Abstract
Ageing and inactivity both contribute to systemic inflammation, but the effects of inactivity on inflammation in healthy elderly individuals have not been elucidated. We hypothesised that 14-day bed rest could affect the pro- and anti-inflammatory markers in young subjects differently than in older adults. A short-term 14-day horizontal bed rest study (BR14) has been used as a model of inactivity in two groups of healthy male volunteers: 7 aged 18–30 years (young) and 16 aged 55–65 years (older adults). The effects of inactivity on inflammation were compared. Key low-grade inflammation mediators, tumour necrosis factor α (TNF-α), interleukin-6 (IL-6), visfatin, resistin, and anti-inflammatory adiponectin were measured in fasting serum samples, collected at baseline (BDC) and post BR14. Young responded to BR14 by increasing serum visfatin and resistin while older adults responded to BR14 by increasing IL-6 and TNF-α. In addition, serum adiponectin increased in all participants. Data from correlation analysis demonstrated positive association between Δ serum visfatin and Δ IL-6 in both groups, while Δ serum adiponectin was negatively associated with Δ TNF-α in young and positively associated with Δ resistin in the older adults. As little as 14 days of complete physical inactivity (BR14) negatively affected markers of low-grade inflammation in both groups, but the inflammation after BR14 was more pronounced in older adults. The effect of BR14 on IL-6 and resistin differed between young and older adults. Inflammatory responses to BR14 in older adults differed from those reported in the literature for obese or subjects in pathological states, suggesting potentially different mechanisms between inactivity- and obesity-induced inflammations.
Keywords: Complete inactivity, Inflammation, Adiponectin, TNF-α, IL-6
Introduction
In the elderly, an acute illness can often cause hospitalisation with prolonged bed rest (Farriols et al. 2009). The combination of ageing plus immobilisation and acute illness can lead to a cascade of events that may lead to dependency (Farriols et al. 2009). Distinguishing between the contributions of the first two (ageing plus immobilisation) and the contribution of the acute illness is essential in order to diminish the possibility of the patient becoming dependent. A bed rest study of healthy elderly subjects would enable us to make such a distinction. Since bed rest and space flight have been proposed as a model of accelerated ageing (Vernikos and Schneider 2010), the contribution of bed rest in elderly to dependency could be substantial.
Horizontal bed rest (BR) is one of the most widely used models for studying the effects of inactivity on the human body. This has been confirmed by BR studies conducted on younger subjects, noting that physical inactivity or prolonged exposure to microgravity causes changes in body composition: a decrease in muscle mass (MM), a decrease in bone mineral density, and an increase in fat mass (FM) (Biolo et al. 2008; de Boer et al. 2008; Pišot et al. 2008; Rittwegger et al. 2009; Vernikos and Schneider 2010). MM and FM changes contribute to an enhanced pro-inflammatory burden by affecting the normal cross-talk of metabolism-regulating hormones.
While regular muscle contraction produces myokines—cytokines, which suppress pro-inflammatory activity (Brüünsgaard 2005)—adipose tissue produces a variety of pro-inflammatory mediators named adipokines that act both locally in the adipose tissue and systemically leading to low-grade inflammation (Havel 2002). Low-grade inflammation in the elderly is associated with higher circulating levels of pro-inflammatory cytokines (tumour necrosis factor α (TNF-α), interleukin-6 (IL-6) and C-reactive protein (CRP)) even in the absence of chronic disease (Brüünsgaard and Pedersen 2003; Krabbe et al. 2004). These pro-inflammatory conditions and processes appear to play a central role in the decline of MM, promote catabolism and develop a cluster of metabolic diseases (Tilg and Moschen 2008). In addition to the well-known pro-inflammatory cytokines, the serum levels of visfatin and resistin secretion and expression are upregulated during inflammation (Stofkova 2010).
Visfatin, originally isolated from peripheral blood lymphocytes as a pre-B cell colony-enhancing factor (Samal et al. 1994), acts as a cytokine with glucose-regulatory and pro-inflammatory properties (Kadoglou et al. 2010). It is produced in several tissues, with higher production in visceral adipose tissue compared to subcutaneous adipose tissue (Fukuhara et al. 2005), suggesting that visfatin plays a key role in various pathophysiological processes. However, the physiological role of visfatin is not completely understood. Resistin, another inflammatory molecule, is primarily expressed in monocytic cells from which it is secreted (Patel et al. 2003). In addition, it is also well known that resistin is more expressed in preadipocytes during adipogenesis than in mature adipocytes (McTernan et al. 2002). Resistin is considered to be a link between obesity, insulin resistance and inflammation (Kusminski et al. 2005), but the pathophysiological role of resistin is not well understood yet.
It has become clear that pro-inflammatory and anti-inflammatory cytokines can be regulated by physical activity (Pedersen and Hoffman-Goetz 2000). Many studies have demonstrated that regular exercise may reduce markers of systemic inflammation (Woods et al. 2012). Both IL-6 and CRP levels are highly regulated by acute exercise (Fischer et al. 2004), but basal circulating IL-6 and CRP levels also appear to be sensitive to the extent of regular physical activity (Panagiotakos et al. 2005). Due to the complexity of visfatin regulation, there are conflicting data concerning the effects of physical activity on its levels (Lee et al. 2010; Jorge et al. 2011). Regular exercise reduces FM and adipose tissue inflammation which is known to contribute to systemic inflammation (Calder et al. 2011). Independent of the loss of FM, exercise also increases muscle production of IL-6, responsible to reduce tumour necrosis factor α (TNF-α) production and increase anti-inflammatory cytokines such as adiponectin (Starkie et al. 2003). On the other hand, the effect of inactivity on serum levels of adipokines and inflammatory markers has not been fully elucidated.
Only a few shorter BR studies of the elderly/older adults have been described in literature so far. The first one did not assess the inflammatory burden of the participants (Kortebein et al. 2008), while the second study assessed the inflammation connected to a decrease in MM, but the 7-day BR (BR7) was too short to show any significant changes of inflammation markers in serum (Drummond et al. 2013). The third study of Coker et al (2014) also did not assess the role of changes in inflammation; however, metabolic dysregulation of free fatty acids and insulin sensitivity were observed after 10-day BR (Coker et al. 2014). The last study of Tanner et al. (2015) demonstrated that older adults were more susceptible than young persons to muscle loss after 5-day BR, partially due to suppression of protein synthesis and a marginal increase in proteolytic markers (Tanner et al. 2015).
Understanding how physical inactivity in the elderly alters body composition and energy requirements and causes low-grade inflammation is of paramount importance because it is implied in the onset of several pathologies related to an increasingly sedentary lifestyle. Knowledge of the effects of physical inactivity on the elderly is also important in cases of acute illness that requires hospitalisation and where a decline in the functional ability of the patients is a frequent outcome (Farriols et al. 2009).
Therefore, the objective of our study was to investigate the effects of a 14-day bed rest on induced inflammation, measured by anti-inflammatory adiponectin and pro-inflammatory visfatin, resistin, TNF-α, and IL-6. The hypothesis was put forward that significant alterations in inflammatory markers would occur during short-term BR in young and older adults.
Methods
Participants
A 14-day experimental bed rest with comparable groups of healthy young (N = 7) men aged 18–30 years and healthy older adult (N = 16) men aged 53–65 years was performed in the Valdoltra Orthopaedic Hospital, Slovenia.
Participants were recruited from Slovenia. Their basic morphological parameters are displayed in Table 1. A medical history and physical examination were performed on each potential participant, as well as routine blood and urine analysis. Exclusion criteria included smokers; regular alcohol consumers; ferromagnetic implants; history of deep vein thrombosis with D-dimer >500 μg/L; acute or chronic skeletal, neuromuscular, metabolic and cardiovascular disease conditions; pulmonary embolism and a Short Physical Performance Battery (SPBB) score <9 (Guralnik et al. 1995).
Table 1.
Mean (SD), baseline (BDC) and post 14-day bed rest (BR14) of anthropometric parameters in young and older adults
| Young (N = 7) | Older adults (N = 16) | |||||
|---|---|---|---|---|---|---|
| BDC | BR14 | p | BDC | BR14 | p | |
| Mass (kg) | 74.8 (8.8) | 71.6 (8.3) | <0.001 | 79.9 (12.3) | 77.5 (11.7) | <0.001 |
| BMI (kg/m2) | 24.0 (2.4) | 22.9 (2.1) | <0.001 | 26.6 (4.4) | 25.8 (4.1) | <0.001 |
| TBW (l) | 44.6 (2.8) | 41.2 (2.8) | 0.005 | 46.0 (6.3) | 43.1 (4.8) | <0.001 |
| FFM (kg) | 60.9 (3.8) | 56.3 (3.9) | 0.004 | 61.8 (9.1) | 58.0 (7.2) | <0.001 |
| FM (kg) | 14.0 (6.2) | 15.3 (7.0) | 0.170 | 18.1 (6.1) | 19.5 (7.0) | 0.049 |
| MM (kg) | 42.5 (3.4) | 39.0 (3.2) | 0.021 | 39.8 (6.8) | 37.5 (4.8) | 0.007 |
Comparison of mass, height, BMI, TBW, FFM, FM and MM between baseline and intervention levels was done with Student t test for dependent variables (paired t test). An alpha level of 0.05 was set for test to indicate significance. No significant differences at baseline and after intervention were observed between groups (independent t test)
BDC baseline data collection, BR14 14-day bed rest, BMI body mass index, TBW total body water, FFM fat-free mass, FM fat mass, MM muscle mass
The subjects were free of any clinical or biochemical disease and did not take any medications 3 months prior to the study. The experimental conditions were well tolerated by all volunteers, and all tests were conducted under close medical supervision. Subjects were informed of the purpose, procedures and potential risk of participation in the study before signing the informed consent. The study was part of a broad-based research project PANGeA: Physical Activity and Nutrition for Good quality Ageing and was approved by the Slovenian National Medical Ethics Committee. The study was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki.
Study design
The participants were housed in hospital rooms and were under constant surveillance and provided with 24-h medical care. The study consisted of three different consecutive phases: (1) 3 days of accommodation to study environment and diet with baseline data collection (BDC). Habitual physical activity of the participants was maintained during this phase. (2) The second phase was BR14 (BR1 to BR14). During this phase, no deviations from the lying position were permitted, and subjects were continuously monitored by video cameras. Subjects were under periodical medical control with physical therapy (passive stretching of lower limbs and low back) on every day and constant nursing assistance. Temperature, blood systolic and diastolic pressure and heart frequency were measured, and blood analysis including D-dimer test and Doppler echography was performed. Neither exercise nor muscle contraction tests were allowed. (3) The third phase was 2 days of ambulatory care (post BR 14 data collection) followed by 28-day recovery that was not a subject of this report.
During BR14, participants performed all daily activities in the horizontal position mostly by the help of one pillow to support the head; the participants were not allowed to raise the head or lift their arms above their head, only to turn on both sides of the body. Moreover, the participants urinated and performed bowel movement in a urine bottle and under body pots, respectively, always lying on their back—they were in a horizontal position. During recruitment medical assessment and on the seventh day of BR14, participants were checked for deep vein thrombosis using D-dimer test and Doppler echography. Preventively, all participants wore elastic socks.
To avoid weight gain due to the reduced activity level, the subjects followed a eucaloric diet during the study. Diet composition and energy intake were monitored daily by an expert dietician, according to the Food and Agriculture Organization/World Health Organization equations (Müller et al. 2004), and dietary energy requirements were designed for each subject multiplying resting energy expenditure by factors 1.4 and 1.2 in the ambulatory period (phases 1 and 3) and BR14 (phase 2), respectively (Biolo et al. 2008). Subjects were required to consume all served food as scheduled by the dietician. The average diet composition reflected typical Slovenian dietary habits to avoid nutrient composition changes. Energy balance maintenance was controlled by body composition analysis in terms of fat mass maintenance.
Every day, subjects received three main meals (breakfast, lunch and dinner). Relative macronutrient content was constantly maintained at 60 % of energy from carbohydrate, 25 % from fat and 15 % from protein during the entire period. Breakfast represented 30 % of dietary energy requirements (DER), lunch 40 % and dinner represented 30 % of the DER. Energy intake was adjusted according to weekly changes in fat mass. The participants had ad libitum access to water throughout the whole study.
Twenty-four subjects were recruited for the study: 8 young and 16 older adults for BR 14. Eight randomly selected older adults participants followed daily 50-min spatial navigation training by navigating through mazes using a hand joystick. As we did not find any significant effects of this intervention in any of the parameters studied, we did not introduce a third group in this report. One young dropped out of the study due to increased values of D-dimer.
Measurements
Anthropometry
Subject height was measured before BR14 to the nearest 0.1 cm in standing position, without shoes, using a Leicester Height Measure (Invicta Plastics Limited, Oadby, England), and the body mass of the participants wearing only underwear was measured to the nearest 0.1 kg. Body mass index (BMI) was calculated using the standard formula.
Fat-free mass (FFM), FM, total body water (TBW) and MM were assessed once per week by bioelectric impedance analysis (BioScan 916S, Maltron, UK) (Dumler and Kilates 2005) performed by a four-electrode measurement system. Bioimpedance analysis was performed on all subjects before, during and after BR.
Biochemical analysis
Fasting venous blood samples were collected in the morning between 7 A.M. and 8 A.M. in 4-ml vacuum test tubes (Becton Dickinson, Rutherford, USA) on the day BDC-1 (baseline) and on the day BR14 (post-intervention). Serum was separated, frozen and stored at −20 °C until subsequent analysis. Analyses were performed as described previously (Jenko-Pražnikar et al. 2013; Jurdana et al. 2013). Briefly, serum concentrations of IL-6, TNF-α, adiponectin, resistin and visfatin were performed in duplicate on a microplate reader (Tecan, Männedorf, Switzerland) using human ELISA Kits for IL-6, TNF-α (both Thermo Fisher Scientific Inc., Rockford, USA), adiponectin, resistin (both BioVendor, Lab. Med. Inc., Brno, Czech Republic) and visfatin (BioVision, Mountain View, CA, USA). The assay sensitivity was 30 pg/ml for visfatin, 10 pg/ml for adiponectin, 33 pg/ml for resistin, <1 pg/ml for IL-6 and <2 pg/ml for TNF-α, and interassay CVs were <10 %. In addition, serum concentrations of C-reactive protein (CRP) were measured using Olympus reagents and performed on an AU 680 analyser (Beckman Coulter), as previously reported (Jenko-Pražnikar et al. 2013).
Statistical analysis
All analyses were carried out using SPSS 20 software (SPSS Inc., Chicago, USA). After checking for normality, non-normally distributed data (TNF-α, IL-6 and CRP) were transformed using logarithmic function. Means and standard deviation were calculated for the baseline and post-intervention data for all parameters. To investigate the effect of BR14 on adipokines and inflammatory markers concentration, subjects were classified in young and older adults. Baseline characteristics of the subjects by groups were compared by independent t tests. Comparison of body composition parameters between baseline and intervention levels was done with Student t test for dependent variables (paired t test). An alpha level of 0.05 was set for the test to indicate significance. To identify possible differences on the variables (adiponectin, IL-6, resistin, visfatin and TNF-α) due to the effect of BR14, two-way analysis of variance for repeated measurements (two-way repeated measures ANOVA) was performed. Covariates were BMI and age. Additionally, Pearson’s correlation coefficient was used on the relative changes of different variables. For all the analyses, the level of statistical significance was set at p < 0.05.
Results
Anthropometrics
The studied BDC and BR14 anthropometric and body composition parameters are given in Table 1. No significant differences at baseline were observed between young and older adult groups. BR14 significantly affected lean body mass, and changes in both groups were observed in body mass (and BMI; p < 0.001), TBW (p < 0.001), FFM (p < 0.001) and MM (young p < 0.05, older adults p < 0.01). There were no significant changes in FM.
Serum adipokines and inflammatory mediators
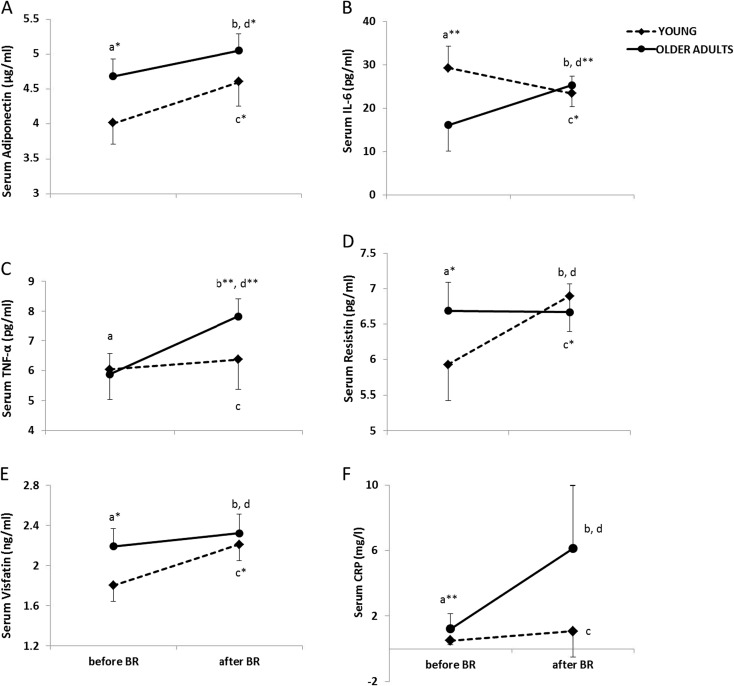
Baseline and after BR14 serum concentrations of inflammatory mediators, adipokines and CRP in young and older adults are presented in Fig. 1.
Fig. 1.
Serum concentrations of adipokines, cytokines and CRP in the basal state before and after short-term 14-day bed rest (BR14) in young (dashed black lines) and older adult (solid black lines) subjects. Before BR: young (N = 7) and older adults (N = 16). After BR: young (N = 7) and older adults (N = 16). a Adiponectin. b IL-6. c TNF-α. d Resistin. e Visfatin. f CRP. a basal state before BR14 in young and older adults, b basal state after BR14 in young and older adults, c basal state before vs. after in young, d basal state before vs. after BR14 in older adults. Data were log-transformed before statistical test for TNF-α and IL-6. Mean data are shown with the SD. Baseline characteristics of the subjects by groups were compared by independent t tests. To identify possible differences on the variables (adiponectin, IL-6, resistin, visfatin and TNF-α) due to the effect of 14 day BR, two-way analysis of variance for repeated measurements (two-way repeated measures ANOVA) was performed. Covariates were BMI and age. *p < 0.05; **p < 0.01.
In contrast to anthropometric parameters, there were already differences in adipokines and inflammatory parameters between young and older adults in baseline. Basal serum adiponectin (p = 0.01), visfatin (p = 0.01) and resistin (p = 0.02) before BR were lower, while levels of basal serum IL-6 (p = 0.001) were higher in young than in older adults. There were no differences in levels of basal serum TNF-α.
In response to BR, serum adiponectin increased in young (by 15 %, p = 0.013, F = 12.10, η2 = 0.669) and older adult subjects (by 9 %, p = 0.041, F = 3.81, η2 = 0.521); on the other hand, serum TNF-α and IL-6 only increased in older adults (by 30 %, p = 0.003, F = 14.11, η2 = 0.485; and for 50 %, p = 0.004, F = 12.56, η2 = 0.456, respectively). Moreover, the young subject’s serum IL-6 decreased and serum visfatin and serum resistin increased (15 %, p = 0.025, F = 8.77, η2 = 0.594; 28 %, p = 0.02, F = 7.07, η2 = 0.550; and 12 %, p = 0.03, F = 5.23, η2 = 0.481, respectively). There were no differences in serum resistin and visfatin in older adult subjects and in serum TNF-α in young subjects in response to BR (Fig. 1).
Correlation analysis
Correlation analysis was performed to investigate the possible associations between changes in adipokines, cytokines and anthropometric parameters in young and older adults. As shown in Table 2, Δ FM significantly correlated with Δ adiponectin in young, and Δ TNF-α in both groups. Only Δ TNF-α negatively correlated with Δ MM in young and older adults.
Table 2.
Associations between changes (baseline minus day 14 BR values) in adipokines, cytokines and anthropometric parameters in young and older adults
| Study group | ||
|---|---|---|
| Δ MM | Δ FM | |
| Δ Adiponectin | ||
| Young | −0.428 | 0.689* |
| Older adults | −0.104 | 0.184 |
| Δ Visfatin | ||
| Young | −0.270 | 0.015 |
| Older adults | −0.050 | 0.070 |
| Δ Resistin | ||
| Young | −0.160 | 0.149 |
| Older adults | −0.027 | 0.023 |
| Δ TNF-α | ||
| Young | −0.598* | 0.420* |
| Older adults | −0.345* | 0.311* |
| Δ IL-6 | ||
| Young | 0.002 | 0.102 |
| Older adults | 0.050 | −0.024 |
N = 7 (young); N = 16 (older adults). Associations between relative changes in adipokines, cytokines and anthropometric parameters were performed using Pearson’s correlation analysis
MM muscle mass, FM fat mass, TNF tumour necrosis factor, IL-6 interleukin 6
*p < 0.05 (associations were statistically significant)
Correlation of study variables
In all participants, Δ serum visfatin was positively associated with Δ IL-6. Δ Adiponectin was negatively associated with Δ TNF-α in young and positively associated with Δ resistin in older adults (Table 3).
Table 3.
Correlations of the study variables
| Variable and range | Δ Visfatin | Δ Resistin | Δ TNF-α | Δ IL-6 |
|---|---|---|---|---|
| Δ Adiponectin | ||||
| Young | 0.256 | 0.021 | −0.254* | −0.439 |
| Older adults | 0.327 | 0.546* | −0.134 | −0.150 |
| Δ Visfatin | ||||
| Young | 1 | 0.369 | 0.074 | 0.742* |
| Older adults | 1 | 0.021 | 0.083 | 0.376* |
| Δ Resistin | ||||
| Young | 1 | 0.050 | 0.472 | |
| Older adults | 1 | −0.085 | −0.125 | |
| Δ TNF-α | ||||
| Young | 1 | 0.552 | ||
| Older adults | 1 | 0.107 | ||
| Δ IL-6 | ||||
| Young | 1 | |||
| Older adults | 1 | |||
N = 7 (young); N = 16 (older adults). Associations between relative changes (baseline minus day 14 BR values) in adipokines and cytokines were performed using Pearson’s correlation analysis
IL-6 interleukin 6, TNF-α tumour necrosis factor α
*p < 0.05 (associations were statistically significant)
Discussion
Physical inactivity induced by BR14 was applied to assess the changes of low-grade inflammation markers, and anti-inflammatory adiponectin in healthy non obese adults; young and older adults were compared. So far, this was the longest BR study with elderly/older adults (Kortebein et al. 2008; Drummond et al. 2013, Coker et al. 2014; Tanner et al. 2015).
There were pronounced differences between the young and older adults, but the key finding of our study was that as little as 14 days of physical inactivity (BR) negatively affected markers of low-grade inflammation, especially in older adults.
It is well known that physical inactivity contributes to an enhanced pro-inflammatory burden (Krabbe et al. 2004) and affects human immune responses (Shearer et al 2009). In healthy subjects, physical inactivity is associated with increased FM, accumulation of visceral fat and decreased FFM, leading to low-grade inflammation and to the development of a cluster of diseases defined as the “diseasome of physical inactivity”, as proposed by Pedersen (2009).
After BR14, serum levels of TNF-α and IL-6 were lower in young than in older adults. TNF-α is a potent stimulator of IL-6 expression and secretion (Rotter et al. 2003) and a potent inhibitor of adiponectin (Bruun et al. 2003). The higher serum TNF-α concentrations observed in older adults may partly explain the greater increase in serum IL-6 and smaller increase in serum adiponectin. A significant relationship was recently demonstrated between intramuscular adipose tissue and muscle IL-6 expression in older, frail individuals with decreased mobility (Addison et al. 2014); therefore, the greater increase in IL-6 in older adults might be additionally explained by increase in intramuscular adipose tissue due to immobility in older adults. Neither TNF-α nor IL-6 serum concentration increased after a 7-day BR study with elderly performed by Drummond et al. (2013), although they reported local increases in IL-6 expression within the muscle tissue. Opposite to the older adults, we did not observe any increase in serum TNF-α in young in response to BR14, and consequently, a greater increase in serum adiponectin was observed. This differs from previous reports, where an increase in serum IL-6 as a response to BR14 with eucaloric diet in younger male subjects was reported, but no increase in response to BR14 with hypocaloric diet (Bosutti et al. 2008).
The effect of physical inactivity on serum TNF-α was pronounced in older adults. Adipose tissue macrophages are a major source of TNF-α (Zeyda and Stulnig 2007), which could indicate that macrophages infiltrate adipose tissue and secrete TNF-α in response to physical inactivity. Adipose tissue of obese individuals contains a higher number of macrophages than adipose tissue of lean subjects (Weisberg et al. 2003). In addition, Bruun et al. (2006) demonstrated that a 15-week lifestyle intervention in terms of hypocaloric diet and daily physical activity reduced the expression of macrophages and inflammatory proteins in adipose tissue of severely obese subjects (Bruun et al. 2006). A negative association between Δ TNF and Δ MM and positive association between Δ TNF and Δ FM found in our study suggest that physical inactivity changes were associated with high circulating levels of TNF-α. TNF-α leads to erosion of MM and induces impairment in muscle protein balance (Reid and Li 2001). Participants of the present study did not gain FM during the BR14 period, so the increase in serum TNF-α in older adults must be ascribed only to physical inactivity, as suggested previously by Højbjerre et al. (2011).
Adiponectin has garnered considerable interest due to its proposed salutary anti-inflammatory properties. Age-related changes in adiponectin levels remain controversial, due to inconsistent normalisation for adiposity and body fat distribution in the literature (Cnop et al. 2003; Andreasson et al. 2012). Recently, Schautz et al. (2012) demonstrated that adiponectin levels increased with age, partially independently of adiposity (Schautz et al. 2012). In accordance with the mentioned study, we found lower serum adiponectin in young in comparison to older adults. However, in both groups, young and older adults, a BR-induced increase in serum adiponectin was observed. Contrary to our findings, Nosova et al. (2014), who demonstrated that short-term physical inactivity impaired vascular function, observed statistically significant decreases in levels of adiponectin after 5-day BR in young subjects and no increase in inflammation (Nosova et al. 2014). We did not measure adiponectin nor inflammatory markers levels after 5-day BR, so we do not know the dynamics of the observed adiponectin and inflammation change. However, the upregulation of serum adiponectin in response to BR14 was probably a compensatory action to the BR-induced inflammation. To maintain homeostasis, this adaptation is rational, but whether the adaptation persists if the period of physical inactivity is prolonged is unknown. In contrast to Batista et al. (2012), who reported that plasma adiponectin concentrations were negatively associated with MM and FM, in the present study, we demonstrated that serum adiponectin levels increased with Δ FM in young subjects.
Furthermore, the anti-inflammatory, antidiabetic and antiatherogenic properties of adiponectin are mediated through a reciprocal association between adiponectin and CRP (Ouchi et al. 2003). A smaller increase in adiponectin concentration after BR14 was observed in older adults compared to the young, which is probably the result of increased serum levels of pro-inflammatory cytokines CRP, TNF-α and IL-6 in older adults. Indeed, a statistically significant negative correlation between Δ adiponectin and Δ TNF-α in young and positive association between Δ adiponectin and Δ resistin in older adults were confirmed.
Several studies have shown significant correlations between circulating serum resistin, visfatin and visceral fat, BMI and inflammation (Zahorska-Markiewicz et al. 2007; Moschen et al. 2007; Schwartz and Lazar 2011). We found a positive correlation between serum visfatin and serum pro-inflammatory IL-6. Previously, we demonstrated that the best significant predictor for serum visfatin levels in male subjects was aerobic capability—they were inversely correlated (Jurdana et al. 2013). In the present study, we observed increases in serum visfatin and resistin levels after BR14 in young, but no change in older adults; Δ visfatin was positively associated with Δ IL-6 in both groups. Data reported in literature about visfatin levels are contradicting. In a study of Kanda et al. (2007) in elderly bedridden patients, visfatin levels seemed to be independent of the time the patients were confined to bed but correlated with inflammation. Visfatin was even proposed as a potential marker of acute inflammation in elderly bedridden patients (Kanda et al. 2007). Our data evidenced that levels of visfatin at physiological condition of inactivity in healthy young might differ from what we observed in the healthy older adults and the results reported from previously mentioned studies which, nevertheless, did not measure healthy elderly subjects. That data suggested that physical inactivity-induced inflammation might differ from obesity-related inflammation in elderly.
Some studies, in particular bed rest at −6° head down tilt (HDTBR), report plasma volume variations (Feuerecker et al. 2013; Mutin-Carnino et al. 2013) which have impact on the inflammatory factors. However, the HDTBR has much more pronounced body volume variations during the course of the experiment than horizontal BR (Montgomery 1993). Therefore, during our bed rest study, without head down position, we did not assess the fluid shifts and consequently the effects of redistribution of body fluids.
We are well aware of the limitations of the study. First, we investigated a small group of participants. This is a characteristic of BR studies where larger groups are logistically impossible. Second, the study is limited to systemic inflammation. Our measurements do not permit to draw conclusions on the origin of the inflammatory markers nor to conclude any mechanisms. Further research to identify the serum inflammatory origin is needed by measuring local inflammatory markers in affected tissues. Third, only men participated in our study—for the female population, recruitment of the young control group should take into account their menstrual cycle phases, which would make the logistical part of the study very difficult. However, due to the differences in adipokine concentrations between men and women, a future female study should be considered.
Conclusions
In conclusion, BR14 caused inflammation in young and older adults, although the inflammatory marker responses differed between the groups. This was the first study to evaluate and compare inflammatory responses between healthy young and older adults, subjected to 14-day BR. Responses in older adults also differed from those reported in the literature for obese or subjects in pathological states, suggesting potentially different mechanisms between inactivity- and obesity-induced inflammations. More studies should be performed to confirm this. Our results also emphasise the particular importance of avoiding even short periods of physical inactivity in older adults.
Acknowledgments
We would like to thank the participants for their time and effort to ensure the success of this study. We acknowledge the excellent assistance of the entire staff of the Valdoltra Orthopaedic Hospital of Ankaran (Koper, Slovenia). Additionally, we thank the research team and the students of Applied Kinesiology of University of Primorska for the help and logistic support and many others who contributed and took care that the study ran smoothly. Special thanks go to Vanja Pahor from Izola General Hospital biochemical laboratory for the support in biochemical analysis. The study was conducted by Slovenian and Italian partners included in the standard project PANGeA: Physical Activity and Nutrition for Quality Ageing which was supported by grant from Cross-border Cooperation Programme Slovenia–Italy 2007-2013 and co-financed by European Regional Development Fund and Slovenian and Italian national funds.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
Footnotes
Mihaela Jurdana, Zala Jenko-Pražnikar and Nina Mohorko equally contributed to this work.
References
- Addison O, Drummond MJ, LaStayo PC, Dibble LE, Wende AR, McClain DA, Marcus RL. Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. J Nutr Health Aging. 2014;18(5):532–538. doi: 10.1007/s12603-014-0019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasson AN, Undén AL, Elofsson S, Brismar K. Leptin and adiponectin: distribution and associations with cardiovascular risk factors in men and women of the general population. Am J Hum Biol. 2012;24(5):595–601. doi: 10.1002/ajhb.22279. [DOI] [PubMed] [Google Scholar]
- Batista ML, Jr, Olivan M, Alcantara PS, Sandoval R, Peres SB, Neves RX, Silverio R, Maximiano LF, Otoch JP, Seelaender M. Adipose tissue-derived factors as potential biomarkers in cachectic cancer patients. Cytokine. 2012 doi: 10.1016/j.cyto.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Biolo G, Agostini F, Simunic B, Sturma M, Torelli L, Preiser JC, Deby-Dupont G, Magni P, Strollo F, di Prampero P, Guarnieri G, Mekjavic IB, Pisot R, Narici MV. Positive energy balance is associated with accelerated muscle atrophy and increased erythrocyte glutathione turnover during 5 wk of bed rest. Am J Clin Nutr. 2008;88(4):950–958. doi: 10.1093/ajcn/88.4.950. [DOI] [PubMed] [Google Scholar]
- Bosutti A, Malaponte G, Zanetti M, Castellino P, Heer M, Guarnieri G, Biolo G. Calorie restriction modulates inactivity-induced changes in the inflammatory markers C-reactive protein and pentraxin-3. J Clin Endocrinol Metab. 2008;93(8):3226–3229. doi: 10.1210/jc.2007-1684. [DOI] [PubMed] [Google Scholar]
- Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, Richelsen B. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab. 2003;285(3):E527–E533. doi: 10.1152/ajpendo.00110.2003. [DOI] [PubMed] [Google Scholar]
- Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290(5):E961–E967. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- Brüünsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin N Am. 2003;23(1):15–39. doi: 10.1016/S0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H. Physical activity and modulation of systemic low-level inflammation. J Leukoc Biol. 2005;78(4):819–835. doi: 10.1189/jlb.0505247. [DOI] [PubMed] [Google Scholar]
- Calder PC, Ahluwalia N, Brouns F, Buetler T, Clement K, Cunningham K, Esposito K, Jönsson LS, Kolb H, Lansink M, Marcos A, Margioris A, Matusheski N, Nordmann H, O’Brien J, Pugliese G, Rizkalla S, Schalkwijk C, Tuomilehto J, Wärnberg J, Watzl B, Winklhofer-Roob BM. Dietary factors and low-grade inflammation in relation to overweight and obesity. Br J Nutr. 2011;106(3):S5–78. doi: 10.1017/S0007114511005460. [DOI] [PubMed] [Google Scholar]
- Coker RH, Hays NP, Williams RH, Xu L, Wolfe RR, Evans WJ. Bed rest worsens impairments in fat and glucose metabolism in older, overweight adults. J Gerontol A Biol Sci Med Sci. 2014;69(3):363–370. doi: 10.1093/gerona/glt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, Retzlaff BM, Knopp RH, Brunzell JD, Kahn SE. Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and gender. Diabetol. 2003;46(4):459–469. doi: 10.1007/s00125-003-1074-z. [DOI] [PubMed] [Google Scholar]
- de Boer MD, Seynnes OR, di Prampero PE, Pisot R, Mekjavić IB, Biolo G, Narici MV. Effect of 5 weeks horizontal bed rest on human muscle thickness and architecture of weight bearing and non-weight bearing muscles. Eur J Appl Physiol. 2008;104(2):401–407. doi: 10.1007/s00421-008-0703-0. [DOI] [PubMed] [Google Scholar]
- Drummond MJ, Timmerman KL, Markofski MM, Walker DK, Dickinson JM, Jamaluddin M, Brasier AR, Rasmussen BB, Volpi E. Short-term bed rest increases TLR4 and IL-6 expression in skeletal muscle of older adults. Am J Physiol Regul Integr Comp Physiol. 2013;305(3):R216–R223. doi: 10.1152/ajpregu.00072.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler F, Kilates C. Prospective nutritional surveillance using bioelectrical impedance in chronic kidney disease patients. J Ren Nutr. 2005;15:148–151. doi: 10.1053/j.jrn.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Farriols C, Bajo L, Muniesa JM, Escalada F, Miralles R. Functional decline after prolonged bed rest following acute illness in elderly patients: is trunk control test (TCT) a predictor of recovering ambulation? Arch Gerontol Geriatr. 2009;49:409–412. doi: 10.1016/j.archger.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Feuerecker M, Feuerecker B, Matzel S, Long M, Strewe C, Kaufmann I, Hoerl M, Schelling G, Rehm M, Choukèr A (2013) Five days of head-down-tilt bed rest induces noninflammatory shedding of L-selectin. J Appl Physiol 115(2):235–242 [DOI] [PubMed]
- Fischer CP, Plomgaard P, Hansen AK, Pilegaard H, Saltin B, Pedersen BK. Endurance training reduces the contraction-induced interleukin-6 mRNA expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;287(6):E1189–E1194. doi: 10.1152/ajpendo.00206.2004. [DOI] [PubMed] [Google Scholar]
- Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307(5708):426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel PJ. Control of energy homeostasis and insulin action by adipocyte hormones: leptin, acylation stimulating protein, and adiponectin. Curr Opin Lipidol. 2002;13(1):51–59. doi: 10.1097/00041433-200202000-00008. [DOI] [PubMed] [Google Scholar]
- Højbjerre L, Sonne MP, Alibegovic AC, Nielsen NB, Dela F, Vaag A, Bruun JM, Stallknecht B. Impact of physical inactivity on adipose tissue low-grade inflammation in first-degree relatives of type 2 diabetic patients. Diabetes Care. 2011;34(10):2265–2272. doi: 10.2337/dc11-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenko-Pražnikar Z, Petelin A, Jurdana M, Ziberna L. Serum bilirubin levels are lower in overweight asymptomatic middle-aged adults: an early indicator of metabolic syndrome? Metabolism. 2013;62(7):976–985. doi: 10.1016/j.metabol.2013.01.011. [DOI] [PubMed] [Google Scholar]
- Jorge ML, de Oliveira VN, Resende NM, Paraiso LF, Calixto A, Diniz AL, Resende ES, Ropelle ER, Carvalheira JB, Espindola FS, Jorge PT, Geloneze B. The effects of aerobic, resistance, and combined exercise on metabolic control, inflammatory markers, adipocytokines, and muscle insulin signaling in patients with type 2 diabetes mellitus. Metabolism. 2011;60(9):1244–1252. doi: 10.1016/j.metabol.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Jurdana M, Petelin A, Černelič Bizjak M, Bizjak M, Biolo G, Jenko-Pražnikar Z (2013) Increased visfatin levels in obesity and its association with anthropometric/biochemical parameters, physical activity and nutrition. e-SPEN Journal 8:e59-e67
- Kadoglou NP, Sailer N, Moumtzouoglou A, Kapelouzou A, Tsanikidis H, Vitta I, Karkos C, Karayannacos PE, Gerasimidis T, Liapis CD. Visfatin (nampt) and ghrelin as novel markers of carotid atherosclerosis in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2010;118(2):75–80. doi: 10.1055/s-0029-1237360. [DOI] [PubMed] [Google Scholar]
- Kanda T, Takahashi T, Sumino H, Nakahashi T, Iwai K, Morimoto S, Matsumoto M. Hepatocyte growth factor and visfatin in elderly bedridden patients. J Am Geriatr Soc. 2007;55(6):963–965. doi: 10.1111/j.1532-5415.2007.01190.x. [DOI] [PubMed] [Google Scholar]
- Kortebein P, Symons TB, Ferrando A, Paddon-Jones D, Ronsen O, Protas E, Conger S, Lombeida J, Wolfe R, Evans WJ. Functional impact of 10 days of bed rest in healthy older adults. J Gerontol A Biol Sci Med Sci. 2008;63(10):1076–1081. doi: 10.1093/gerona/63.10.1076. [DOI] [PubMed] [Google Scholar]
- Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in elderly. Experim Gerontol. 2004;39(5):687–699. doi: 10.1016/j.exger.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Kusminski CM, McTernan PG, Kumar S (2005) Role of resistin in obesity, insulin resistance and Type II diabetes. Clin Sci (Lond) 109(3):243–256 [DOI] [PubMed]
- Lee KJ, Shin YA, Lee KY, Jun TW, Song W. Aerobic exercise training-induced decrease in plasma visfatin and insulin resistance in obese female adolescents. Int J Sport Nutr Exerc Metab. 2010;20(4):275–281. doi: 10.1123/ijsnem.20.4.275. [DOI] [PubMed] [Google Scholar]
- McTernan PG, McTernan CL, Chetty R, Jenner K, Fisher FM, Lauer MN, Crocker J, Barnett AH, Kumar S (2002) Increased resistin gene and protein expression in human abdominal adipose tissue. J Clin Endocrinol Metab 87(5):2407 [DOI] [PubMed]
- Montgomery LD. Body volume changes during simulated microgravity. II: comparison of horizontal and head-down bed rest. Aviat Space Environ Med. 1993;64(10):899–904. [PubMed] [Google Scholar]
- Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, Tilg H. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J Immunol. 2007;178(3):1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- Mutin-Carnino M, Carnino A, Roffino S, Chopard A. Effect of muscle unloading, reloading and exercise on inflammation during a head-down bed rest. Int J Sports Med. 2013;35(1):28–34. doi: 10.1055/s-0033-1343407. [DOI] [PubMed] [Google Scholar]
- Müller MJ, Bosy-Westphal A, Klaus S, Kreymann G, Lührmann PM, Neuhäuser-Berthold M, Noack R, Pirke KM, Platte P, Selberg O, Steiniger J. World Health Organization equations have shortcomings for predicting resting energy expenditure in persons from a modern, affluent population: generation of a new reference standard from a retrospective analysis of a German database of resting energy expenditure. Am J Clin Nutr. 2004;80:1379–1390. doi: 10.1093/ajcn/80.5.1379. [DOI] [PubMed] [Google Scholar]
- Nosova EV, Yen P, Chong KC, Alley HF, Stock EO, Quinn A, Hellmann J, Conte MS, Owens CD, Spite M, Grenon SM. Short-term physical inactivity impairs vascular function. J Surg Res. 2014;190(2):672–682. doi: 10.1016/j.jss.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Funahashi T, Matsuzawa Y, Walsh K. Obesity, adiponectin and vascular inflammatory disease. Curr Opin Lipidol. 2003;14(6):561–566. doi: 10.1097/00041433-200312000-00003. [DOI] [PubMed] [Google Scholar]
- Panagiotakos DB, Pitsavos C, Chrysohoou C, Kavouras S, Stefanadis C (2005) The associations between leisure-time physical activity and inflammatory and coagulation markers related to cardiovascular disease: the ATTICA Study Prev Med 40(4):432–437 [DOI] [PubMed]
- Patel L, Buckels AC, Kinghorn IJ, Murdock PR, Holbrook JD, Plumpton C, Macphee CH, Smith SA (2003) Resistin is expressed in human macrophages and directly regulated by PPAR gamma activators. Biochem Biophys Res Commun 300(2):472–476 [DOI] [PubMed]
- Pedersen BK. The diseasome of physical inactivity—and the role of myokines in muscle—fat cross talk. J Physiol. 2009;587(Pt 23):5559–5568. doi: 10.1113/jphysiol.2009.179515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Hoffman-Goetz L. Exercise and the immune system: regulation, integration, and adaptation. Physiol Rev. 2000;80(3):1055–1081. doi: 10.1152/physrev.2000.80.3.1055. [DOI] [PubMed] [Google Scholar]
- Pisot R, Narici MV, Simunic B, De Boer M, Seynnes O, Jurdana M, Biolo G, Mekjavić IB. Whole muscle contractile parameters and thickness loss during 35-day bed rest. Eur J Appl Physiol. 2008;104(2):409–414. doi: 10.1007/s00421-008-0698-6. [DOI] [PubMed] [Google Scholar]
- Reid MB, Li YP. Tumor necrosis factor-α and muscle wasting: a cellular perspective. Respir Res. 2001;2:269–272. doi: 10.1186/rr67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittweger J, Simunic B, Bilancio G, De Santo NG, Cirillo M, Biolo G, Pisot R, Eiken O, Mekjavic IB, Narici M. Bone loss in the lower leg during 35 days of bed rest is predominantly from the cortical compartment. Bone. 2009;44(4):612–618. doi: 10.1016/j.bone.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278(46):45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14(2):1431–1437. doi: 10.1128/MCB.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schautz B, Later W, Heller M, Peters A, Müller MJ, Bosy-Westphal A. Impact of age on leptin and adiponectin independent of adiposity. Br J Nutr. 2012;108(2):363–370. doi: 10.1017/S0007114511005605. [DOI] [PubMed] [Google Scholar]
- Schwartz DR, Lazar MA. Human resistin: found in translation from mouse to man. Trends Endocrinol Metab. 2011;22(7):259–265. doi: 10.1016/j.tem.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer WT, Ochs HD, Lee BN, Cohen EN, Reuben JM, Cheng I, Thompson B, Butel JS, Blancher A, Abbal M, Aviles H, Sonnenfeld G. Immune responses in adult female volunteers during the bed-rest model of spaceflight: antibodies and cytokines. J Allergy Clin Immunol. 2009;123(4):900–905. doi: 10.1016/j.jaci.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17(8):884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- Stofkova A. Resistin and visfatin: regulators of insulin sensitivity, inflammation and immunity. Endocr Regul. 2010;44(1):25–36. doi: 10.4149/endo_2010_01_25. [DOI] [PubMed] [Google Scholar]
- Tanner RE, Brunker LB, Agergaard J, Barrows KM, Briggs RA, Kwon OS, Young LM, Hopkins PN, Volpi E, Marcus RL, LaStayo PC, Drummond MJ (2015) Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol doi:10.1113/JP270699. [Epub ahead of print] [DOI] [PMC free article] [PubMed]
- Tilg H, Moschen AR. Role of adiponectin and PBEF/visfatin as regulators of inflammation: involvement in obesity-associated diseases. Clin Sci (Lond) 2008;18:275–288. doi: 10.1042/CS20070196. [DOI] [PubMed] [Google Scholar]
- Vernikos J, Schneider VS. Space, gravity and the physiology of aging: parallel or convergent disciplines? A mini-review. Gerontol. 2010;56(2):157–166. doi: 10.1159/000252852. [DOI] [PubMed] [Google Scholar]
- Zahorska-Markiewicz B, Olszanecka-Glinianowicz M, Janowska J, Kocelak P, Semik-Grabarczyk E, Holecki M, Dabrowski P, Skorupa A. Serum concentration of visfatin in obese women. Metabolism. 2007;56:1131–1134. doi: 10.1016/j.metabol.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Zeyda M, Stulnig TM. Adipose tissue macrophages. Immunol Lett. 2007;112(2):61–67. doi: 10.1016/j.imlet.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods JA, Wilund KR, Martin SA, Kistler BM. Exercise, inflammation and aging. Aging Dis. 2012;3(1):130–140. [PMC free article] [PubMed] [Google Scholar]