Abstract
With considerable variation including potential sex-specific differential rate of skeletal muscle loss, identifying modifiable factors for sarcopenia will be pivotal to guide targeted interventions. This study seeks to identify clinical and biological correlates of sarcopenia in community-dwelling older adults, with emphasis on the role of anabolic and catabolic stimuli, and special reference to gender specificity. In this cross-sectional study involving 200 community-dwelling and functionally independent older adults aged ≥50 years, sarcopenia was defined using the Asian Working Group for Sarcopenia criteria. Comorbidities, cognitive and functional performance, physical activity and nutritional status were routinely assessed. Biochemical parameters included haematological indices, lipid panel, vitamin D level, anabolic hormones [insulin-like growth factor-1 (IGF-1), free testosterone (males only)] and catabolic markers [inflammatory markers (interleukin-6, C-reactive protein) and myostatin]. Multiple logistic regression was performed to identify independent predictors for sarcopenia. Age was associated with sarcopenia in both genders. Malnutrition conferred significantly higher odds for sarcopenia in women (OR = 5.71, 95 % CI 1.13–28.84.44, p = 0.035) while higher but acceptable range serum triglyceride was protective in men (OR = 0.05, 95 % CI 0.00–0.52, p = 0.012). Higher serum myostatin independently associated with higher odds for sarcopenia in men (OR = 1.11, 95 % CI 1.00–1.24, p = 0.041). Serum IGF-1 was significantly lower amongst female sarcopenic subjects, with demonstrable trend for protective effect against sarcopenia in multiple regression models, such that each 1 ng/ml increase in IGF-1 was associated with 1 % decline in odds of sarcopenia in women (p = 0.095). Our findings support differential pathophysiological mechanisms for sarcopenia that, if corroborated, may have clinical utility in guiding sex-specific targeted interventions for community-dwelling older adults.
Keywords: Sarcopenia, Nutrition, Insulin-like growth factor-1, Myostatin
Introduction
Age-related decline in skeletal muscle mass eventually culminating in loss of strength and function is now recognized as a distinct clinical phenotype referred to as sarcopenia (Fielding et al. 2011). The implications of sarcopenia in the older adult have been reported extensively, including impairment in physical performance, mobility limitations, frailty and its consequences of falls, fractures and hospitalizations Clark and Manini (2010). Although progressive loss of muscle mass appears inevitable, with annual rate of decline of 1–2 % from as early as age 50, and muscle strength decline of 1.5 % per year between ages 50 and 60 increasing to 3 % annually thereafter (von Haehling et al. 2010), there exists considerable variation in the rate of skeletal muscle mass loss (Korostishevsky et al. 2015) which, importantly, remains potentially reversible as even the most frail of older adults had exhibited improvements with exercise interventions (Fiatarone et al. 1994).
There is emerging evidence for sarcopenia being a multi-factorial process, driven by hormonal alterations, nutritional factors, inflammation and disease states (Cruz-Jentoft et al. 2014; Landi et al. 2012). Myostatin, a member of the transforming growth factor-β superfamily, has also received attention for its role as an inhibitor of skeletal muscle growth and satellite cell proliferation (Trendelenburg et al. 2009). Further, it has been postulated that the progressive loss of muscle mass may be consequent to the imbalance between muscle tissue anabolism and catabolism, although the specific contribution of individual pathways in the complex pathogenesis of muscle wasting remains to be delineated. With an ageing population and estimated direct healthcare cost attributable to sarcopenia amounting to $18.5 billion in the USA in the year 2000 (Janssen et al. 2004), the identification of modifiable factors for sarcopenia will be pivotal to developing therapeutic interventions to counteract the cascade from sarcopenia through frailty and eventual disability.
Epidemiological data for discordance in sarcopenia prevalence between older men and women have been conflicting (Landi et al. 2012; Patel et al. 2013; Lee et al. 2013). Several studies had suggested differential sex-specific rate of absolute muscle loss, being greater in men than in women, which could not be attributed merely to the larger initial muscle mass in men (Payette et al. 2003). The need for improved insights into potential differential sex-specific mechanisms driving sarcopenia is further heightened by the recently observed higher mortality risk conferred by sarcopenia in older women despite its lower prevalence compared to their male counterparts (Batsis et al. 2014). Indeed, data from the Framingham Heart Study had suggested that longitudinal decline in fat-free mass was consequent to a withdrawal of anabolic stimuli in men but reflecting an increase in catabolic stimuli represented by interleukin-6 (IL-6) in women (Payette et al. 2003).
This study sought to identify clinical and biological correlates of sarcopenia in a representative cohort of community-dwelling and functionally independent older adults, with emphasis on the role of anabolic hormones [insulin-like growth factor-1 (IGF-1) and free testosterone (in men)] and catabolic stimuli (inflammation and myostatin), and special reference to sex specificity.
Methods
Study population
The “Longitudinal Assessment of Biomarkers for characterization of early Sarcopenia and predicting frailty and functional decline in community-dwelling Asian older adults Study” (GERI-LABS) is a prospective cohort study involving cognitively intact and functionally independent adults aged 50 years and older residing within the community. We have completed the study’s target recruitment of 200 subjects between August 2013 and July 2014, with all subjects having undergone baseline clinical and blood biomarker assessments.
Informed written consent was obtained from the participant, and the study was approved by the Domain Specific Review Board (DSRB) of the National Healthcare Group (NHG).
Eligibility criteria
Subjects were eligible if they were (i) aged 50 to 99 years at study enrollment, (ii) community-dwelling, and (iii) independent in both activities of daily living (ADLs) and instrumental ADLs (iADLs). We excluded subjects with a known history of dementia or evidence of cognitive impairment [defined as Chinese Mini-Mental State Examination (CMMSE) score ≤21] (Sahadevan et al. 2000), and inability to walk at least 4.5 m independently. Residents of sheltered or nursing homes were similarly excluded.
Data collection
Clinical assessments
Demographic data and comorbid vascular risk factors—hypertension, hyperlipidemia, diabetes mellitus, atrial fibrillation, peripheral vascular disease, smoking and alcohol history, stroke or transient ischemic attack and ischemic heart disease—were captured at baseline, based on self-report or the use of disease-specific medications. The presence of chronic inflammatory disease and active treatment with steroids or immunosuppressant medication, malignancy, other endocrine disorders and evidence of advanced organ failure was routinely documented.
Standing height, body weight and waist circumference were measured, and body mass index (BMI) was calculated. With increasing recognition for the limitation of BMI as an indicator of obesity as it includes lean mass in its calculation without discriminating between muscle and fat distribution, A Body Shape Index (ABSI) was calculated for each individual [ABSI = Waist circumference/(BMI2/3Height1/2)], which allows for better representation of central body volume (Krakauer and Krakauer 2012).
The locally validated CMMSE was administered for assessment of cognitive performance. Functional ability was evaluated using Barthel’s basic activities of daily living (ADL) index and Lawton and Brody’s instrumental ADL (iADL) index (Mahoney and Barthel 1965; Barberger-Gateau et al. 1992). Physical activity level was captured using the Frenchay activity scale (Wade et al. 1985). Nutrition was systematically assessed using the locally validated Mini Nutritional Assessment (MNA) questionnaire (Chan et al. 2010).
Grip strength was measured using the hydraulic hand dynamometer (North Coast@ Hydraulic Hand Dynamometer), with two trials of grip strength for each hand and all four trials averaged to yield strength. Gait speed was based on the time to walk 3 m, and physical performance was measured using the Short Physical Performance Battery (SPPB) (Guralnik et al. 1994).
Laboratory investigations
Fasting venous blood sample was obtained at baseline for measurement of serum albumin, glucose, lipid profile, C-reactive protein, full blood count and 25-hydroxy vitamin D, with additional analysis for free testosterone level in male subjects, performed within the clinical laboratory of Tan Tock Seng Hospital, Singapore. Vitamin D deficiency was defined as serum concentration <20 ng/ml based on modified Holick’s classification (Thacher and Clarke 2011), while C-reactive protein levels in excess of 5 mg/l were considered elevated.
In addition, serum obtained following centrifugation at 3000 rpm for 10 min was aliquoted and stored at −80 °C until analysis for Interleukin-6 (IL-6) (eBioscience, San Diego, CA, USA), myostatin (Immundiagnostik AG, Bensheim) and insulin-like growth factor-1 (IGF-1) (BioVendor, Ceska republika). All assays were performed according to the manufacturers’ recommendations, and measured in duplicates, with detection limits of 0.04 pg/ml for IL-6, 0.6 ng/ml for myostatin and 1 ng/ml for IGF-1.
Muscle and fat measures
Percentage body fat and lean mass measures were obtained via a dual-energy X-ray absorptiometry system (Discovery™ APEX 13.3; Hologic, Bedford, MA, USA). Appendicular skeletal mass was derived from the summation of muscle mass measurements in the four limbs.
Sarcopenia was defined using the Asian Working Group for Sarcopenia criteria, employing recommended gender specific cut-off values for muscle mass as measured on DXA, as well as grip strength and gait speed (Chen et al. 2014).
Statistical analyses
Descriptive data are presented as means (±SD) or median (interquartile range, IQR) for quantitative variables and as absolute and relative frequencies for categorical variables. We performed univariate analyses comparing sarcopenic and non-sarcopenic subjects in baseline demographics, clinical measures of cognitive, functional and physical performance, nutritional status, comorbid medical conditions and biochemical parameters using independent-sample t test and Wilcoxon rank-sum test for parametric and non-parametric continuous variables, respectively, and chi-square and Fisher’s exact tests for categorical variables. Analyses were first performed on the whole cohort, followed by subgroup analyses according to gender. To identify independent factors contributing to sarcopenia, we performed multiple logistic regression, adjusting for age and significant univariate variables.
Statistical analyses were performed using STATA version 12 (Stata Corp., College Station, TX). All statistical tests were two-tailed, with p value ≤0.05 considered statistically significant.
Results
Clinical characteristics of study cohort
Two hundred and thirty-one healthy older adults fulfilled eligibility criteria, of whom 200 provided written informed consent and were recruited. The mean age of enrolled subjects was 67.9 ± 7.9 years, with female predominance (68.5 %) and majority of Chinese ethnicity (92 %). There was no difference in age and gender distribution between study participants and non-participants.
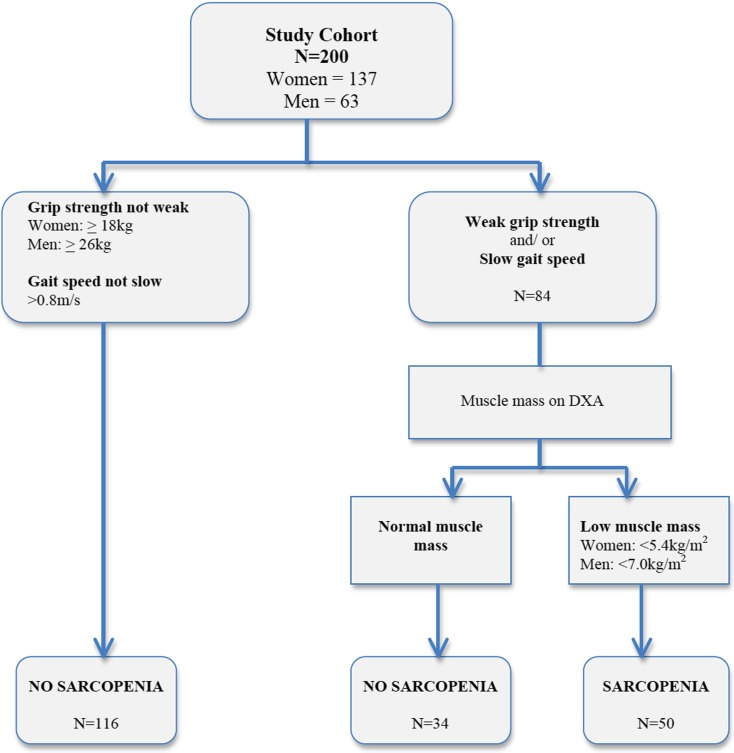
Fifty (25 %) subjects fulfilled criteria for sarcopenia at baseline (Fig. 1), and they were significantly older than their non-sarcopenic counterparts (72.0 ± 8.1 vs 66.6 ± 7.3, p < 0.001), but gender distribution was similar between groups. Amongst the individual medical comorbidities, only cerebrovascular disease was significantly different, being higher in prevalence amongst sarcopenic subjects (8 vs 0.7 %, p = 0.014). While serum albumin level was similar and none of the participants was overtly malnourished based on MNA total score <17, sarcopenic subjects were significantly more likely to be at risk of malnutrition (MNA total score 17–23.5: 14 % vs 4.7 %, p = 0.025). Sarcopenic subjects had significantly lower BMI (21.7 ± 2.4 vs 24.7 ± 3.8, p < 0.001), but their ABSI was higher than their non-sarcopenic counterparts (8.55 ± 0.64 vs 8.27 ± 0.47, p = 0.002) (Table 1). Functional ability and physical performance was similar between sarcopenic and non-sarcopenic subjects, but sarcopenic subjects exhibited significantly lower physical activity level (FAI: 29.9 ± 5.6 vs 32.8 ± 4.7, p = 0.0003).
Fig. 1.
Application of the Asian Working Group Algorithm for Sarcopenia
Table 1.
Clinical characteristics of study cohort (N = 200)
| Sarcopenic | Non-sarcopenia | p value | |
|---|---|---|---|
| (n = 50) | (n = 150) | ||
| Demographics | |||
| Age (years), mean (SD) | 72.0 (8.1) | 66.6 (7.3) | <0.001 |
| Gender (female), n (%) | 34 (68 %) | 103 (68.7 %) | 0.930 |
| Race (Chinese), n (%) | 50 (100 %) | 134 (89.3 %) | 0.294 |
| Comorbidities (n, %) | |||
| Hypertension | 27 (54 %) | 69 (46 %) | 0.327 |
| Hyperlipidaemia | 32 (64 %) | 99 (66 %) | 0.797 |
| Diabetes mellitus | 10 (20 %) | 35 (23.3 %) | 0.625 |
| IHD | 2 (4 %) | 2 (1.3 %) | 0.261 |
| Atrial fibrillation | 2 (4 %) | 7 (4.7 %) | 1.00 |
| Stroke/TIA | 4 (8 %) | 1 (0.7 %) | 0.014 |
| Smoker | 7 (14 %) | 14 (9.3 %) | 0.510 |
| Ethanol use | 5 (10 %) | 6 (4 %) | 0.013 |
| Inflammatory diseasea | 2 (4 %) | 1 (0.7 %) | 0.155 |
| Advanced organ failure | 0 | 1 (0.7 %) | 1.00 |
| Malignancy | 3 (6 %) | 9 (6 %) | 1.00 |
| Other endocrine | 5 (10 %) | 9 (6 %) | 0.337 |
| Cognitive performance | |||
| CMMSE (median, IQR) | 27 (26–28) | 27 (25–28) | 0.577 |
| Functional performance | |||
| MBI (median, IQR) | 100 (100–100) | 100 (100–100) | 0.487 |
| iADL (median, IQR) | 9 (9–9) | 9 (9–9) | 0.245 |
| Physical activity and performance | |||
| FAI (mean, SD) | 29.9 (5.6) | 32.8 (4.7) | <0.001 |
| SPPB (median, IQR) | 12 (10–12) | 12 (11–12) | 0.120 |
| Nutritional status | |||
| BMI (kg/m2), mean (SD) | 21.7 (2.4) | 24.7 (3.8) | <0.001 |
| ABSI, mean (SD) | 8.55 (0.64) | 8.27 (0.49) | 0.002 |
| Albumin (g/l), mean (SD) | 38.3 (2.4) | 39.2 (2.6) | 0.369 |
| MNA total, mean (SD) | 26.1 (2.0) | 27.3 (1.9) | <0.001 |
| At risk of malnutrition, n (%) | 7 (14 %) | 7 (4.7 %) | 0.025 |
ABSI a body shape index, BMI body mass index, CMMSE Chinese Mini-Mental State Examination, FAI Frenchay Activity Index, iADL Lawton and Brody’s instrumental activities of daily living, IHD ischemic heart disease, MBI modified Barthel Index, MNA Mini Nutrition Assessment, SPPB short physical performance battery
aInflammatory disease: myasthenia gravis, on immunosuppressant therapy (n = 2); ulcerative colitis (n = 1)
Biochemical parameters of study cohort
Haemoglobin level was significantly lower in sarcopenic subjects (12.9 ± 1.3 vs 13.3 ± 1.2 g/dl, p = 0.038), who also had lower serum triglyceride levels (0.97 ± 0.41 vs 1.27 ± 0.61 mmol/l, p = 0.001) despite there being no difference in prevalence of dyslipidaemia or use of cholesterol-lowering medications. Total cholesterol, fasting glucose and serum vitamin D levels were similar between groups.
Serum markers of inflammation were similar between sarcopenic and non-sarcopenic subjects (even following exclusion of subjects with inflammatory disease or immunosuppressant therapy), and there was no significant difference in myostatin levels. However, sarcopenic subjects had significantly lower serum levels of IGF-1 [100.1 (IQR 77.7–117.6) vs 117.6 ng/ml (IQR 88.2–147), p = 0.007] (Table 2).
Table 2.
Biochemical correlates of sarcopenia (N = 200)
| Sarcopenic | Non-sarcopenia | p value | |
|---|---|---|---|
| (n = 50) | (n = 150) | ||
| Full blood count, mean (SD) | |||
| Haemoglobin (g/dl) | 12.9 (1.3) | 13.3 (1.2) | 0.038 |
| White blood cell count (×109/l) | 5.7 (1.7) | 5.8 (1.3) | 0.628 |
| Neutrophils (×109/l) | 3.4 (1.3) | 3.4 (1.0) | 0.852 |
| Lymphocytes (×109/l) | 1.7 (0.7) | 1.8 (0.5) | 0.132 |
| Monocytes (×109/l) | 0.41 (0.13) | 0.41 (0.12) | 0.675 |
| Fasting glucose (mmol/l), median (IQR) | 5.2 (4.9–5.8) | 5.4 (5–6.3) | 0.094 |
| Lipid panel (mmol/l), mean (SD) | |||
| Total cholesterol | 4.95 (1.05) | 5.13 (1.00) | 0.274 |
| Low density lipoprotein | 2.93 (0.84) | 3.11 (0.83) | 0.191 |
| High density lipoprotein | 1.58 (0.42) | 1.47 (0.37) | 0.079 |
| Triglyceride | 0.97 (0.41) | 1.27 (0.61) | 0.001 |
| Inflammatory markers | |||
| Raised C-reactive protein (>5 mg/l), n (%) | 4 (8 %) | 4 (8 %) | 1.00 |
| Interleukin-6 (pg/ml), median (IQR) | 1.07 (0.60–1.54) | 1.0 (0.64–1.7) | 0.762 |
| Myostatin (ng/ml), median (IQR) | 29.75 (22.5–33.5) | 27.68 (22.3–32.51) | 0.257 |
| Hormonal | |||
| Vitamin D (μg/l), mean (SD) | 29.5 (9.6) | 29.3 (9.6) | 0.930 |
| Insulin-like growth factor-1 (ng/ml), median (IQR) | 100.1 (77.7–117.6) | 117.6 (88.2–147) | 0.007 |
Clinical and biochemical parameters associated with sarcopenia: gender-specific)
The prevalence of sarcopenia was 24.8 % in women and 25.4 % in men. While the risk of malnutrition was significantly higher in female sarcopenic subjects (17.7 vs 2.9 %, p = 0.007), nutritional status was not associated with sarcopenia in men. Additionally, we observed that female sarcopenic subjects had significantly higher ABSI (8.52 ± 0.75 vs 8.22 ± 0.53, p = 0.012) despite their significantly lower BMI, while sarcopenia was associated with ABSI but not BMI in men (Table 3). In both genders, serum triglyceride level was significantly lower in sarcopenic subjects. There was no association between vitamin D and sarcopenia in both genders.
Table 3.
Gender-specific clinical parameters associated with sarcopenia—female (N = 137) and male (N = 63)
| Female (N = 137) | p value | Male (N = 63) | p value | |||
|---|---|---|---|---|---|---|
| Sarcopenia (n = 34) | Non-sarcopenia (n = 103) | Sarcopenia (n = 16) | Non-sarcopenia (n = 47) | |||
| Demographics | ||||||
| Age, mean (SD) | 70.7 (8.7) | 65.2 (6.7) | <0.001 | 74.8 (5.7) | 69.5 (7.9) | 0.016 |
| Race (Chinese) | 34 (100 %) | 93 (90.3 %) | 0.478 | 16 (100 %) | 41 (87.2 %) | 1.00 |
| Comorbidities | ||||||
| Hypertension | 18 (52.9 %) | 42 (40.8 %) | 0.215 | 9 (56.3 %) | 27 (57.5 %) | 0.933 |
| Hyperlipidaemia | 22 (64.7 %) | 68 (66.0 %) | 0.889 | 10 (62.5 %) | 31 (66.0 %) | 1.00 |
| Diabetes mellitus | 5 (14.7 %) | 23 (22.3 %) | 0.339 | 5 (31.3 %) | 12 (25.5 %) | 0.747 |
| IHD | 2 (5.9 %) | 0 | 0.060 | 0 | 2 (4.3 %) | 1.00 |
| Atrial fibrillation | 2 (5.9 %) | 3 (2.9 %) | 0.598 | 0 | 4 (8.5 %) | 0.564 |
| Stroke/TIA | 0 | 1 (0.97 %) | 1.00 | 4 (25 %) | 0 | 0.003 |
| Smoker | 2 (5.8 %) | 2 (1.9 %) | 0.117 | 5 (31.3 %) | 12 (25.5 %) | 0.872 |
| Ethanol use | 2 (5.9 %) | 0 | 0.060 | 3 (18.8 %) | 5 (10.6 %) | 0.214 |
| Inflammatory diseasea | 0 | 1 (0.97 %) | 1.00 | 2 (12.5 %) | 0 | 0.061 |
| Advanced organ failure | 0 | 1 (1.0 %) | 1.00 | 0 | 0 | – |
| Malignancy | 2 (2.9 %) | 6 (5.8 %) | 0.681 | 2 (12.5 %) | 3 (6.4 %) | 0.594 |
| Other endocrine | 5 (14.7 %) | 6 (5.8 %) | 0.098 | 0 | 3 (6.4 %) | 0.564 |
| Cognitive | ||||||
| CMMSE (median, IQR) | 27 (26–28) | 27 (25–28) | 0.880 | 27 (26–28) | 27 (25–27) | 0.413 |
| Functional | ||||||
| MBI (median, IQR) | 100 (100–100) | 100 (100–100) | 0.534 | 100 (100–100) | 100 (100–100) | 0.746 |
| iADL (median, IQR) | 9 (9–9) | 9 (9–9) | 0.408 | 9 (9–9) | 9 (9–9) | 0.420 |
| Physical activity and performance | ||||||
| FAI (mean, SD) | 32.0 (3.7) | 33.3 (4.3) | 0.116 | 25.3 (6.2) | 31.7 (5.4) | <0.001 |
| SPPB (median, IQR) | 12 (11–12) | 12 (11–12) | 0.265 | 12 (10–12) | 12 (11–12) | 0.245 |
| Nutritional | ||||||
| BMI (mean, SD) | 21.4 (2.1) | 24.9 (3.9) | <0.001 | 22.6 (2.8) | 24.4 (3.6) | 0.076 |
| ABSI (mean, SD) | 8.52 (0.75) | 8.22 (0.53) | 0.012 | 8.60 (0.26) | 8.37 (0.37) | 0.027 |
| Albumin (mean, SD) | 39.0 (2.3) | 39.0 (2.6) | 0.938 | 38.6 (2.4) | 39.7 (2.6) | 0.143 |
| MNA total (mean, SD) | 25.8 (2.0) | 27.2 (1.8) | <0.001 | 26.8 (1.6) | 27.4 (2.0) | 0.298 |
| At risk of malnutrition, n (%) | 6 (17.7 %) | 3 (2.9 %) | 0.007 | 1 (6.3 %) | 4 (8.5 %) | 1.00 |
ABSI a body shape index, BMI body mass index, CMMSE Chinese Mini-Mental State Examination, FAI Frenchay Activity Index, iADL Lawton and Brody’s instrumental activities of daily living, IHD ischemic heart disease, MBI modified Barthel Index, MNA Mini Nutrition Assessment, SPPB short physical performance battery
aInflammatory disease: myasthenia gravis, on immunosuppressant therapy (n = 2); ulcerative colitis (n = 1)
Male sarcopenic subjects had significantly lower haemoglobin and lymphocyte counts than their non-sarcopenic counterparts, a difference that was not observed in women. Amongst the individual biomarkers representative of a catabolic state, there was a trend for higher serum myostatin in male sarcopenic subjects [30.72 (IQR 23.75–34.05) vs 25 (21–32.27) ng/ml, p = 0.057], but without demonstrable difference in women (Table 4). Serum levels of inflammatory cytokine (IL-6) were similar between sarcopenic and non-sarcopenic subjects in both genders, with no observed association with CRP.
Table 4.
Gender-specific biochemical parameters associated with sarcopenia—female (N = 137) and male (N = 63)
| Female (N = 137) | p value | Male (N = 63) | p value | |||
|---|---|---|---|---|---|---|
| Sarcopenia (n = 34) | Non-sarcopenia (n = 103) | Sarcopenia (n = 16) | Non-sarcopenia (n = 47) | |||
| FBC, mean (SD) | ||||||
| Hb (g/dl) | 12.6 (1.3) | 12.9 (1.1) | 0.242 | 13.4 (1.0) | 14.2 (1.1) | 0.013 |
| WBC (×109/l) | 5.81 (1.86) | 5.73 (1.23) | 0.768 | 5.51 (1.33) | 6.04 (1.42) | 0.197 |
| Neutrophils | 3.40 (1.43) | 3.27 (0.92) | 0.548 | 3.41 (1.02) | 3.59 (1.13) | 0.590 |
| Lymphocytes | 1.81 (0.76) | 1.86 (0.47) | 0.665 | 1.42 (0.45) | 1.74 (0.53) | 0.034 |
| Monocytes | 0.41 (0.14) | 0.38 (0.11) | 0.230 | 0.41 (0.14) | 0.38 (0.11) | 0.230 |
| Glucose, mmol/l, median (IQR) | 5.2 (4.7–5.6) | 5.4 (5–6.1) | 0.066 | 5.6 (4.9–5.9) | 5.6 (4.9–6.4) | 0.710 |
| Lipid panel, mmol/l, mean (SD) | ||||||
| Total cholesterol | 5.17 (1.08) | 5.25 (0.96) | 0.686 | 4.47 (0.80) | 4.86 (1.06) | 0.178 |
| LDL | 3.09 (0.91) | 3.15 (0.83) | 0.698 | 2.61 (0.59) | 3.03 (0.83) | 0.068 |
| HDL | 1.65 (0.43) | 1.53 (0.38) | 0.126 | 1.44 (0.36) | 1.34 (0.32) | 0.331 |
| TG | 0.96 (0.44) | 1.28 (0.62) | 0.011 | 0.94 (0.34) | 1.26 (0.59) | 0.043 |
| Inflammatory | ||||||
| Raised CRP (>5 mg/l), n (%) | 2 (5.6 %) | 9 (8.7 %) | 0.731 | 2 (12.5 %) | 3 (6.4 %) | 0.594 |
| IL-6, pg/ml, median (IQR) | 1.06 (0.60–1.5) | 0.96 (0.59–1.58) | 0.921 | 1.12 (0.671–1.69) | 1.2 (0.7–3.8) | 0.372 |
| Myostatin, ng/ml, median (IQR) | 28.86 (22.1–33.5) | 28.5 (22.8–32.74) | 0.992 | 30.72 (23.75–34.05) | 25 (21–32.27) | 0.057 |
| Hormonal | ||||||
| Vitamin D, ug/l, mean (SD) | 27.8 (10.3) | 27.8 (9.0) | 0.987 | 33.13 (6.94) | 32.70 (10.17) | 0.878 |
| IGF1, ng/ml, median (IQR) | 94.11 (75.6–112.35) | 117.6 (88.2–141.71) | 0.002 | 111.87 (94.35–143.85) | 117.6 (88.2–151.2) | 0.740 |
| Free testosterone, pmol/l, mean (SD) | 23.02 (7.10) | 25.99 (6.47) | 0.136 | |||
FBC full blood count, CRP C-reactive protein, Hb haemoglobin, HDL high density lipoprotein, IGF-1 insulin-like growth factor-1, IL-6 interleukin-6, LDL low density lipoprotein, TG triglyceride, WBC white blood cell count
Within hormonal pathways, serum IGF-1 was significantly lower in female sarcopenic subjects [94.11 (IQR 75.6–112.35) vs 117.6 ng/ml (IQR 88.2–141.71), p = 0.002]. However, neither IGF-1 nor free testosterone level differentiated between male sarcopenic and non-sarcopenic subjects.
Differential predictors for sarcopenia: gender-specific
As individual factors contributing to sarcopenia were different in men and women, separate multiple logistic regression models were performed according to gender.
In women, we performed multiple logistic regression for sarcopenia as the outcome variable, selecting as independent variables age, risk for malnutrition, serum IGF-1 level, and triglyceride level. BMI and ABSI were excluded owing to collinearity with MNA score. In the first model, the odds for sarcopenia increased significantly with age (OR = 1.08, 95 % CI 1.02–1.15), while being at risk of malnutrition conferred sixfold higher odds for sarcopenia (OR = 6.23, 95 % CI 1.13–34.44) (Table 5). Neither serum IGF-1 nor triglyceride significantly associated with the odds of being sarcopenic. However, as earlier studies had linked IGF-1 to alterations in lipid metabolism (Eggert et al. 2014), we excluded triglyceride from the final model to delineate the independent effect of serum IGF-1 on sarcopenia status. Age and nutritional status remained significant predictors of sarcopenia, while each 1 ng/ml increase in serum IGF-1 was associated with 1 % decline in odds of sarcopenia, although not achieving statistical significance (p = 0.095).
Table 5.
Multiple logistic regression model for sarcopenia in women (N = 137)
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| OR (95 % CI) | p value | OR (95 % CI) | p value | |
| Age | 1.08 (1.02–1.15) | 0.014 | 1.08 (1.02–1.15) | 0.013 |
| MNA-risk of malnutrition | 6.23 (1.13–34.44) | 0.036 | 5.71 (1.13–28.84) | 0.035 |
| Triglyceride | 0.40 (0.16–1.03) | 0.058 | ||
| IGF-1 | 0.99 (0.98–1.00) | 0.212 | 0.99 (0.98–1.00) | 0.095 |
Model 1: R 2 = 16.7 %; model 2: R 2 = 14.4 %
In the first-step multiple logistic regression model in men, we included age and significant univariate variables of ABSI, triglyceride level, serum myostatin, haemoglobin and lymphocyte count. Taking into consideration the limited sample size in men, only age and variables with p value of <0.10 (myostatin and triglyceride) were retained in the final model (model 2). The odds for sarcopenia increased significantly with age (OR = 1.12, 95 % CI 1.01–1.25), with protective effect conferred by incremental serum triglyceride level (p = 0.012). Each 1 ng/ml increase in serum myostatin increased the odds for sarcopenia by 11 % (p = 0.041) (Table 6). Nearly 28 % of the variance in likelihood of being sarcopenic in an older male adult could be attributed to the combination of age, serum myostatin and triglyceride level.
Table 6.
Multiple logistic regression model for sarcopenia in men (N = 63)
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| OR (95 % CI) | p value | OR (95 % CI) | p value | |
| Age | 1.08 (0.96–1.22) | 0.219 | 1.12 (1.01–1.25) | 0.040 |
| ABSI | 5.29 (0.54–52.27) | 0.154 | ||
| Myostatin | 1.11 (0.99–1.23) | 0.065 | 1.11 (1.00–1.23) | 0.041 |
| Triglyceride | 0.10 (0.01–1.33) | 0.081 | 0.05 (0.00–0.52) | 0.012 |
| Haemoglobin | 0.61 (0.30–1.24) | 0.174 | ||
| Lymphocytes | 0.51 (0.11–2.43) | 0.400 | ||
Model 1: R 2 = 33.9 %; model 2: R 2 = 27.6 %
Discussion
This study contributes to the evolving literature for sarcopenia etiopathogenesis, thereby confirming the sex-specific differences in mechanisms underlying age-related sarcopenia. While older age is a common risk factor, sarcopenia appears to be driven by the catabolic influence of myostatin in men, with anabolic decline represented by reduced IGF-1 potentially contributing to sarcopenia in women. Additionally, we observed a protective effect conferred by nutrition in women and serum triglyceride in men.
The growth hormone/IGF-1 axis has been widely examined as a potential mediator of skeletal muscle loss (Perrini et al. 2010; Harridge 2003; Scicchitano et al. 2009). In a recent application of the EWGSOP criteria for sarcopenia in community-dwelling older people, IGF-1 in the lowest tertile was associated with approximately fourfold higher odds for sarcopenia, a finding that was initially corroborated in our study (Volpato et al. 2014). However, subgroup analysis by gender revealed that IGF-1 was associated with sarcopenia only in women, with similar levels being observed in sarcopenic and non-sarcopenic male subjects, although subsequent multiple regression models suggested that the observed influence of IGF-1 in women was largely driven by the effect of age. The gender-specific association between body composition and IGF-1 has been contradictory (Payette et al. 2003; Hofmann et al. 2015; Goodman-Gruen and Barrett-Connor 1997; Harris et al. 1997) . Contrary to the earlier studies that had defined sarcopenia based simply on muscle mass measurements, we had incorporated performance-based measures in defining sarcopenia, and our observed sex-specific influence of IGF-1, albeit not fulfilling statistical significance in the multiple regression models, is in line with an earlier study demonstrating correlation between muscle power and IGF-1 in older women but not in men (Kostka et al. 2000). There is thus greater impetus to examine the differential effects of IGF-1, potentially remediable through exercise and nutritional interventions (Yamada et al. 2015).
Myostatin has received recognition as a potent inhibitor of skeletal muscle growth (Elliot et al. 2012), further supported by animal models evidencing muscle hypertrophy with myostatin gene deletion (McPherron and Lee 1997; McPherron et al. 1997) while its over-expression in transgenic mice was associated with lower muscle mass (Reisz-Porszasz et al. 2003). Indeed, myostatin is already emerging as a promising therapeutic target for muscle health, with myostatin antibody demonstrating increase in muscle mass amongst persons with muscle dystrophy, and its pharmacological inhibition leading to improved lean mass in patients undergoing androgen deprivation therapy for prostate cancer (Wagner et al. 2008; Padhi et al. 2014). Yet, data for the relationship between circulating myostatin and age-related muscle loss have been scarce and conflicting. While an earlier study reported significant elevations in serum myostatin levels with advancing age and declining lean mass (Yarasheski et al. 2002), more recent studies had failed to support these findings (Hofmann et al. 2015; Ratkevicius et al. 2011). Intriguingly, we have observed myostatin to confer deleterious effects for sarcopenia only in older male adults that, if corroborated, has important clinical significance in selection of individuals most likely to benefit from myostatin blocking therapy.
The influence of nutritional status for optimal muscle performance has been further reinforced, evident by the clearly detrimental impact of malnutrition conferring greater than fivefold higher risk for sarcopenia in women. Interestingly, we observed that higher serum triglyceride was independently associated with reduced odds for sarcopenia in men even after adjusting for the use of lipid-lowering agents, running contrary to prior hypotheses for the negative impact of adiposity on muscle quality and physical performance (Kleevil et al. 2015; Newman et al. 2003), and the observed univariate associations between central adiposity represented by ABSI and sarcopenia. Nonetheless, our findings parallel the recently observed linear relationship between a composite profile comprising measures of adiposity and metabolic markers (including total cholesterol and triglycerides) with skeletal muscle mass (Perna et al. 2015). However, it is worthy to mention that the serum lipid parameters in our male cohort as well as the previous study had been within appropriate reference ranges, suggesting that the observed influence of serum triglyceride may therefore merely reflect its role as a mediator of the effect of malnutrition on muscle mass loss (Rondanelli et al. 2014).
Contrary to the widely recognized role of chronic inflammation in driving sarcopenia and frailty (Brinkley et al. 2009; Michaud et al. 2013), we failed to confirm the detrimental effect of inflammatory biomarkers, even after taking into consideration the presence of chronic inflammatory conditions or immunosuppressant treatment, although this will need to be clarified in our ongoing follow-up. Further, we failed to detect significant correlations between ABSI which is representative of adiposity and serum IL-6 levels. The longitudinal tracking of inflammatory markers with sarcopenic status will be especially pertinent given the pleiotropic nature of IL-6 (Fontes et al. 2015), along with its recognition as a myokine, being secreted by skeletal muscle with potential benefits that include improved skeletal muscle glucose uptake and insulin sensitivity in response to exercise (Pal et al. 2014).
Our study’s strengths include a well-characterized cohort of older adults with complete clinical, imaging and biochemical parameters, incorporating functional performance measures beyond mere muscle mass in defining sarcopenia, thereby according due recognition that mere measurement of quantitative muscle mass may not accurately reflect important changes impacting muscle performance. However, several limitations are acknowledged, including the cross-sectional design that does not allow us to definitively conclude the temporal relationship between sarcopenia and its risk factors, limiting confidence in dismissing potential for alterations in the measured blood biomarkers being consequent to changes in body composition. Further, it has been suggested that measured serum myostatin levels may not reflect myostatin activity, owing to its secretion as a precursor protein and regulation by its antagonist follistatin (White and LeBrasseur 2014). Our sample size of 200 subjects had been calculated for the longitudinal follow-up of adverse outcomes associated with sarcopenia, and we acknowledge that this may not be adequately powered for the cross-sectional analysis due to the limited number of participants fulfilling sarcopenia criteria at baseline, with consequent type II error in the multiple regression models. We have also recruited a cohort of relatively well and functionally independent older adults residing within the community, warranting caution in generalizability to a wider population of heterogeneous older adults including those at the end of the frailty spectrum.
In conclusion, our findings support sex-specific pathophysiological mechanisms for sarcopenia, that have potential important clinical utility in guiding targeted interventions for older men and women, respectively. Malnutrition appears to be a common modifiable risk factor for sarcopenia, reflected in different surrogate measures of MNA score in women and higher but appropriate range serum triglyceride levels in men. While therapies that block myostatin signalling may be relevant in older male adults, the role of IGF-1 agonists may hold greater promise in women. Finally, differing underlying physiology supports examining therapeutic effects by gender in clinical trials for the prevention and treatment of sarcopenia.
Acknowledgments
We thank Ms. Yang Jun, Department of Pharmacology, National University of Singapore for her assistance in the blood biomarker analysis, as well as Mr. Samuel Neo, medical social worker, Department of Continuing and Community Care, Tan Tock Seng Hospital, for his assistance in recruitment through the various Senior Activity Centres (SAC). We extend our appreciation to the following SACs [Wesley SAC, Care Corner SAC, Xin Yuan Community Service, Potong Pasir Wellness Centre, Tung Ling Community Services (Marine Parade and Bukit Timah), Viriya Community Services-My Centre@Moulmein, House of Joy) and the study participants who have graciously consented to participate in the study.
Compliance with ethical standards
Informed written consent was obtained from the participant, and the study was approved by the Domain Specific Review Board (DSRB) of the National Healthcare Group (NHG).
Funding support
This study was funded by Lee Foundation Grant 2013.
Conflict of interest
The authors declare that they have no competing interests.
References
- Barberger-Gateau P, Commenges D, Gagnon M, et al. Instrumental activities of daily living as a screening tool for cognitive impairment and dementia in elderly community dwellers. J Am Geriatr Soc. 1992;40:1129–1134. doi: 10.1111/j.1532-5415.1992.tb01802.x. [DOI] [PubMed] [Google Scholar]
- Batsis JA, Mackenzie TA, Barre LK, et al. Sarcopenia, sarcopenic obesity and mortality in older adults: results from the National Health and Nutrition Examination Survey III. Eur J Clin Nutr. 2014;68:1001–1007. doi: 10.1038/ejcn.2014.117. [DOI] [PubMed] [Google Scholar]
- Brinkley BE, Leng X, Miller ME, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci. 2009;64:455–461. doi: 10.1093/gerona/gln038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan M, Lim YP, Ernest A, Tan TL. Nutritional assessment in an Asian nursing home and its association with mortality. J Nutr Health Aging. 2010;14:23–28. doi: 10.1007/s12603-010-0005-1. [DOI] [PubMed] [Google Scholar]
- Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- Clark BC, Manini TM. Functional consequences of sarcopenia and dynapenia in the elderly. Curr Opin Clin Nutr Metab Care. 2010;13:271–276. doi: 10.1097/MCO.0b013e328337819e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS) Age Ageing. 2014;43:748–759. doi: 10.1093/ageing/afu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert ML, Wallaschofski H, Grotevendt A, et al. Cross-sectional and longitudinal relation of IGF and IGF-binding protein 3 with lipid metabolism. Eur J Endocrinol. 2014;171:9–19. doi: 10.1530/EJE-13-1017. [DOI] [PubMed] [Google Scholar]
- Elliot B, Renshaw D, Getting S, Mackenzie R. The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol. 2012;205:324–340. doi: 10.1111/j.1748-1716.2012.02423.x. [DOI] [PubMed] [Google Scholar]
- Fiatarone MA, O’Neill EF, Ryan ND, et al. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- Fielding RA, Vellas B, Evans WJ, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes JA, Rose NR, Cihakova D. The varying faces of IL-6: from cardiac protection to cardiac failure. Cytokine. 2015;74:62–68. doi: 10.1016/j.cyto.2014.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman-Gruen D, Barrett-Connor E. Epidemiology of insulin-like growth factor-I in elderly men and women. The Rancho Bernardo Study. Am J Epidemiol. 1997;145:970–976. doi: 10.1093/oxfordjournals.aje.a009065. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferruci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.M85. [DOI] [PubMed] [Google Scholar]
- Harridge SD. Ageing and local growth factors in muscle. Scand J Med Sci Sports. 2003;13:34–39. doi: 10.1034/j.1600-0838.2003.20235.x. [DOI] [PubMed] [Google Scholar]
- Harris TB, Kiel D, Roubenoff R, et al. Association of insulin-like growth factor-I with body composition, weight history, and past health behaviors in the very old: the Framingham Heart Study. J Am Geriatr Soc. 1997;45:133–139. doi: 10.1111/j.1532-5415.1997.tb04497.x. [DOI] [PubMed] [Google Scholar]
- Hofmann M, Halper B, Franzke B, et al. Serum concentrations of insulin-like growth factor-1, members of the TGF-beta superfamily and follistatin do not reflect different stages of dynapenia and sarcopenia in elderly women. Exp Gerontol. 2015;64:35–45. doi: 10.1016/j.exger.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- Kleevil VL, Luben R, Dalzell N, et al. Cross-sectional associations between different measures of obesity and muscle strength in men and women in a British cohort study. J Nutr Health Aging. 2015;19:3–11. doi: 10.1007/s12603-014-0492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korostishevsky M, Steves CJ, Malkin I, et al. Genomics and metabolomics of muscular mass in community-based sample of UK females. Eur J Hum Genet. 2015 doi: 10.1038/ejhg.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostka T, Arsac LM, Patricot MC, et al. Leg extensor power and dehydroepiandrosterone sulfate, insulin-like growth factor-I and testosterone in healthy active elderly people. Eur J Appl Physiol. 2000;82:83–90. doi: 10.1007/s004210050655. [DOI] [PubMed] [Google Scholar]
- Krakauer NY, Krakauer JC. A new body shape index predicts mortality hazard independently of body mass index. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0039504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landi F, Liperoti R, Fusco D, et al. Prevalence and risk factors of sarcopenia among nursing home older residents. J Gerontol A Biol Sci Med Sci. 2012;67:48–55. doi: 10.1093/gerona/glr035. [DOI] [PubMed] [Google Scholar]
- Lee WJ, Liu LK, Peng LN, et al. Comparisons of sarcopenia defined by IWGS and EWGSOP criteria among older people: results from the I-Lan longitudinal aging study. J Am Med Dir Assoc. 2013;14:528.e1–7. doi: 10.1016/j.jamda.2013.03.019. [DOI] [PubMed] [Google Scholar]
- Mahoney FI, Barthel D. Functional evaluation: the Barthel Index. Md State Med J. 1965;14:61–65. [PubMed] [Google Scholar]
- McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci U S A. 1997;94:12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- Michaud M, Balardy L, Moulis G, et al. Proinflammatory cytokines, aging and age-related diseases. J Am Med Dir Assoc. 2013;14:877–82. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Newman AB, Haggerty CL, Goodpaster B, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc. 2003;51:323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- Padhi D, Higano CS, Shore ND, et al. Pharmacological inhibition of myostatin and changes in lean body mass and lower extremity muscle size in patients receiving androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2014;99:E1967–E1975. doi: 10.1210/jc.2014-1271. [DOI] [PubMed] [Google Scholar]
- Pal M, Febbraio MA, Whitham M. From cytokine to myokine: the emerging role of interleukin-6 in metabolic regulation. Immunol Cell Biol. 2014;92:331–339. doi: 10.1038/icb.2014.16. [DOI] [PubMed] [Google Scholar]
- Patel HP, Syddall HE, Jameson K, et al. Prevalence of sarcopenia in community-dwelling older people in the UK using the European Working Group on Sarcopenia in Older People (EWGSOP) definition: findings from the Hertfordshire Cohort Study (HCS) Age Ageing. 2013;42:378–384. doi: 10.1093/ageing/afs197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payette H, Roubenoff R, Jacques PF, et al. Insulin-like growth factor-1 and Interleukin 6 predict sarcopenia in very old community-living men and women: the Framingham Heart Study. J Am Geriatr Soc. 2003;51:1237–1243. doi: 10.1046/j.1532-5415.2003.51407.x. [DOI] [PubMed] [Google Scholar]
- Perna S, Guido D, Grassi M, Rondanelli M. Association between muscle mass and adipo-metabolic profile: a cross-sectional study in older subjects. Clin Interv Aging. 2015;10:499–504. doi: 10.2147/CIA.S67872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrini S, Laviola L, Carreira MC, et al. The GH/IGF1 axis and signalling pathways in muscle and bone: mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol. 2010;205:201–210. doi: 10.1677/JOE-09-0431. [DOI] [PubMed] [Google Scholar]
- Ratkevicius A, Joyson A, Selmer I, et al. Serum concentrations of myostatin and myostatin-interacting proteins do not differ between young and sarcopenic elderly men. J Gerontol A Biol Sci Med Sci. 2011;66:620–626. doi: 10.1093/gerona/glr025. [DOI] [PubMed] [Google Scholar]
- Reisz-Porszasz S, Bhasin S, Artaza JN, et al. Lower skeletal muscle mass in male transgenic mice with muscle-specific overexpression of myostatin. Am J Physiol Endocrinol Metab. 2003;285:E876–E888. doi: 10.1152/ajpendo.00107.2003. [DOI] [PubMed] [Google Scholar]
- Rondanelli M, Guido D, Opizzi A, et al. A path model of sarcopenia on bone loss in elderly subjects. J Nutr Health Aging. 2014;18:15–22. doi: 10.1007/s12603-013-0357-4. [DOI] [PubMed] [Google Scholar]
- Sahadevan S, Lim PP, Tan NJ, Chan SP. Diagnostic performance of two mental status tests in the older Chinese: influence of education and age on cut-off values. Int J Geriatr Psychiatry. 2000;15:234–241. doi: 10.1002/(SICI)1099-1166(200003)15:3<234::AID-GPS99>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Scicchitano BM, Rizutto E, Musaro A. Counteracting muscle wasting in aging and neuromuscular disease: the critical role of IGF-1. Aging. 2009;13(1):451–457. doi: 10.18632/aging.100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thacher TD, Clarke BL. Vitamin D insufficiency. Mayo Clin Proc. 2011;86:50–60. doi: 10.4065/mcp.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg AU, Meyer A, Rohner D, et al. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am J Physiol Cell Physiol. 2009;296:C1258–1270. doi: 10.1152/ajpcell.00105.2009. [DOI] [PubMed] [Google Scholar]
- Volpato S, Bianchi L, Cherubini A, et al. Prevalence and clinical correlates of sarcopenia in community-dwelling older people: application of the EWGSOP definition and diagnostic algorithm. J Gerontol A Biol Sci Med Sci. 2014;69:438–446. doi: 10.1093/gerona/glt149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Haehling S, Morley JE, Anker SD. An overview of sarcopenia: facts and numbers on prevalence and clinical impact. J Cachexia Sarcopenia Muscle. 2010;1:129–133. doi: 10.1007/s13539-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade DT, Legh-Smith J, Langton Hewer R. Social activities after stroke: measurement and natural history using the Frenchay Activities Index. Int Rehabil Med. 1985;7:176–181. doi: 10.3109/03790798509165991. [DOI] [PubMed] [Google Scholar]
- Wagner KR, Fleckenstein JL, Amato AA, et al. A phase I/II trial of MYO-029 in adult subjects with muscular dystrophy. Ann Neurol. 2008;63:561–571. doi: 10.1002/ana.21338. [DOI] [PubMed] [Google Scholar]
- White TA, LeBrasseur NK. Myostatin and sarcopenia: opportunities—a mini-review. Gerontology. 2014;60:289–293. doi: 10.1159/000356740. [DOI] [PubMed] [Google Scholar]
- Yamada M, Nishiguchi S, Fukutani N, et al. Mail-based intervention for sarcopenia prevention increased anabolic hormone and muscle mass in community-dwelling Japanese older adults: the INE (Intervention by Nutrition and Exercise) study. J Am Med Dir Assoc. 2015;16:654–660. doi: 10.1016/j.jamda.2015.02.017. [DOI] [PubMed] [Google Scholar]
- Yarasheski KE, Bhasin S, Sinha-Hikim I, et al. Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J Nutr Health Aging. 2002;6:343–348. [PubMed] [Google Scholar]