Abstract
Aging is associated with a decline in function in many of the stem cell niches of the body. An emerging body of literature suggests that one of the reasons for this decline in function is due to cell non-autonomous influences on the niche from the body. For example, studies using the technique of parabiosis have demonstrated a negative influence of blood from aged mice on muscle satellite cells and neurogenesis in young mice. We examined if we could reverse this effect of aged serum on stem cell proliferation by treating aged rats with NT-020, a dietary supplement containing blueberry, green tea, vitamin D3, and carnosine that has been shown to increase neurogenesis in aged rats. Young and aged rats were administered either control NIH-31 diet or one supplemented with NT-020 for 28 days, and serum was collected upon euthanasia. The serum was used in cultures of both rat hippocampal neural progenitor cells (NPCs) and rat bone marrow-derived mesenchymal stem cells (MSCs). Serum from aged rats significantly reduced cell proliferation as measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and 5-bromo-2′-deoxyuridine (BrdU) assays in both NPCs and MSCs. Serum from aged rats treated with NT-020 was not different from serum from young rats. Therefore, NT-020 rescued the effect of serum from aged rats to reduce stem cell proliferation.
Keywords: Aging, Stem cells, Proliferation, NT-020
Introduction
Aging is a complex process that involves cellular senescence, a gradual loss of tissue homeostasis and decline in organ function. Emerging evidence suggests that stem cell niches within the body may be particularly sensitive to aging. At this time, there are two major areas under investigation in relation to stem cell senescence: one is cell autonomous alterations and the involvement of senescence-related genes such as p16INK4A, ARF, p21, p53, and the WNT/β-catenin pathways (Sharpless and Depinho 2007). Alternately, there is also strong evidence for cell non-autonomous effects on stem cell niches, with much of the early evidence for this coming from studies using heterochronic parabiosis (Conboy et al. 2005; Villeda et al. 2011) and early studies in our lab using the technique of in oculo transplantation (Granholm et al. 1987; Willis et al. 2005).
With age, there is a decline in stem cell regenerative capacity that is observed in stem cell niches both in the periphery and the CNS. For example, although hematopoietic stem cells (HSCs) from aged mice show increased numbers of HSCs, there is a reduced repopulating ability in serial bone marrow transplant studies (Chambers et al. 2007; Harrison and Astle 1982; Mayack et al. 2010; Sharpless and Depinho 2007). However, in some strains, HSCs do not decline with age (Harrison and Doubleday 1975). Changes in stem cell function in the central nervous system have also been extensively studied and have been the subject of review (Lazarov et al. 2010). This reduction in stem cell function with age may underlie some aspects of cognitive decline (Encinas et al. 2011; Lazarov et al. 2010).
Cell non-autonomous or systemic milieu influences on stem cell senescence have been elucidated. Using the technique of heterochronic parabiosis, the systemic circulation of a young mouse and an old mouse is connected. Using this technique, researchers have demonstrated that the systemic milieu of aged mice reduces muscle satellite stem cell function, liver stem cell function, hematopoietic stem cell function, and neural stem cell function (Conboy et al. 2005; Mayack et al. 2010; Villeda et al. 2011). Furthermore, when serum from old rats or mice is used in cultures of stem cells from various niches, the serum from old animals has a negative impact on stem cell proliferation and there are changes in fate determination that recapitulate aging (Mayack et al. 2010; Villeda et al. 2014, 2011). Several possible changes in old blood have been identified as possible negative and positive influences on the stem cell niches. For example, Villeda et al. initially suggested that an increase in CCL11 (eotaxin) is increased similarly in human plasma and parabiont plasma and may be one of the negative regulators of the aged blood on neurogenesis (Villeda et al. 2011), and a recent paper suggests beta2-microglobulin is also a pro-aging factor (Smith et al. 2015). Several reports have suggested that growth differentiation protein 11 (GDF-11) is decreased with age and treatment with GDF-11 has a positive effect on several stem cell niches (Katsimpardi et al. 2014; Sinha et al. 2014). Recently, a different group has reported the opposite (Egerman et al. 2015); the difference may lie in the methods used to detect GDF-11. As we have shown, the use of a nutraceutical also has a positive impact on stem cell aging (Acosta et al. 2010; Bickford et al. 2006; Shytle et al. 2007), we examined if there was a benefit of treatment of aged rats with NT-020 on the ex vivo effects of serum on mesenchymal stem cells (MSC) and neural progenitor cell (NPC) survival and proliferation.
Methods
Fischer 344 rats of 3–6 and 20–22 months were obtained from the NIA contract colony and housed in the USF AAALAC-accredited animal facility. All procedures were approved by the institutional IACUC. The rats were fed ad lib either a NIH-31 standard lab chow or NIH-31 lab chow supplemented with NT-020 plus Biovin (a proprietary grape seed extract (Cyvex)) (supplement provided by Natura Therapeutics) at 0.5 % to deliver a dose of NT-020 of 135 mg/kg/day. Body weight and food consumption were measured three times a week. No differences were observed between the groups in terms of body weight or food consumption. After 28 days, the rats were euthanized with CO2 and serum obtained by heart puncture into blood collection tubes and let sit then spun at 3000 rpm for 10 min. The serum was aliquoted and frozen at −80 °C until used for cell culture or ELISA experiments.
Measurement of stem cell function
MTT assay
Four thousand rat hippocampal neural progenitor cells (NPCs) (Millipore) were cultured in laminin-coated 96-well plates with proliferation media containing essential growth factors. After 24 h, the media was exchanged for fresh media containing young, old, or old-NT serum. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) labeling reagent (Roche) was added after 48 h of treatment, and the relative absorbency was measured using an ELISA microplate reader (BioTek Instruments).
Three thousand rat MSCs were cultured in poly-l-lysine-coated 96-well plates (BD 354516) with Dulbecco’s modified Eagle medium (DMEM), 10 % fetal bovine serum, and 1 % streptomycin/penicillin. Following 24-h incubation, the media was removed and replaced with young, old, young-NT-020, or old-NT-020 serum and fresh media. After 48 h, the serum was removed and standard protocol for the MTT assay was followed (Roche, USA, Cat # 11465007001). The optical density of solubilized purple formazan was measured at 570 nm on a Synergy HT plate reader (BioTek).
BrdU
Ten thousand NPCs were cultured in laminin-coated eight-well microscope slides with proliferation media containing essential growth factors. After 24 h, the media was exchanged for fresh media containing a young, old, or old-NT serum; 10 μM 5-bromo-2′-deoxyuridine (BrdU; Sigma) was added after 44 h, and cells were treated for four additional hours. Immunocytochemistry staining was performed, and photomicrographs were taken using a fluorescence microscope (Zeiss) at ×20 magnification from at least 30 different fields of vision.
Three thousand rat MSCs were cultured in poly-l-lysine-coated 96-well plates (BD 354516) with DMEM, 10 % fetal bovine serum, and 1 % streptomycin/penicillin. Following 24-h incubation, the media was removed and replaced with young, old, young-NT-020, or old-NT-020 serum and fresh media. Bromodeoxyuridine (5-bromo-2′-deoxyuridine (BrdU)) was measured using cell proliferation enzyme-linked immunosorbent assay following standard procedure (ELISA; Roche, USA, Cat # 11647229001). The optical density was measured approximately 5–10 min after the addition of the substrate solution. Absorbance was measured at 370 and 492 nm using a Synergy HT plate reader (BioTek), with the change between these two measures used for subsequent analyses.
CRP (RayBiotech), CCL11 (Quantikine) and CCL2 (RayBiotech), and BDNF (Booster Biologics) ELISA was conducted according to manufacturer’s instructions. Briefly, serum samples were diluted 1:50,000(CRP), 1:4 (CCL11), 1:1000 (CCL2), and 1:2 (BDNF) in assay diluent. One hundred microliters of sample was added to the plate and allowed to incubate overnight at 4 °C. The plate was then washed with the provided buffer four times, with careful attention to removing all excess liquid between steps. One hundred microliters of antibody solution was allowed to incubate for 1–2.5 h depending on target and then washed. After 45-min incubation with one hundred microliters of streptavidin, the plate was then washed another four times and then incubated with the TMB One substrate solution for 30 min. All incubation steps were conducted while oscillating at 60 RPM at room temperature unless otherwise noted.
Results
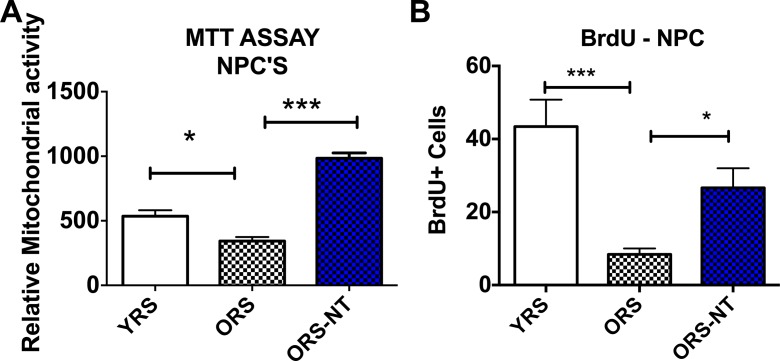
NT-020 treatment reduces the negative impact of aged serum on proliferation of hippocampal neural progenitor cells. We examined serum from young rats (4 months) and aged rats (21 months) with or without supplementation with NT-020 (135 mg/kg) for 1 month. NPCs were grown in proliferation media for 24 h prior to addition of 10 % rat serum for 48 h. Figure 1a illustrates that treatment of NPCs with old rat serum reduces the MTT signal which can be correlated with reduced numbers of cells at the time of the assay as it measures mitochondrial activity, whereas the serum from old rats treated with NT-020 is significantly higher than that found with old rat serum (one-way ANOVA F(2,12) = 65.45 followed by Sidak’s multiple comparison test *p < 0.05; **p < 0.001). As the MTT assay is an indirect measure of cell proliferation and could be increased with treatments that increase mitochondrial function, we also measured the numbers of dividing cells with BrdU. A similar effect is observed in Fig. 1b when examining BrdU-positive cells after 48-h treatment with 10 % serum. There is a significant negative effect of old rat serum on the number of dividing stem cells, and this is reversed with the serum from old rats fed NT-020 (one-way ANOVA F(2,17) = 13.64, followed by Sidak’s multiple comparison test ***p < 0.001 and *p < 0.05).
Fig. 1.
a Treatment of NPCs with old rat serum (ORS, N = 5) reduces relative cell proliferation as measured by mitochondrial MTT when compared with young rat serum (YRS, N = 5), whereas the serum from old rats treated with NT-020 (ORS-NT, N = 5) is significantly higher than that found with old rat serum (one-way ANOVA F(2,12) = 65.45 followed by Sidak’s multiple comparison test *p < 0.05; **p < 0.001). A similar effect is observed in (b) when examining BrdU-positive cells after 48-h treatment with 10 % serum. There is a significant negative effect of old rat serum on the number of BrdU-positive cells, and this is increased in NPCs treated with serum from old rats fed NT-020 (one-way ANOVA F(2,17) = 13.64, followed by Sidak’s multiple comparison test ***p < 0.001; *p < 0.05)
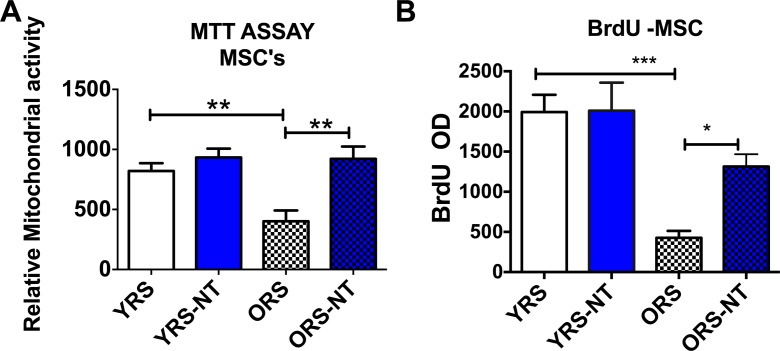
We next examined if this effect would also be observed with bone marrow-derived mesenchymal stem cells including serum from young rats treated with NT-020. As can be observed in Fig. 2a, old rat serum again reduced the relative cell proliferation when compared to young rat serum. Treatment of aged rats, but not young rats, significantly increased the relative cellular proliferation in MSCs treated with serum from these rats (one-way ANOVA F(3,16) = 8.746 followed by Sidak’s multiple comparison test **=p < 0.01). In Fig. 2b, it can be observed that old rat serum decreases proliferation of MSCs when compared with young serum. Serum from aged rats treated with NT-020 increased the proliferation of MSCs when compared with serum from aged controls (one-way ANOVA F(3,12) = 11.22 followed by Sidak’s multiple comparison ***p < 0.001; *p < 0.05).
Fig. 2.
a Bar graphs demonstrating that treatment of aged rats with NT-020, but not young rats, significantly increased the relative cellular proliferation in MSCs treated with serum from these rats (one-way ANOVA F(3,16) = 8.746 followed by Sidak’s multiple comparison test **=p < 0.01). b It can be observed that old rat serum decreases proliferation of MSCs when compared with young serum when measured using BrdU. Serum from aged rats treated with NT-020 increased the proliferation of MSCs when compared with serum from aged controls (one-way ANOVA F(3,12) = 11.22 followed by Sidak’s multiple comparison ***p < 0.001; *p < 0.05)
We assessed a few potential biomarkers in the serum to examine their contribution to stem cell proliferation and mitochondrial activity. We measured CCL11, as it had been previously described as one circulating factor in aged serum that explained the negative effect (Villeda et al. 2011). There was a significant increase in CCL11 (eotaxin) with age; however, treatment with NT-020 did not significantly decrease the levels (Table 1). Similarly, a related chemokine CCL2 (MCP-1) was increased with age in serum, but treatment with NT-020 did not significantly change the circulating levels of CCL2. Thus, although there are trends in the data, no significant reduction in these chemokines explains the effects of NT-020 treatment in the aged rats. We also examined BDNF and C-reactive protein (CRP) and found no significant age or treatment effects (Table 1).
Table 1.
Serum metabolite measures
| Young mean ± SEM (N) |
Young NT-020 mean ± SEM (N) |
Old mean ± SEM (N) |
Old NT-020 mean ± SEM (N) |
|
|---|---|---|---|---|
| CCL11 (eotaxin) (pg/ml) | 256.6 ± 7.0 (6) | 266.7 ± 9.0 (6) | 317.8 ± 15.0 (5)* | 308.0 ± 12.6 (6) |
| CCL2 (MCP-1) (pg/ml) | 2156 ± 121.3 (6) | 2565 ± 158.5 (6) | 3946 ± 760.6 (6)* | 2875 ± 217.3 (6) |
| BDNF (pg/ml) | 85.8 ± 9.5 (6) | 88.2 ± 9.3 (4) | 73.5 ± 3.5 (4) | 84.4 ± 7.8 (6) |
| CRP (ng/ml) | 20.8 ± 2.5 (10) | 21.7 ± 1.4 (10) | 15.59 ± 3.3 (9) | 20.98 ± 4.5 (9) |
*p < 0.01 young versus old
Discussion
We demonstrate that treatment of old rats with NT-020 eliminates the negative impact of the serum on stem cell proliferation when examined in vitro as serum from aged rats on the NT-020-supplemented diet did not reduce proliferation of either NPCs and MSCs. This is consistent with earlier reports that NT-020 increases neurogenesis in vivo (Acosta et al. 2010). Interestingly, this was not observed in young animals treated with NT-020, suggesting that this is indeed an effect on the aged serum and not simply the presence of NT-020 in the serum.
The literature suggests that CCL11 may be one of the negative regulators of this effect of aged serum to have a negative impact on the neurogenic niche; however, according to our data, this does not explain the effect of NT-020. We did observe an increase in CCL11 with age, but this was not significantly reduced with NT-020 treatment. It is possible that there are differences between mice and rats, as the previous report examined neurogenesis in mice (Villeda et al. 2011). We also examined a few other factors that have been suggested to play a role in neurogenesis such as BDNF; however, we were not able to identify a single factor that was responsible for the effect of NT-020, it is likely that there is a cumulative effect of multiple factors or changes in factors that were not measured. As there has been significant literature published on the positive effects of GDF-11 as a potential factor that increases both muscle and neural stem cell proliferation in aged mice (Katsimpardi et al. 2014; Loffredo et al. 2013; Sinha et al. 2014), we also attempted to measure GDF-11. However, GDF-11 was undetectable in our assay; this may have been the result of a technical problem as the samples had been in the freezer longer than the suggested 6 months (CUSABIO Biotech). Additional studies will need to be performed to examine this further. At this time, the literature suggests that with age, there are both increases in negative regulators of stem cell proliferation in the serum as well as loss of potential positive regulators. Studies using parabiosis in fact do report a mixture of effects, as in some niches, the young parabionts have a decline in stem cell proliferation when connected to aged partners as well as the positive effect of the young partner on the aged parabiont (Conboy et al. 2005; Villeda et al. 2011).
The area of research examining the effects of young blood has exploded in the past few years, and there seems to be a search for the “fountain of youth” factor that may be responsible for this effect. The data on parabiosis or injections of young blood or neonatal blood are compelling and have important effects on the neurogenic niche and other niches within the body. One study used umbilical cord blood demonstrating that a single injection was sufficient to increase neurogenesis in aged rats (Bachstetter et al. 2008). A follow-up study examined which cells within the umbilical cord blood might be responsible, and it was suggested that naïve T cells were the fraction responsible for this effect (Shahaduzzaman et al. 2013). Thus, it is clear that multiple factors and cells within young/neonatal blood may have beneficial effects on the stem cell niches of aged subjects. Others have also examined serum from caloric restricted mice and monkeys as a potential in vitro model of caloric restriction (CR) as CR has been shown to increase maximal life span in many species (de Cabo et al. 2003). In these studies, the authors present data showing that the serum from young CR mice reduced proliferation of FaO rat hepatoma cells. At first, this may seem in contrast to our data suggesting that increasing cell proliferation is a positive outcome; however, the studies examine very different cell types. It seems that proliferation of cancer cells and progenitor cells is regulated by similar mechanisms as many tumor suppressor genes also regulate stem cell proliferation (Sharpless 2005). With aging, it is desirable to increase neurogenesis as this has been associated with increased neural plasticity and cognitive function (Dupret et al. 2007; Lazarov et al. 2010; van Praag et al. 2002). One advantage of a nutraceutical approach is that it targets multiple mechanisms (Mandel et al. 2012). Although in this study we have not identified the mechanism, future studies using proteomics and flow cytometry to examine a larger set of protein and cellular changes would likely point to multiple changes in circulating factors that combined together leading to the beneficial effect of the NT-020 treatment.
Acknowledgments
Conflict of interest
PCB is co-founder of Natura Therapeutics, Inc. PCB, JT, RDS, and CDS are inventors on patents relating to NT-020. JT and RDS are consultants for Natura Therapeutics.
References
- Acosta S., Jernberg J., Sanberg C. D., Sanberg P. R., Small B. J., Gemma C., Bickford P. C. NT-020, a natural therapeutic approach to optimize spatial memory performance and increase neural progenitor cell proliferation and decrease inflammation in the aged rat. Rejuvenation Res. 2010;13(5):581–588. doi: 10.1089/rej.2009.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachstetter A.D., Pabon M.M., Cole M.J., Hudson C.E., Sanberg P.R., Willing A.E., Bickford PC, Gemma C. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC Neurosci. 2008;9:22. doi: 10.1186/1471-2202-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford P.C., Tan J., Shytle R.D., Sanberg C.D., El-Badri N., Sanberg P.R. Nutraceuticals synergistically promote proliferation of human stem cells. Stem Cells Dev. 2006;15(1):118–123. doi: 10.1089/scd.2006.15.118. [DOI] [PubMed] [Google Scholar]
- Chambers S.M., Shaw C.A., Gatza C., Fisk C.J., Donehower L.A., Goodell M.A. Aging hematopoietic stem cells decline in function and exhibit epigenetic dysregulation. PLoS Biol. 2007;5(8):e201. doi: 10.1371/journal.pbio.0050201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy I.M., Conboy M.J., Wagers A.J., Girma E.R., Weissman I.L., Rando T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- de Cabo R., Furer-Galban S., Anson R. M., Gilman C., Gorospe M., Lane M. A. An in vitro model of caloric restriction. Exp Gerontol. 2003;38(6):631–639. doi: 10.1016/S0531-5565(03)00055-X. [DOI] [PubMed] [Google Scholar]
- Dupret D., Fabre A., Dobrossy M. D., Panatier A., Rodriguez J. J., Lamarque S., Lemaire V., Oliet S.H., Piazza P.V., Abrous D. N. Spatial learning depends on both the addition and removal of new hippocampal neurons. PLoS Biol. 2007;5(8):e214. doi: 10.1371/journal.pbio.0050214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egerman M. A., Cadena S. M., Gilbert J. A., Meyer A., Nelson H. N., Swalley S. E., Mallozzi C., Jacobi C., Jennings L.L., Clay I., Laurent G., Ma S., Brachat S., Lach-Trifilieff E., Shavlakadze T., Trendelenburg A.U., Brack A.S., Glass D. J. GDF11 increases with age and inhibits skeletal muscle regeneration. Cell Metab. 2015;22(1):164–174. doi: 10.1016/j.cmet.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas J.M., Michurina T.V., Peunova N., Park J.H., Tordo J., Peterson D.A., Fishell G, Koulakov A, Enikolopov G. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8(5):566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm A.C., Gerhardt G.A., Eriksdotter-Nilsson M., Bickford-Wimer P.C., Palmer M.R., Seiger A., Olson L, Hoffer B.J. Age-related changes in cerebellar noradrenergic pre- and postsynaptic mechanisms: intrinsic vs extrinsic determinants evaluated with brain grafts in oculo. Brain Res. 1987;423:71–78. doi: 10.1016/0006-8993(87)90826-2. [DOI] [PubMed] [Google Scholar]
- Harrison D.E., Astle C.M. Loss of stem cell repopulating ability upon transplantation. Effects of donor age, cell number, and transplantation procedure. J ExpMed. 1982;156(6):1767–1779. doi: 10.1084/jem.156.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D.E., Doubleday J.W. Normal function of immunologic stem cells from aged mice. J Immunol. 1975;114(4):1314–1317. [PubMed] [Google Scholar]
- Katsimpardi L., Litterman N. K., Schein P. A., Miller C. M., Loffredo F. S., Wojtkiewicz G. R., Chen J.W., Lee R.T., Wagers A.J., Rubin L. L. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344(6184):630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov O., Mattson M.P., Peterson D.A., Pimplikar S.W., van Praag H. When neurogenesis encounters aging and disease. Trends Neurosci. 2010;33(12):569–579. doi: 10.1016/j.tins.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loffredo F. S., Steinhauser M. L., Jay S. M., Gannon J., Pancoast J. R., Yalamanchi P., Sinha M., Dall’Osso C., Khong D., Shadrach J.L., Miller CM, Singer B.S., Stewart A., Psychogios N., Gerszten R.E., Hartigan A.J., Kim M.J., Serwold T., Wagers A.J., Lee R. T. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell. 2013;153(4):828–839. doi: 10.1016/j.cell.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel S. A., Weinreb O., Amit T., Youdim M. B. Molecular mechanisms of the neuroprotective/neurorescue action of multi-target green tea polyphenols. Front Biosci (Schol Ed) 2012;4:581–598. doi: 10.2741/S286. [DOI] [PubMed] [Google Scholar]
- Mayack S.R., Shadrach J.L., Kim F.S., Wagers A.J. Systemic signals regulate ageing and rejuvenation of blood stem cell niches. Nature. 2010;463(7280):495–500. doi: 10.1038/nature08749. [DOI] [PubMed] [Google Scholar]
- Shahaduzzaman M., Golden J. E., Green S., Gronda A. E., Adrien E., Ahmed A., Sanberg P.R., Bickford P.C., Gemma C, Willing A. E. A single administration of human umbilical cord blood T cells produces long-lasting effects in the aging hippocampus. Age (Dordr) 2013;35(6):2071–2087. doi: 10.1007/s11357-012-9496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless N.E. INK4a/ARF: a multifunctional tumor suppressor locus. Mutat Res. 2005;576(1–2):22–38. doi: 10.1016/j.mrfmmm.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Sharpless N.E., Depinho R.A. How stem cells age and why this makes us grow old Nat. J ExpMed. 2007;8(9):703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- Shytle R.D., Ehrhart J., Tan J., Vila J., Cole M., Sanberg C.D., Sanberg P.R., Bickford P.C. Oxidative stress of neural, hematopoietic, and stem cells: protection by natural compounds. Rejuvenation Res. 2007;10(2):173–178. doi: 10.1089/rej.2006.0515. [DOI] [PubMed] [Google Scholar]
- Sinha M., Jang Y. C., Oh J., Khong D., Wu E. Y., Manohar R., Miller C., Regalado S.G., Loffredo F.S., Pancoast J.R., Hirshman M.F., Lebowitz J., Shadrach J.L., Cerletti M., Kim M.J., Serwold T., Goodyear L.J., Rosner B., Lee R.T., Wagers A. J. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344(6184):649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. K., He Y., Park J. S., Bieri G., Snethlage C. E., Lin K., Gontier G., Wabl R., Plambeck K.E., Udeochu J., Wheatley E.G., Bouchard J., Eggel A., Narasimha R., Grant J.L., Luo J., Wyss-Coray T., Villeda S. A. Beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. 2015 doi: 10.1038/nm.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H., Schinder A.F., Christie B.R., Toni N., Palmer T.D., Gage F.H. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda S. A., Plambeck K. E., Middeldorp J., Castellano J. M., Mosher K. I., Luo J., Smith L.K., Bieri G., Lin K., Berdnik D., Wabl R., Udeochu J., Wheatley E.G., Zou B., Simmons D.A., Xie X.S., Longo F.M., Wyss-Coray T. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20(6):659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda S.A., Luo J., Mosher K.I., Zou B., Britschgi M., Bieri G., Stan T.M., Fainberg N, Ding Z., Eggel A., Lucin K.M., Czirr E., Park J.S., Couillard-Després S., Aigner L., Li G., Peskind E.R., Kaye J.A., Quinn J.F., Galasko D.R., Xie X.S., Rando T.A., Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477(7362):90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis L, Bickford PC, Zaman V, Moore A, Granholm A-C (2005) Bleuberry extract enhances survival of intraocular hippocampal transplants. Cell Transplantation [DOI] [PubMed]