Abstract
The purpose of this study was to analyze the effects of a progressive resistance training (RT) program on C-reactive protein (CRP), blood glucose (GLU), and lipid profile in older women with differing levels of RT experience. Sixty-five older women (68.9 ± 6.1 years, 67.1 ± 13.1 kg) were separated according to RT experience: an advanced group composed by 35 participants who previously carried out 24 weeks of RT and a novice group composed by 30 participants without previous experience in RT (n = 30). Both groups performed a RT program comprised of eight exercises targeting all the major muscles. Training was carried out 3 days/week for 8 weeks. Serum triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), GLU, and CRP concentrations were determined pre- and post- intervention after 12 h fasting. A significant group by time interaction (P < 0.05) for the TC (novice = −1.9 % vs. advanced = 1.0 %), and CRP (novice = −22.9 % vs. advanced = −54.5 %) was observed. A main effect of time (P < 0.05) was identified for the GLU (novice = −2.6 % vs. advanced = −6.6 %), TG (novice = −12.9 % vs. advanced = −5.7 %), HDL-C (novice = +6.7 % vs. advanced = +2.6 %), and LDL-C (novice = −34.0 % vs. advanced = −25.4 %). These results suggest that RT improves the metabolic profile of older women and that training for a longer period of time seems to produce more pronounced reductions mainly on CRP.
Keywords: Aging, C-reactive protein, Lipoproteins, Strength training
Introduction
Biological aging is associated with morphological and biochemical alterations that increase the risk of developing cardiovascular disease, which is the major cause of morbidity and mortality in the older adult (Zaslavsky and Gus 2002). Among the risk factors, serum blood levels of glucose and lipids profile have been shown to be of a greater impact on cardiovascular disease risk in women compared to men (Roeters van Lennep et al. 2002; Tan et al. 2010). Chronic inflammation has also been implicated as playing a role in cardiovascular disease (Davison and Davis 2003). To this end, elevations in C-reactive protein (CRP), an inflammation biomarker, may be considered an important independent indicator of mortality for cardiovascular and metabolic diseases (Kengne et al. 2012; Pai et al. 2004).
Resistance training (RT) has been promoted as a means to attenuate the deleterious effects of aging (American College of Sports Medicine 2009; Garber et al. 2011). In addition to its effects on enhancing muscular strength, RT provides numerous additional benefits to older adults’ health that may directly impact cardiovascular disease risk, including positive changes in body composition (American College of Sports Medicine 2009; Garber et al. 2011), improvements in metabolic profile (Conceicao et al. 2013; Kelley and Kelley 2009; Maesta et al. 2007; Williams et al. 2011), and a reduction in inflammatory markers (de Salles et al. 2010; Lera Orsatti et al. 2014; Phillips et al. 2012; Stewart et al. 2007).
Metabolic and inflammatory changes induced by RT may be dependent on the specific characteristics of the program (Calle and Fernandez 2010; Lira et al. 2010; Sheikholeslami Vatani et al. 2011). In addition, training status may play an important role in metabolic and inflammatory adaptations induced by RT (Calle and Fernandez 2010; Pedersen and Febbraio 2008). Compared to novice trainees, subjects previously exposed to RT have a greater skeletal muscle mass, which is the primary metabolic target tissue for glucose (GLU) and triglycerides metabolism (Strasser and Schobersberger 2011) and acts as an endocrine organ releasing myokines with anti-inflammatory properties (Pedersen and Febbraio 2008).
Although previous research has investigated the effects of RT on metabolic and inflammatory changes in older women, it remains undetermined whether these changes are influenced by distinct levels of previous experience with RT. Therefore, the purpose of this study was to analyze the effects of a comprehensive RT program on CRP, blood GLU, and lipid profile of older women with differing levels of RT experience. We hypothesized that the experience in RT for a longer period could improve the metabolic and inflammatory responses in older women.
Methods
Participants
Sixty-five older women (≥60 years old) volunteered to participate in this study. Recruitment was carried out through newspaper and radio advertisings and home delivery of leaflets in the central area and residential neighborhoods. Participants were assigned to one of the two groups according to their previous RT experience: a group of advanced participants (n = 35, 70.0 ± 6.1 years, 68.9 ± 14.7 kg, 156.0 ± 6.5 cm, 28.2 ± 5.3 kg/m2) that previously carried out 24 weeks of RT, and a group of novice participants in RT (n = 30, 67.6 ± 5.7 years, 64.9 ± 10.6 kg, 154.7 ± 5.5 cm, 27.0 ± 4.1 kg/m2). All participants completed health history and physical activity questionnaires and met the following inclusion criteria: non-hypertensive (systolic blood pressure ≤ 140 mmHg, and diastolic blood pressure ≤ 90 mmHg), non-diabetic, free from cardiac or renal dysfunction, nonsmokers, not receiving hormonal replacement therapy, and not performing any regular physical exercise for more than once a week over the 6 months preceding the beginning of the study. Participants passed a diagnostic, graded exercise stress test with 12-lead electrocardiogram reviewed by a cardiologist and were released with no restrictions for participation in this study. Adherence to the program was satisfactory, with all participants performing >85 % of the total sessions. Written informed consent was obtained from all participants after a detailed description of study procedures was provided. This investigation was conducted according to the Declaration of Helsinki and was approved by the local University Ethics Committee.
Experimental design
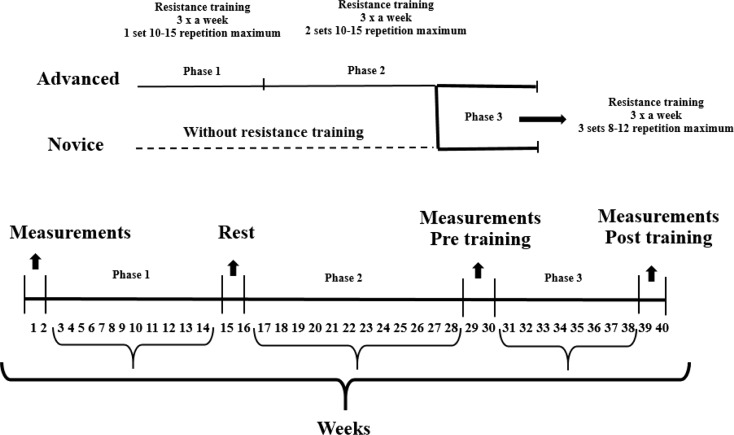
The study was carried out over a period of 40 weeks that were divided into three phases. The first two phases consisted of 12-week periods where participants who were ultimately placed in the advanced group underwent a RT program for 24 weeks (weeks 3–14 and 17–28). In the third phase of the experiment, new participants were recruited to form the group comprised of novices, and all participants then performed 8 weeks of RT (weeks 31–38). At the beginning of phases 1 and 2, and at the end of the third phase, 2 weeks were used for evaluations consisting of anthropometric measures, one repetition maximum tests (1RM), body composition analysis by dual-energy X-ray absorptiometry (DXA), and blood sampling for biochemical analysis. The experimental design is displayed in Fig. 1.
Fig. 1.
Experimental design
Anthropometry
Body mass was measured to the nearest 0.1 kg using a calibrated electronic scale (Balmak, Laboratory Equipment Labstore, Curitiba, PR, Brazil), with subjects wearing light workout clothing and no shoes. Height was measured using a wooden stadiometer to the nearest 0.1 cm while subjects were standing without shoes. Body mass index was calculated as the body mass in kilograms divided by the square of the height in meters.
Body composition
Body composition measurements were carried out using a whole-body DXA scan (Lunar Prodigy, model NRL 41990, GE Lunar, Madison, WI). Appendicular lean-soft tissue mass was estimated by the sum of the upper and lower limbs lean-soft tissue on both the right and the left sides. The appendicular lean-soft tissue value was then used to estimate skeletal muscle mass using the prediction equation developed and cross-validated by Kim et al. (2002). Prior to scanning, participants were instructed to remove all objects containing metal. Scans were performed with the participants lying in the supine position along the table’s longitudinal centerline axis. Feet were taped together at the toes to immobilize the legs while the hands were maintained in a pronated position within the scanning region. Participants remained motionless during the entire scanning procedure. Both calibration and analysis were carried out by a skilled laboratory technician. The equipment calibration followed the manufacturer’s recommendations. The software generated standard lines that set apart the limbs from the trunk and head. These lines were adjusted by the same technician using specific anatomical points determined by the manufacturer. Analyses during the intervention were performed by the same technician who was blinded to intervention time point. Previous test-retest scans resulted in a standard error of measurement of 0.29 kg and intraclass correlation coefficient of 0.997 for skeletal muscle mass and a standard error of measurement of 0.90 kg and intraclass correlation coefficient of 0.980 for %fat.
Muscular strength
Maximal dynamic strength was evaluated using the 1RM test assessed on chest press, knee extension, and preacher curl performed in this exact order. Testing for each exercise was preceded by a warm-up set (6–10 repetitions), with approximately 50 % of the estimated load used in the first attempt of the 1RM. This warm-up was also used to familiarize the subjects with the testing equipment and lifting technique. The testing procedure was initiated 2 min after the warm-up. The participants were instructed to try to accomplish two repetitions with the imposed load in three attempts in both exercises. The rest period was 3 to 5 min between each attempt and 5 min between exercises. The 1RM was recorded as the last resistance lifted in which the subject was able to complete only one single maximal execution (Amarante do Nascimento et al. 2013). Execution technique for each exercise was standardized and continuously monitored to ensure reliability. All 1RM testing sessions were supervised by two researchers for greater safety and integrity of the participants. Verbal encouragement was given throughout each test. Three 1RM sessions were performed separated by 48 h (ICC ≥ 0.96). The highest load achieved among the three sessions was used for analysis in each exercise. Total strength was determined by the sum of the three exercises.
Biochemical analysis
Serum levels of high-sensitivity CRP, GLU, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglycerides (TG) were determined after 12 h fasting by a laboratory technician. The blood was taken from the antecubital vein. The participants were instructed not to perform vigorous exercise for the preceding 24 h and to avoid alcohol or caffeinated beverages 72 h before collection. Measurements were performed by standard methods in a specialized laboratory at the university hospital. Samples were deposited in vacuum tubes with a gel separator without anticoagulant and were centrifuged for 10 min at 1008×g for serum separation. Thereafter, the CRP, TG, and HDL-C concentrations were determined. The LDL-C was calculated using the Friedewald, Levy, and Fredrickson (Friedewald et al. 1972) equation, where LDL-C = TC − (HDL-C + TGL/5). The analyses were carried out using a biochemical autoanalyzer system (Dimension RxL Max—Siemens Dade Behring) according to established methods in the literature consistent with the manufacturer’s recommendations.
Dietary intake
Participants were instructed by a dietitian to complete a food record on three nonconsecutive days (two weekdays and one weekend day) pre- and post-training. Participants were given specific instructions regarding the recording of portion sizes and quantities to identify all food and fluid intake, in addition to viewing food models in order to enhance precision. Total energy intake, protein, carbohydrate, and lipid content were calculated using nutrition analysis software (Avanutri Processor Nutrition Software, Rio de Janeiro, Brazil; version 3.1.4). All participants were asked to maintain their normal diet throughout the study period.
Resistance training program
Supervised RT was performed during the morning hours in the university facilities. The protocol was based on recommendations for RT in an older population to improve muscular strength and hypertrophy (American College of Sports Medicine 2009; Garber et al. 2011). All participants were personally supervised by physical education professionals with substantial RT experience to help ensure consistent and safe performance. Participants performed RT using a combination of free weights and machines.
The sessions took place three times per week on Mondays, Wednesdays, and Fridays. The RT program was a whole body program with eight exercises comprising one exercise with free weights and seven with machines performed in the following order: chest press, horizontal leg press, seated row, knee extension, preacher curl (free weights), leg curl, triceps pushdown, and seated calf raise. Participants of the advanced group performed one set of 10–15 repetitions maximum during the first 12-week phase and then two sets of 10–15 repetitions maximum in the second 12-week phase. In the third phase, participants in both groups performed three sets of 8–12 repetitions maximum. Participants were instructed to inhale during the eccentric phase and exhale during the concentric phase while maintaining a constant velocity of movement at a ratio of approximately 1:2 (concentric and eccentric phases, respectively). Participants were afforded 1 to 2 min of rest interval between sets and 2 to 3 min between each exercise. Instructors adjusted the loads of each exercise according to the participant’s abilities and improvements in exercise capacity throughout the study in order to ensure that they were exercising with as much resistance as possible while maintaining proper exercise technique. Progression was planned when the upper limits of the repetitions-zone were completed for two consecutive training sessions then weight was increased 2–5 % for the upper limb exercises and 5–10 % for the lower limb exercises in the next session (American College of Sports Medicine 2009).
Statistical analyses
Two-way analysis of covariance (ANCOVA) for repeated measures was applied for comparisons, with baseline scores used as covariates. For total energy and macronutrients intake comparisons, two-way analysis of variance (ANOVA) for repeated measures was applied. When F-ratio was significant, Bonferroni’s post hoc test was employed to identify the mean differences. The effect size (ES) was calculated as the post-training mean minus the pre-training mean divided by the pooled standard deviation of pre- and post-training, in order to verify the magnitude of the differences, where an ES of 0.20–0.40 was considered as small, 0.50–0.79 as moderate, and >0.80 as large (Cohen 1988). For all statistical analyses, significance was accepted at P < 0.05. The data were analyzed using STATISTICA software version 10.0 (StatSoft Inc., Tulsa, OK, USA).
Results
Total energy and macronutrients daily intake at pre- and post-training are shown in Table 1. There were no significant (P > 0.05) main effects, indicating that the relative daily energy and macronutrients intake were not different between groups and did not change over time.
Table 1.
Dietary intake at pre- and post-training according to resistance training experience. Data are expressed as mean and standard deviation
| Advanced (n = 35) |
Novice (n = 30) |
Effects ANOVA |
F | P | |
|---|---|---|---|---|---|
| Carbohydrate (g/kg/d) | Group | 0.02 | 0.87 | ||
| Pre | 2.6 ± 0.8 | 2.9 ± 1.3 | Time | 1.11 | 0.30 |
| Post | 3.0 ± 1.8 | 2.9 ± 1.2 | Interaction | 0.95 | 0.33 |
| Protein (g/kg/d) | Group | 0.01 | 0.89 | ||
| Pre | 0.8 ± 0.4 | 0.8 ± 0.3 | Time | 1.04 | 0.31 |
| Post | 0.8 ± 0.4 | 0.9 ± 0.6 | Interaction | 1.36 | 0.25 |
| Lipids (g/kg/d) | Group | 0.22 | 0.64 | ||
| Pre | 0.6 ± 0.3 | 0.5 ± 0.2 | Time | 0.27 | 0.23 |
| Post | 0.6 ± 0.4 | 0.6 ± 0.5 | Interaction | 1.52 | 0.23 |
| Total energy intake (kcal/kg/d) | Group | 0.01 | 0.97 | ||
| Pre | 19.7 ± 6.7 | 19.0 ± 8.0 | Time | 0.90 | 0.35 |
| Post | 21.2 ± 12.4 | 21.8 ± 12.0 | Interaction | 0.08 | 0.76 |
Table 2 depicts the baseline scores of the participants who composed the advance group. The muscular strength and body composition outcomes are presented in Table 3. Statistically significant interactions (P < 0.05) were observed for 1RM in the three exercises and for the total strength (sum of the three exercises), in which the novice group presented greater increases compared with the advanced group. Statistical significance (P < 0.05) was also observed for body composition, wherein the novice group showed a higher increase in skeletal muscle mass than the advanced group, while for body fat, only the novice altered the scores after 8 weeks of RT.
Table 2.
General characteristics of the advanced group at baseline (n = 35)
| Mean | Standard deviation | 95 % confidence interval | |
|---|---|---|---|
| Chest press (kg) | 37.2 | 5.7 | 34.6–39.9 |
| Knee extension (kg) | 41.8 | 9.4 | 37.8–45.7 |
| Preacher curl (kg) | 19.3 | 3.3 | 18.1–20.5 |
| Total strength (kg) | 98.4 | 15.7 | 91.6–105.1 |
| Body fat (%) | 38.8 | 7.4 | 36.2–41.3 |
| Skeletal muscle mass (kg) | 18.4 | 2.9 | 16.9–19.1 |
| Glucose (mg/dL) | 106.2 | 21.5 | 98.8–113.6 |
| Total cholesterol (mg/dL) | 194.2 | 38.6 | 180.9–207.5 |
| Triglycerides (mg/dL) | 115.2 | 45.3 | 93.6–125.2 |
| HDL-C (mg/dL) | 55.1 | 19.2 | 46.9–56.4 |
| LDL-C (mg/dL) | 115.7 | 40.0 | 102.0–129.5 |
| CRP (mg/L) | 3.3 | 2.4 | 2.4–4.1 |
HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, CRP C-reactive protein
Table 3.
Muscular strength, body fat, and skeletal muscle mass of older women at pre- and post-training according to resistance training experience. Data are expressed as mean, standard deviation, and confidence interval
| Advanced (n = 35) |
Novice (n = 30) |
Effects ANCOVA |
F | P | |
|---|---|---|---|---|---|
| Chest press (kg) | |||||
| Pre | 44.8 ± 8.0 (41.9–47.7) | 35.5 ± 5.1 (33.6–37.8) | Time | 0.17 | 0.67 |
| Post | 46.0 ± 7.9* (43.2–48.9) | 40.0 ± 6.9* (37.4–42.5) | Interaction | 23.96 | <0.001 |
| Δ% | +2.7 | +12.7 | |||
| Effect size | 0.15 | 0.75 | |||
| Knee extension (kg) | |||||
| Pre | 52.4 ± 12.2 (48.0–56.7) | 39.0 ± 7.0 (36.4–41.5) | Time | 4.51 | 0.03 |
| Post | 55.7 ± 12.3* (51.4–60.1) | 45.4 ± 8.7* (42.2–48.5) | Interaction | 7.76 | 0.01 |
| Δ% | +6.3 | +16.4 | |||
| Effect size | 0.27 | 0.82 | |||
| Preacher curl (kg) | |||||
| Pre | 24.3 ± 4.4 (22.7–25.9) | 19.5 ± 3.1 (18.3–20.6) | Time | 1.62 | 0.20 |
| Post | 25.5 ± 4.6* (23.8–27.2) | 21.9 ± 3.9* (20.5–23.3) | Interaction | 5.56 | 0.02 |
| Δ% | +4.9 | +12.3 | |||
| Effect size | 0.26 | 0.67 | |||
| Total strength (kg) | |||||
| Pre | 121.8 ± 21.9 (113.9–129.7) | 94.0 ± 13.3 (89.2–98.8) | Time | 0.71 | 0.40 |
| Post | 127.6 ± 22.2* (119.6–135.6) | 107.7 ± 17.5* (101.0–113.6) | Interaction | 22.48 | <0.001 |
| Δ% | +4.8 | +14.6 | |||
| Effect size | 0.26 | 0.89 | |||
| Body fat (%) | |||||
| Pre | 37.3 ± 8.7 (34.2–40.9) | 38.7 ± 7.6 (35.9–41.5) | Time | 4.06 | 0.04 |
| Post | 37.2 ± 9.1 (34.0–40.5) | 37.5 ± 5.1* (34.6–40.3) | Interaction | 13.18 | <0.001 |
| Δ% | −0.3 | −3.1 | |||
| Effect size | −0.01 | −0.19 | |||
| Skeletal muscle mass (kg) | |||||
| Pre | 19.2 ± 3.5 (17.9–20.4) | 16.4 ± 2.1 (15.7–17.2) | Time | 5.14 | 0.02 |
| Post | 19.4 ± 3.4* (18.1–20.6) | 17.1 ± 2.1* (16.3–17.9) | Interaction | 8.79 | 0.01 |
| Δ% | +1.0 | +4.3 | |||
| Effect size | 0.06 | 0.33 | |||
*P < 0.05 vs. pre
Table 4 displays the metabolic parameters at pre- and post-training. There was a significant interaction (P < 0.05) for TC, in which only the novice group reduced their scores after training. A main effect of time (P < 0.05) was found for GLU, TG, HDL-C, and LDL-C, with both groups showing similar improvements (decrease of GLU, TG, and LDL-C and increase of HDL-C).
Table 4.
Metabolic profile of older women at pre- and post-training according to resistance training experience. Data are expressed as mean, standard deviation, and confidence interval
| Advanced (n = 35) |
Novice (n = 30) |
Effects ANCOVA |
F | P | |
|---|---|---|---|---|---|
| Glucose (mg/dL) | |||||
| Pre | 99.9 ± 22.1 (92.0–107.7) | 95.8 ± 10.9 (91.8–99.7) | Time | 31.28 | <0.001 |
| Post | 93.3 ± 14.3* (88.2–98.4) | 93.3 ± 14.2* (88.3–98.5) | Interaction | 0.47 | 0.49 |
| Δ% | −6.6 | −2.6 | |||
| Effect size | −0.36 | −0.20 | |||
| Total cholesterol (mg/dL) | |||||
| Pre | 186.5 ± 29.2 (176.1–196.0) | 212.2 ± 44.4 (196.1–228.2) | Time | 60.12 | <0.001 |
| Post | 188.3 ± 23.6 (179.9–196.6) | 208.2 ± 40.1* (193.8–222.7) | Interaction | 3.77 | 0.05 |
| Δ% | +1.0 | −1.9 | |||
| Effect size | 0.07 | −0.09 | |||
| Triglycerides (mg/dL) | |||||
| Pre | 110.1 ± 52.9 (91.3–128.9) | 133.7 ± 68.7 (108.9–158.7) | Time | 67.20 | <0.001 |
| Post | 103.8 ± 44.0* (88.1–119.4) | 116.5 ± 46.7* (99.6–133.3) | Interaction | 1.02 | 0.31 |
| Δ% | −5.7 | −12.9 | |||
| Effect size | −0.13 | −0.30 | |||
| HDL-C (mg/dL) | |||||
| Pre | 57.4 ± 17.7 (51.1–63.7) | 52.6 ± 12.2 (48.2–57.1) | Time | 41.95 | <0.001 |
| Post | 58.9 ± 16.3* (53.1–64.6) | 56.1 ± 14.0* (51.0–61.2) | Interaction | 0.20 | 0.64 |
| Δ% | +2.6 | +6.7 | |||
| Effect size | 0.09 | 0.27 | |||
| LDL-C (mg/dL) | |||||
| Pre | 107.0 ± 32.0 (98.8–115.2) | 132.7 ± 37.1 (119.3–146.1) | Time | 48.94 | <0.001 |
| Post | 79.8 ± 15.0* (74.4–85.1) | 87.6 ± 23.3* (79.1–96.0) | Interaction | 0.75 | 0.38 |
| Δ% | −25.4 | −34.0 | |||
| Effect size | −1.16 | −1.49 | |||
HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol
*P < 0.05 vs. pre
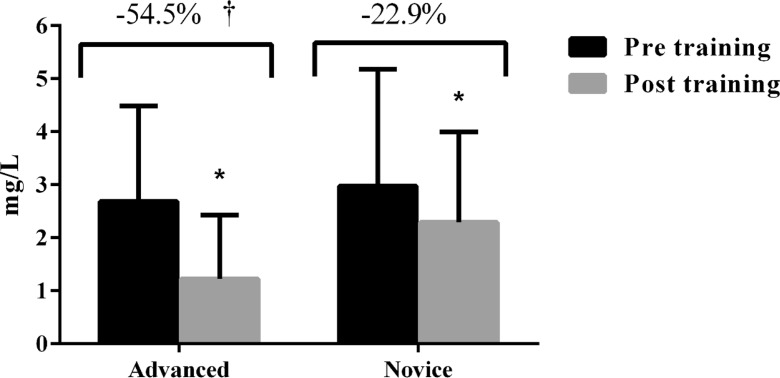
The changes in CRP from pre to post training are displayed in Fig. 2. A significant interaction was observed (F = 7.73, P < 0.01) with the advanced group showing a higher decrease in CRP scores after the intervention period in comparison with the novice group (advanced = 2.6 ± 1.8 vs. 1.2 ± 1.1 mg/L, ES = −0.97; novice = 2.9 ± 2.2 vs. 2.2 ± 1.7 mg/L, ES = −0.35). The covariate means as well as the adjusted post-training scores are presented in Table 5.
Fig. 2.
C-reactive protein in advanced (n = 35) and novice (n = 30) resistance trained older women after 8 weeks of intervention. *P < 0.05 vs. pre. † P < 0.05 vs. novice
Table 5.
Adjusted mean by ANCOVA to post-test
| Covariate mean | Advanced (n = 35) | Novice (n = 30) | |||
|---|---|---|---|---|---|
| Mean | 95 % CI | Mean | 95 % CI | ||
| Chest press (kg) | 40.2 | 41.1 | 40.4–42.1 | 44.9 | 43.9–45.9 |
| Knee extension (kg) | 45.8 | 49.1 | 47.8–50.6 | 52.2 | 50.7–53.7 |
| Preacher curl (kg) | 21.9 | 23.0 | 22.3–23.7 | 24.3 | 23.6–27.1 |
| Total strength (kg) | 107.9 | 112.9 | 110.7–115.5 | 121.9 | 119.5–124.0 |
| Body fat (%) | 38.0 | 37.9 | 37.5–38.4 | 36.7 | 36.2–37.2 |
| Skeletal muscle mass (kg) | 17.9 | 18.1 | 17.9–18.2 | 18.4 | 18.3–18.6 |
| Glucose (mg/dL) | 97.9 | 92.3 | 88.2–96.4 | 94.3 | 90.2–98.5 |
| Total cholesterol (mg/dL) | 199.1 | 189.9 | 178.0–201.5 | 206.6 | 194.4–217.9 |
| Triglycerides (mg/dL) | 121.8 | 104.3 | 87.9–120.3 | 116.0 | 99.8–132.5 |
| HDL-C (mg/dL) | 55.1 | 58.4 | 53.1–63.7 | 56.6 | 51.2–61.9 |
| LDL-C (mg/dL) | 119.7 | 81.4 | 74.2–88.5 | 85.9 | 78.6–93.3 |
| CRP (mg/L) | 2.82 | 1.26 | 0.75–1.77 | 2.25 | 1.73–2.73 |
HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, CRP C-reactive protein
Discussion
The main and novel finding of this investigation was that the longer experience in RT may influence CRP responses. We hypothesized that previously trained older women would have superior metabolic and inflammatory biomarker reductions; the hypothesis was confirmed for the used biomarker inflammatory outcome. To the authors’ knowledge, this was the first study to compare the adaptive responses from RT in older women with different levels of RT experience.
Previous studies have found reductions in CRP after a RT program in novice participants (Buresh and Berg 2014; Calle and Fernandez 2010; Donges et al. 2010; Lee et al. 2015; Lera Orsatti et al. 2014; Mavros et al. 2014; Phillips et al. 2012). For example, Lera Orsatti et al. (Lera Orsatti et al. 2014) showed that RT performed three times a week was able to reduce CRP levels in sedentary obese older women after 16 weeks of training. Similarly, Phillips et al. (Phillips et al. 2012) observed a 33 % reduction on CRP in obese postmenopausal women after 12 weeks of RT. Nevertheless, some authors have reported no effect of RT on CRP in older adults (Nikseresht et al. 2014; Simonavice et al. 2014). These conflicting results may be at least partly related to methodological issues between studies, such as training protocols that used different volumes and/or intensities, differences in characteristics of the participants (e.g., obese vs. non-obese), and differences in baseline CRP levels (Calle and Fernandez 2010; Donges et al. 2013; Lera Orsatti et al. 2014).
Although the exact mechanisms by which RT leads to CRP reduction are not fully understood, we can speculate on several possibilities. Muscle contraction produces myokines that have anti-inflammatory effects antagonizing the pro-inflammatory cytokines, thus favoring a reduction in inflammation and CRP (Pedersen and Febbraio 2008). Moreover, changes in some body composition components, such as body fat and skeletal muscle mass may play an important role for the reductions in inflammatory levels (Buresh and Berg 2014; Calle and Fernandez 2010; Donges et al. 2010; Lee et al. 2015; Mavros et al. 2014; Selvin et al. 2007), and RT has a positive impact on both of these outcomes. On the other hand, some investigations observed that the decrease in CRP is independent of body composition changes (Lera Orsatti et al. 2014; Phillips et al. 2012). While the novice group in the present study displayed greater improvements in body composition, the advanced group had a better response in inflammatory profile. These findings suggest that factors other than body composition changes are responsible for CRP decreases in those with RT experience. The greater reductions in CRP observed in the advanced group may be related to the amount of muscle mass involved in the contractile activity (Pedersen and Febbraio 2008). Moreover, myokines are sensitive to exercise intensity (Pedersen and Febbraio 2008), and although the trained and the novice participants performed the exercise at the same relative intensity, the advanced group achieved higher absolute workloads.
Another metabolic profile variable that showed different adaptive responses between advanced and novice was the TC, in which only the beginner group reduced their values after the 8-week intervention period. Thus, while RT can play an important role in decreasing TC during the initial stages of training, no additive effect is seen over time. However, it is noteworthy that the TC includes both LDL-C and HDL-C, and given the antagonistic differences between these two lipoproteins on health, analysis of TC itself can lead to misinterpretations. Regarding specific lipoproteins fractions and TG, we observed beneficial changes to both HDL-C and LDL-C as well as to the TG irrespective of training status. Previous studies have also observed positive effects of RT on lipoprotein fractions in older women (Conceicao et al. 2013; Maesta et al. 2007; Williams et al. 2011), conceivably by mediating LDL-C plasma removal, and lipid oxidation via an increase in lipoprotein lipase (Mann et al. 2014). RT also can increase the ability of skeletal muscle to use fat, thereby reducing the levels of plasma lipids (Mann et al. 2014). Conversely, other studies found no improvement in lipoprotein after a period of RT (Lera Orsatti et al. 2014; Marques et al. 2009). Discrepancies between findings are not clear and, therefore, require further investigation.
With respect to fasting blood GLU, our experiment noted modest but significant reductions after participation in the RT program. This decrease is logical, given that RT primarily relies on the glycolytic pathway for energy (Lambert and Flynn 2002), and thus, the immediate effects from the exercise bout itself helps to improve GLU homeostasis. Moreover, RT enhances insulin sensitivity via multiple mechanisms, including an increase of skeletal muscle mass and qualitative improvement in muscle metabolic properties, such as increased density of GLUT-4 transporters and an increase in the content/activity of the enzyme glycogen synthase (Phillips and Winett 2010). It therefore is not surprising that the present study observed reductions on blood GLU.
Our results showed that muscular strength and hypertrophy adaptations from a RT program are greater in novice lifters compared with those who have previous training experience. Research indicates that the acute increase in muscular protein synthesis after a RT bout is modulated by an individual’s training status (Damas et al. 2015), with evidence showing that untrained subjects have higher mixed muscular protein synthesis rates compared to trained individuals (Phillips et al. 1999).
In a recent review on this topic, Damas et al. (2015) pointed out that exercise-induced increases in muscular protein synthesis are longer-lived and peak later in the untrained state than in the trained state, resulting in greater overall muscular protein synthesis. Thus, a heightened anabolic environment seemingly enhances the ability for novice RT participants to achieve greater net protein accretion compared to trained individuals. In support of this hypothesis, Ahtiainen et al. (Ahtiainen et al. 2003) compared the quadriceps femoris cross-sectional area following 21 weeks of heavy RT in untrained and trained subjects. After 21 weeks, only the untrained group demonstrated significant hypertrophy, while the trained group had reached a plateau in muscular adaptations.
It is reasonable to speculate that the systematic manipulation of RT program variables in the present study helped to promote significant increases in skeletal muscle mass in the trained group, while preventing a plateau. Regarding muscular strength, the greater increases observed in the novice group may be a result of neural adaptations that occur along the training continuum, such as increased motor recruitment and decreased co-activation of antagonist muscles (Gabriel et al. 2006).
The study has some limitations. For one, the results are specific to older women and should not be extrapolated to other populations. The lack of pro- and anti-inflammatory markers can be considered a limitation as well. Moreover, we were not able to monitor physical activity levels outside of the study environment, which potentially may have confounded results. On the other hand, to our knowledge, the present study is the first to investigate the effect of differing levels of RT experience on muscular strength, body composition, and metabolic and inflammatory profiles in older women and thus represents an important contribution to the current body of literature. Furthermore, the improvements in muscular strength, body composition, and metabolic and inflammatory markers observed in this investigation occurred without changes in the dietary habits of the participants, which suggest that the macronutrients and total energy intake during the progressive RT program in this study were sufficient to support adaptations derived from the RT.
Conclusion
The results suggest that RT is an effective strategy to reduce CRP levels as well as to improve blood GLU and lipid profile of older women. In addition, the magnitude of the changes in CRP concentration, muscular strength, and body composition is dependent on individual training status.
Compliance with ethical standards
Written informed consent was obtained from all participants after a detailed description of study procedures was provided. This investigation was conducted according to the Declaration of Helsinki and was approved by the local University Ethics Committee.
References
- American College of Sports Medicine position stand Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41:687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- Ahtiainen JP, Pakarinen A, Alen M, Kraemer WJ, Hakkinen K. Muscle hypertrophy, hormonal adaptations and strength development during strength training in strength-trained and untrained men. Eur J Appl Physiol. 2003;89:555–563. doi: 10.1007/s00421-003-0833-3. [DOI] [PubMed] [Google Scholar]
- Amarante do Nascimento M, Borges Januario RS, Gerage AM, Mayhew JL, Cheche Pina FL, Cyrino ES. Familiarization and reliability of one repetition maximum strength testing in older women. J Strength Cond Res. 2013;27:1636–1642. doi: 10.1519/JSC.0b013e3182717318. [DOI] [PubMed] [Google Scholar]
- Buresh R, Berg K. Role of exercise on inflamation and chronic disease. Strength Cond J. 2014;36:87–93. doi: 10.1519/SSC.0000000000000071. [DOI] [Google Scholar]
- Calle MC, Fernandez ML. Effects of resistance training on the inflammatory response. Nutr Res Pract. 2010;4:259–269. doi: 10.4162/nrp.2010.4.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Erlbaum Associate; 1988. [Google Scholar]
- Conceicao MS, et al. Sixteen weeks of resistance training can decrease the risk of metabolic syndrome in healthy postmenopausal women. Clin Interv Aging. 2013;8:1221–1228. doi: 10.2147/CIA.S44245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas F, Phillips S, Vechin FC, Ugrinowitsch C. A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Med. 2015;45:801–807. doi: 10.1007/s40279-015-0320-0. [DOI] [PubMed] [Google Scholar]
- Davison S, Davis SR. New markers for cardiovascular disease risk in women: impact of endogenous estrogen status and exogenous postmenopausal hormone therapy. J Clin Endocrinol Metab. 2003;88:2470–2478. doi: 10.1210/jc.2002-021929. [DOI] [PubMed] [Google Scholar]
- de Salles BF, Simao R, Fleck SJ, Dias I, Kraemer-Aguiar LG, Bouskela E. Effects of resistance training on cytokines. Int J Sports Med. 2010;31:441–450. doi: 10.1055/s-0030-1251994. [DOI] [PubMed] [Google Scholar]
- Donges CE, Duffield R, Drinkwater EJ. Effects of resistance or aerobic exercise training on interleukin-6, C-reactive protein, and body composition. Med Sci Sports Exerc. 2010;42:304–313. doi: 10.1249/MSS.0b013e3181b117ca. [DOI] [PubMed] [Google Scholar]
- Donges CE, Duffield R, Guelfi KJ, Smith GC, Adams DR, Edge JA. Comparative effects of single-mode vs. duration-matched concurrent exercise training on body composition, low-grade inflammation, and glucose regulation in sedentary, overweight, middle-aged men. Appl Physiol Nutr Metab. 2013;38:779–788. doi: 10.1139/apnm-2012-0443. [DOI] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Gabriel DA, Kamen G, Frost G. Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. 2006;36:133–149. doi: 10.2165/00007256-200636020-00004. [DOI] [PubMed] [Google Scholar]
- Garber CE, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Kelley GA, Kelley KS. Impact of progressive resistance training on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Prev Med. 2009;48:9–19. doi: 10.1016/j.ypmed.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Kengne AP, Batty GD, Hamer M, Stamatakis E, Czernichow S. Association of C-reactive protein with cardiovascular disease mortality according to diabetes status: pooled analyses of 25,979 participants from four U.K. prospective cohort studies. Diabetes Care. 2012;35:396–403. doi: 10.2337/dc11-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76:378–383. doi: 10.1093/ajcn/76.2.378. [DOI] [PubMed] [Google Scholar]
- Lambert CP, Flynn MG. Fatigue during high-intensity intermittent exercise: application to bodybuilding. Sports Med. 2002;32:511–522. doi: 10.2165/00007256-200232080-00003. [DOI] [PubMed] [Google Scholar]
- Lee JS, Kim CG, Seo TB, Kim HG, Yoon SJ. Effects of 8-week combined training on body composition, isokinetic strength, and cardiovascular disease risk factors in older women. Aging Clin Exp Res. 2015;27:179–186. doi: 10.1007/s40520-014-0257-4. [DOI] [PubMed] [Google Scholar]
- Lera Orsatti F, Nahas EA, Maesta N, Nahas Neto J, Lera Orsatti C, Vannucchi Portari G, Burini RC. Effects of resistance training frequency on body composition and metabolics and inflammatory markers in overweight postmenopausal women. J Sports Med Phys Fitness. 2014;54:317–325. [PubMed] [Google Scholar]
- Lira FS, et al. Low and moderate, rather than high intensity strength exercise induces benefit regarding plasma lipid profile. Diabetol Metab Syndr. 2010;2:31. doi: 10.1186/1758-5996-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maesta N, Nahas EA, Nahas-Neto J, Orsatti FL, Fernandes CE, Traiman P, Burini RC. Effects of soy protein and resistance exercise on body composition and blood lipids in postmenopausal women. Maturitas. 2007;56:350–358. doi: 10.1016/j.maturitas.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014;44:211–221. doi: 10.1007/s40279-013-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques E, Carvalho J, Soares JM, Marques F, Mota J. Effects of resistance and multicomponent exercise on lipid profiles of older women. Maturitas. 2009;63:84–88. doi: 10.1016/j.maturitas.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Mavros Y, et al. Reductions in C-reactive protein in older adults with type 2 diabetes are related to improvements in body composition following a randomized controlled trial of resistance training. J Cachexia Sarcopenia Muscle. 2014;5:111–120. doi: 10.1007/s13539-014-0134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikseresht M, Sadeghifard N, Agha-Alinejad H, Ebrahim K. Inflammatory markers and adipocytokine responses to exercise training and detraining in men who are obese. J Strength Cond Res. 2014;28:3399–3410. doi: 10.1519/JSC.0000000000000553. [DOI] [PubMed] [Google Scholar]
- Pai JK, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Phillips MD, Patrizi RM, Cheek DJ, Wooten JS, Barbee JJ, Mitchell JB. Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med Sci Sports Exerc. 2012;44:2099–2110. doi: 10.1249/MSS.0b013e3182644984. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Ferrando AA, Wolfe RR. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol. 1999;276:E118–E124. doi: 10.1152/ajpendo.1999.276.1.E118. [DOI] [PubMed] [Google Scholar]
- Phillips SM, Winett RA. Uncomplicated resistance training and health-related outcomes: evidence for a public health mandate. Curr Sports Med Rep. 2010;9:208–213. doi: 10.1249/JSR.0b013e3181e7da73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeters van Lennep JE, Westerveld HT, Erkelens DW, van der Wall EE. Risk factors for coronary heart disease: implications of gender. Cardiovasc Res. 2002;53:538–549. doi: 10.1016/S0008-6363(01)00388-1. [DOI] [PubMed] [Google Scholar]
- Selvin E, Paynter NP, Erlinger TP. The effect of weight loss on C-reactive protein: a systematic review. Arch Intern Med. 2007;167:31–39. doi: 10.1001/archinte.167.1.31. [DOI] [PubMed] [Google Scholar]
- Sheikholeslami Vatani D, Ahmadi S, Ahmadi Dehrashid K, Gharibi F. Changes in cardiovascular risk factors and inflammatory markers of young, healthy, men after six weeks of moderate or high intensity resistance training. J Sports Med Phys Fitness. 2011;51:695–700. [PubMed] [Google Scholar]
- Simonavice E, Liu PY, Ilich JZ, Kim JS, Arjmandi B, Panton LB. The effects of a 6-month resistance training and dried plum consumption intervention on strength, body composition, blood markers of bone turnover, and inflammation in breast cancer survivors. Appl Physiol Nutr Metab. 2014;39:730–739. doi: 10.1139/apnm-2013-0281. [DOI] [PubMed] [Google Scholar]
- Stewart LK, et al. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med Sci Sports Exerc. 2007;39:1714–1719. doi: 10.1249/mss.0b013e31811ece1c. [DOI] [PubMed] [Google Scholar]
- Strasser B, Schobersberger W. Evidence for resistance training as a treatment therapy in obesity. J Obes. 2011 doi: 10.1155/2011/482564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan YY, Gast GC, van der Schouw YT. Gender differences in risk factors for coronary heart disease. Maturitas. 2010;65:149–160. doi: 10.1016/j.maturitas.2009.09.023. [DOI] [PubMed] [Google Scholar]
- Williams AD, Almond J, Ahuja KD, Beard DC, Robertson IK, Ball MJ. Cardiovascular and metabolic effects of community based resistance training in an older population. J Sci Med Sport. 2011;14:331–337. doi: 10.1016/j.jsams.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Zaslavsky C, Gus I. The elderly. heart disease and comorbidities. Arq Bras Cardiol. 2002;79:635–639. doi: 10.1590/S0066-782X2002001500011. [DOI] [PubMed] [Google Scholar]