by counting the number of lines that the rats crossed during the test, as described in previous studies (Arakawa 2005; Pintana et al. 2013). The assessment of cognitive function was performed by using the MWM with two assessments including the acquisition test which was carried out for five consecutive days, and the probe test which was performed on day 6 (Pintana et al. 2013; Vorhees and Williams 2006). Data analysis of the MWM test was done manually from video tape recordings by the investigators, who were blinded to all experimental groups.
Statistical analysis
Data were expressed as mean ± SEM. For all comparisons, the significance of the differences in peripheral biochemical parameters was calculated using the Mann–Whitney U test. The comparisons in the percentage of insulin-induced LTD, the percentage of LTP, brain mitochondrial function, immunoblot, the OFT tests and the MWM tests for the probe test between groups were performed using the one-way ANOVA test, followed by post hoc LSD analysis. Comparisons among groups in MWM tests for the acquisition test were performed using a two-way ANOVA, followed by post hoc LSD analysis. P < 0.05 was considered statistically significant.
Results
Obesity, but not testosterone deprivation, caused peripheral insulin resistance
Testosterone deprivation by orchiectomy was confirmed by reduced plasma testosterone levels in NDO rats treated with castor oil (NDOOil) and HFO rats treated with castor oil (HFOOil) (Table 1). Testosterone replacement increased plasma testosterone levels in both NDO rats treated with testosterone (NDOTes) and HFO rats treated with testosterone (HFOTes), when compared to that of NDOOil and HFOOil rats (Table 1). NDOOil rats had significantly decreased body weight and less visceral fat, without any significant alteration in plasma insulin, HOMA index, plasma glucose area under the curve of oral glucose tolerance test (AUCg), total cholesterol, plasma LDL cholesterol levels as well as plasma HDL cholesterol levels, when compared with those of NDS rats. Furthermore, the body weight and visceral fat in NDOTes rats were significantly decreased, when compared with those in NDS rats and NDOOil rats. The plasma insulin, HOMA index, plasma glucose area under the curve of oral glucose tolerance test (AUCg), total cholesterol, plasma LDL cholesterol levels as well as plasma HDL cholesterol levels in NDOTes rats were not significantly different, when compared with those in NDS rats and NDOOil rats. These findings indicated that peripheral insulin resistance was not observed in the NDOOil and NDOTes rats.
Table 1.
Metabolic parameters after 4 weeks of testosterone treatment
| Metabolic parameters | Treatment group | ||||
|---|---|---|---|---|---|
| NDS | NDOOil | NDOTes | HFOOil | HFOTes | |
| Body weight (g) | 506.8 ± 10.4 | 423.7 ± 13.8a,b | 441.8 ± 13.8a,b | 508.3 ± 18.6 | 515.7 ± 9.7 |
| Plasma testosterone levels (ng/ml) | 0.79 ± 0.05 | <0.025 | 4.19 ± 0.79b,c | <0.025 | 4.32 ± 0.27b,c |
| Visceral fat (g) | 28.3 ± 1.0 | 15.6 ± 1.1*,b | 16.1 ± 0.7a,b | 28.5 ± 1.7 | 29.5 ± 1.9 |
| Plasma glucose (mg/dl) | 134.9 ± 11.5 | 135.7 ± 8.6 | 130.3 ± 9.5 | 131.7 ± 10.7 | 131.9 ± 8.2 |
| Plasma insulin (ng/ml) | 2.73 ± 0.31 | 3.30 ± 0.52 | 2.88 ± 0.33 | 5.45 ± 1.01a | 4.58 ± 0.29a |
| HOMA index | 22.7 ± 2.9 | 26.1 ± 4.9 | 24.8 ± 3.7 | 45.9 ± 10.0a | 41.1 ± 5a |
| Plasma glucose AUC (AUCg) (mg/dlxminx104) | 4.6 ± 0.18 | 4.8 ± 0.5 | 4.7 ± 0.1 | 6.0 ± 0.4a | 5.0 ± 0.1b |
| Plasma total cholesterol (mg/dl) | 75.9 ± 6.4 | 76.4 ± 6.2 | 73.6 ± 3.9 | 115.3 ± 7.6a | 95.2 ± 5.0a,b |
| Plasma triglyceride (mg/dl) | 53.2 ± 2.2 | 51.8 ± 5.8 | 57.8 ± 5.5 | 50.9 ± 7.9 | 55.0 ± 7.1 |
| HDL cholesterol (mg/dl) | 7.0 ± 0.4 | 7.3 ± 0.5 | 7.1 ± 0.5 | 4.8 ± 0.7a | 6.0 ± 0.2 |
| LDL cholesterol (mg/dl) | 56.2 ± 7.4 | 56.6 ± 6.6 | 56.2 ± 6.1 | 105.2 ± 6.9a | 82.2 ± 5.6a,b |
aCompared to NDS
bCompared to HFOOil
cCompared to NDOOil
HFOOil rats demonstrated the characteristics of peripheral insulin resistance, including increased plasma insulin, HOMA index, plasma AUCg, total cholesterol, plasma LDL cholesterol levels as well as decreased plasma HDL cholesterol levels, when compared with those of NDS rats. Interestingly, HFOTes rats had significantly decreased plasma AUCg, plasma total cholesterol and plasma LDL cholesterol levels, when compared with those of HFOOil rats (Table 1). However, plasma glucose and plasma triglyceride levels were not significantly different between all groups. All of these findings suggest that testosterone replacement attenuated peripheral insulin resistance in testosterone-deprived obese rats via improved peripheral insulin sensitivity as indicated by improved OGTT and reduced lipid profiles.
Obesity, but not testosterone deprivation, caused the impairment of brain insulin receptor function
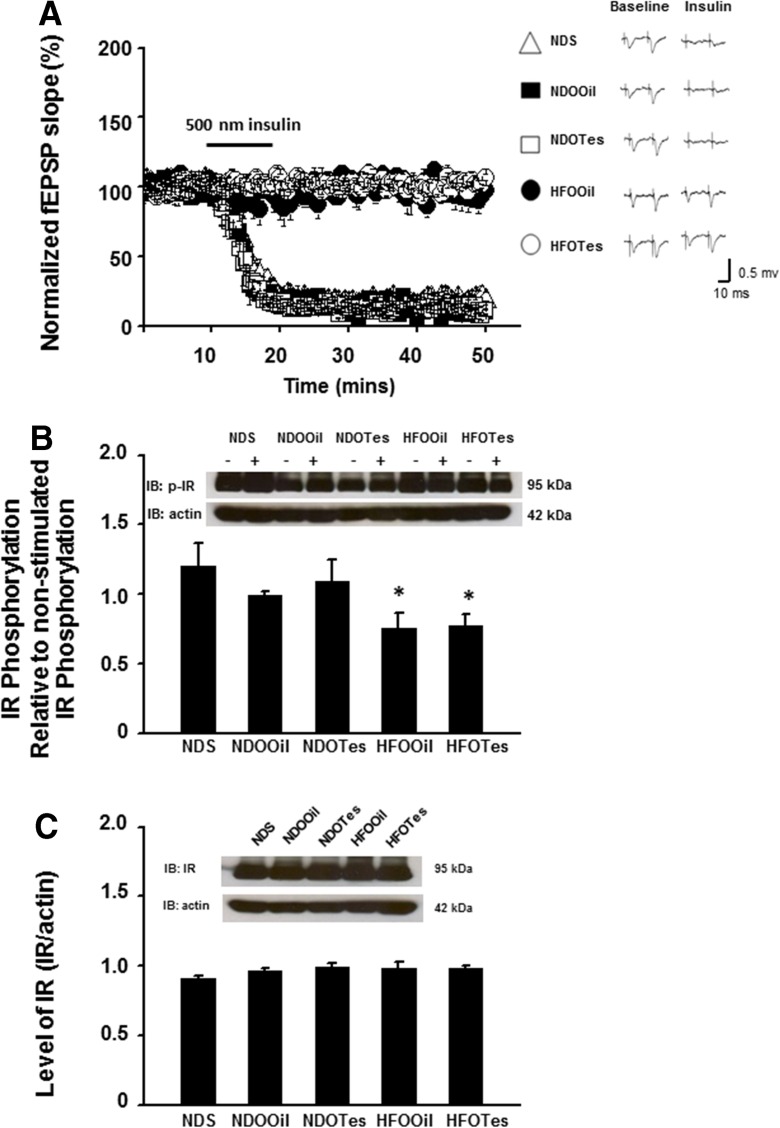
Brain insulin receptor function was tested by measuring insulin-induced LTD and brain insulin signaling. For the insulin-induced LTD study, the degrees of insulin-mediated LTD in ND-fed rats (NDS, NDOOil, and NDOTes) were not significantly different between groups (n = 2–3 independent slices/animal, n = 6 animals/group Fig. 2a). However, the degree of insulin-induced LTD was significantly reduced in HFOOil rats, when compared with that of NDS, NDOOil, and NDOTes rats (n = 2–3 independent slices/animal, n = 6 animals/group, Fig. 2a). Testosterone replacement did not reverse that impairment in HFOTes rats (n = 2–3 independent slices/animal, n = 6 animals/rats, Fig. 2a).
Fig. 2.
The effects of testosterone replacement on brain insulin receptor function, including insulin-induced LTD, the phosphorylation of insulin receptor (p-IR), and the expression of insulin receptors (IR) in testosterone-deprived rats with or without obesity. Insulin-induced LTD in the CA1 hippocampus and the phosphorylation of IR are significantly decreased in rats in the HFOOil group, when compared with that of the NDS group (a, b). Testosterone replacement could not improve the ability of insulin-induced LTD and the phosphorylation of IR in rats in the HFOTes group, when compared with that of HFOOil rats (a, b). No differences in the expression of IR between all groups were found (c). NDS sham-operated rats with normal diet feeding, NDOOil orchiectomized-operated rats with normal diet feeding and treated with castor oil, NDOTes orchiectomized-operated rats with normal diet feeding and treated with testosterone, HFOOil orchiectomized-operated rats with high-fat diet feeding and treated with castor oil, HFOTes orchiectomized-operated rats fed with a high-fat diet and treated with testosterone; *p < 0.05 vs NDS band densities of phosphorylated IR were represented as a ratio of insulin stimulation (+) and no insulin stimulation (−) and were normalized to total IR
As regards the brain insulin signaling study, the phosphorylation of insulin receptors (p-IR) showed no significant difference between rats in the groups of ND-fed rats (NDS, NDOOil, and NDOTes) (Fig. 2b). However, levels of p-IR of HFOOil rats were significantly decreased, when compared with those of ND-fed rats (Fig. 2b). In addition, the expression of insulin receptors (IR) in all groups showed no significant difference between all groups (Fig. 2c). Testosterone replacement did not reverse these impaired effects in HFOTes rats (Fig. 2b).
All of these findings suggest that the obese insulin-resistant condition, but not testosterone deprivation, caused the impairment of brain insulin receptor function and brain insulin signaling. Moreover, testosterone replacement failed to restore the impairment of brain insulin receptor function as well as brain insulin signaling in the testosterone-deprived obese condition.
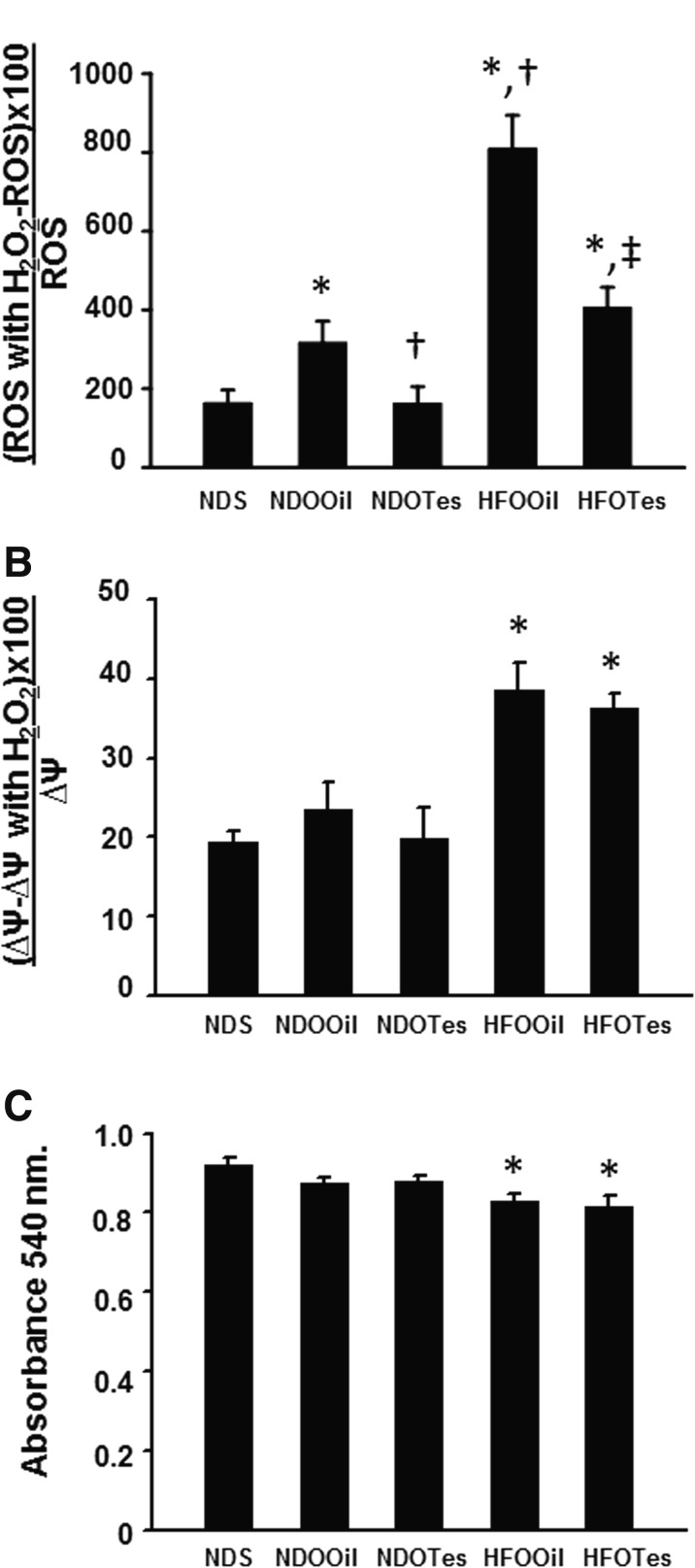
Testosterone deprivation with obesity caused brain mitochondrial dysfunction and testosterone replacement reduced brain mitochondrial ROS production
Brain mitochondrial function was determined by assessing changes in brain mitochondrial ROS production and brain ∆Ψm after H2O2 stimulation, and brain mitochondrial swelling. The results show that brain mitochondrial ROS production was significantly increased in NDOOil rats, when compared with NDS rats, whereas NDOTes rats had significantly decreased brain mitochondrial ROS production, when compared with NDOOil rats (Fig. 3a). However, brain mitochondrial membrane potential change and brain mitochondrial swelling were not significantly different between groups of ND-fed rats (NDS, NDOOil, and NDOTes) (Fig. 3b, c). Furthermore, HFOOil and HFOTes rats had significantly increased brain mitochondrial ROS production, brain mitochondrial depolarization, and brain mitochondrial swelling, when compared with those of NDS rats (Fig. 3a–c). Interestingly, the brain mitochondrial ROS production in the HFOOil group was significantly higher than that in NDOOil rats. This result suggests that the combination of high-fat diet and orchiectomy aggravates the severity of brain mitochondrial ROS production. In addition, testosterone replacement significantly decreased brain ROS production in HFOTes rats, when compared with that of HFOOil rats (Fig. 3a). However, testosterone replacement did not attenuate brain mitochondrial depolarization and brain mitochondrial swelling in HFOTes rats (Fig. 3b, c).
Fig. 3.
The effects of testosterone replacement on brain mitochondrial function in testosterone-deprived rats with or without obesity. Rats in the NDOOil group show significantly increased brain mitochondrial ROS production following H2O2 application (a). Rats in the HFOOil group demonstrated brain mitochondrial dysfunction, as indicated by increased brain mitochondrial ROS production following H2O2 application (a), increased brain mitochondrial membrane potential change following H2O2 application (b), and decreased absorbance values, indicating brain mitochondrial swelling (c). Testosterone replacement significantly decreased brain mitochondrial ROS production in NDOTes and HFOTes rat groups (a). However, testosterone did not improve brain mitochondrial membrane potential change and brain mitochondrial swelling in rats in the HFOTes group (b, c). NDS sham-operated rats with normal diet feeding, NDOOil orchiectomized-operated rats with normal diet feeding and treated with castor oil, NDOTes orchiectomized-operated rats with normal diet feeding and treated with testosterone, HFOOil orchiectomized-operated rats with high-fat diet feeding and treated with castor oil, HFOTes orchiectomized-operated rats fed with a high-fat diet and treated with testosterone; *p < 0.05 vs NDS, †p < 0.05 vs NDOOil, and ‡p < 0.05 vs HFOOil
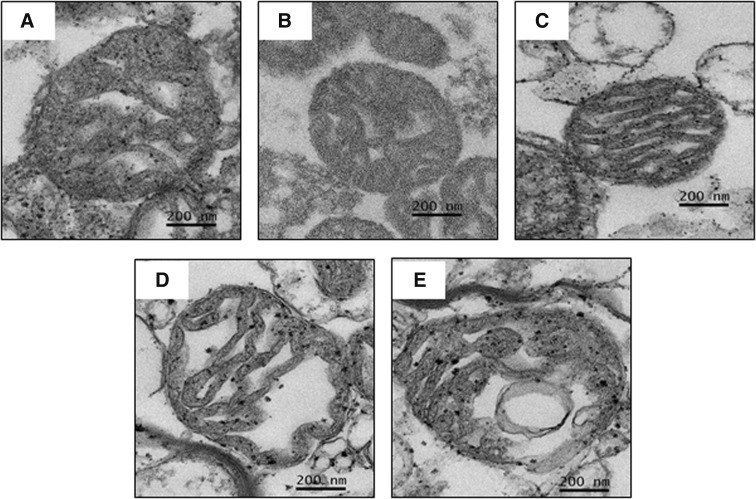
The morphological changes of brain mitochondria from all groups are shown in Fig. 4. Intact brain mitochondrial morphology with apparent folded cristae was observed in all ND-fed rats (NDS, NDOOil, and NDOTes) (Fig. 4a–c). Brain mitochondrial swelling was observed in both the HFOOil and HFOTes groups as indicated by markedly unfolded cristae (Fig. 4d, e). All of these findings suggest that testosterone deprivation, with or without obesity, caused an increase in brain mitochondrial ROS production. Obesity alone, but not testosterone deprivation, caused brain mitochondrial dysfunction via increased brain ROS production, brain mitochondrial depolarization and brain mitochondrial swelling. In addition, testosterone replacement reduced brain ROS production in the testosterone-deprived rats with or without obesity, but it did not restore other mitochondrial functions.
Fig. 4.
Representative images of brain mitochondrial morphology by transmission electron microscopy (JEM-2200FS field emission electron microscope, original magnification ×20,000) after testosterone treatment. Normal folding of cristae in brain mitochondrial morphology was shown among all ND-fed rats (a–c). However, brain mitochondrial swelling, as indicated by unfolded cristae, of both HFOOil and HFOTes groups was observed (d, e). NDS sham-operated rats with normal diet feeding, NDOOil orchiectomized-operated rats with normal diet feeding and treated with castor oil, NDOTes orchiectomized-operated rats with normal diet feeding and treated with testosterone, HFOOil orchiectomized-operated rats with high-fat diet feeding and treated with castor oil, HFOTes orchiectomized-operated rats fed with a high-fat diet and treated with testosterone
Testosterone deprivation with or without obesity caused an increase in brain lipid peroxidation, and testosterone replacement attenuated those impairments
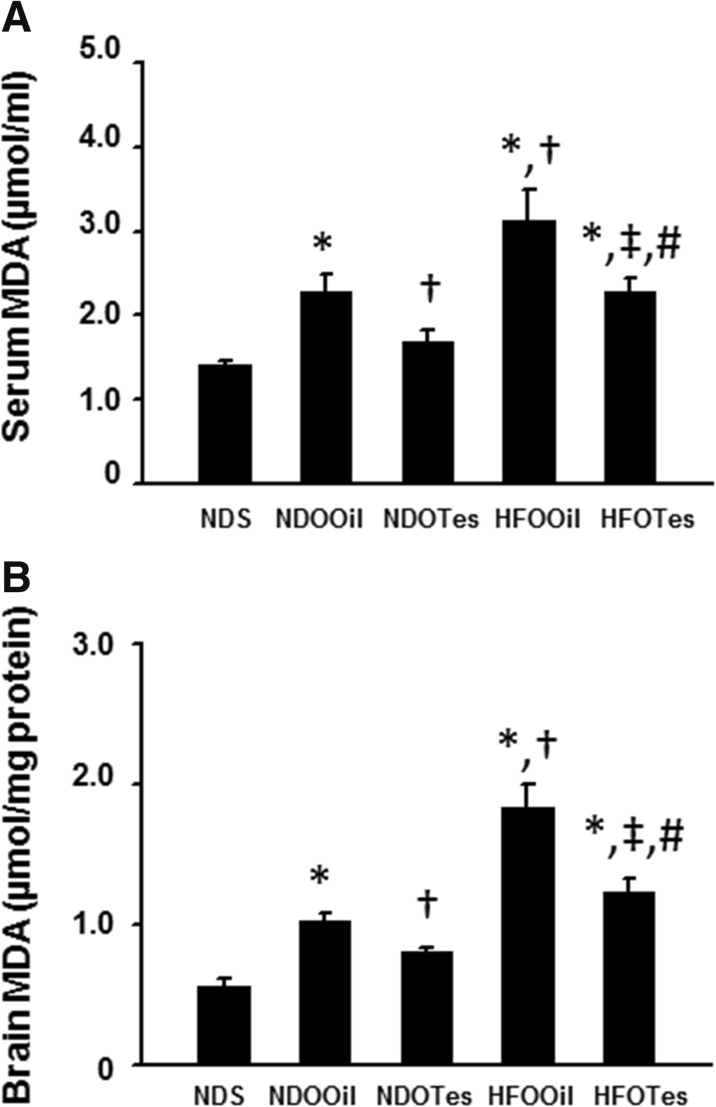
Circulation and brain oxidative stress were determined by assessing serum MDA and brain MDA levels. The results demonstrated that both circulating MDA and brain MDA levels significantly increased in NDOOil, HFOOil, and HFOTes rats compared to NDS rats (Fig. 5a, b). Furthermore, rats in the HFOOil group showed both significantly increased circulating and brain MDA levels compared to NDOOil rats (Fig. 5a, b). Testosterone replacement significantly decreased both circulating MDA and brain MDA levels in NDOTes and HFOTes, but the levels of plasma and brain MDA in NDOTes rats were significantly lower than those in HFOTes rats.
Fig. 5.
The effects of testosterone replacement on serum and brain malondialdehyde (MDA) levels in testosterone deprived rats with or without obesity. Both NDOOil and HFOOil treatments significantly increased serum and brain MDA levels (a, b). Testosterone replacement significantly decreased both serum and brain MDA levels in rats in the NDOTes and HFOTes groups (a, b). NDS sham-operated rats with normal diet feeding, NDOOil orchiectomized-operated rats with normal diet feeding and treated with castor oil, NDOTes orchiectomized-operated rats with normal diet feeding and treated with testosterone, HFOOil orchiectomized-operated rats with high-fat diet feeding and treated with castor oil, HFOTes orchiectomized-operated rats fed with a high-fat diet and treated with testosterone; *p < 0.05 vs NDS, †p < 0.05 vs NDOOil, ‡p < 0.05 vs HFOOil, and #p < 0.05 vs NDOTes
These findings suggest that testosterone deprivation, with or without obesity, increased both circulating and brain lipid peroxidation levels, and testosterone replacement attenuated those impairments.
Testosterone deprivation causes cognitive impairment, and testosterone replacement restores this impairment in non-obese condition
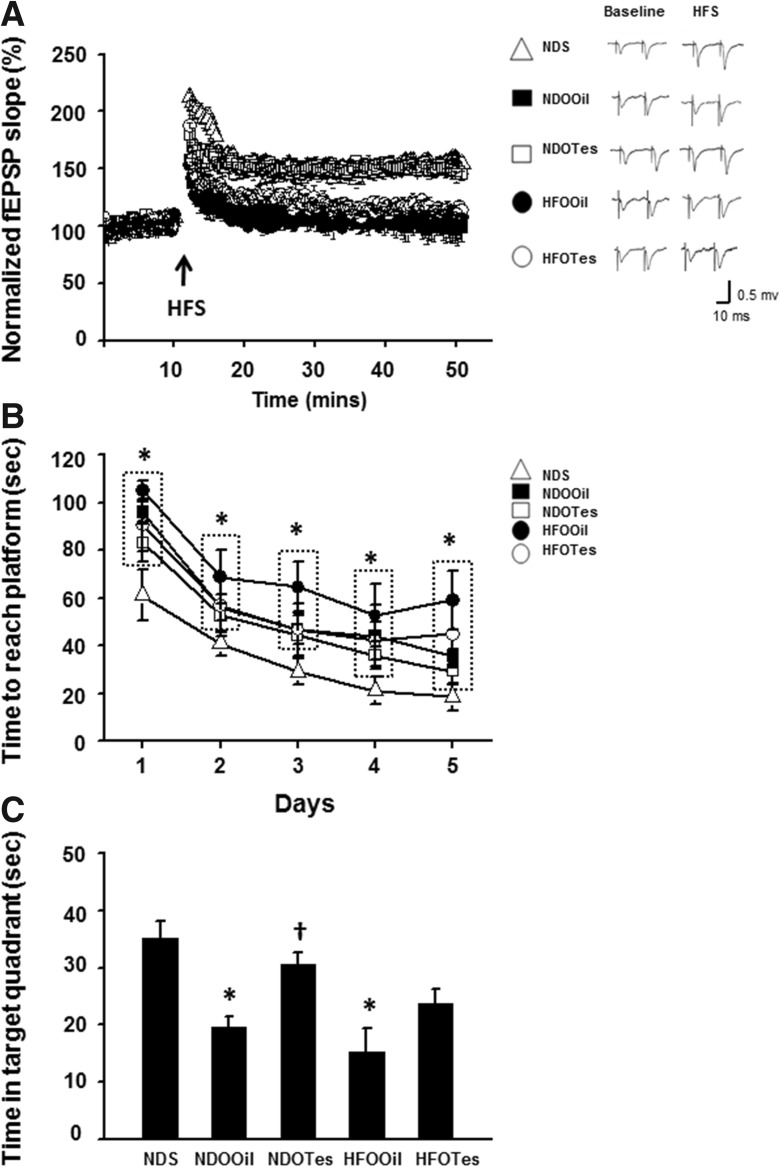
To study hippocampal synaptic plasticity, electrical-induced LTP was determined. The results demonstrated that the degree of electrical-induced LTP was significantly decreased in both NDOOil and HFOOil rats, when compared with that of NDS rats. The reductions of electrical-induced LTP in both NDOOil and HFOOil rats (n = 2–3 independent slices/animal, n = 6 animals/group Fig. 6a) were not significantly different. In addition, testosterone replacement improved the degree of electrical-induced LTP in only NDOTes rats. These findings indicate that testosterone deprivation with or without obesity caused the impairment of hippocampal synaptic plasticity. However, testosterone replacement restored that impairment in testosterone deprivation without obesity.
Fig. 6.
The effects of testosterone replacement on hippocampal synaptic long-term potentiation (LTP) and cognitive function determined by the Morris water maze (MWM) test in testosterone-deprived rats with or without obesity. Both orchiectomized-operated groups (NDOOil and HFOOil rat groups) showed significantly a reduced percentage of normalized fEPSPs, when compared with that of NDS rats (a). Testosterone replacement improves the percentage of normalized fEPSPs in only rats in the NDOTes group, when compared with that of rats in the HFOOil group (a). The rats in the HFOOil group showed a significantly increased time to reach the platform in the acquisition test as well as having a decreased time spent in target quadrant in the probe test, when compared with that of the NDS rat group (b, c). Rats in the NDOOil group showed a significantly decreased time spent in the target quadrant in the probe test, but there was no change in the time taken to reach the platform in the acquisition test (b, c). Testosterone replacement restored the time spent in the target quadrant in NDOTes rats, but not in HFOTes rats (c). NDS sham-operated rats with normal diet feeding, NDOOil orchiectomized-operated rats with normal diet feeding and treated with castor oil, NDOTes orchiectomized-operated rats with normal diet feeding and treated with testosterone, HFOOil orchiectomized-operated rats fed on a high-fat diet and treated with castor oil, HFOTes orchiectomized-operated rats fed on a high-fat diet and treated with testosterone, HFS high-frequency stimulation. *p < 0.05 vs NDS and †p < 0.05 vs NDOOil
The OFT was used to screen locomotor activity. The results showed that there was no significant difference between all groups, indicating that the locomotor activity did not differ between the groups (data not shown). In addition, this study determined cognitive function by using the MWM test. For the acquisition test, the time to reach the platform of NDOOil and HFOOil rats was significantly longer than that of NDS rats (Fig. 6b). In addition, testosterone administration could not improve the time to reach the platform in these rats (Fig. 6b).
For the probe test, the time spent in the target quadrant of NDOOil and HFOOil rats was significantly decreased, when compared with that of NDS rats (Fig. 6c). However, no difference was found for this parameter between NDOOil and HFOOil rats (Fig. 6c). Testosterone replacement significantly increased the time spent in the target quadrant of NDOTes rats but not that of HFOTes rats (Fig. 6c).
These findings suggest that testosterone deprivation alone could cause cognitive decline, and testosterone replacement could help restore this impairment. However, testosterone failed to improve cognitive decline when testosterone deprivation was under obese condition. Table 2 summarizes all findings reported in the present study.
Table 2.
Summary of findings in rats with and without testosterone treatment
| Parameters | Treatment groups | ||||
|---|---|---|---|---|---|
| NDS | NDOOil | NDOTes | HFOOil | HFOTes | |
| Peripheral insulin sensitivity | Control | – | – | X | ✔ |
| Brain insulin sensitivity | Control | – | – | X | X |
| Brain mitochondrial function | Control | – | – | X | X |
| Circulation and brain oxidative stress | Control | X | ✔ | XX | ✔ |
| Hippocampal synaptic plasticity | Control | X | ✔ | X | X |
| Cognitive function | Control | X | ✔ | X | X |
– no change compared with NDS group, X impairment compared with NDS and NDO groups, ✔ improvement when compared with the impaired group of the same diet type, XX greater impairment compared with NDO group
Discussion
The major findings of the present study are as follows: (1) testosterone deprivation alone causes the impairment of hippocampal synaptic plasticity and cognitive decline, without causing the impairment of peripheral insulin sensitivity and brain insulin sensitivity, and brain mitochondrial function; (2) testosterone replacement effectively restores the hippocampal synaptic plasticity and cognitive function in these testosterone-deprived rats; (3) testosterone deprivation with obesity causes both peripheral and brain insulin sensitivity, brain mitochondrial dysfunction, and impaired hippocampal synaptic plasticity, resulting in cognitive decline; (4) testosterone replacement improves peripheral insulin sensitivity in these testosterone-deprived obese rats, but fails to restore brain insulin receptor function and cognitive function in testosterone-deprived obese rats; (5) regarding lipid peroxidation, testosterone deprivation increases circulating and brain lipid peroxidation, and obesity aggravates this condition in testosterone-deprived rats; and (6) testosterone replacement causes a decrease in both circulation and brain lipid peroxidation in both testosterone-deprived rats and testosterone-deprived obese rats, but at a greater degree in testosterone deprivation alone.
There is growing evidence that testosterone deprivation is associated with cognitive decline in both men and animal models (Hogervorst et al. 2004; Hogervorst et al. 2001; Sakata et al. 2000; Spritzer et al. 2011). A previous study demonstrated that testosterone deprivation in orchiectomized male rats caused increased oxidative damage and a reduction of antioxidants in the hippocampus (Meydan et al. 2010). In addition, several previous studies showed that testosterone plays an important role in cognitive function (Naghdi et al. 2005; Sakata et al. 2000; Sandstrom et al. 2006; Spritzer et al. 2011). The present study confirms previous findings that rats with testosterone deprivation alone developed cognitive decline, impaired hippocampal synaptic plasticity and showed an increase in brain lipid peroxidation. However, additional findings in this study further demonstrated that there were no alterations in brain insulin sensitivity and brain mitochondrial function in these testosterone-deprived rats. Since brain lipid peroxidation and brain mitochondrial function have been shown to play an important role in brain insulin sensitivity (Muriach et al. 2014; Pipatpiboon et al. 2013), the possible explanation of no alteration of brain insulin sensitivity and brain mitochondrial function in these rats could be due to the fact that the level of brain mitochondrial ROS production and brain lipid peroxidation in these testosterone-deprived rats was not sufficiently high to reach the critical threshold in order to cause brain mitochondrial dysfunction, thus no impaired brain insulin sensitivity was observed in these rats (Aon et al. 2006; Cortassa et al. 2004). Previous studies demonstrated that testosterone deficiency is associated with the impairment of insulin sensitivity (Christoffersen et al. 2010; Georgiev et al. 2011). Orchiectomized rats in that study had significant weight gain. However, in our study and others (Axell et al. 2006; Borst and Conover 2006; Chai et al. 1999; Erben et al. 2000; Gao et al. 2005; Gentry and Wade 1976; Kakolewski et al. 1968), no impairment of insulin sensitivity was found. This could be due partly to the fact that no weight gain was observed in those orchiectomized rats.
Previous studies demonstrated that HFD-induced obesity caused the development of peripheral insulin resistance and brain insulin resistance in male rats (Akiyama et al. 1996; Pratchayasakul et al. 2011; Stranahan et al. 2008). In addition, our previous studies suggest that brain mitochondrial dysfunction and increased brain oxidative stress caused the impairment of brain insulin sensitivity and cognitive decline in obese-insulin resistant rats (Pintana et al. 2013; Pipatpiboon et al. 2013; Pipatpiboon et al. 2012). The present study further demonstrated that it is the obese condition that caused peripheral insulin resistance, brain insulin resistance, and brain mitochondrial dysfunction in testosterone-deprived rats. Similarly to testosterone-deprived rats, these obese testosterone-deprived rats also had increased brain lipid peroxidation with impaired hippocampal synaptic plasticity and showed cognitive decline. All of these findings indicate that both testosterone and obesity play an important role in cognitive function.
Although several studies indicated that testosterone would be pro-oxidant (Siddiqui et al. 2005; Warner et al. 2004), our study showed that testosterone replacement exerted antioxidative effects, as indicated by a reduction in brain mitochondrial ROS level and MDA level in NDO and HFO rats. In the present study, testosterone replacement restored cognitive function, synaptic plasticity, and decreased lipid peroxidation in testosterone-deprived rats. However, testosterone replacement improved only peripheral insulin sensitivity but failed to improve brain insulin sensitivity, hippocampal synaptic plasticity, and cognition in testosterone-deprived obese rats. The beneficial effect of testosterone on metabolic parameters in obese condition was also supported by other previous reports (Isidori et al. 2005; Marin et al. 1992). For cognitive function, although testosterone replacement restored cognitive function possibly through the antioxidant effect in testosterone-deprived rats, we found that this treatment failed to restore cognition, hippocampal synaptic plasticity, and brain insulin sensitivity in testosterone-deprived obese rats. This could be due to the fact that testosterone replacement in the present study could reduce brain mitochondrial ROS production (Fig. 3a) and brain lipid peroxidation levels (Fig. 5b) to a lesser degree in testosterone-deprived obese rats when compared to rats with testosterone deprivation alone. These reductions were not sufficient to restore brain mitochondrial function, increase brain insulin sensitivity, and improve hippocampal synaptic plasticity and cognitive function in testosterone-deprived rats with obesity.
In conclusion, the present study indicates that in conditions of testosterone deprivation with obesity, testosterone replacement could only attenuate peripheral insulin sensitivity, improve lipid profiles, and decrease brain mitochondrial ROS production, as well as circulating and brain lipid peroxidation levels, without restoring hippocampal synaptic plasticity and improving cognitive function. In cases of testosterone deprivation alone, impaired hippocampal synaptic plasticity and cognitive function can be restored by testosterone replacement.
Funding
This work was supported by grants from the Thailand Research Fund TRF-BRG5780016 (SC), TRF-TRG5680018 (WP), the Royal Golden Jubilee PhD program (PHD/0025/2555 HP&SC), National Research Council of Thailand (SC), a NSTDA Research Chair Grant from the National Science and Technology Development Agency Thailand (NC), and Chiang Mai University Excellent Center Award (NC).
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Akiyama T, Tachibana I, Shirohara H, Watanabe N, Otsuki M. High-fat hypercaloric diet induces obesity, glucose intolerance and hyperlipidemia in normal adult male Wistar rat. Diabetes Res Clin Pract. 1996;31:27–35. doi: 10.1016/0168-8227(96)01205-3. [DOI] [PubMed] [Google Scholar]
- Aon MA, Cortassa S, Akar FG, O'Rourke B. Mitochondrial criticality: a new concept at the turning point of life or death. Biochim Biophys Acta. 2006;1762:232–240. doi: 10.1016/j.bbadis.2005.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H. Age dependent effects of space limitation and social tension on open-field behavior in male rats. Physiol Behav. 2005;84:429–436. doi: 10.1016/j.physbeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Axell AM, et al. Continuous testosterone administration prevents skeletal muscle atrophy and enhances resistance to fatigue in orchidectomized male mice. Am J Physiol Endocrinol Metab. 2006;291:E506–E516. doi: 10.1152/ajpendo.00058.2006. [DOI] [PubMed] [Google Scholar]
- Aydilek N, Aksakal M. Effects of testosterone on lipid peroxidation, lipid profiles and some coagulation parameters in rabbits. J Vet Med A Physiol Pathol Clin Med. 2005;52:436–439. doi: 10.1111/j.1439-0442.2005.00764.x. [DOI] [PubMed] [Google Scholar]
- Bassil N, Morley JE. Late-life onset hypogonadism: a review. Clin Geriatr Med. 2010;26:197–222. doi: 10.1016/j.cger.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Borst SE, Conover CF. Orchiectomized Fischer 344 male rat models body composition in hypogonadal state. Life Sci. 2006;79:411–415. doi: 10.1016/j.lfs.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Buletko AB, Dluzen DE, McDermott JL, Darvesh AS, Geldenhuys WJ. Markers associated with testosterone enhancement of methamphetamine-induced striatal dopaminergic neurotoxicity. Neurotoxicol Teratol. 2012;34:338–343. doi: 10.1016/j.ntt.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Candan N, Tuzmen N. Very rapid quantification of malondialdehyde (MDA) in rat brain exposed to lead, aluminium and phenolic antioxidants by high-performance liquid chromatography-fluorescence detection. Neurotoxicology. 2008;29:708–713. doi: 10.1016/j.neuro.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Chai JK, Blaha V, Meguid MM, Laviano A, Yang ZJ, Varma M. Use of orchiectomy and testosterone replacement to explore meal number-to-meal size relationship in male rats. Am J Physiol. 1999;276:R1366–R1373. doi: 10.1152/ajpregu.1999.276.5.R1366. [DOI] [PubMed] [Google Scholar]
- Christoffersen BO, Gade LP, Golozoubova V, Svendsen O, Raun K. Influence of castration-induced testosterone and estradiol deficiency on obesity and glucose metabolism in male Gottingen minipigs. Steroids. 2010;75:676–684. doi: 10.1016/j.steroids.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Cortassa S, Aon MA, Winslow RL, O'Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys J. 2004;87:2060–2073. doi: 10.1529/biophysj.104.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham GR, Toma SM. Clinical review: why is androgen replacement in males controversial? J Clin Endocrinol Metab. 2010;96:38–52. doi: 10.1210/jc.2010-0266. [DOI] [PubMed] [Google Scholar]
- Cunningham RL, Giuffrida A, Roberts JL. Androgens induce dopaminergic neurotoxicity via caspase-3-dependent activation of protein kinase Cdelta. Endocrinology. 2009;150:5539–5548. doi: 10.1210/en.2009-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donatucci C, Cui Z, Fang Y, Muram D. Long-term treatment patterns of testosterone replacement medications. J Sex Med. 2014;11:2092–2099. doi: 10.1111/jsm.12608. [DOI] [PubMed] [Google Scholar]
- Emamian S, Naghdi N, Sepehri H, Jahanshahi M, Sadeghi Y, Choopani S. Learning impairment caused by intra-CA1 microinjection of testosterone increases the number of astrocytes. Behav Brain Res. 2010;208:30–37. doi: 10.1016/j.bbr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Erben RG, Eberle J, Stahr K, Goldberg M. Androgen deficiency induces high turnover osteopenia in aged male rats: a sequential histomorphometric study. J Bone Miner Res. 2000;15:1085–1098. doi: 10.1359/jbmr.2000.15.6.1085. [DOI] [PubMed] [Google Scholar]
- Gao W, Reiser PJ, Coss CC, Phelps MA, Kearbey JD, Miller DD, Dalton JT. Selective androgen receptor modulator treatment improves muscle strength and body composition and prevents bone loss in orchidectomized rats. Endocrinology. 2005;146:4887–4897. doi: 10.1210/en.2005-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry RT, Wade GN. Androgenic control of food intake and body weight in male rats. J Comp Physiol Psychol. 1976;90:18–25. doi: 10.1037/h0077264. [DOI] [PubMed] [Google Scholar]
- Georgiev IP, et al. Effects of castration-induced visceral obesity and antioxidant treatment on lipid profile and insulin sensitivity in New Zealand white rabbits. Res Vet Sci. 2011;90:196–204. doi: 10.1016/j.rvsc.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Grossmann M, et al. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93:1834–1840. doi: 10.1210/jc.2007-2177. [DOI] [PubMed] [Google Scholar]
- Haffner SM, Karhapaa P, Mykkanen L, Laakso M. Insulin resistance, body fat distribution, and sex hormones in men. Diabetes. 1994;43:212–219. doi: 10.2337/diab.43.2.212. [DOI] [PubMed] [Google Scholar]
- Harooni HE, Naghdi N, Sepehri H, Rohani AH. Intra hippocampal injection of testosterone impaired acquisition, consolidation and retrieval of inhibitory avoidance learning and memory in adult male rats. Behav Brain Res. 2008;188:71–77. doi: 10.1016/j.bbr.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Williams J, Budge M, Barnetson L, Combrinck M, Smith AD. Serum total testosterone is lower in men with Alzheimer's disease. Neuro Endocrinol Lett. 2001;22:163–168. [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for Alzheimer's disease. Exp Gerontol. 2004;39:1633–1639. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Isidori AM, et al. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin Endocrinol (Oxf) 2005;63:280–293. doi: 10.1111/j.1365-2265.2005.02339.x. [DOI] [PubMed] [Google Scholar]
- Janowsky JS. Thinking with your gonads: testosterone and cognition. Trends Cogn Sci. 2006;10:77–82. doi: 10.1016/j.tics.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Kakolewski JW, Cox VC, Valenstein ES. Sex differences in body-weight change following gonadectomy of rats. Psychol Rep. 1968;22:547–554. doi: 10.2466/pr0.1968.22.2.547. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Fabregas G, Song C, Biskup B, Bellantonio S. Effects of testosterone on behavior, depression, and cognitive function in older men with mild cognitive loss. J Gerontol A Biol Sci Med Sci. 2004;59:75–78. doi: 10.1093/gerona/59.1.M75. [DOI] [PubMed] [Google Scholar]
- Khorshidahmad T, et al. Interactive effects of a protein kinase AII inhibitor and testosterone on spatial learning in the Morris water maze. Behav Brain Res. 2012;228:432–439. doi: 10.1016/j.bbr.2011.12.028. [DOI] [PubMed] [Google Scholar]
- Lu PH, et al. Effects of testosterone on cognition and mood in male patients with mild Alzheimer disease and healthy elderly men. Arch Neurol. 2006;63:177–185. doi: 10.1001/archneur.63.2.nct50002. [DOI] [PubMed] [Google Scholar]
- Maki PM, et al. Intramuscular testosterone treatment in elderly men: evidence of memory decline and altered brain function. J Clin Endocrinol Metab. 2007;92:4107–4114. doi: 10.1210/jc.2006-1805. [DOI] [PubMed] [Google Scholar]
- Margo K, Winn R. Testosterone treatments: why, when, and how? Am Fam Physician. 2006;73:1591–1598. [PubMed] [Google Scholar]
- Marin P, et al. The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes Relat Metab Disord. 1992;16:991–997. [PubMed] [Google Scholar]
- Mateos R, Lecumberri E, Ramos S, Goya L, Bravo L. Determination of malondialdehyde (MDA) by high-performance liquid chromatography in serum and liver as a biomarker for oxidative stress. Application to a rat model for hypercholesterolemia and evaluation of the effect of diets rich in phenolic antioxidants from. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827:76–82. doi: 10.1016/j.jchromb.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Meydan S, et al. Effects of testosterone on orchiectomy-induced oxidative damage in the rat hippocampus. J Chem Neuroanat. 2010;40:281–285. doi: 10.1016/j.jchemneu.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Muriach M, Flores-Bellver M, Romero FJ, Barcia JM. Diabetes and the brain: oxidative stress, inflammation, and autophagy. Oxid Med Cell Longev. 2014;2014:102158. doi: 10.1155/2014/102158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghdi N, Asadollahi A. Genomic and nongenomic effects of intrahippocampal microinjection of testosterone on long-term memory in male adult rats. Behav Brain Res. 2004;153:1–6. doi: 10.1016/j.bbr.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Naghdi N, Majlessi N, Bozorgmehr T. The effect of intrahippocampal injection of testosterone enanthate (an androgen receptor agonist) and anisomycin (protein synthesis inhibitor) on spatial learning and memory in adult, male rats. Behav Brain Res. 2005;156:263–268. doi: 10.1016/j.bbr.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Pintana H, Apaijai N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci. 2012;91:409–414. doi: 10.1016/j.lfs.2012.08.017. [DOI] [PubMed] [Google Scholar]
- Pintana H, Apaijai N, Chattipakorn N, Chattipakorn SC. DPP-4 inhibitors improve cognition and brain mitochondrial function of insulin resistant rats. J Endocrinol. 2013;218:1–11. doi: 10.1530/JOE-12-0521. [DOI] [PubMed] [Google Scholar]
- Pintana H, Sripetchwandee J, Supakul L, Apaijai N, Chattipakorn N, Chattipakorn S. Garlic extract attenuates brain mitochondrial dysfunction and cognitive deficit in obese-insulin resistant rats. Appl Physiol Nutr Metab. 2014;39:1373–1379. doi: 10.1139/apnm-2014-0255. [DOI] [PubMed] [Google Scholar]
- Pipatpiboon N, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. PPARgamma agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets. Endocrinology. 2012;153:329–338. doi: 10.1210/en.2011-1502. [DOI] [PubMed] [Google Scholar]
- Pipatpiboon N, Pintana H, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. DPP4-inhibitor improves neuronal insulin receptor function, brain mitochondrial function and cognitive function in rats with insulin resistance induced by high-fat diet consumption. Eur J Neurosci. 2013;37:839–849. doi: 10.1111/ejn.12088. [DOI] [PubMed] [Google Scholar]
- Pongkan W, Chattipakorn SC, Chattipakorn N. Chronic testosterone replacement exerts cardioprotection against cardiac ischemia-reperfusion injury by attenuating mitochondrial dysfunction in testosterone-deprived rats. PLoS One. 2015;10 doi: 10.1371/journal.pone.0122503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratchayasakul W, Kerdphoo S, Petsophonsakul P, Pongchaidecha A, Chattipakorn N, Chattipakorn SC. Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci. 2011;88:619–627. doi: 10.1016/j.lfs.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Sakata K, Tokue A, Kawai N. Altered synaptic transmission in the hippocampus of the castrated male mouse is reversed by testosterone replacement. J Urol. 2000;163:1333–1338. doi: 10.1016/S0022-5347(05)67773-7. [DOI] [PubMed] [Google Scholar]
- Sandstrom NJ, Kim JH, Wasserman MA. Testosterone modulates performance on a spatial working memory task in male rats. Horm Behav. 2006;50:18–26. doi: 10.1016/j.yhbeh.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Shi Z, Araujo AB, Martin S, O'Loughlin P, Wittert GA. Longitudinal changes in testosterone over five years in community-dwelling men. J Clin Endocrinol Metab. 2013;98:3289–3297. doi: 10.1210/jc.2012-3842. [DOI] [PubMed] [Google Scholar]
- Siddiqui IA, Raisuddin S, Shukla Y. Protective effects of black tea extract on testosterone induced oxidative damage in prostate. Cancer Lett. 2005;227:125–132. doi: 10.1016/j.canlet.2004.10.046. [DOI] [PubMed] [Google Scholar]
- Spritzer MD, Daviau ED, Coneeny MK, Engelman SM, Prince WT, Rodriguez-Wisdom KN. Effects of testosterone on spatial learning and memory in adult male rats. Horm Behav. 2011;59:484–496. doi: 10.1016/j.yhbeh.2011.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripetchwandee J, Pipatpiboon N, Chattipakorn N, Chattipakorn S. Combined therapy of iron chelator and antioxidant completely restores brain dysfunction induced by iron toxicity. PLoS One. 2014;9 doi: 10.1371/journal.pone.0085115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB. Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men: prospective results from the Massachusetts male aging study. Diabetes Care. 2000;23:490–494. doi: 10.2337/diacare.23.4.490. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus. 2008;18:1085–1088. doi: 10.1002/hipo.20470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan C, Goldstein FC, Tenover JL. Exogenous testosterone alone or with finasteride does not improve measurements of cognition in healthy older men with low serum testosterone. J Androl. 2007;28:875–882. doi: 10.2164/jandrol.107.002931. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner DS, Sheng H, Batinic-Haberle I. Oxidants, antioxidants and the ischemic brain. J Exp Biol. 2004;207:3221–3231. doi: 10.1242/jeb.01022. [DOI] [PubMed] [Google Scholar]
- Wolf OT, Preut R, Hellhammer DH, Kudielka BM, Schurmeyer TH, Kirschbaum C. Testosterone and cognition in elderly men: a single testosterone injection blocks the practice effect in verbal fluency, but has no effect on spatial or verbal memory. Biol Psychiatry. 2000;47:650–654. doi: 10.1016/S0006-3223(99)00145-6. [DOI] [PubMed] [Google Scholar]