Abstract
Serum uric acid (sUA) level may be associated with cognitive impairment/dementia. It is possible this relationship varies with dementia subtype, particularly between vascular dementias (VaD) and Alzheimer’s (AD) or Parkinson’s disease (PDD)-related dementia. We aimed to present a synthesis of all published data on sUA and relationship with dementia/cognition through systematic review and meta-analysis. We included studies that assessed the association between sUA and any measure of cognitive function or a clinical diagnosis of dementia. We pre-defined subgroup analyses for patients with AD, VaD, PDD, mild cognitive impairment (MCI), and mixed or undifferentiated. We assessed risk of bias/generalizability, and where data allowed, we performed meta-analysis to describe pooled measures of association across studies. From 4811 titles, 46 papers (n = 16,688 participants) met our selection criteria. Compared to controls, sUA was lower in dementia (SDM −0.33 (95%CI)). There were differences in association by dementia type with apparent association for AD (SDM −0.33 (95%CI)) and PDD (SDM −0.67 (95%CI)) but not in cases of mixed dementia (SDM 0.19 (95%CI)) or VaD (SDM −0.05 (95%CI)). There was no correlation between scores on Mini-Mental State Examination and sUA level (summary r 0.08, p = 0.27), except in patients with PDD (r 0.16, p = 0.003). Our conclusions are limited by clinical heterogeneity and risk of bias in studies. Accepting this caveat, the relationship between sUA and dementia/cognitive impairment is not consistent across all dementia groups and in particular may differ in patients with VaD compared to other dementia subtypes.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-016-9871-8) contains supplementary material, which is available to authorized users.
Keywords: Uric acid, Dementia, Cognition, Systematic review, Meta-analysis
Introduction
Despite increasing absolute numbers with dementia/cognitive impairment, our understanding of the syndrome is limited and we have few therapeutic options. Identification of modifiable risk factors is critical, as this will allow for better understanding of pathophysiology, risk stratification, and potential interventions. Serum uric acid (sUA) has been suggested as a risk marker and possible therapeutic target for a number of common chronic diseases, particularly cardiovascular diseases (Dawson and Walters 2006).
The association of sUA and dementia is less clear. There are several published studies, but results have been equivocal or conflicting. The syndrome of dementia comprises a variety of distinct pathologies, and this may explain the mixed results from studies of sUA. As sUA has strong hydrophilic antioxidant properties, potential neuroprotective properties have been suggested that could be important in neurodegenerative diseases, such as Alzheimer’s disease dementia (ADD) or dementias associated with Parkinson’s disease (PDD). (Yu et al. 1998) The association of sUA with accelerated vascular disease could contribute to cognitive decline through clinical and occult cerebrovascular disease (Vanorsdall et al. 2008). The association between sUA and cognitive impairment/dementia may therefore differ between dementia subtypes. We sought to test this hypothesis through systematic review and meta-analysis to collate all available evidence on sUA and the relationship with dementia/cognitive impairment. Our aims were to describe association between sUA and a diagnosis of dementia and with measures of cognitive function.
Methods
Our study followed the conduct and reporting guidance as described in meta-analysis of observational studies in epidemiology (MOOSE) (Stroup et al. 2000) and the PROGRESS group guidance on prognosis based research. (Hemingway et al. 2013) We created a search protocol made available on an open access website (PROSPERO register, registration number CRD42014014898).
Eligibility
All studies that reported sUA level in relation to a measure of cognitive function or in relation to a dementia diagnosis in human participants were potentially eligible with no restrictions based on language or year. We did not prespecify a preferred study methodology but formulated an analysis plan that studies would either be as follows:
Case control studies where sUA level was compared in patients with and without cognitive dysfunction;
Prospective studies of the relationship between incident dementia or cognitive decline and sUA; and
Cross-sectional studies of the relationship between sUA level and measures of cognitive function.
We did not limit the measures of cognitive function to any particular test or specify the method of sUA measurement. We excluded conference proceedings, theses, and case studies. Where the same data was presented in more than one publication, we used the primary (first) publication.
Search strategy: our search was conducted between November 2012 and July 2014
All aspects of study selection, extraction, and assessment were performed by two reviewers working independently (AK, JD) with recourse to a third arbitrator if required. Chinese language studies were reviewed by YF. We reviewed multiple international and cross-disciplinary electronic databases: EMBASE (OvidSP), CINAHL (EBSCO), MEDLINE (OvidSP), LILACS (Bireme), and ALOIS (Cochrane Dementia and Cognitive improvement Group) and included Chinese language medical databases (Chinese National Knowledge Infrastructure (CNKI) database, VIP and Wanfang databases). We used a concepts-based approach for creation of search terms; concepts of interest were around sUA and cognition/dementia (full search strategy in Supplementary information).
We reviewed all study titles from database searches. Abstracts of potentially relevant titles were assessed and full text of potentially eligible studies reviewed. We used forward and backward citation searching of relevant papers and repeated the process until no new titles were generated. As a test of validity of the search strategy, a researcher not involved in the original searches identified five exemplar papers that should be included in the analysis. We assessed if our search strategy identified all of these titles.
Data extraction and assessment
For those studies not published in English language, translation services were employed, or in the case of Chinese papers, they were translated by one of the authors (YF). We independently extracted data using a specific data extraction form (Supplementary materials).
We categorized diagnoses using the following labels: AD, MCI, mixed or undifferentiated, PDD, VaD. We used the diagnostic classification described in the primary paper. Under our rubric of VaD, we included vascular cognitive impairment and post-stroke cognitive impairment. Under our rubric of PDD, we included those diseases with a predominantly parkinsonian phenotype.
We assessed generalizability (external validity) and risk of bias of each study using a pre-specified, bespoke tool based on Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidance (von Elm et al. 2008), Cochrane tools (Higgin et al. 2011), and the Newcastle-Ottawa scales (Wells GA) for cohort and case control studies (Supplementary materials).
Quantitative analysis
We attempted meta-analysis where there were three or more eligible studies. For case-control data, we calculated pooled mean differences for sUA (standardized difference in means (SDM) and associated 95 % confidence interval (95 %)) or the odds of dementia according to sUA. For papers describing correlation of sUA and a cognitive test score (e.g., Folstein’s Mini-Mental State Examination (Folstein et al. 1975 Nov)), we described summary correlation coefficient.
We assessed heterogeneity with visual inspection of forest plots and with the I2 statistic, taking a value of >50 % to define substantial heterogeneity. In cases of substantial heterogeneity, we used random effects models.
We assessed publication bias using Egger’s plots (funnel plots) for analyses where more than five papers were included. We used a one-tailed p value of <0.1 for Egger’s regression intercept for quantitative assessment of potential publication bias.
All analyses were performed using Comprehensive Meta-analysis software (version 3.0 CMA group New Jersey USA). Given the large numbers of included papers and their heterogeneity, we present results grouped by study methodology and within this, by dementia type.
Results
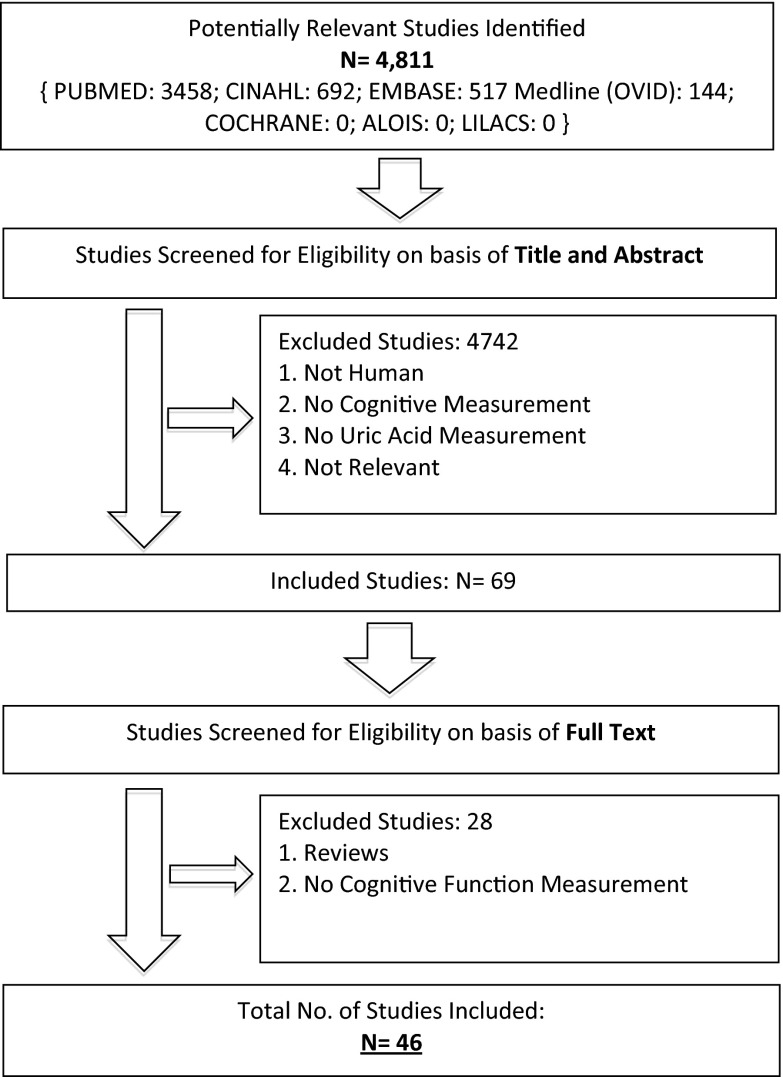
Our search strategy identified 4811 titles. Of these, 69 papers were selected for full text review and 46 were eligible for inclusion. These 46 papers included 16,688 participants (Fig. 1). Five studies were written in Chinese (Wei et al. 2012; Qin and Yang 2009; Shang et al. 2009; Wang et al. 2009; You and Liu 2012), one in Japanese (Matsubayashi et al. 1988), and one in Turkish (Cankurtaran et al. 2013). Four authors were contacted for additional data and three responded. Our internal validity checks confirmed rigor of our search strategy, with all five exemplar papers included in the first search. Twenty-two studies were judged to be low risk of bias (eTable 1). The main sources of bias were performance bias and lack of adjustment for potential confounders.
Fig. 1.
PRISMA diagram
Twenty-two papers included patients with AD (n = 1194, Table 1) (Ahlskog et al. 1995; Baldieras et al. 2008; Bowman et al. 2010; Can et al. 2013; Cankurtaran et al. 2013; Cascalheira et al. 2009; Cervelatti et al. 2013; Foy et al. 1999; Gackowski et al. 2008; Irizarry et al. 2009; Iuliano et al. 2010; Kasa et al. 1989; Kim et al. 2006; Maesaka et al. 1993; Polidori and Meococci 2002; Polidori et al. 2004; Pulido et al. 2005; Rinaldi et al. 2003; Shang et al. 2009; Tohgi et al. 1993; Wikkelso et al. 1981; Zafrilla et al. 2006); 12 included patients with PDD (Afsar et al. 2011; Ahlskog et al. 1995; Annanmaki et al. 2011; Annanmaki et al. 2008; Ascherio et al. 2009; Foy et al. 1999; González-Aramburu et al. 2014; Maetzler et al. 2011; Moccia et al. 2014; Pan et al. 2013; Wang et al. 2009; You and Liu 2012) (n = 1404, Table 2); and 5 included patients with VaD (Foy et al. 1999; Maesaka et al. 1993; Matsubayashi et al. 1988; Tohgi et al. 1993; Wikkelso et al. 1981) (n = 121, Table 1).
Table 1.
Studies including patients with Alzheimer’s Disease and Vascular Dementia
| Ref. | Disease population | Country | Design | Control population | n, cases/controls | SUA compared in cases/controls | Cog. function related to SUA | Longitudinal follow-up |
|---|---|---|---|---|---|---|---|---|
| Ahlskog et al. (1995) | AD; PD | USA | Case-control | Normal controls | 71/15 | Yes | No | No |
| Baldieras et al. (2008) | AD; MCI | Portugal | Case-control | Healthy age matched | 127/37 | Yes | No | No |
| Bowman et al. (2010) | AD | USA | Cohort | No | 32/NA | No | Yes | Yes |
| Can et al. (2013) | AD | Turkey | Case-control | Age / gender matched | 32/32 | Yes | Yes | No |
| Cankurtaran et al. (2013) | AD | Turkey | Case-control | Age and gender matched | 143/1553 | Yes | Yes | No |
| Cascalheira et al. (2009) | AD | Portugal | Case-control | Healthy older adults | 19/36 | Yes | No | No |
| Cervelatti et al. (2013) | AD; MCI | Italy | Case-control | Older adults | 235/99 | Yes | No | No |
| Foy et al. (1999) | AD; VD; PDD | UK | Case-control | Age matched | 134/58 | Yes | No | No |
| Gackowski et al. (2008) | AD | Poland | Case-control | Age matched | 18/33 | Yes | No | No |
| Irizarry et al. (2009) | AD | USA / Canada | Cohort | No | 204/NA | No | Yes | Yes |
| Iuliano et al. (2010) | AD; MCI | Italy | Case-control | Healthy controls | 90/24 | Yes | No | No |
| Kasa et al. (1989) | AD | USA | Case-control | Other dementias | 47/71 | Yes | No | No |
| Kim et al. (2006) | AD | Korea | Cross-sectional | Healthy controls | 101/101 | Yes | No | No |
| Wei et al. (2012) | Stroke, CI | China | Case-control | Stroke, no CI | 34/88 | Yes | Yes | No |
| Maesaka et al. (1993) | AD; VD | USA | Case-control | Healthy controls | AD 18 VD 6/11 |
Yes | No | No |
| Matsubayashi et al. (1988) | VD | Japan | Case-control | Cerebral infarcts no dementia | 24/27 | Yes | No | No |
| Polidori and Meococci (2002) | AD, female | Germany | Cross-sectional | Age matched healthy females | 35/40 | Yes | No | No |
| Polidori et al. (2004) | AD; VD | Germany | Cohort | Healthy controls | AD 63 VD 23/55 |
Yes | No | No |
| Pulido et al. (2005) | AD | Spain | Case-control | No cognitive damage | 20/22 | Yes | No | No |
| Qin and Yang (2009) | Stroke, CI | China | Cross-sectional | Stroke, CI | 26/72 | Yes | No | No |
| Rinaldi et al. (2003) | AD; MCI | Italy | Case-control | Elderly adults attending hospital | AD 63 MCI 25/56 |
Yes | No | No |
| Shang et al. (2009) | AD | China | Case-control | Healthy Controls | 30/30 | Yes | No | No |
| Tohgi et al. (1993) | AD; VD | Japan | Case-control | Not defined | 25/14 | Yes | No | No |
| Wen et al. (2012) | Delirium, dementia, others | China | Case-control | Healthy controls | 64/42 | Yes | No | No |
| Wikkelso et al. (1981) | AD; VD | Sweden | Cross-sectional | No | 38 | No | No | No |
| Zafrilla et al. (2006) | AD | Spain | Case-control | Healthy controls | 66/27 | Yes | Yes | No |
AD Alzheimer’s disease, VD Vascular Dementia, PD Parkinson’s Disease, CI cognitive impairment, MCI mild cognitive impairment, NA not applicable
AD Alzheimer’s disease, VD Vascular Dementia, PD Parkinson’s Disease, CI cognitive impairment, MCI mild cognitive impairment, NA not applicable
Table 2.
Studies Including Patients with PD
| Ref. | Disease population | Country | Design | Control population | n, Cases/controls | SUA compared in cases/controls | Cog function related to SUA | Longitudinal follow-up |
|---|---|---|---|---|---|---|---|---|
| Ahlskog et al. (1995) | AD; PD | America | Case Control | Normal controls | 71/15 | Yes | No | No |
| Annanmaki et al. (2008) | PD | Finland | Cross Sectional | No | 40/NA | No | Yes | No |
| Annanmaki et al. (2011) | PD | Finland | Cohort | Yes | 28/12 | Yes | Yes | Yes |
| Ascherio et al. (2009) | PD | America | Clinical Trial Analysis | No | 774/NA | No | Yes | No |
| Foy et al. (1999) | AD; VD; PD with dementia | UK | Case Control | Age matched | 134/41 | Yes | No | No |
| González-Aramburu et al. (2014) | PD, CI | Spain | Case Control | PD, no CI | 72/271 | Yes | Yes | No |
| Maetzler et al. (2011) | Lewy-Body Disorders | Germany | Case Control | Healthy controls | 171/76 | Yes | Yes | No |
| Moccia et al. (2014) | PD | Italy | Cross Sectional | No | 80/NA | No | Yes | No |
| Pan et al. (2013) | PD | China | Case Control | Age matched | 160/80 | No | Yes | No |
| Tohgi et al. (1993) | PD | Japan | Case Control | Age matched | 26/14 | Yes | Yes | No |
| Wang et al. (2009) Jun 16 | PD, CI | China | Case Control | PD, no CI | 54/54 | Yes | Yes | No |
| You and Liu 2012 | PD with depression or CI | China | Case Control | PD without depression or CI | 29/26 | Yes | No | No |
AD Alzheimer’s disease, VD Vascular Dementia, PD Parkinson’s Disease, CI cognitive impairment, NA not applicable
Five studies (Cankurtaran et al. 2013; Cascalheira et al. 2009; Cicero et al. 2014; Li et al. 2010; Ruggiero Cherubini et al. 2009) (n = 3281 (489 with cognitive impairment/dementia + 2792 without)) described the relationship between sUA and incident dementia or cognition decline over time.
Case-control data
Thirty-two studies (Ahlskog et al. 1995; Annanmaki et al. 2011; Baldieras et al. 2008; Can et al. 2013; Cankurtaran et al. 2013; Cascalheira et al. 2009; Cervelatti et al. 2013; Cicero et al. 2014; Foy et al. 1999; Gackowski et al. 2008; González-Aramburu et al. 2014; Iuliano et al. 2010; Kasa et al. 1989; Kim et al. 2006; Li et al. 2010; Wei et al. 2012; Maesaka et al. 1993; Maetzler et al. 2011; Matsubayashi et al. 1988; Polidori and Meococci 2002; Polidori et al. 2004; Pulido et al. 2005; Qin and Yang 2009; Rinaldi et al. 2003; Ruggiero Cherubini et al. 2009; Shang et al. 2009; Tohgi et al. 1993; Wang et al. 2009; Wen et al. 2012; You and Liu 2012; Zafrilla et al. 2006) (n = 7021 participants) included a comparison of sUA between cases of cognitive impairment/dementia (n = 2681) and non-dementia controls (eTable 2). Five studies (Cankurtaran et al. 2013; Cascalheira et al. 2009; Cicero et al. 2014; Li et al. 2010; Ruggiero Cherubini et al. 2009) reported odds of dementia according to sUA; the remainder compared absolute measures of sUA between groups (eTable 3). There was substantial statistical heterogeneity in most analyses. There was a suggestion of possible publication bias (eFigure 1, p = 0.04 on Egger’s regression intercept) across all studies, but not evident in analyses restricted to dementia subgroups (eFigures 2 to 4).
All cause dementia/cognitive impairment
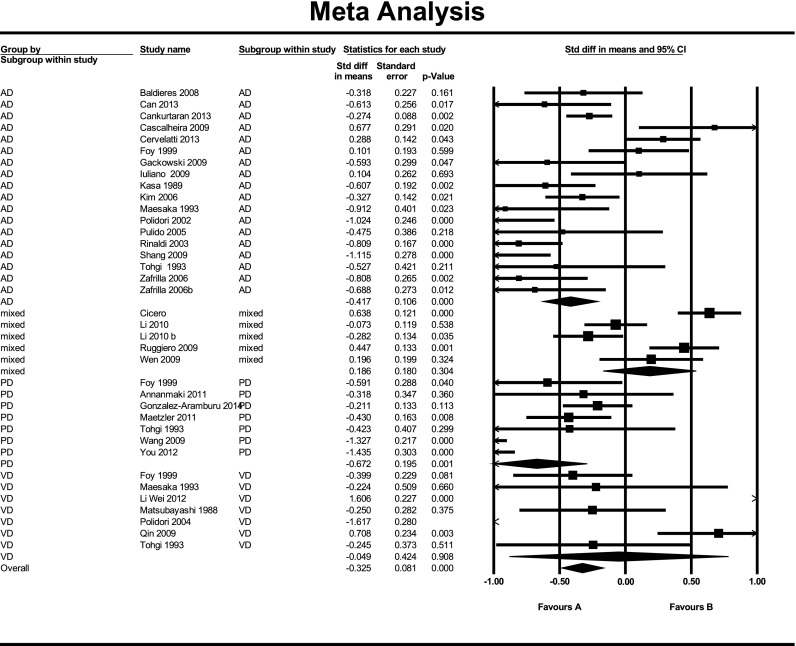
Across 31 studies, sUA was lower in cases of dementia compared to non-dementia controls with SDM −0.33 (95 %CI, p < 0.001) (Fig. 2). The summary odds of dementia on adjusted logistic regression analysis across five studies suggested no association with increasing sUA, OR 1.18 (95 % CI 0.96 to 1.46, p = 0.12) (eFigure 6).
Fig. 2.
Meta analysis of sUA level in cases of dementia versus controls by dementia group
AD
There was variation in the reported association with 14 studies describing sUA as lower in cases; (Baldieras et al. 2008; Can et al. 2013; Cankurtaran et al. 2013; Gackowski et al. 2008; Kasa et al. 1989; Kim et al. 2006; Maesaka et al. 1993; Maetzler et al. 2011; Polidori and Meococci 2002; Polidori et al. 2004; Rinaldi et al. 2003; Shang et al. 2009; Tohgi et al. 1993; Zafrilla et al. 2006) 3 studies reported the converse (Cascalheira et al. 2009; Cervelatti et al. 2013; Foy et al. 1999) and 2 reported no difference (Ahlskog et al. 1995; Iuliano et al. 2010). On pooled analysis, sUA was lower in AD compared to controls with SDM −0.42 (95 %CI) (Fig. 2).
VaD
Four studies found sUA levels to be lower in cases (Foy et al. 1999; Maesaka et al. 1993; Polidori et al. 2004; Tohgi et al. 1993), and one found no difference (Matsubayashi et al. 1988). In studies including patients with stroke, sUA levels were higher in cases (Wei et al. 2012; Qin and Yang 2009). There was no apparent difference in sUA between cases of VaD and controls across seven studies (SDM −0.05) (95 %CI, p = 0.908) (Fig. 2).
PDD
Five studies found sUA levels to be lower in cases (Foy et al. 1999; Maetzler et al. 2011; Tohgi et al. 1993; Wen et al. 2012; You and Liu 2012), and three found no difference (Ahlskog et al. 1995; Annanmaki et al. 2011; González-Aramburu et al. 2014). Pooled analysis of seven studies suggested lower sUA in PDD with SDM −0.67 (95 %CI, p = 0.001) (Fig. 2).
MCI
Two studies found sUA levels to be lower in cases (Baldieras et al. 2008; Rinaldi et al. 2003); one found levels to be higher (Cervelatti et al. 2013) and one found no difference (Iuliano et al. 2010). There was no apparent difference on pooled analysis (SMD −0.24) (95 %CI) (Supplementary materials).
Mixed or unspecified dementia
Three studies found levels to be higher in cases (Cicero et al. 2014; Ruggiero Cherubini et al. 2009; Wen et al. 2012). One paper (Li et al. 2010) studied males and females independently: in the male population, sUA levels were found to be lower and within the female population, no difference was seen. Across four studies, there was no apparent difference between groups (SDM 0.19) (95 %CI, p = 0.304) (Fig. 2).
Association of sUA and measures of cognitive function
Twenty-four cross-sectional studies (Vanorsdall et al. 2008; Afsar et al. 2011; Annanmaki et al. 2008; Ascherio et al. 2009; Baldieras et al. 2008; Bowman et al. 2010; Can et al. 2013; Cankurtaran et al. 2013; Cicero et al. 2014; González-Aramburu et al. 2014; Irizarry et al. 2009; Li et al. 2010; Wei et al. 2012; Madan et al. 2007; Maetzler et al. 2011; Moccia et al. 2014; Pan et al. 2013; Ruggiero Cherubini et al. 2009; Schretlen et al. 2007; Verhaaren et al. 2013; Wang et al. 2009; Wu et al. 2013; Yoldas et al. 2010; Zafrilla et al. 2006) (n = 9999 with 6045 with cognitive impairment/dementia) compared sUA to a measure of cognitive function (eTable 4). Two papers describing PDD used the same cohort and so only the primary dataset was used in analysis. Various approaches were used to describe association of sUA and cognition.
All cause dementia/cognitive impairment
Results were conflicting, with two papers (Pan et al. 2013; Wu et al. 2013) suggesting a positive correlation between sUA and MMSE, three papers (Afsar et al. 2011; Cicero et al. 2014; Wei et al. 2012) suggested negative correlation, and six papers (Annanmaki et al. 2008; Baldieras et al. 2008; Bowman et al. 2010; González-Aramburu et al. 2014; Maetzler et al. 2011) suggested no correlation. Two papers described levels of sUA across dementia severity groupings, with one paper (Cankurtaran et al. 2013) suggesting lower sUA in severe disease and the other reporting no association (Zafrilla et al. 2006). Four papers performed regression analysis with cognitive function as the dependent variable and sUA as a predictor, one study (Moccia et al. 2014) found higher sUA to be associated with better performance in cognitive tests, two found higher sUA to be associated with poorer performance (Madan et al. 2007; Wen et al. 2012) (n = 1508), and one found no relationship (Zafrilla et al. 2006). Twelve papers (n = 4134 participants) presented a form of correlation analysis using Pearson product moment correlation coefficient (Afsar et al. 2011; Annanmaki et al. 2011; Annanmaki et al. 2008; Baldieras et al. 2008; Bowman et al. 2010; Can et al. 2013; Cicero et al. 2014; González-Aramburu et al. 2014; Li et al. 2010; Wei et al. 2012; Maetzler et al. 2011; Pan et al. 2013; Wu et al. 2013), 11 of which used the MMSE and were suitable for pooled analysis (Afsar et al. 2011; Annanmaki et al. 2008; Baldieras et al. 2008; Bowman et al. 2010; Can et al. 2013; Cicero et al. 2014; González-Aramburu et al. 2014; Li et al. 2010; Wei et al. 2012; Maetzler et al. 2011; Pan et al. 2013; Wu et al. 2013) (eTable 5). Included patients were heterogeneous (AD n = 2 studies; PDD n = 4 studies; “healthy” older adults n = 2). There was no suggestion of correlation between sUA and MMSE across the body of studies (r = −0.084) (p = 0.274) (Supplementary materials).
PDD
Across four studies with PDD (Annanmaki et al. 2008; González-Aramburu et al. 2014; Maetzler et al. 2011; Pan et al. 2013), a positive correlation between sUA and MMSE was apparent (r = 0.155; p = 0.003) (eTable 6). There was no evidence of potential publication bias across the ten studies (p = 0.326 on Egger’s regression intercept) (Supplementary materials).
Time series analyses of sUA and cognitive function
Four studies reported the relationship between sUA and cognitive function over time (Table 3). The heterogeneity in study methodology and reporting precluded quantitative analyses. One paper (Gackowski et al. 2008) (n = 4618 participants) reported lower risk of incident dementia across increasing quartiles of sUA in a healthy population cohort. One paper (Kasa et al. 1989) (n = 747 participants) reported risk of incident dementia across quintiles of sUA in an MCI cohort with no relationship reported. The remaining two papers were modest in size ((Annanmaki et al. 2011) (n = 40 participants) (Cankurtaran et al. 2013) (n = 32 participants)) and described no association with sUA and change in cognitive function over time.
Table 3.
Time series analyses of serum and cognitive function
| Ref | Population studied | Number of subjects | Analysis performed | Summary result |
|---|---|---|---|---|
| Euser et al. (2009) | Population cohort | 4618 | Cox proportional hazards model for HR for sUA (quartiles) and risk of dementia | HR for dementia 0.73 (95 % CI 0.55 to 0.97) for highest vs. lowest quartile of sUA |
| Annanmaki et al. (2011) | PD | 28 | Correlation between baseline sUA and cognitive function at 3 years (neuropsychological battery). | No correlation with any measure |
| Irizarry et al. (2009) | MCI | 747 | 1. Survival analysis for survival free of AD across quintiles of sUA 2. Interaction between sUA and rate of cognitive decline (ADAS-cog) |
1. No relationship between sUA and progression to AD. 2. Low plasma urate associated with faster cognitive decline (p = 0.008 for interaction term sUA × time) |
| Bowman et al. (2010) | AD | 32 | Correlation between baseline sUA and annual change in cognition (ADAS, MMSE, CDR) | No correlation |
HR hazard ratio, sUA serum Uric Acid, ADAS Alzheirmer’s disease Assessment Scale, MMSE Mini-mental state exam, CDR Clinical Dementia Rating
Discussion
Using systematic review and meta-analyses, we offer a synthesis of the literature describing the relationship between sUA and dementia/cognitive impairment. Although many relevant papers were available, there was substantial heterogeneity and risk of various biases. Accepting this important caveat, we can draw some cautious conclusions. Association between sUA and dementia/cognitive impairment was weak across undifferentiated dementia groups; however, when described according to underlying pathology, there appeared to be a stronger association with AD and PDD than with VAD. This relationship was seen in both case-control and correlational analyses.
Our findings would support an association between sUA and cognitive function/dementia but the relationship is complex with sUA potentially damaging in context of vascular disease (stroke, small vessel cerebrovascular disease) and potentially neuroprotective (hydrophilic antioxidant properties) in other settings. This paradox has a biological plausibility. In vivo, ingested and endogenously synthesized purines are metabolized, via the action of xanthine oxidase, to xanthine and then sUA. The action of xanthine oxidase yields hydroxyl free radicals and hydrogen peroxide which can add to or initiate oxidative stress. Thus, despite sUA itself being antioxidant, its generation in vivo is associated with an oxidative stress (Dawson and Walters 2006).
Strengths and limitations of included studies
The majority of included studies were graded as high risk of bias. The weakest study design for investigating association is case-control due to the potential to inflate estimates by including phenotypic extremes. The majority of data available used a case-control approach. Correlation of sUA with a cognitive test score provides useful data but offers no information on potential direction of association or causation. A more informative study design would be prospective follow-up of a cohort free from dementia at baseline. Few studies used this approach, where sUA was related to temporal change in cognitive function; the largest (and highest quality) study in our review suggested that higher sUA level was associated with reduced risk of incident dementia.
Association between sUA and cognitive outcomes could be confounded by a number of other related factors, for example, age, diet, and medication can all impact on sUA and cognition (Choi et al. 2004; Reyes 2003). Few studies adequately corrected for confounders. It is interesting that while simple case-control studies suggested an association, pooled analysis of studies that used multivariable regression to assess for independent associations reported a neutral result.
Strengths and limitations of the review and analyses
We used a robust search strategy informed by an experienced team and employing validated search strings. We followed best practice guidance in conduct and reporting and included multiple internal and external “quality control” measures. We were aware that several Chinese studies had been conducted in the field and so imposed no language restriction and purposively searched specific Chinese resources.
There are limitations in our approach. Our dementia phenotyping was necessarily pragmatic. We used those diagnostic labels employed in the original papers. Diagnostic classification has evolved over time and clinical diagnosis is often inexact, for example, many labeled AD may have a vascular component. To facilitate summary analyses, we grouped diagnostic labels, but we recognize that this approach is potentially problematic, for example, we classed studies of post-stroke dementia under the rubric “vascular dementia” although the two states are not synonymous and we grouped dementias with parkinsonian phenotype together, but accept that within this group there is potential pathophysiological heterogeneity. It is of interest that the two studies that specifically looked at post-stroke cognitive decline suggested an association with sUA that was the converse of the other studies.
We used various meta-analytical techniques to offer a summary of the complex literature. With the various biases and heterogeneity, these analyses need to be treated with caution and should be regarded as hypothesis-generating, rather than definitive. Even when pooling studies, total numbers may still have been too small to show real but modest associations.
Implications for research and practice
Further study of sUA and cognition is warranted, but basic approaches such as uncorrected case-control analyses are unlikely to progress our understanding. Given the potential issues of confounding, there may be a role for a Mendelian randomization approach, incorporating fixed genetic information into the traditional epidemiological study design to provide suggestive information on causality free from the usual lifestyle and environmental confounders. Use of large clinical registries may also be informative; linking prescribing data and national morbidity/mortality records have allowed investigators to describe links between sUA lowering medications and cardiovascular outcomes across whole populations. A similar paradigm could be used for cognitive outcomes.
Our findings offer potential new avenues for investigating the pathophysiology of cognitive decline; however, data are not sufficiently robust to suggest direct clinical applications. Randomized controlled trials of sUA lowering and vascular outcomes are ongoing, and it will be of interest to see cognitive outcomes in these studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(DOCX 80 kb)
Acknowledgments
Dr. J Dawson has access to all relevant project data and takes full responsibility for the data, the analyses and interpretation, and the conduct of the research. There are no conflicts of interest to note.
Dr. Quinn is supported by a joint Stroke Association/Chief Scientist Office Senior Clinical Lectureship.
This project was supported by a Scottish Society of Physicians Research Grant.
Author contributions
Dr Khan—study design, data acquisition, analysis, interpretation, critical revision
Dr. Quinn—study design, data acquisition, analysis, interpretation, critical revision
Dr. Hewitt—study design, interpretation, critical revision
Dr. Fan—data acquisition, interpretation, and critical revision
Dr. Dawson—study design, data acquisition, analysis, interpretation, critical revision, and study supervision
Compliance with ethical standards
Disclosure
Dr Khan reports no disclosures.
Dr Quinn reports no disclosures.
Dr Hewitt reports no disclosures.
Dr Fan reports no disclosures.
Dr Dawson reports no disclosures.
References
- Afsar B, Elsurer R, Covic A, et al. Relationship between uric acid and subtle cognitive dysfunction in chronic kidney disease. Am J Nephrol. 2011;34:49–54. doi: 10.1159/000329097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlskog JE, Uitti RJ, Low PA, et al. No evidence for systemic oxidant stress in Parkinson’s or Alzheimer’s disease. Mov Disord. 1995;10(5):566–573. doi: 10.1002/mds.870100507. [DOI] [PubMed] [Google Scholar]
- Annanmaki T, Pessala-Driver A, Hokkanen L, et al. Uric acid associates with cognition in Parkinson’s disease. Parkinsonism Relat Disord. 2008;14:576–578. doi: 10.1016/j.parkreldis.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Annanmaki T, Pohja M, Parviainn T, et al. Uric acid and cognition in Parkinson’s disease: a follow-up study. Parkinsonism Relat Disord. 2011;17:333–337. doi: 10.1016/j.parkreldis.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Ascherio A, LeWitt PA, Xu K (2009) Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol 66 (12) [DOI] [PMC free article] [PubMed]
- Baldieras I, Santana I, Proenca MT, et al. Peripheral oxidative damage in mild cognitive impairment and mild Alzheimer’s disease. J Alzheimers Dis. 2008;15:117–128. doi: 10.3233/jad-2008-15110. [DOI] [PubMed] [Google Scholar]
- Bowman GL, Shannon J, Frei B. Uric acid as a CNS antioxidant. J Alzheimers Dis. 2010;19(4):1331–1336. doi: 10.3233/JAD-2010-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can M, Varlibas F, Guven B. Ischemia modified albumin and plasma oxidative stress markers in Alzheimer’s disease. Eur Neurol. 2013;69:377–380. doi: 10.1159/000339006. [DOI] [PubMed] [Google Scholar]
- Cankurtaran M, Yesil Y, Kuyumcu ME, et al. Altered levels of homocysteine and serum natural antioxidants links oxidative damage to Alzheimer’s disease. J Alzheimers Dis. 2013;33(4):1051–1058. doi: 10.3233/JAD-2012-121630. [DOI] [PubMed] [Google Scholar]
- Cascalheira JF, Joao SS, Pinhancos SS. Serum homocysteine: interplay with other circulating and genetic factors in association to Alzheimer’s type dementia. Clin Biochem. 2009;42:783–790. doi: 10.1016/j.clinbiochem.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Cervelatti C, Cremonini E, Bosi C. Systemic oxidative stress in older patients with mild cognitive impairment or late onset Alzheimer’s disease. Curr Alzheimer Res. 2013;10:000–000. doi: 10.2174/1567205011310040003. [DOI] [PubMed] [Google Scholar]
- Choi HK, Atkinson K, Karlson EW, Willett W, Curhan G. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350:1093–1103. doi: 10.1056/NEJMoa035700. [DOI] [PubMed] [Google Scholar]
- Cicero AF, Desideri G, Grossi G (2014) Serum uric acid and impaired cognitive function in a cohort of healthy young elderly: data from the Brisighella study. Intern Emerg Med [DOI] [PubMed]
- Dawson J, Walters M. Uric acid and xanthine oxidase: future therapeutic targets in the prevention of cardiovascular disease? Br J Clin Pharmacol. 2006;62:633–644. doi: 10.1111/j.1365-2125.2006.02785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Euser SM, Hofman A, Westendrop RGJ. Serum uric acid and cognitive function and dementia. Brain. 2009;132:377–382. doi: 10.1093/brain/awn316. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Fosltein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foy CJ, Passmore AP, Vahidassr MD, et al. Plasma chain-breaking antioxidants in Alzheimer’s disease, vascular dementia and Parkinson’s disease. Q J Med. 1999;92:39–45. doi: 10.1093/qjmed/92.1.39. [DOI] [PubMed] [Google Scholar]
- Gackowski D, Rozalski R, Siomek A. Oxidative stress and oxidative DNA damage is characteristic for mixed Alzheimer disease/vascular dementia. J Neurol Sci. 2008;266:57–62. doi: 10.1016/j.jns.2007.08.041. [DOI] [PubMed] [Google Scholar]
- González-Aramburu I, Sánchez-Juan P, Sierra M. Serum uric acid and risk of dementia in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20(6):637–639. doi: 10.1016/j.parkreldis.2014.02.023. [DOI] [PubMed] [Google Scholar]
- Hemingway H, Croft P, Perel P. Prognosis research strategy (PROGRESS) 1: a framework for researching clinical outcomes. BMJ. 2013;346:e5595. doi: 10.1136/bmj.e5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgin JPT, Altman DB, Sterne JAC (2011) Chapter 8: assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 Updated March
- Irizarry MC, Raman R, Schwarzschild MA. Plasma urate and progression of mild cognitive impairment. Neurodegener Dis. 2009;6:23–28. doi: 10.1159/000170883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iuliano L, Monticolo R, Straface G. Vitamin E and enzymatic/oxidative stress-driven oxysterols in amnestic mild cognitive impairment subtypes and Alzheimer’s disease. J Alzheimers Dis. 2010;21:1383–1392. doi: 10.3233/jad-2010-100780. [DOI] [PubMed] [Google Scholar]
- Kasa M, Bierma TJ, Waterstraat F, Jr, et al. Routine blood chemistry screen: a diagnostic aid for Alzheimer’s disease. Neuroepidemiology. 1989;8(5):254–261. doi: 10.1159/000110191. [DOI] [PubMed] [Google Scholar]
- Kim TS, Pae CU, Yun SJ. Decreased plasma antioxidants in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2006;21:344–348. doi: 10.1002/gps.1469. [DOI] [PubMed] [Google Scholar]
- Li J, Dong BR, Lin P. Association of cognitive function with serum uric acid level among Chinese nonagenarians and centenarians. Exp Gerontol. 2010;45(5):331–335. doi: 10.1016/j.exger.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Li W, Zhao P, Zhao X-J, Li Z-K. Clinic Focus. 2012;27:1508–1509. [Google Scholar]
- Madan P, Kalra OP, Agarwal S, et al. Cognitive impairment in chronic kidney disease. Nephrol Dial Transplant. 2007;22:440–444. doi: 10.1093/ndt/gfl572. [DOI] [PubMed] [Google Scholar]
- Maesaka JK, Wolf-Klein G, Piccione JM, et al. Hypouricemia, abnormal renal tubular urate transport, and plasma natriuretic factor(s) in patients with Alzheimer's disease. J Am Geriatr Soc. 1993;41(5):501–506. doi: 10.1111/j.1532-5415.1993.tb01885.x. [DOI] [PubMed] [Google Scholar]
- Maetzler W, Stapf AK, Schulte C, et al. Serum and cerebrospinal fluid uric acid levels in Lewy body disorders: associations with disease occurrence and amyloid-B pathway. J Alzheimers Dis. 2011;27:119–126. doi: 10.3233/JAD-2011-110587. [DOI] [PubMed] [Google Scholar]
- Matsubayashi K, Matsumoto M, Kawamoto A, et al. Hematocrit, serum lipids and cardiovascular indices as risk factors in vascular dementia. Jpn J Geriatr. 1988;25:576–580. doi: 10.3143/geriatrics.25.576. [DOI] [PubMed] [Google Scholar]
- Moccia M, Piccilo M, Erro R, et al. I s serum uric acid related to non-motor symptoms in de-novo Parkinson’s disease patients? Parkinsonism Relat Disord. 2014;20(7):772–775. doi: 10.1016/j.parkreldis.2014.03.016. [DOI] [PubMed] [Google Scholar]
- Pan M, Gao H, Long L, et al. Serum uric acid in patients with Parkinson’s disease and vascular parkinsonism: a cross-sectional stud. Neuroimmunomodulation. 2013;20:19–28. doi: 10.1159/000342483. [DOI] [PubMed] [Google Scholar]
- Polidori MC, Meococci P. Plasma susceptibility to free radical-induced al1tioxidant consumptiol1 and lipid peroxidation is increased in very old subjects with Alzheimer disease. J Alzheimers Dis. 2002;4(6):517–522. doi: 10.3233/jad-2002-4608. [DOI] [PubMed] [Google Scholar]
- Polidori MC, Mattioli P, Aldred S. Plasma antioxidant status, immunoglobulin g oxidation and lipid peroxidation in demented patients: relevance to Alzheimer disease and vascular dementia. Dement Geriatr Cogn Disord. 2004;18(3-4):265–270. doi: 10.1159/000080027. [DOI] [PubMed] [Google Scholar]
- Pulido R, Jimenez-Escrig A, Orensanz L, et al. Study of plasma antioxidant status in Alzheimer’s disease. Eur J Neurol. 2005;12:531–535. doi: 10.1111/j.1468-1331.2005.01000.x. [DOI] [PubMed] [Google Scholar]
- Qin JB, Yang MH. Clin Focus. 2009;24:57–58. [Google Scholar]
- Reyes AJ. Cardiovascular drugs and serum uric acid. Cardiovasc Drugs Ther. 2003;17:397–414. doi: 10.1023/B:CARD.0000015855.02485.e3. [DOI] [PubMed] [Google Scholar]
- Rinaldi P, Polidori MC, Metastasio X, et al. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiol Aging. 2003;24:915–919. doi: 10.1016/S0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Ruggiero Cherubini A, Lauretani F, et al. Uric acid and dementia in community-dwelling older persons. Dement Geriatr Cogn Disord. 2009;27:382–389. doi: 10.1159/000210040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schretlen DJ, Inscore AB, Vannorsdall TD, et al. Serum uric acid and brain ischemia in normal elderly adults. Neurology. 2007;69:1418–1423. doi: 10.1212/01.wnl.0000277468.10236.f1. [DOI] [PubMed] [Google Scholar]
- Shang X, Zhang F, Meng L et al (2009) Relationship between the level of serum uric acid and alzheimer’s disease. J China Med Univ 38(5)
- Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- Tohgi H, Abe T, Takahashi S, et al. The urate and xanthine concentrations in the cerebrospinal fluid in patients with vascular dementia of the Binswanger type, Alzheimer type dementia, and Parkinson’s disease. J Neural Transm. 1993;6:119–126. doi: 10.1007/BF02261005. [DOI] [PubMed] [Google Scholar]
- Vanorsdall TD, Jinnah HA, Gordon B, Kraut M, Schrelten D. Cerebral ischemia mediates the effect of serum uric acid on cognitive function. Stroke. 2008;39:3418–3420. doi: 10.1161/STROKEAHA.108.521591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaaren BF, Vernooji MW, Dehghen A, et al. The relation of uric acid to brain atrophy and cognition: the Rotterdam Scan Study. Neuroepidemiology. 2013;41(1):29–34. doi: 10.1159/000346606. [DOI] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Wang X, Luo W, Wang L, et al. Study on uric acid and the related factors associated with cognition in the patients with Parkinson’s disease. Zhonghua Yi Xue Za Zhi. 2009;89(23):1633–1635. [PubMed] [Google Scholar]
- Wells GA, Shea B, O’Connell D et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available from: URL: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- Wen S, Minfeng C, Houliang W, et al. Serum uric acid levels and the clinical characteristics of depression. Clin Biochem. 2012;45:49–53. doi: 10.1016/j.clinbiochem.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Wikkelso C, Blomstrand C, Nordquist P. Cerebrospinal fluid investigations in multi-infarct dementia and senile dementia. Acta Neurol Scand. 1981;64:1–11. doi: 10.1111/j.1600-0404.1981.tb04380.x. [DOI] [PubMed] [Google Scholar]
- Wu Y, Zhang D, Pang Z, et al. Association of serum uric acid level with muscle strength and cognitive function among Chinese aged 50–74 years. Geriatr Gerontol Int. 2013;13:672–677. doi: 10.1111/j.1447-0594.2012.00962.x. [DOI] [PubMed] [Google Scholar]
- Yoldas TK, Keklikoglu H, Zengin O, et al. Relation of serum uric acid level with cognitive functions and number of plaques in patients with relapsing remitting multiple sclerosis. Nöropsikiyatri Arşivi. 2010;47:333–337. [Google Scholar]
- You Z, Liu D (2012) The relationship of serum uric acid level with depression and cognitive dysfunction in elderly patients with PD. Chinese J Rehabil 27(4)
- Yu ZF, Bruce-Keller AJ, Goodman Y, Mattson MP. Uric acid protects neurons against excitotoxic and metabolic insults in cell culture, and against focal ischemic brain injury in vivo. J Neurosci Res. 1998;53:613–625. doi: 10.1002/(SICI)1097-4547(19980901)53:5<613::AID-JNR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Zafrilla P, Mulero J, Xandri JM et al (2006) Oxidative stress in alzheimer patients in different stages of the disease. Current I("iI,,'illal Chemistr) 13:1075-1 OS] [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 80 kb)