Abstract
Higher intra-individual lap time variation (LTV) of the 400-m walk is cross-sectionally associated with poorer attention in older adults. Whether higher LTV predicts decline in executive function and whether the relationship is accounted for by slower walking remain unanswered. The main objective of this study was to examine the relationship between baseline LTV and longitudinal change in executive function. We used data from 347 participants aged 60 years and older (50.7 % female) from the Baltimore Longitudinal Study of Aging. Longitudinal assessments of executive function were conducted between 2007 and 2013, including attention (Trails A, Digit Span Forward Test), cognitive flexibility and set shifting (Trails B, Delta TMT: Trials B minus Trials A), visuoperceptual speed (Digit Symbol Substitution Test), and working memory (Digit Span Backward Test). LTV and mean lap time (MLT) were obtained from the 400-m walk test concurrent with the baseline executive function assessment. LTV was computed as variability of lap time across ten 40-m laps based on individual trajectories. A linear mixed-effects model was used to examine LTV in relation to changes in executive function, adjusted for age, sex, education, and MLT. Higher LTV was associated with greater decline in performance on Trails B (β = 4.322, p < 0.001) and delta TMT (β = 4.230, p < 0.001), independent of covariates. Findings remained largely unchanged after further adjustment for MLT. LTV was not associated with changes in other executive function measures (all p > 0.05). In high-functioning older adults, higher LTV in the 400-m walk predicts executive function decline involving cognitive flexibility and set shifting over a long period of time. High LTV may be an early indicator of executive function decline independent of MLT.
Keywords: Lap time variation, Executive function, Longitudinal study, Aging
Introduction
In community-dwelling older adults, slower gait is associated with poorer executive function from both cross-sectional (Ble et al., 2005; Doi et al., 2014; Holtzer et al., 2006; Rosano et al., 2005) and longitudinal studies (Inzitari et al., 2007; Mielke et al., 2013). There may be a shared neuropathology underlying the motor and executive deficits. In addition to brain atrophy in the prefrontal and selected subcortical areas (Pugh and Lipsitz, 2002; Roriz-Cruz et al., 2007), white matter lesions and disrupted white matter microstructure (Kuo and Lipsitz, 2004) influence both motor performance and executive function.
Executive function involves complex processing among cognitive, sensory, and motor systems. There are various constructs within executive function, such as attention, cognitive flexibility and set shifting (Anderson et al., 2001), visuoperceptual speed, as well as working memory (Fisk and Sharp, 2004). Intact executive function is necessary for an individual’s independence, even with the loss of other cognitive abilities in older age (Lezak et al., 2004). Thus, executive function is a critical contributor to an older person’s quality of life and functional independence. Many aspects of executive function substantially decline at age 60 (Hedden and Gabrieli, 2004; Park et al., 2002). Worsened executive function is associated with subcortical ischemic vascular disease (Jokinen et al., 2009), cerebral microbleeds (Yamashiro et al., 2014), and cerebral microangiopathy (Schroeter et al., 2007). Poorer executive function is also associated with fall risks (Herman et al., 2010; Mirelman et al., 2012) which may lead to subsequent mobility loss and disability. Identifying clinical indicators of executive decline, such as variable and slow gait, may contribute to anticipating cognitive impairment and dementia. A further understanding of the relationship between abnormal gait and executive function is valuable, especially for older adults who have not yet experienced cognitive impairment.
While gait speed is related to executive function, few studies have explored associations of other parameters of gait with executive function, especially among community-dwelling older adults. Abnormal gait, reflected in stride-to-stride variability, may provide prognostic information about worsened neurological processes caused either by age or neuropathological conditions (Beauchet et al., 2013). Greater stride-to-stride gait variability is cross-sectionally associated with poorer global executive function in older adults (Brach et al., 2008; Ijmker and Lamoth, 2012; Martin et al., 2013), and one study shows that greater gait variability is associated with a specific subdomain—information updating and monitoring (Beauchet et al., 2012). One attractive way to measure gait variability is to use fast-paced walking. Fast-paced walking is a stressful and demanding task. Measuring variability during fast-paced walking may address the potential confounding effects of biomechanical abnormalities during usual-paced walking, detects low-functioning individuals, and captures additional physiological differences in fitness and function (Beauchet et al., 2013; Deshpande et al., 2009; Fitzpatrick et al., 2007). Compared to stride-to-stride gait variability, a simpler, more clinically accessible approach is assessing variation across repeated walking laps (Vestergaard et al., 2009). Our group recently reported that lap time variation (LTV) during a 400-m fast-paced walking was associated with poorer executive function, specifically attention, in the Baltimore Longitudinal Study of Aging (Tian et al., 2015). To control for fatigability, in that study, we defined LTV as variability in lap time based on individual trajectories. The relationship with executive function was not affected by mean lap time, which might have been suggested to play an important role in the relation between LTV and executive function. High LTV under fast-paced walking may potentially indicate a vulnerable stage of central nervous system. LTV may serve as an early and sensitive indicator of poorer executive function decline, but to date, this hypothesis has not been tested in longitudinal studies. Further, it is not clear which constructs within executive function are specifically associated with gait disturbance.
Thus, the objective of this study was to examine the relationship between LTV and longitudinal changes in various constructs of executive function, including attention, cognitive flexibility and set shifting, visuoperceptual speed, and working memory in adults aged 60 and older. We hypothesize that high LTV was prospectively associated with greater rate of decline in executive function. We also hypothesized that the relationship would be independent of mean lap time. To determine the specificity of the findings for executive function/attention, we also examined associations with changes in other cognitive functions (verbal memory, language, and visuospatial ability) and manual dexterity.
Methods
Study population
Participants were drawn from the Baltimore Longitudinal Study of Aging (BLSA). BLSA is a prospective study of community dwellers mostly from the Baltimore–Washington area. BLSA participants aged 60 to 79 return to the National Institute on Aging clinical research unit every 2 years for a variety of clinical, medical, and neuropsychological testing, while participants aged 80 and older return every year for testing.
A total of 813 participants aged 60 and older completed an initial 400-m walk test between 2007 and 2013. Since 1986, participants aged 60 and older in the BLSA completed an expanded neuropsychological test battery which was used to characterize age-related changes in cognitive function. Of the 813 participants, 349 had at least two measures of executive function conducted between 2007 and 2013. Most also had repeated measures of other cognitive functions (verbal memory, language, and visuospatial ability) and manual dexterity. All cognitive and manual dexterity measures used in the present study were concurrent with and subsequent to the time of the initial 400-m walk test. The follow-up period refers to the time from the initial 400-m walk test to all subsequent assessments of cognitive function. The average number of visits per participant was 2.6 (ranged from 1 to 6).
Based on standardized consensus diagnostic procedures for the BLSA (Driscoll et al., 2006), 347 participants were free of cognitive impairment or dementia at the time of the initial 400-m walk test. Seven of 347 were diagnosed with mild cognitive impairment over the course of the study between 2007 and 2013. As this study focused on normal aging, data of cognitive function at and following the onset of dementia were excluded. Dementia was diagnosed by consensus diagnostic conferences using the Diagnostic and Statistical Manual of Mental Disorders, Third Edition, revised criteria for dementia and the National Institute of Neurological and Communicative Disorders and Stroke–Alzheimer’s disease and Related Disorders Association criteria for Alzheimer’s Disease using neuropsychological diagnostic tests and clinical data (McKhann et al., 1984). The diagnosis of dementia required evidence of a progressive cognitive syndrome, including memory decline.
The study protocol was approved by the institutional review board of record at the time of data collection. All participants provided written informed consent.
Mobility measures
LTV was obtained from the 400-m walk test. Details of the 400-m walk test were described elsewhere (Simonsick et al., 2001). Participants were excluded from the 400-m walk if they had medical contraindications (Newman et al. 2006). Briefly, participants were instructed to walk for ten 40-m laps as quickly as possible and they received feedback and encouragement after completion of each lap. Participants may not be able to complete the entire ten laps due to fatigue or symptoms. Lap time was recorded after the completion of each lap using a stop watch by an examiner. For those who completed the 400-m walk, LTV was calculated as standard deviation of lap time residuals based on individual trajectories (Tian et al., 2015). First, an individual trajectory of lap time across ten laps was computed for each participant. Second, an estimated lap time at each lap was computed based on the individual trajectory. The residual was obtained by subtracting the actual lap time from the estimated lap time. Then the standard deviation of the residuals was obtained as LTV. This measure accounted for the possible effect of fatigability as the lap number increased (Simonsick et al., 2014; Tian et al., 2015). Mean lap time was obtained as the average of lap time across ten laps.
Usual gait speed was measured on a 6-m course in an uncarpeted corridor. Participants were asked to walk at their usual and comfortable pace. Time to complete the 6-m course was measured. Two trials were completed and the faster gait speed was used in the study in meter/s.
Cognitive and manual dexterity measures
Executive function, other cognitive functions (verbal memory, language, and visuospatial ability), and manual dexterity were assessed by trained examiners. It was expected to see that LTV was associated only with longitudinal change in executive function and not with changes in verbal memory, language, visuospatial ability, or manual dexterity.
Executive function was measured by a variety of neuropsychological tests, including the Trail Making Test (TMT), the Digit symbol substitution test (DSST), and the Digit Span Test. Specifically, attention and motor speed were assessed by Trails A and Digit Span Forward Test provided another measure of attention and concentration (Lezak, 1995). Cognitive flexibility and set shifting was assessed by the Trails B and Delta TMT (i.e., Trails B minus Trails A) (Kortte et al., 2002; Lezak, 1995). Visuoperceptual speed was assessed by the DSST (Wechsler, 1981). Working memory was assessed by the Digit Span Backward Test (Wechsler, 1981).
Verbal memory was measured by the California Verbal Learning Test (Delis, 1991) and Benton Visual Retention Test, respectively (Benton, 1965). Language and verbal fluency were measured by the letter (Thurstone, 1938) and category fluency test (Rosen, 1980). Visuospatial ability was measured by the card rotation test (Ekstrom et al., 1976). Manual dexterity was assessed by the Purdue Pegboard Test (Tiffin, 1968), and it was included in this study because it required sensorimotor function.
Statistical analysis
Bivariate associations between sample characteristics and LTV were examined using the Pearson correlation coefficients or independent t tests as appropriate. Bivariate associations between baseline executive function measures and LTV were examined using the Pearson correlation coefficients. Associations between LTV and changes in executive function, other cognitive functions, and manual dexterity were examined using the linear mixed-effects model which accounted for repeated measures (SAS v. 9.3; SAS Institute, Cary, NC).
The model was first adjusted for age, sex, education, and body mass index. Each cognitive measure was modeled as a dependent variable. LTV, interval (i.e., the time period in years between baseline cognitive measurement and each follow up visit), baseline age, sex, education, and their interaction with interval were modeled as fixed effects. The intercept and interval were modeled as random effects. The full model was set up as: cognitive measure = β0 + β1 × LTV + β2 × interval + β3 × LTV × interval + β4 × age + β5 × sex + β6 × education + β7 × age × interval + β8 × sex × interval + β9 × education × interval + β10 × body mass index + β11 × body mass index × interval.
To examine whether walking speed would affect the relationship, the model was further adjusted for mean lap time and its interaction with interval. The adjusted model was set up as: cognitive measure = β0 + β1 × LTV + β2 × interval + β3 × LTV × interval + β4 × age + β5 × sex + β6 × education + β7 × age × interval + β8 × sex × interval + β9 × education × interval + + β10 × body mass index + β11 × body mass index × interval + β12 × mean lap time + β13 × mean lap time × interval.
In the linear mixed-effects model, the regression coefficient of LTV represented the cross-sectional association between LTV and baseline cognitive functions and manual dexterity. The regression coefficient of the interaction between LTV and interval represented longitudinal associations between LTV and changes in cognitive functions and manual dexterity during follow-up.
Although most had more than one cognitive function measures during follow-up, we conducted a sensitivity analysis by excluding those who had only one baseline assessment (Digit Span Tests: n = 345; Purdue Pegboard Test dominant hand: n = 337; non-dominant hand: n = 335; CVLT immediate free recall: n = 343; CVLT long-delay free recall: n = 343; CVLT short-delay free recall: n = 342; BVRT: n = 345; Card Rotation Test: n = 342).
Results
Table 1 describes baseline sample characteristics and mobility measures. Higher LTV was associated with older age and shorter height. Those who reported less than 150 min per week moderate to vigorous exercise had higher LTV than those who reported more. Higher body mass index, longer mean lap time, and slower usual gait speed were associated with higher LTV. Other sample characteristics, such as sex, race, education, body weight, and smoking status, were not associated with LTV. Table 2 describes baseline values of executive function, other cognitive functions, and manual dexterity. Bivariate analysis of baseline data showed that higher LTV was correlated with poorer performance on the Trails A, DSST, Purdue Pegboard Test, BVRT, and Cart Rotation Test (Table 2). Bivariate associations between LTV and other cognitive function measures were not significant (Table 2).
Table 1.
Baseline sample characteristics and their correlations with lap time variation (LTV) (n = 347)
| Mean ± SD or N (%) | Range | Correlations with LTV: r (p value) or MD ± SE (p value) | |
|---|---|---|---|
| Demographics | |||
| Age, years | 70.7 ± 7.7 | 60.0–94.7 | 0.233 (<0.001) |
| Female | 176 (50.7) | – | 0.05 ± 0.04 (0.263) |
| Education > college | 193 (55.6) | – | −0.02 ± 0.04 (0.593) |
| Black | 101 (29.1) | – | 0.01 ± 0.05 (0.856) |
| Height, cm | 169.6 ± 8.9 | 149.4–194.9 | −0.139 (0.009) |
| Weight, kg | 78.1 ± 15.0 | 44.7–149.6 | 0.034 (0.532) |
| Health-related conditions | |||
| Current smokers or quit < 10 years | 12 (3.5) | – | 0.03 ± 0.12 (0.803) |
| Moderate-to-vigorous activity > 150 min/week | 138 (39.8) | – | −0.10 ± 0.04 (0.014) |
| Body mass index, kg/m2 | 27.1 ± 4.4 | 18.3–45.7 | 0.132 (0.014) |
| Usual gait speed, meter/s | 1.17 ± 0.20 | 0.44–1.73 | −0.271 (<0.001) |
| Mobility measures | |||
| Lap time variation, s | 0.8 ± 0.4 | 0.2–4.1 | – |
| Mean lap time, s | 27.0 ± 5.1 | 18.0–57.7 | 0.418 (<0.001) |
MD ± SE denotes mean difference ± standard error
Table 2.
Measures of executive function, other cognitive functions, and manual dexterity (n = 347)
| Measures | Total number of visits | Repeat visits, Mean ± SD range | First visit, mean score | Correlations with LTV, r (p value) | |
|---|---|---|---|---|---|
| Executive function (hypothesized domain) | Attention | ||||
| Trails A, s | 953 | 2.7 ± 0.8 (2-6) | 31 ± 10 | 0.252 (<0.001) | |
| Digit span forward | 944 | 2.7 ± 0.8 (1-6) | 8.3 ± 2.4 | 0.014 (0.789) | |
| Cognitive flexibility and set shifting | |||||
| Trails B, s | 953 | 2.7 ± 0.8 (2-6) | 76 ± 32 | 0.084 (0.116) | |
| Delta TMT, seconds | 953 | 2.7 ± 0.8 (2-6) | 45 ± 28 | 0.003 (0.949) | |
| Visuoperceptual speed | |||||
| Digit symbol substitution test | 925 | 2.7 ± 0.8 (2-5) | 48 ± 10 | –0.148 (0.006) | |
| Working memory | |||||
| Digit span backward | 945 | 2.7 ± 0.8 (1-6) | 7.1 ± 2.4 | –0.070 (0.197) | |
| Other cognitive functions and manual dexterity | Verbal memory | ||||
| CVLT immediate free recall (sum of 5 trials) | 927 | 2.7 ± 0.8 (2-6) | 53.7 ± 11.5 | –0.041 (0.453) | |
| CVLT long-delay free recall | 925 | 2.7 ± 0.8 (1-6) | 10.9 ± 3.2 | –0.035 (0.522) | |
| CVLT short-delay free recall | 922 | 2.7 ± 0.8 (1-6) | 11.5 ± 3.3 | –0.047 (0.385) | |
| BVRT | 943 | 2.7 ± 0.8 (1-6) | 5.9 ± 3.9 | 0.139 (0.010) | |
| Language | |||||
| Letter fluency | 953 | 2.7 ± 0.8 (2-6) | 14.6 ± 4.1 | –0.049 (0.364) | |
| Category fluency | 953 | 2.7 ± 0.8 (2-6) | 16.4 ± 3.6 | –0.109 (0.042) | |
| Visuospatial ability | |||||
| Card rotation | 919 | 2.6 ± 0.8 (1–6) | 93.8 ± 40.0 | −0.129 (0.017) | |
| Manual dexterity | |||||
| Pegboard dominant | 919 | 2.6 ± 0.8 (2–5) | 12.2 ± 1.8 | −0.161 (0.003) | |
| Pegboard non-dominant | 898 | 2.6 ± 0.8 (2–5) | 11.9 ± 1.7 | −0.117 (0.032) | |
LTV lap time variation, Delta TMT Trails B minus Trails A, CVLT California verbal learning test, BVRT Benton visual retention test
After adjustment for age, sex, education, and body mass index, the cross-sectional association between LTV and Trails A remained significant (Table 3, cross-sectional, model 1). This association persisted after further adjustment for mean lap time (Table 3, cross-sectional, model 2). Cross-sectional associations of LTV with other cognitive function measures or manual dexterity were not significant after adjustment for age, sex, education, body mass index, and mean lap time (Table 3, cross-sectional, model 1 and 2).
Table 3.
Associations between lap time variation (LTV) and changes in executive function, other cognitive functions, and manual dexterity (n = 347)
| Cross-sectional: LTV | Longitudinal: LTV × interval | |||
|---|---|---|---|---|
| Model 1 | Model 2 | Model 1 | Model 2 | |
| β (SE), p value | β (SE), p value | |||
| Attention | ||||
| Trails A | 4.563* (1.489) 0.002 | 3.487* (1.540) 0.024 | 0.041 (0.334) 0.902 | −0.243 (0.350) 0.489 |
| Digit span backward | −0.204 (0.315) 0.518 | −0.129 (0.331) 0.698 | −0.069 (0.081) 0.399 | −0.070 (0.086) 0.412 |
| Cognitive flexibility and set shifting | ||||
| Trails B | −0.781 (4.822) 0.872 | −4.202 (5.040) 0.405 | 5.701* (1.124) <0.001 | 5.758* (1.185) <0.001 |
| Delta TMT | −5.263 (4.145) 0.205 | −7.532 (4.353) 0.085 | 5.680* (1.135) <0.001 | 6.002* (1.196) <0.001 |
| Visuoperceptual speed | ||||
| Digit symbol substitution test | −1.384 (1.246) 0.267 | −0.369 (1.297) 0.777 | −0.093 (0.220) 0.673 | −0.120 (0.232) 0.606 |
| Working memory | ||||
| Digit span forward | 0.231 (0.327) 0.481 | 0.309 (0.344) 0.370 | −0.081 (0.082) 0.324 | −0.127 (0.086) 0.139 |
| Verbal memory | ||||
| CVLT immediate free recall | 0.127 (1.548) 0.935 | 0.959 (1.622) 0.555 | 0.085 (0.345) 0.806 | 0.221 (0.363) 0.542 |
| CVLT long-delay free recall | 0.089 (0.437) 0.839 | 0.200 (0.459) 0.664 | 0.021 (0.094) 0.822 | 0.088 (0.099) 0.373 |
| CVLT short-delay free recall | 0.116 (0.434) 0.790 | 0.284 (0.455) 0.533 | −0.049 (0.094) 0.603 | −0.045 (0.099) 0.648 |
| BVRT | 0.451 (0.471) 0.339 | 0.352 (0.494) 0.477 | 0.104 (0.110) 0.345 | 0.074 (0.115) 0.522 |
| Language | ||||
| Letter fluency | −0.588 (0.535) 0.273 | –0.217 (0.558) 0.698 | −0.060 (0.090) 0.502 | −0.015 (0.094) 0.876 |
| Category fluency | −0.166 (0.451) 0.713 | 0.262 (0.469) 0.577 | −0.003 (0.082) 0.972 | −0.009 (0.087) 0.919 |
| Visuospatial ability | ||||
| Card Rotations | −3.196 (5.137) 0.534 | –0.537 (5.379) 0.921 | −0.987 (0.696) 0.157 | −0.828 (0.734) 0.259 |
| Manual dexterity | ||||
| Pegboard dominant | −0.117 (0.186) 0.549 | 0.174 (0.189) 0.357 | −0.027 (0.047) 0.562 | −0.029 (0.049) 0.560 |
| Pegboard non- dominant | −0.022 (0.191) 0.910 | 0.217 (0.197) 0.272 | 0.019 (0.048) 0.685 | 0.017 (0.050) 0.733 |
Model 1: adjusted for age, sex, education, body mass index, and their interaction with interval
Model 2: model 1 + mean lap time + mean lap time × interval
*p < 0.05, significant associations
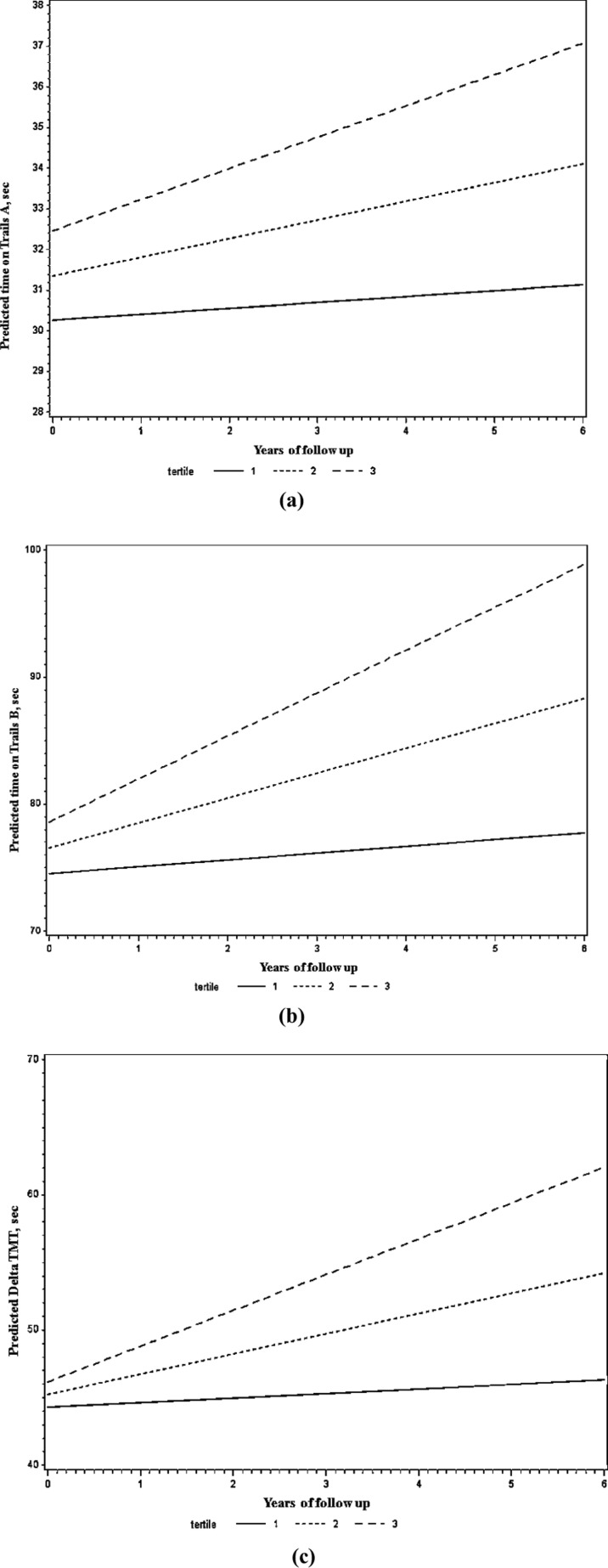
Higher LTV was prospectively associated with greater decline in performance on the Trails B and Delta TMT during follow-up, after adjustment for age, sex, education, and body mass index (Table 3, longitudinal, model 1; Fig. 1). These associations remained significant after further adjustment for mean lap time (Table 3, longitudinal, model 2). LTV was not associated with changes in other executive function measures or with changes in manual dexterity, verbal, language, and visuospatial ability (Table 3, longitudinal).
Fig. 1.
Predicted values of Trails A (a), Trails B (b), and Delta TMT (c) as a function of follow-up time, stratified by tertiles of baseline lap time variation
Results remained largely unchanged when analyses were restricted to participants with repeated cognitive assessments during follow-up (data not shown).
Discussion
Among high-functioning older adults, higher LTV predicts a greater rate of decline in executive function specifically involving cognitive flexibility and set shifting. We did not find associations with changes in other executive function measures, verbal memory, language, visuospatial ability, or manual dexterity.
This study extended previous research by examining longitudinal patterns of executive function and gait disturbance. The link between executive function and LTV in this study is in line with previous cross-sectional findings (Brach et al., 2008; Ijmker and Lamoth, 2012; Martin et al., 2013; Tian et al., 2015). Initial neuroimaging studies support the notion that poor motor and executive functions share a common neuropathology (Bolandzadeh et al., 2014; Nadkarni et al., 2014; Rosano et al., 2012). Among various subdomains of executive function, we observed a strong association between LTV and declines in cognitive flexibility and set shifting, captured by the Trails B and Delta TMT. The Trails B requires alternating attention and mental tracking between tasks and contains a working memory component. Delta TMT adjusts for the motor speed component and provides a clearer index of cognitive flexibility and set shifting than the Trails B alone. The ability to alternate attention and switch tasks is essential for daily performance, such as walking while talking, watching TV while making dinner. Intact cognitive flexibility and set shifting is critical for walking and is related to fall risks.
We are aware that prior studies also showed associations of gait variability with manual dexterity (Brach et al., 2008), visuospatial ability (Martin et al., 2013), and memory (Li et al., 2001). The fact that we did not observe associations of LTV with these factors may be due to different measures of gait parameters, different study populations, and with or without accounting for the effect of walking speed. For instance, double-support phase variability was shown to be associated with visuospatial ability (Martin et al., 2013). Visuospatial ability influences postural control which may alter double-support phase variability. Another study showed stance time variability was shown to be associated with manual dexterity measured by finger tapping, without consideration of walking speed (Brach et al., 2008). This relationship was found only in slow walkers, but not in fast walkers.
The observed associations were robust, independent of walking speed. Some prior studies focusing on gait variability and executive function did not consider the influence of walking speed. Mounting evidence shows slower gait is associated with poorer executive function both cross-sectionally and longitudinally. In the present study, walking speed was highly correlated with LTV. Thus, it is important to account for walking speed because it is related to both the predictor and the outcome. Further, we hypothesize that variable gait may precede overall slowing, not vice versa. Prior research suggests that slow walking is more likely to be the consequence of variable walking (Kang and Dingwell, 2008). Thus, it is essential to understand whether LTV is an early indicator of executive decline, independent of walking speed. After accounting for walking speed, our results remained largely unchanged. This suggests that LTV may be an independent, early indicator of executive decline over time.
One novel aspect of the study is the use of a measure of LTV. LTV is simple to measure and clinically accessible and has several advantages. Compared to conventional stride-to-stride gait variability, LTV is easy to implement in large population-based studies and clinical settings with low cost. It does not require a motion analysis system or force plate. Further, LTV is informative, high levels of which predict mortality (Vestergaard et al., 2009). As fatigue might cause progressive slowing across laps, a simple standard deviation would not account for decreased trend toward slowing, which is not the same as variability. LTV was calculated around individual trajectories in our study. Compared to the measure of lap time variability used in the prior study (Vestergaard et al. 2009), our approach accounted for fatigability and more accurately reflects intra-individual variations in motor performance. It is worth noting that LTV is measured under fast-paced walking, which requires maximal effort. The added stress allows it well, characterizing individuals with low physical function. This approach reduces the potential biomechanical effects during usual-paced walking. It would be interesting to investigate LTV under different speeds and distances in relation to executive decline in future research.
This study has limitations. The study sample is composed of high-functioning elderly limited to those who can complete the 400-m walk test. Participants in the BLSA tend to be very healthy compared to general population. Findings may not be generalizable to a broader population including those with mobility and cognitive impairments. Further, findings from this study do not imply any causal relationship between motor and executive deficits. While we observed LTV was associated with changes in executive function over time, a possible reverse causation cannot be ruled out. It is still not known whether motor deficit predicts the subsequent executive deficit, or vice versa. Future studies are warranted to examine the temporal sequence of motor and executive functions in a contemporary period.
In conclusion, high-functioning older adults with higher LTV may have greater decline in executive function, primarily involving cognitive flexibility, and set shifting. LTV may be used in a clinical context as an important early indicator of executive decline for older adults.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institute on Aging.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- Anderson VA, et al. Development of executive functions through late childhood and adolescence in an Australian sample. Dev Neuropsychol. 2001;20:385–406. doi: 10.1207/S15326942DN2001_5. [DOI] [PubMed] [Google Scholar]
- Beauchet O, et al. Gait control: a specific subdomain of executive function? J Neuroeng Rehabil. 2012;9:12. doi: 10.1186/1743-0003-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O, et al. Gait variability at fast-pace walking speed: a biomarker of mild cognitive impairment? J Nutr Health Aging. 2013;17:235–9. doi: 10.1007/s12603-012-0394-4. [DOI] [PubMed] [Google Scholar]
- Benton A (1965) Manuel pour l’application du test de rétention visuelle. Applications cliniques et expérimentales. 2nd edition., Vol., Paris: Editions du Centre de Psychologie Appliquée.
- Ble A, et al. Executive function correlates with walking speed in older persons: the InCHIANTI study. J Am Geriatr Soc. 2005;53:410–5. doi: 10.1111/j.1532-5415.2005.53157.x. [DOI] [PubMed] [Google Scholar]
- Bolandzadeh N, et al. Pathways linking regional hyperintensities in the brain and slower gait. Neuroimage. 2014;99:7–13. doi: 10.1016/j.neuroimage.2014.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brach JS, et al. Stance time and step width variability have unique contributing impairments in older persons. Gait Posture. 2008;27:431–9. doi: 10.1016/j.gaitpost.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, et al. Profiles of demented and amnesic patients on the California Verbal Learning Test: Implications for the assessment of memory disorders. Psychological Assessment. 1991;3:19–26. doi: 10.1037/1040-3590.3.1.19. [DOI] [Google Scholar]
- Deshpande N, et al. Gait speed under varied challenges and cognitive decline in older persons: a prospective study. Age Ageing. 2009;38:509–14. doi: 10.1093/ageing/afp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, et al. Cognitive function and gait speed under normal and dual-task walking among older adults with mild cognitive impairment. BMC Neurol. 2014;14:67. doi: 10.1186/1471-2377-14-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll I, et al. Impact of Alzheimer’s pathology on cognitive trajectories in nondemented elderly. Ann Neurol. 2006;60:688–95. doi: 10.1002/ana.21031. [DOI] [PubMed] [Google Scholar]
- Ekstrom RB et al (1976) Manual for the kit offactor-referenced cognitive tests, Princeton, NJ: Educational Testing Service
- Fisk JE, Sharp CA. Age-related impairment in executive functioning: updating, inhibition, shifting, and access. J Clin Exp Neuropsychol. 2004;26:874–90. doi: 10.1080/13803390490510680. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick AL, et al. Associations of gait speed and other measures of physical function with cognition in a healthy cohort of elderly persons. J Gerontol A Biol Sci Med Sci. 2007;62:1244–51. doi: 10.1093/gerona/62.11.1244. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Herman T, et al. Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2010;65:1086–92. doi: 10.1093/gerona/glq077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer R, et al. Cognitive processes related to gait velocity: results from the Einstein Aging Study. Neuropsychology. 2006;20:215–23. doi: 10.1037/0894-4105.20.2.215. [DOI] [PubMed] [Google Scholar]
- Ijmker T, Lamoth CJ. Gait and cognition: the relationship between gait stability and variability with executive function in persons with and without dementia. Gait Posture. 2012;35:126–30. doi: 10.1016/j.gaitpost.2011.08.022. [DOI] [PubMed] [Google Scholar]
- Inzitari M, et al. Gait speed predicts decline in attention and psychomotor speed in older adults: the health aging and body composition study. Neuroepidemiology. 2007;29:156–62. doi: 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokinen H, et al. Longitudinal cognitive decline in subcortical ischemic vascular disease—the LADIS Study. Cerebrovasc Dis. 2009;27:384–91. doi: 10.1159/000207442. [DOI] [PubMed] [Google Scholar]
- Kang HG, Dingwell JB. Separating the effects of age and walking speed on gait variability. Gait Posture. 2008;27:572–7. doi: 10.1016/j.gaitpost.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Kortte KB, Horner MD, Windham WK. The trail making test, part B: cognitive flexibility or ability to maintain set? Appl Neuropsychol. 2002;9:106–9. doi: 10.1207/S15324826AN0902_5. [DOI] [PubMed] [Google Scholar]
- Kuo HK, Lipsitz LA. Cerebral white matter changes and geriatric syndromes: is there a link? J Gerontol A Biol Sci Med Sci. 2004;59:818–26. doi: 10.1093/gerona/59.8.M818. [DOI] [PubMed] [Google Scholar]
- Lezak MD (1995) Neuropsychological Assessment, 3rd edition., Vol., New York: Oxford University Press.
- Lezak MD et al (2004) Neuropsychological assessment. 4th ed, Vol., Oxford University Press, New York, NY, US
- Li S, et al. Short-term fluctuations in elderly people’s sensorimotor functioning predict text and spatial memory performance: The Macarthur Successful Aging Studies. Gerontology. 2001;47:100–16. doi: 10.1159/000052782. [DOI] [PubMed] [Google Scholar]
- Martin KL, et al. Cognitive function, gait, and gait variability in older people: a population-based study. J Gerontol A Biol Sci Med Sci. 2013;68:726–32. doi: 10.1093/gerona/gls224. [DOI] [PubMed] [Google Scholar]
- McKhann G, et al. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–44. doi: 10.1212/WNL.34.7.939. [DOI] [PubMed] [Google Scholar]
- Mielke MM, et al. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J Gerontol A Biol Sci Med Sci. 2013;68:929–37. doi: 10.1093/gerona/gls256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman A, et al. Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition. PLoS One. 2012;7:e40297. doi: 10.1371/journal.pone.0040297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni NK, et al. Association between cerebellar gray matter volumes, gait speed, and information-processing ability in older adults enrolled in the Health ABC study. J Gerontol A Biol Sci Med Sci. 2014;69:996–1003. doi: 10.1093/gerona/glt151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB et al (2006) Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 295:2018--26 [DOI] [PubMed]
- Park DC, et al. Models of visuospatial and verbal memory across the adult life span. Psychol Aging. 2002;17:299–320. doi: 10.1037/0882-7974.17.2.299. [DOI] [PubMed] [Google Scholar]
- Pugh KG, Lipsitz LA. The microvascular frontal-subcortical syndrome of aging. Neurobiol Aging. 2002;23:421–31. doi: 10.1016/S0197-4580(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Roriz-Cruz M, et al. Cognitive impairment and frontal-subcortical geriatric syndrome are associated with metabolic syndrome in a stroke-free population. Neurobiol Aging. 2007;28:1723–36. doi: 10.1016/j.neurobiolaging.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Rosano C, et al. Association between physical and cognitive function in healthy elderly: the health, aging and body composition study. Neuroepidemiology. 2005;24:8–14. doi: 10.1159/000081043. [DOI] [PubMed] [Google Scholar]
- Rosano C, et al. Slower gait, slower information processing and smaller prefrontal area in older adults. Age Ageing. 2012;41:58–64. doi: 10.1093/ageing/afr113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen WG. Verbal fluency in aging and dementia. Journal of Clinical Neuropsychology. 1980;2:135–146. doi: 10.1080/01688638008403788. [DOI] [Google Scholar]
- Schroeter ML, et al. Neurovascular coupling is impaired in cerebral microangiopathy—An event-related Stroop study. Neuroimage. 2007;34:26–34. doi: 10.1016/j.neuroimage.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, et al. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc. 2001;49:1544–8. doi: 10.1046/j.1532-5415.2001.4911247.x. [DOI] [PubMed] [Google Scholar]
- Simonsick EM, et al. Assessing fatigability in mobility-intact older adults. J Am Geriatr Soc. 2014;62:347–51. doi: 10.1111/jgs.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurstone LL (1938) Primary mental abilities. Psychometric Monographs, No. 1, Vol., Chicago: Univ. Chicago Press.
- Tian Q et al (2015) Lap time variation and executive function in older adults: the Baltimore Longitudinal Study of Aging. Age Ageing doi:10.1093/ageing/afv076 [DOI] [PMC free article] [PubMed]
- Tiffin J (1968) Purdue Pegboard Examiner Manual, Vol., Science Research Associates, Chicago, IL
- Vestergaard S, et al. Characteristics of 400-meter walk test performance and subsequent mortality in older adults. Rejuvenation Res. 2009;12:177–84. doi: 10.1089/rej.2009.0853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D (1981) WAIS-R manual: Wechsler adult intelligence scale-revised. Psychological Corporation, Vol
- Yamashiro K, et al. Cerebral microbleeds are associated with worse cognitive function in the nondemented elderly with small vessel disease. Cerebrovasc Dis Extra. 2014;4:212–20. doi: 10.1159/000369294. [DOI] [PMC free article] [PubMed] [Google Scholar]