Abstract
Aging is the natural process of decline in physiological structure and function of various molecules, cells, tissues, and organs. Growing evidence indicates that increased immune genetic diversity and dysfunction of immune system cause aging-related pathophysiological process with the growth of age. In the present study, we observed that LPS-induced higher activation of cyclooxygenase (COX)-2 promoter is associated with the upregulated binding activity of nuclear factor kappa B (NF-κB) in peritoneal macrophages of aged mice than young ones. Additionally, COX-2 is a direct target of miR-101b and miR-26b in the macrophages. Significant upregulation of miR-101b and miR-26b effectively prevented LPS-induced excessive expression of COX-2 in the young mice. Because these negative regulatory factors were unresponsive to LPS stimulation, the levels of COX-2 were markedly higher in the macrophages of aged mice. Further study showed that NF-κB activation contributed to the increase in the expression of miR-101b and miR-26b in the LPS-stimulated macrophages of young mice, but not aged ones. Moreover, histone deacetylase (HDAC) inhibitor trichostatin A (TSA) upregulated expression of miR-101b and miR-26b in the aged mouse macrophages only, but not the young cells. This demonstrated that HDAC suppressed the expression of miR-101b and miR-26b in the LPS-treated macrophages of aged mice and contributed to the aging process. TSA-induced increased expression of miR-101b and miR-26b could further suppress COX-2 expression. These findings provide novel evidence on the regulation of immune senescence and miR-101b and miR-26b, which might be promising targets in treating aged-related inflammatory diseases. Epigenetic regulation of the microRNAs (miRNAs) provides an important evidence for the treatment of innate inflammatory disease with HDAC inhibitors in elderly.
Keywords: miR-101b, miR-26b, COX-2, Aging, Immunosenescence, Macrophage
Introduction
RL Walford first proposed the immunologic theory of aging during the 1960s. Briefly, Walford hypothesized that increased immune genetic diversity and dysfunction of immune system cause aging-related pathophysiological processes with the growth of the age, termed immunosenescence (Walford 1962, 1964). Indeed, numerous clinical studies have indicated a decline in immune function with aging (Frasca and Blomberg 2009; Haynes and Maue 2009; Linton and Dorshkind 2004). Macrophage, a bridge linking innate and adaptive immune system, shows the dysregulation of chemokine and cytokine secretion, which may result in poor responses to infection and protracted repair processes with aging (Plowden et al. 2004). Several experimental evidences have demonstrated that LPS-activated macrophages from aged mice and humans produced more prostaglandin E2 (PGE2) than those from younger individuals (Beharka et al. 1997; Wu et al. 1998). The increased PGE2 may lead to dysfunctional immune responses including a declined T cell function in the elderly (Beharka et al. 1997).
Cyclooxygenase (COX) is the rate-limiting enzyme in the biosynthesis of PGE2. Two isoenzymes, COX-1 and COX-2, have been identified. COX-1 displays the traits of a “housekeeping” gene and involves in the maintenance of normal physiological function of several tissues, whereas COX-2 would undergo rapid induction in response to phorbol esters, LPS, and a myriad of proinflammatory factors (Morita 2002). The age-associated increase in PGE2 synthesis stemmed from enhanced expression of inducible COX-2 in macrophages (Hayek et al. 1997). However, the molecular mechanism underlying the upregulation of COX-2 in the macrophages of aged animals/individuals is presently unclear. A study has suggested that LPS upregulates COX-2 transcription in the macrophages of aged mice by increasing the activation of transcription factor nuclear factor kappa B (NF-κB) (Wu et al. 2003). However, to date, the regulation of COX-2 at the level of post-transcription in macrophages has not been demonstrated.
MicroRNAs (miRNAs), a large family of gene expression regulatory factors, play a key role in the regulation of gene expression at the post-transcriptional level. miRNAs usually control gene expression through perfect or partial complementary to the 3′ untranslated region (UTR) of target messenger RNA (mRNA), which causes the degradation of the mRNAs and/or the translation suppression, and moreover, off-target transcripts containing sites of partial complementarity to the miRNAs might be regulated by multiple miRNAs (Engels and Hutvagner 2006). COX-2 mRNA translation inhibition could be mediated by several miRNAs. For example, mmu-miR-101a and mmu-miR-199a* affect embryo implantation of mice by post-transcriptional regulation of COX-2 (Chakrabarty et al. 2007) and miR-26b could regulate the expression of COX-2 in desferrioxamine-treated CNE cells (Ji et al. 2010).
Recently, researchers began to link miRNAs to immunosenescence. Although studies found that the expression levels of miRNAs in certain immune cells changed with the aging process (Hackl et al. 2010; Li et al. 2012; Park et al. 2013), whether these differentially expressed miRNAs are involved in regulating the biological function of the immune cells in the aging body is not fully understood. The expression profiles of miRNAs in the peritoneal macrophages of young and aged mice in LPS-induced acute inflammatory response were, for the first time, performed in our previous study using the miRNA microarray analysis. A series of differentially expressed miRNAs were screened and confirmed that abnormal expression of miR-146a could lead to the altered expression levels of IL-1β and IL-6 in the macrophages of aged mice (Jiang et al. 2012). However, whether the differentially expressed miRNAs are involved in the regulation of the biological function of macrophages in the aged body remains to be clarified.
In this study, we demonstrated for the first time that the inflammatory factor COX-2 expression was regulated by miR-101b and miR-26b coordinately in the macrophages. The increased expression of inflammatory factor COX-2, which was caused by these negative regulatory factors unresponsive to LPS in peritoneal macrophages of senescence mice, may eventually accelerate the process of aging. Further study showed that there existed a NF-κB-dependent induction of miR-101b and miR-26b expression. Moreover, histone deacetylase (HDAC) inhibitor trichostatin A (TSA) suppressed aged-related increase in LPS-induced COX-2 expression by upregulating both miR-101b and miR-26b. In a sentence, our findings identify a novel mechanism of regulating macrophage-related and immunosenescence-associated miRNAs.
Materials and methods
Mice and reagents
Male young (2 months) and aged (20 months) BALB/c mice were obtained from the Institute of Zoological Sciences, Chinese Academy of Medical Sciences, Beijing, and bred in specific pathogen-free conditions. All animal experiments were approved by the Committee on the Use and Care of Animals, Chinese Academy of Medical Sciences. RPMI 1640 medium, DMEM, and FBS were obtained from GIBCO. LPS (Escherichia coli 0111:B4), CpG oligodeoxynucleotide (ODN), poly(I:C), and TSA were purchased from Sigma-Aldrich. Antibodies against COX-2, nuclear factor of kappa light polypeptide gene enhancer in B cell inhibitor alpha (IκBα), and phosphorylated IκBα (p-IκBα) were from Cayman Chemical Co. and Cell Signaling Technology Co., respectively. The secondary antibodies conjugated to horseradish peroxidase were purchased from Zhongshan Golden Bridge Biotechnology. BAY 11-7082, an inhibitor of IκBα phosphorylation, was acquired from Santa Cruz Biotechnology. Probes for miR-101b, miR-26b, U6 small nuclear RNA (snRNA), TaqMan MicroRNA Reverse Transcription kit, and TaqMan Universal PCR Master Mix were purchased from Applied Biosystems. miR-101b mimics, miR-26b mimics, negative control (NC) mimics (double-stranded RNA (dsRNA) oligonucleotides), miR-101b inhibitors, miR-26b inhibitors, and NC inhibitors (single-stranded chemically modified oligonucleotides) were from GenePharma (Shanghai, China). Cells were transfected with RNAs using INTERFERin (Polyplus-Transfection SA, Illkirch, France) according to the manufacturer’s instructions.
Peritoneal macrophage isolation
The detailed method for isolating peritoneal macrophages from BALB/c mice was described in the previous reports (Jiang et al. 2012). Briefly, mice were intraperitoneally (i.p.) injected with 2 mL of 3 % thioglycollate (Difco). Three days later, peritoneal exudate cells were collected by peritoneal lavage and washed three times with complete culture medium. Then, cells were cultured in endotoxin-free RPMI 1640 medium containing 5 % FCS and allowed to adhere at 37 °C in 5 % CO2. After 2 h of incubation, non-adherent cells were removed by washing three times with warm Hank’s balanced salt solution. The adherent cells were used as mouse peritoneal macrophages.
RNA extraction and quantitative real-time PCR
Total RNA was extracted using the TRIzol reagent (Invitrogen) according to the manufacturer’s introductions. To measure mRNA expression of COX-2, total RNA was reversely transcribed using the M-MLV Reverse Transcription System (Invitrogen) and oligo-dT primer and then the quantitative real-time PCR (q-PCR) was carried out with specific primer pairs and SYBR Green PCR Master Mix (TaKaRa). Primers of COX-2 for q-PCR were forward 5′-TACCGCAAACGC TTCTCCCT-3′ and reverse 5′-TGGTCTCCCCAAAGATAGCA-3′. The expression of miR-101b and miR-26b was measured using the TaqMan miRNA assay system (Applied Biosystems) following the manufacturer’s instructions. COX-2 mRNA expression was normalized to β-actin mRNA levels, while miRNA expression was normalized to mouse U6 RNA levels. The relative quantities were calculated using the 2−ΔΔCT method.
Western blot assay
Cells were collected and lysed in ice-cold lysis buffer for 30 min. The protein concentration of the lysate was determined by BCA assay. After boiling in loading buffer, each sample containing 50 μg of denatured proteins was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (5 % stacking gel and 12 % separating gel). The proteins in the gel were then transferred to polyvinylidene difluoride (PVDF) membrane (Amersham Biosciences) in a semidry transfer system. The PVDF membrane was incubated with specific primary antibodies and HRP-conjugated secondary antibodies. Immunoblotting proteins were visualized using an enhanced chemiluminescence (ECL) reagent (Amersham Biosciences) and followed by exposure to X-ray film. Band intensity on X-ray film was estimated using Gel-Pro Analyzer software. Normalization was made against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression.
PGE2 measurement
PGE2 concentration in the culture supernatants was detected using an enzyme-linked immunosorbent assay (ELISA) using a PGE2 ELISA kit (R&D Systems) according to the manufacturer’s instructions.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) assay was carried out using a ChIP assay kit (Millipore, Billerica, MA, USA) in accordance with the manufacturer’s instructions. Approximately, 1 × 107 cells were harvested and followed by sonication to yield DNA fragments with a length of 200–1000 bp. After centrifugation for 10 min, the cleared supernatant was collected and 20 μl was reserved as input control. Cross-link was carried out with 1 % formaldehyde solution for 10 min at 37 °C. Immunoprecipitation analysis was then performed using the anti-NF-κB p65 antibody or anti-mouse IgG (a control for nonspecific binding) at 4 °C overnight. The primer pairs (forward, 5′-CACCAGTACAGATGTGGAC-3′C reverse, 5′-AGGTGGTGCC AAGAGAGC-3′) amplified a DNA fragment that contains NF-κB binding site I of the COX-2 gene promoter, and the primer pairs (forward, 5′-CCCGGAGGGTAGTTCCATGAAAGA CTTCAAC-3′; reverse, 5′-GGTGGAGCTGGCAGGATGCAGT-3′) amplified a DNA fragment that encompasses NF-κB binding site II of the COX-2 gene promoter. The input DNA was analyzed by q-PCR using the same primers.
Dual-Luciferase reporter assay
The whole 3′ UTR fragment of Mus musculus COX-2 mRNA and its mutants was cloned into the pMIR-REPORT™ luciferase reporter plasmid (Life Technologies). The 293T cells were seeded in a 24-well plate at 4 × 104 cells/well and, on the next day, co-transfected with pMIR-REPORT™ plasmids encoding either wild-type COX-2 3′ UTR or mutant COX-2 3′ UTR and pRL-TK plasmids combined with miR-101b or miR-26b mimics or NC mimics, using Lipofectamine 2000 (Invitrogen). After 48 h of incubation, cells were lysed and the luciferase activity was determined using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer’s protocol. Data were normalized through dividing firefly luciferase activity with Renilla luciferase activity.
Statistical analysis
Each experiment was performed separately in triplicate, and data were expressed as mean value ± SD of three experiments. Student’s t test was used to assess the differences between the two groups. Differences were considered statistically significant when the P value was less than 0.05.
Results
LPS enhances expression of COX-2 and PGE2 in aged mouse peritoneal macrophages
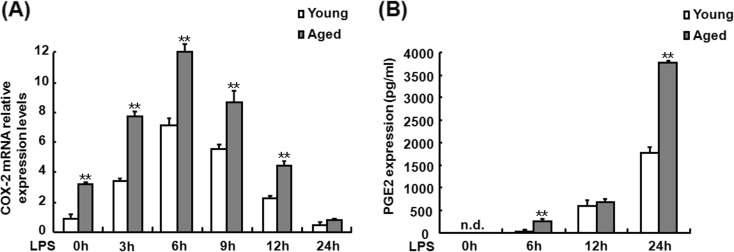
The effects of LPS on the levels of COX-2 and PGE2 expression in the peritoneal macrophages from young and aged mice were examined firstly. The peritoneal macrophages from the young and old mice were isolated and exposed to LPS for 0, 3, 6, 9, 12, and 24 h. COX-2 mRNA and PGE2 production in the cells or supernatants was measured by RT-PCR and ELISA, respectively. As shown in Fig. 1a, COX-2 mRNA expression in the cells from young and aged mice was significantly increased at 3 h and peaked at 6 h and then gradually reduced to the basal level at 24 h after LPS challenge but the levels were markedly higher in the macrophages from aged mice than those in the macrophages from the young mice at each time point after LPS treatment. PGE2 production in LPS-treated peritoneal macrophages of old mice was also prominently higher than that in those of young mice (Fig. 1b). These data demonstrate that LPS enhances the expression of COX-2 and PGE2 in aged mouse peritoneal macrophages.
Fig. 1.
Effects of LPS on the levels of COX-2 mRNA and PGE2 synthesis in the peritoneal macrophage from young and aged mice. Macrophages were isolated and cultured in the absence or presence of LPS for the indicated time, and q-PCR was used to measure the expression of COX-2 mRNA (a). Supernatants were also collected at the indicated time points after LPS stimulation, and then, PGE2 levels were determined in the culture supernatant using a PGE2 assay kit (b). Similar results were obtained from three independent experiments. Data are the mean ± SD (n = 3) of three independent experiments. The fold change is relative to young cells without LPS. *P < 0.05; **P < 0.01, compared with young cells under the same conditions
LPS-induced high expression of COX-2 in aged murine peritoneal macrophages is associated with NF-κB activation
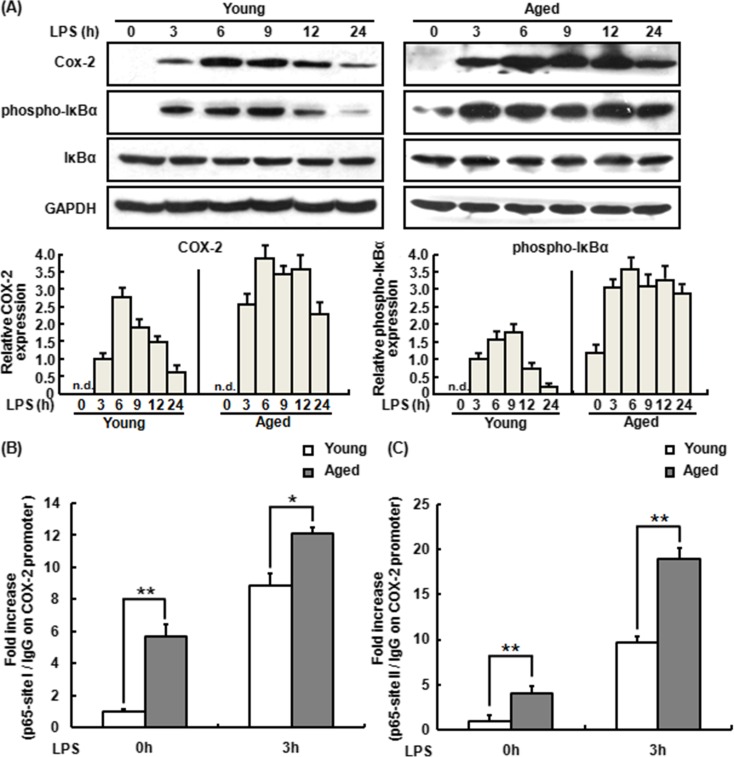
Appleby et al. (1994) reported that human COX-2 promoter contains two NF-κB binding sites. Indeed, many studies confirmed that NF-κB signaling pathways modulate COX-2 expression (Kim et al. 2012; Wu et al. 2003). To validate whether the increased expression of COX-2 in aged mouse peritoneal macrophages is associated with the activation of NF-κB, the level of phosphorylated IκBα (p-IκBα) expression was detected by Western blot assay. As shown in Fig. 2a, LPS induced higher levels of COX-2 protein in the peritoneal macrophages from aged mice than in those from young mice and this was accompanied by higher NF-κB activity in LPS-treated aged murine macrophages. Chromatin immunoprecipitation (ChIP) was further performed to evaluate NF-κB binding activity in the LPS-treated macrophages from young and aged mice. As shown in Fig. 2b, c, LPS increased the binding of NF-κB p65 subunit to the two sites in the COX-2 promoter in both young and aged murine macrophages. Together, NF-κB was a critical contributor to the LPS-induced high expression of COX-2 in aged murine peritoneal macrophages.
Fig. 2.
Regulation of LPS-induced COX-2 expression by NF-κB activation in the macrophage from young and aged mice. a Expression of COX-2 and activation of NF-κB in the macrophage from young and aged mice by LPS stimulation. The macrophages were treated with LPS for a time course (0 to 24 h), and the cell lysates were subjected to SDS-PAGE. Western blot analysis was performed using the antibodies specific for COX-2, p-IκBα, and total IκBα. GAPDH was used as a control. One representative Western blot image out of three independent experiments was shown. The ratio of COX-2 and phospho-IκBα to GAPDH band intensity for each lysate was normalized to the control ratio (young macrophage cells treated with LPS for 3 h). b, c NF-κB p65 subunit binding to two sites upstream of COX-2 transcriptional unit in the macrophage isolated from young and aged mice by LPS stimulation. ChIP assay was applied to evaluate p65 association with the COX-2 gene promoter after 3 h in the absence or presence of LPS. Formaldehyde-cross-linked chromatin was sonicated and immunoprecipitated by p65 Ab or control IgG, followed by q-PCR analysis with specific primers set specific for the two different p65 binding sites in the COX-2 promoter at site I (b) and site II (c). Non-immunoprecipitated chromatin was used as control. Similar results were obtained from three independent experiments. Data are the mean ± SD (n = 3) of three independent experiments. *P < 0.05; **P < 0.01, compared with young macrophage cells under the same conditions
miR-101b and miR-26b are differentially expressed in LPS-treated macrophages from young and aged mice
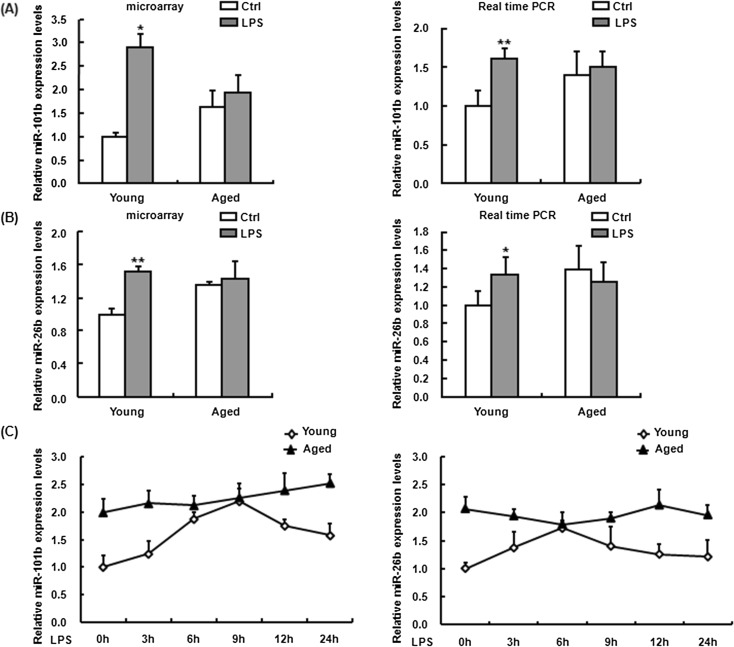
In many cases, the altered miRNA level could reflect COX-2 expression at the post-transcriptional level (Harper and Tyson-Capper 2008). Previously, we have profiled the expression of miRNAs in LPS-treated and untreated macrophages from young and aged mice using the microarray technology and showed that miR-101b and miR-26b were differentially expressed in the two age groups (Jiang et al. 2012) (Fig. 3a, b, left). It has been reported that the two miRNAs are involved in the regulation of COX-2 expression in certain cell lines (Hao et al. 2011; Ji et al. 2010; Li et al. 2013; Strillacci et al. 2009). Therefore, we here focused on miR-101b and miR-26b expression in the mouse peritoneal macrophages.
Fig. 3.
The expression of miR-101b and miR-26b in LPS-treated young and aged mouse macrophage. Mice were sacrificed 24 h after intraperitoneal injection of 3 mg/kg LPS, and macrophages were isolated. Total RNA was purified from the respective cell pellets, followed by the microarray analysis for the expression of miR-101b (a, left) and miR-26b (b, left) in untreated and LPS-treated macrophage of two age groups. Confirmation of the expression of miR-101b (a, right) and miR-26b (b, right) by q-PCR. Macrophages isolated from young and old mouse were treated with LPS in vitro for the indicated time (0, 3, 6, 9, 12, and 24 h), and RNA samples from these cells were used for q-PCR analysis, respectively (c). Individual miRNA level in each sample was normalized against the expression of U6. Similar results were obtained from three independent experiments. Data are the mean ± SD (n = 3) of three independent experiments. *P < 0.05; **P < 0.01, compared with young control cells (Ctrl)
To validate the results of microarray in mouse macrophages, total RNA was extracted from the peritoneal macrophages of young and aged mice following intraperitoneal injection of LPS and then q-PCR was performed to evaluate the expression of miR-101b and miR-26b. As shown in the right of Fig. 3a, b, the expression of both miR-101b and miR-26b was upregulated about 1.5-fold in the macrophages from aged mice without LPS stimulation as compared to young mice. Upon LPS stimulation, the expression levels of miR-101b and miR-26b in the macrophages from young mice were increased 3-fold but the macrophages from aged mice lacked of response to LPS stimulation in the expression of the two miRNAs, consisting with the results of microarray assay.
To examine whether LPS treatment affects the expression of miR-101b and miR-26b in the macrophages from young and aged mice, the peritoneal macrophages from young and aged mice were stimulated with LPS for 0, 3, 6, 9, 12, and 24 h in vitro and then miR-101b and miR-26b expression was measured by q-PCR. As shown in Fig. 3c, without LPS treatment, the expression of both miR-101b and miR-26b was much higher in the macrophages from aged mice than that in those from young mice and, with LPS treatment, miR-101b and miR-26b were expressed in a time-dependent manner in the macrophages from young mice; however, the expression of both miRNAs was almost not changed in aged mouse macrophages upon LPS treatment.
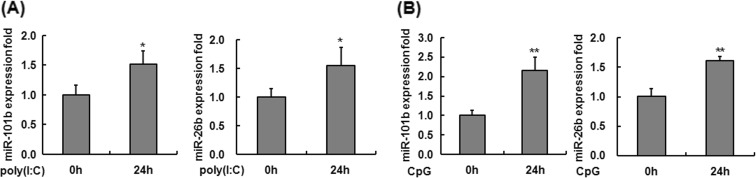
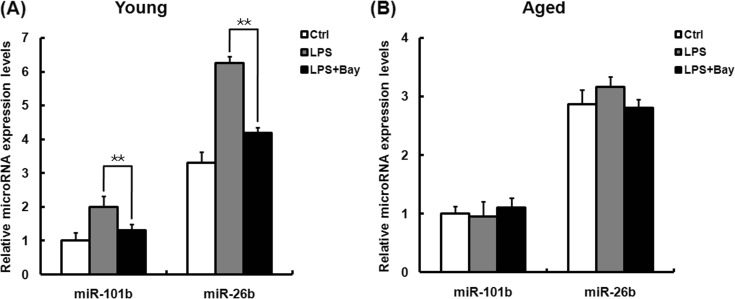
To investigate whether miR-101b and miR-26b could be induced in the peritoneal macrophages from young mice by other Toll-like receptor (TLR) ligands, young mice were intraperitoneally injected with poly(I:C), a TLR3 ligand, and CpG ODN, a TLR9 ligand, and the expression levels of miR-101b and miR-26b in the peritoneal macrophages were detected by q-PCR. As shown in Fig. 4a, b, stimulation with poly(I:C) or CpG ODN induced the expression of both miR-101b and miR-26b in young mouse macrophages, which is consistent with the LPS-treated macrophages. These results indicate that miR-101b and miR-26b might constitutively present in murine macrophages and be upregulated by different TLR ligands and then play a crucial role in the regulation of immune response to microbial infection.
Fig. 4.
Expression levels of miR-101b and miR-26b in the macrophage from young mice upon incubation with TLR agonists. Mice were sacrificed 24 h after intraperitoneal administration of PBS or poly(I:C) (a) or CpG ODN (at a dose of 5 or 10 mg/kg body weight, respectively) (b). The expression of both miR-101b and miR-26b was measured by q-PCR. Expression levels were normalized to housekeeping gene U6, and fold changes were calculated relative to non-treated cells. Similar results were obtained from three independent experiments. Data are the mean ± SD (n = 3) of three independent experiments. *P < 0.05; **P < 0.01, compared with control cells (cells in the PBS treatment group)
Both miR-101b and miR-26b regulate COX-2 expression in mouse macrophages
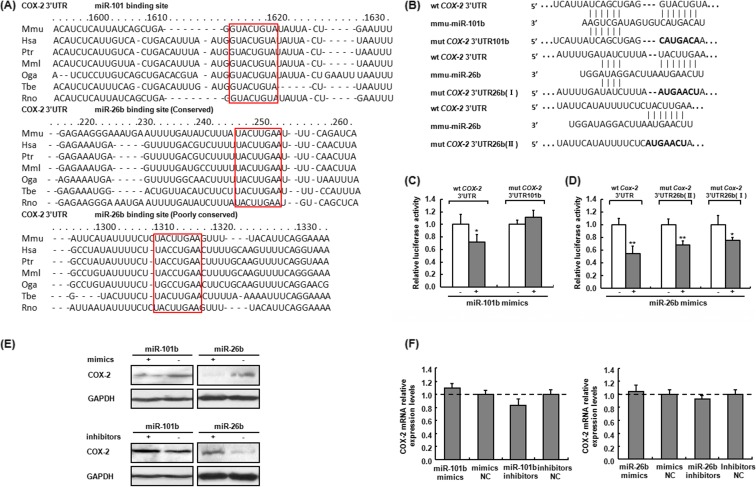
We further analyzed the binding sites of miR-101b and miR-26b in the 3′ UTR of COX-2 by using online program, TargetScan (http://www.targetscan.org/). As shown in Fig. 5, miR-101b possessed one 7-mer target seed region and miR-26b possessed two conserved 7-mer target seed regions starting from 1310 and 246 bp (in red block diagram), respectively, in 3′ UTR of COX-2 in different species. Luciferase reporter assay using the vectors containing either wild-type 3′ UTR of COX-2 (wt COX-2 3′ UTR) or the 3′ UTR with point mutations in the miRNA binding sites (mut COX-2 3′ UTR/101b or mut COX-2 3′ UTR/26b) showed that transfection with miR-101b or miR-26b mimics markedly suppressed the luciferase activity of the wild-type 3′ UTR of COX-2 (Fig. 5b), while miR-101b mimics did not affect the luciferase activity of the mut COX-2 3′ UTR/101b compared to the negative mimics (Fig. 5c). The luciferase activity of wt 3′ UTR of COX-2 vector was downregulated, while the activity of both mutants of COX-2 3′ UTR was significantly changed by miR-26b mimics (Fig. 5d), suggesting that the binding of miR-26b to both site I and site II in COX-2 3′ UTR might result in the inhibition of COX-2 expression.
Fig. 5.
miR-101b and miR-26b target COX-2 and repress the translation of COX-2 mRNA in mouse macrophage. a Sequence alignment of miR-101b and miR-26b and their target sites in 3′ UTR of COX-2. miR-101b and miR-26b were predicted to regulate COX-2 expression among different species by TargetScan (targetscan.org). b–d miR-101b and miR-26b repress COX-2 expression through an interaction with the 3′ UTR of COX-2. miR-101b binding site and two miR-26b binding sites in the 3′ UTR of COX-2 were mutated as shown in b. Mutant vectors with seven-nt substitutions disrupting base pairing with the seed region of miR-101b or miR-26b were constructed. Luciferase reporter assays were done by transfecting 293T cells with wt COX-2 3′ UTR or mut COX-2 3′ UTR vectors together with 10 nM miR-101b (c) or miR-26b (d) mimics or NC mimics (control dsRNA) for 48 h. *P < 0.05; **P < 0.01, compared with NC mimic-transfected cells. e, f miR-101b and miR-26b regulated COX-2 expression. Macrophages were transfected with 10 nM miR-101b and miR-26b mimics or inhibitors or corresponding NC (control), respectively. After 48 h of incubation, the COX-2 expression was analyzed by Western blot (e) and q-PCR (f). GAPDH was served as control. Similar results were obtained from three independent experiments. Data are the mean ± SD (n = 3) of three independent experiments
To further evaluate the effect of miR-101b and miR-26b on COX-2 expression, miR-101b and miR-26b were overexpressed (mimics) or suppressed (inhibitors) in murine peritoneal macrophages using miR mimics or inhibitors and then COX-2 mRNA level and protein expression were determined by q-PCR and Western blot assay, respectively. As shown in Fig. 5e, f, transfection of miR-101b or miR-26b mimics caused a decrease in COX-2 protein expression, but not its mRNA levels, while transfection of miR-101b or miR-26b inhibitors notably elevated COX-2 protein expression, but not mRNA levels, indicating that both miR-101b and miR-26b negatively regulated COX-2 expression by suppressing the translation of COX-2 rather than mRNA stability.
NF-κB signaling pathway regulates LPS-induced miR-101b and miR-26b expression in young mouse macrophages
We have demonstrated that activation of NF-κB is proved to be age-dependent induction in both young and aged murine macrophages and expression of both miR-101b and miR-26b is time-dependent alterations in LPS-treated macrophages from young mice. To estimate whether the increase in miR-101b and miR-26b expression in young mouse macrophages treated with LPS is associated with the activation of NF-κB, the macrophages from young mice were pretreated with IκBα phosphorylation inhibitor BAY 11-7082 and then stimulated with LPS for 6 h, followed by measurement of miR-101b and miR-26b expression. As shown in Fig. 6a, BAY 11-7082 almost completely prevented LPS-induced upregulation of miR-101b and miR-26b expression in macrophages from young only, but not aged mice (Fig. 6b). These data suggest that NF-κB activation contributes to the increase in the expression of miR-101b and miR-26b in the LPS-stimulated macrophages of young mice, but not aged ones.
Fig. 6.
NF-κB-dependent induction of miR-101b and miR-26b expression in the macrophages isolated from mice after treatment with LPS. Peritoneal macrophages from young (a) and aged (b) mice were pretreated with IκB phosphorylation inhibitor BAY 11-7082 for 1 h and then stimulated with LPS for 6 h. The isolated total RNAs were used to determine the expression levels of miR-101b and miR-26b by q-PCR, normalizing to U6 snRNA. Similar results were obtained from three independent experiments. Data are the mean ± SD (n = 3) of three independent experiments. **P < 0.01, compared with LPS-stimulated cells without BAY 11-7082 pretreatment
TSA suppresses COX-2 protein expression by upregulation of miR-101b and miR-26b in aged mouse macrophages
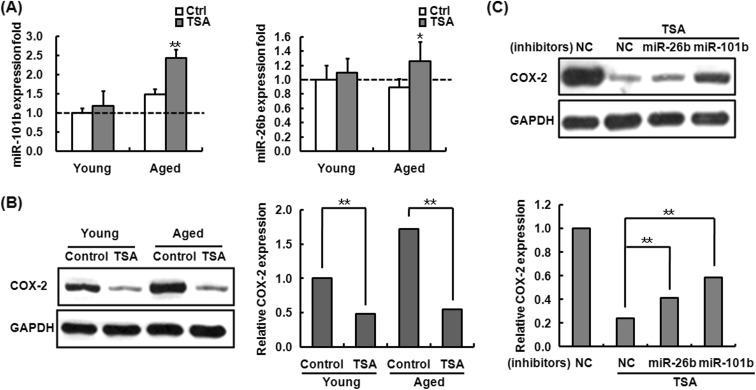
Epigenetic modification has been demonstrated to be important in aging and age-related immune dysfunction (Calvanese et al. 2009; Grolleau-Julius et al. 2010; Issa 2003). There is growing evidence of epigenetic regulation on miRNA expression (Sato et al. 2011). To examine whether histone acetylation is involved in the regulation of miR-101b and miR-26b expression in aged mouse macrophages, the peritoneal macrophages from young and aged mice were pre-treated with histone deacetylase (HDAC) inhibitor trichostatin A (TSA) before LPS challenge and then the levels of miR-101b and miR-26b were determined by q-PCR. As shown in Fig. 7a, LPS-stimulated aged mouse cells exhibited elevated miR-101b expression which increased by 1.6-fold and miR-26b by 1.4-fold in aged cells, while no changes in the levels of miR-101b and miR-26b in the cells from young mice, suggesting that miR-101b and miR-26b expression is modulated by histone acetylation in aged murine macrophages.
Fig. 7.
Histone acetylation involved in the regulation of miR-101b, miR-26b, and COX-2 expression in the aged macrophage. a The effect of TSA on miR-101b and miR-26b expression in LPS-treated macrophages from young and aged mice. Macrophages from young and aged mice were pretreated with TSA for 18 h and then treated with LPS. After 6 h of incubation, total RNA was extracted to do the q-PCR. miR-101b and miR-26b abundance was normalized to the U6. The error bars represent the standard deviation of the mean values for triplicate experiments. *P < 0.05; **P < 0.01, compared with old control cells (LPS-stimulated aged cells without TSA pretreatment). b, c The effect of TSA on COX-2 expression in LPS-treated macrophages from young and aged mice. Mouse peritoneal macrophages from aged mice were transfected with miR-101b and miR-26b inhibitor and its control. After 24 h of transfection, cells were treated with TSA for 48 h and then treated with medium or LPS for 6 h. The protein levels of COX-2 were determined by Western blot. The band intensity was quantitated using Gel-Pro Analyzer software and normalized to the control (GAPDH). Similar results were obtained from three independent experiments. Data are the mean ± SD (n = 3) of three independent experiments. *P < 0.05; **P < 0.01, compared with control
Furthermore, we determined whether TSA-induced expression of miR-101b and miR-26b can suppress COX-2 expression by Western blot assay. As shown in Fig. 7b, TSA treatment decreased COX-2 expression in LPS-stimulated macrophages, and the inhibitory effect was more significant in the macrophages from aged mice than young ones. Notably, COX-2 expression was upregulated in the aged mouse macrophages transfected with miR-101b or miR-26b inhibitor before TSA treatment, compared to the controls (Fig. 7c), indicating that TSA inhibited LPS-induced expression of COX-2 by upregulation of miR-101b and miR-26b in aged mouse macrophages.
Collectively, these data demonstrated that LPS can induce NF-κB activation in the macrophage of young mice. The activation of NF-κB activity not only directly upregulates COX-2 expression but also increases the levels of miR-101b and miR-26b which function as negative feedback modulators of LPS-induced COX-2 expression and avoid excessive inflammatory response in the young body. LPS-stimulated aged mice cells exhibited high amounts of NF-κB DNA binding activity. Thus, COX-2 expression and PGE2 production in these cells were significantly higher than those in macrophages from young mice. In addition, aged-related degeneration of histone modification patterns could contribute to dysfunction of negative regulatory factors such as miR-101b and miR-26b, which further enhance the expression of inflammatory factor COX-2 and may eventually accelerate the aging process.
Discussion
LPS, a component of the Gram-negative bacterial cell wall, could induce acute inflammation. In response to LPS stimulation, COX-2 is induced rapidly, leading to the production of prostaglandin E2 (PGE2). Data obtained from several reports clearly indicate an age-related increase in the expression of COX-2 and PGE2 (Baek et al. 2001; Hayek et al. 1997; Kim et al. 2000), which is consistent with our results in the present study. Over-production of PGE2 eventually gives rise to the age-associated diseases, such as atherosclerosis, Alzheimer’s disease, and arthritis (FitzGerald 2003; Mukherjee et al. 2001; Pasinetti and Aisen 1998). Therefore, there might exist disordered regulation of COX-2 expression in the aging body. Wu et al. (2003) demonstrated that NF-κB is a critical positive regulator in the age-associated LPS-induced upregulation of COX-2 activation in macrophages. To confirm this and to also ascertain the role of NF-κB in the age-associated increase of COX-2 expression, we demonstrated that NF-κB activation caused by LPS stimulation in macrophages was mirrored by those in COX-2 expression. In agreement with the result, we further found in ChIP assay that old macrophages gave significantly stronger binding of NF-κB p65 subunit to the COX-2 promoter compared with the young macrophages. However, interestingly, although without stimulation of LPS, the old macrophages possessed a much higher binding activity of p65 subunit and expression of COX-2 mRNA than the young ones and there was no expression of COX-2 protein in young and aged mouse macrophages. Thus, we could presume that multiple regulatory mechanisms including post-transcriptional events might control the expression of COX-2.
MicroRNAs appear to be a central class of post-transcriptional regulators that function as negative regulators of gene expression. It has been demonstrated that miR-26b and miR-16 regulate COX-2 expression in some cancer cells (Ji et al. 2010; Young et al. 2012). However, to our knowledge, so far, there is no insight into the relationship between COX-2 upregulation and altered miRNA profile in macrophages of senescence mice. We previously detected LPS-induced inflammatory miRNA expression patterns in young mouse macrophages compared with aged group by miRNA array chip assay (Jiang et al. 2012). In the present study, we aimed at those miRNAs stimulated by LPS in young mouse macrophages, but not in aged ones, which might contribute to age-related dysfunction of macrophages. Among these candidate miRNAs, miR-101b and miR-26b were predicted to be base paired with the sequence of 3′ UTR of COX-2. Further study revealed that LPS could upregulate the expression level of miR-101b and miR-26b in the macrophages of young mice, but there was no effect on the miRNA expression in macrophages of old mice. Moreover, we demonstrated, for the first time, that miR-101b and miR-26b could downregulate COX-2 protein expression without affecting COX-2 mRNA levels in the macrophages, suggesting that significant upregulation of miR-101b and miR-26b effectively prevents LPS-induced excessive expression of COX-2 in the young mice, which could avoid excessive inflammatory response. However, because miR-101b and miR-26b are unresponsive to LPS stimulation, old macrophages have less LPS-induced negative regulation mediated by these miRNAs, which subsequently results in a greater inflammatory process and more extensive pathological lesions in the elderly. Furthermore, to answer whether LPS-induced differential expression of miR-101b and miR-26b is a phenomenon specific for LPS, we observed a similar effect of Toll-like receptor agonists poly(I:C) and CpG ODN on miR-101b and miR-26b expression in the macrophages of young mice, suggesting that miR-101b and miR-26b may function as universal regulators of TLR-associated signaling events in macrophages. These observations provide a support for the involvement of miRNAs as a mechanism underlying the precise control of inflammatory response, which finally ensures an appropriate grade of inflammation.
Age-related changes in DNA binding activity of transcription factors have not been well studied; particularly, the exact mechanism linking these age-associated changes with miR-101b and miR-26b expression was unclear in macrophages. Numerous studies have proposed that LPS could induce the activation of NF-κB, contributing to the upregulated expression of several genes in macrophages (Hwang et al. 1997; Shakhov et al. 1990). Moreover, NF-κB DNA binding activity is altered in the rat liver and brain and mouse macrophages with aging (Korhonen et al. 1997; Radak et al. 2004; Sarkar and Fisher 2006), indicating that NF-κB might be involved in modulating the differentially expressed miR-101b and miR-26b. Data from this study show that IκBα phosphorylation inhibitor BAY 11-7082 suppressed miR-101b and miR-26b expression in young macrophages. More importantly, blocking NF-κB activation has no effect on the expression of miR-101b and miR-26b in the old macrophages. Aberrant NF-κB binding to miR-101b and miR-26b promoter with aging could explain, in some extent, why these miRNAs are unresponsive to the stimulation of LPS.
Histone modifications and DNA methylation are important epigenetic mechanisms for the control of gene expression. Epigenetic changes during aging have also been reported (Calvanese et al. 2009; Fraga et al. 2005). We hypothesized that aged-related degeneration of epigenetic patterns must be functionally associated with age-associated dysfunction of macrophages. Results in this study demonstrated that HDAC inhibitor TSA upregulated the expression of miR-101b and miR-26b in LPS-treated macrophages from the aged mice, but not from the young mice, suggesting that HDAC suppresses the expression of miR-101b and miR-26b in the macrophages of aged mice. Deng et al. (2004) reported that LPS could increase p300 HAT binding to COX-2 promoter and subsequently upregulate COX-2 protein levels. In the present study, we firstly observed that TSA strongly reduced the expression of COX-2 protein in response to LPS stimulation in aged mouse macrophages. Although the current view realized that TSA has a positive effect on LPS-mediated high levels of COX-2 in RAW264.7 cells, Liu et al. (2010) found that TSA attenuated the recruitment of C/EBPδ and c-Jun to the COX-2 promoter region. Yamaguchi et al. (2005) reported that TSA blocked c-Jun binding to the COX-2 promoter, resulting in reduced COX-2 expression in esophageal squamous cell carcinoma. These data suggest that the distinct mechanisms of regulation of COX-2 synthesis by TSA are cell type dependent and TSA might suppress LPS-induced expression of COX-2 through the abovementioned mechanisms in primary mouse peritoneal macrophages. Moreover, TSA-mediated acetylation of miR-101b and miR-26b restrained aged-related increase in LPS-induced COX-2 expression. Thus, age-dependent epigenetic regulation of the miRNAs and their targets may then be the very molecular force driving the age-related immune disorders. Additionally, HDAC inhibitors have emerged as potent contenders for treatment of inflammatory diseases at present (Camelo et al. 2005; Halili et al. 2009; Kim et al. 2007). The anti-inflammatory effect of HDAC inhibitor observed in this study is more potent in the elderly. Thus, HDAC inhibitors might be useful in relieving aged-related inflammatory progress.
In summary, this study reveals a new mechanism underlying the aged-related increase in LPS-induced COX-2 expression in mouse macrophages. These findings provide novel evidence for the studies on the regulation of macrophage-related immune senescence, and miR-101b and miR-26b are expected to be promising antiaging targets in the treatment of aged-related inflammatory diseases.
Acknowledgments
This work was partially supported by the State Key Basic Research Program of China (Grant No. 2013CB530805) and the Natural Science Foundation of China (Grant Nos. 81001315 and 30972684).
Contributor Information
Minghong Jiang, Phone: +86 10 6915 6409, Email: jiangminghong@163.com.
Dexian Zheng, Phone: +86 10 6915 6409, Email: zhengdx2008@gmail.com.
References
- Appleby S. B., Ristimaki A., Neilson K., Narko K., Hla T. Structure of the human cyclo-oxygenase-2 gene. Biochem J. 1994;302(Pt 3):723–727. doi: 10.1042/bj3020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek B. S., Kim J. W., Lee J. H., Kwon H. J., Kim N. D., Kang H. S., Yoo M. A., Yu B. P., Chung H. Y. Age-related increase of brain cyclooxygenase activity and dietary modulation of oxidative status. J Gerontol A Biol Sci Med Sci. 2001;56(10):B426–B431. doi: 10.1093/gerona/56.10.B426. [DOI] [PubMed] [Google Scholar]
- Beharka A. A., Wu D., Han S. N., Meydani S. N. Macrophage prostaglandin production contributes to the age-associated decrease in T cell function which is reversed by the dietary antioxidant vitamin E. Mech Ageing Dev. 1997;93(1–3):59–77. doi: 10.1016/S0047-6374(96)01819-2. [DOI] [PubMed] [Google Scholar]
- Calvanese V., Lara E., Kahn A., Fraga M. F. The role of epigenetics in aging and age-related diseases. Ageing Res Rev. 2009;8(4):268–276. doi: 10.1016/j.arr.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Camelo S., Iglesias A. H., Hwang D., Due B., Ryu H., Smith K., Gray S. G., Imitola J., Duran G., Assaf B., Langley B., Khoury S. J., Stephanopoulos G., De Girolami U., Ratan R. R., Ferrante R. J., Dangond F. Transcriptional therapy with the histone deacetylase inhibitor trichostatin A ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;164(1–2):10–21. doi: 10.1016/j.jneuroim.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A., Tranguch S., Daikoku T., Jensen K., Furneaux H., Dey S. K. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc Natl Acad Sci U S A. 2007;104(38):15144–15149. doi: 10.1073/pnas.0705917104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W. G., Zhu Y., Wu K. K. Role of p300 and PCAF in regulating cyclooxygenase-2 promoter activation by inflammatory mediators. Blood. 2004;103(6):2135–2142. doi: 10.1182/blood-2003-09-3131. [DOI] [PubMed] [Google Scholar]
- Engels B. M., Hutvagner G. Principles and effects of microRNA-mediated post-transcriptional gene regulation. Oncogene. 2006;25(46):6163–6169. doi: 10.1038/sj.onc.1209909. [DOI] [PubMed] [Google Scholar]
- FitzGerald G. A. COX-2 and beyond: approaches to prostaglandin inhibition in human disease. Nat Rev Drug Discov. 2003;2(11):879–890. doi: 10.1038/nrd1225. [DOI] [PubMed] [Google Scholar]
- Fraga M. F., Ballestar E., Paz M. F., Ropero S., Setien F., Ballestar M. L., Heine-Suner D., Cigudosa J. C., Urioste M., Benitez J., Boix-Chornet M., Sanchez-Aguilera A., Ling C., Carlsson E., Poulsen P., Vaag A., Stephan Z., Spector T. D., Wu Y. Z., Plass C., Esteller M. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102(30):10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasca D., Blomberg B. B. Effects of aging on B cell function. Curr Opin Immunol. 2009;21(4):425–430. doi: 10.1016/j.coi.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grolleau-Julius A., Ray D., Yung R. L. The role of epigenetics in aging and autoimmunity. Clin Rev Allergy Immunol. 2010;39(1):42–50. doi: 10.1007/s12016-009-8169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackl M., Brunner S., Fortschegger K., Schreiner C., Micutkova L., Muck C., Laschober G. T., Lepperdinger G., Sampson N., Berger P., Herndler-Brandstetter D., Wieser M., Kuhnel H., Strasser A., Rinnerthaler M., Breitenbach M., Mildner M., Eckhart L., Tschachler E., Trost A., Bauer J. W., Papak C., Trajanoski Z., Scheideler M., Grillari-Voglauer R., Grubeck-Loebenstein B., Jansen-Durr P., Grillari J. miR-17, miR-19b, miR-20a, and miR-106a are down-regulated in human aging. Aging Cell. 2010;9(2):291–296. doi: 10.1111/j.1474-9726.2010.00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halili M. A., Andrews M. R., Sweet M. J., Fairlie D. P. Histone deacetylase inhibitors in inflammatory disease. Curr Top Med Chem. 2009;9(3):309–319. doi: 10.2174/156802609788085250. [DOI] [PubMed] [Google Scholar]
- Hao Y., Gu X., Zhao Y., Greene S., Sha W., Smoot D. T., Califano J., Wu T. C., Pang X. Enforced expression of miR-101 inhibits prostate cancer cell growth by modulating the COX-2 pathway in vivo. Cancer Prev Res (Phila) 2011;4(7):1073–1083. doi: 10.1158/1940-6207.CAPR-10-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper K. A., Tyson-Capper A. J. Complexity of COX-2 gene regulation. Biochem Soc Trans. 2008;36(Pt 3):543–545. doi: 10.1042/BST0360543. [DOI] [PubMed] [Google Scholar]
- Hayek M. G., Mura C., Wu D., Beharka A. A., Han S. N., Paulson K. E., Hwang D., Meydani S. N. Enhanced expression of inducible cyclooxygenase with age in murine macrophages. J Immunol. 1997;159(5):2445–2451. [PubMed] [Google Scholar]
- Haynes L., Maue A. C. Effects of aging on T cell function. Curr Opin Immunol. 2009;21(4):414–417. doi: 10.1016/j.coi.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang D., Jang B. C., Yu G., Boudreau M. Expression of mitogen-inducible cyclooxygenase induced by lipopolysaccharide: mediation through both mitogen-activated protein kinase and NF-kappaB signaling pathways in macrophages. Biochem Pharmacol. 1997;54(1):87–96. doi: 10.1016/S0006-2952(97)00154-8. [DOI] [PubMed] [Google Scholar]
- Issa J. P. Age-related epigenetic changes and the immune system. Clin Immunol. 2003;109(1):103–108. doi: 10.1016/S1521-6616(03)00203-1. [DOI] [PubMed] [Google Scholar]
- Ji Y., He Y., Liu L., Zhong X. MiRNA-26b regulates the expression of cyclooxygenase-2 in desferrioxamine-treated CNE cells. FEBS Lett. 2010;584(5):961–967. doi: 10.1016/j.febslet.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Jiang M., Xiang Y., Wang D., Gao J., Liu D., Liu Y., Liu S., Zheng D. Dysregulated expression of miR-146a contributes to age-related dysfunction of macrophages. Aging Cell. 2012;11(1):29–40. doi: 10.1111/j.1474-9726.2011.00757.x. [DOI] [PubMed] [Google Scholar]
- Kim H. G., Han E. H., Jang W. S., Choi J. H., Khanal T., Park B. H., Tran T. P., Chung Y. C., Jeong H. G. Piperine inhibits PMA-induced cyclooxygenase-2 expression through downregulating NF-kappaB, C/EBP and AP-1 signaling pathways in murine macrophages. Food Chem Toxicol. 2012;50(7):2342–2348. doi: 10.1016/j.fct.2012.04.024. [DOI] [PubMed] [Google Scholar]
- Kim H. J., Kim K. W., Yu B. P., Chung H. Y. The effect of age on cyclooxygenase-2 gene expression: NF-kappaB activation and IkappaBalpha degradation. Free Radic Biol Med. 2000;28(5):683–692. doi: 10.1016/S0891-5849(99)00274-9. [DOI] [PubMed] [Google Scholar]
- Kim H. J., Rowe M., Ren M., Hong J. S., Chen P. S., Chuang D. M. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: multiple mechanisms of action. J Pharmacol Exp Ther. 2007;321(3):892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- Korhonen P., Helenius M., Salminen A. Age-related changes in the regulation of transcription factor NF-kappa B in rat brain. Neurosci Lett. 1997;225(1):61–64. doi: 10.1016/S0304-3940(97)00190-0. [DOI] [PubMed] [Google Scholar]
- Li G., Yu M., Lee W. W., Tsang M., Krishnan E., Weyand C. M., Goronzy J. J. Decline in miR-181a expression with age impairs T cell receptor sensitivity by increasing DUSP6 activity. Nat Med. 2012;18(10):1518–1524. doi: 10.1038/nm.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Kong X., Zhang J., Luo Q., Li X., Fang L. MiRNA-26b inhibits proliferation by targeting PTGS2 in breast cancer. Cancer Cell Int. 2013;13(1):7. doi: 10.1186/1475-2867-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton P. J., Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5(2):133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- Liu Y. W., Wang S. A., Hsu T. Y., Chen T. A., Chang W. C., Hung J. J. Inhibition of LPS-induced C/EBP delta by trichostatin A has a positive effect on LPS-induced cyclooxygenase 2 expression in RAW264.7 cells. J Cell Biochem. 2010;110(6):1430–1438. doi: 10.1002/jcb.22682. [DOI] [PubMed] [Google Scholar]
- Morita I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002;68-69:165–175. doi: 10.1016/S0090-6980(02)00029-1. [DOI] [PubMed] [Google Scholar]
- Mukherjee D., Nissen S. E., Topol E. J. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 2001;286(8):954–959. doi: 10.1001/jama.286.8.954. [DOI] [PubMed] [Google Scholar]
- Park S., Kang S., Min K. H., Woo Hwang K., Min H. Age-associated changes in MicroRNA expression in bone marrow derived dendritic cells. Immunol Investig. 2013;42(3):179–190. doi: 10.3109/08820139.2012.717328. [DOI] [PubMed] [Google Scholar]
- Pasinetti G. M., Aisen P. S. Cyclooxygenase-2 expression is increased in frontal cortex of Alzheimer’s disease brain. Neuroscience. 1998;87(2):319–324. doi: 10.1016/S0306-4522(98)00218-8. [DOI] [PubMed] [Google Scholar]
- Plowden J., Renshaw-Hoelscher M., Engleman C., Katz J., Sambhara S. Innate immunity in aging: impact on macrophage function. Aging Cell. 2004;3(4):161–167. doi: 10.1111/j.1474-9728.2004.00102.x. [DOI] [PubMed] [Google Scholar]
- Radak Z., Chung H. Y., Naito H., Takahashi R., Jung K. J., Kim H. J., Goto S. Age-associated increase in oxidative stress and nuclear factor kappaB activation are attenuated in rat liver by regular exercise. FASEB J. 2004;18(6):749–750. doi: 10.1096/fj.03-0509fje. [DOI] [PubMed] [Google Scholar]
- Sarkar D., Fisher P. B. Molecular mechanisms of aging-associated inflammation. Cancer Lett. 2006;236(1):13–23. doi: 10.1016/j.canlet.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Sato F., Tsuchiya S., Meltzer S. J., Shimizu K. MicroRNAs and epigenetics. FEBS J. 2011;278(10):1598–1609. doi: 10.1111/j.1742-4658.2011.08089.x. [DOI] [PubMed] [Google Scholar]
- Shakhov A. N., Collart M. A., Vassalli P., Nedospasov S. A., Jongeneel C. V. Kappa B-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor alpha gene in primary macrophages. J Exp Med. 1990;171(1):35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strillacci A., Griffoni C., Sansone P., Paterini P., Piazzi G., Lazzarini G., Spisni E., Pantaleo M. A., Biasco G., Tomasi V. MiR-101 downregulation is involved in cyclooxygenase-2 overexpression in human colon cancer cells. Exp Cell Res. 2009;315(8):1439–1447. doi: 10.1016/j.yexcr.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Walford R. L. Auto-immunity and aging. J Gerontol. 1962;17:281–285. doi: 10.1093/geronj/17.3.281. [DOI] [PubMed] [Google Scholar]
- Walford R. L. The immunologic theory of aging. Gerontologist. 1964;4:195–197. doi: 10.1093/geront/4.4.195. [DOI] [PubMed] [Google Scholar]
- Wu D., Marko M., Claycombe K., Paulson K. E., Meydani S. N. Ceramide-induced and age-associated increase in macrophage COX-2 expression is mediated through up-regulation of NF-kappa B activity. J Biol Chem. 2003;278(13):10983–10992. doi: 10.1074/jbc.M207470200. [DOI] [PubMed] [Google Scholar]
- Wu D., Mura C., Beharka A. A., Han S. N., Paulson K. E., Hwang D., Meydani S. N. Age-associated increase in PGE2 synthesis and COX activity in murine macrophages is reversed by vitamin E. Am J Physiol. 1998;275(3 Pt 1):C661–C668. doi: 10.1152/ajpcell.1998.275.3.C661. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Lantowski A., Dannenberg A. J., Subbaramaiah K. Histone deacetylase inhibitors suppress the induction of c-Jun and its target genes including COX-2. J Biol Chem. 2005;280(38):32569–32577. doi: 10.1074/jbc.M503201200. [DOI] [PubMed] [Google Scholar]
- Young L. E., Moore A. E., Sokol L., Meisner-Kober N., Dixon D. A. The mRNA stability factor HuR inhibits microRNA-16 targeting of COX-2. Mol Cancer Res. 2012;10(1):167–180. doi: 10.1158/1541-7786.MCR-11-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]