Abstract
Aging leads to several anatomical and functional deficits in circadian timing system. In previous works, we observed morphological alterations with age in hypothalamic suprachiasmatic nuclei, one central component of this system. However, there are few data regarding aging effects on other central components of this system, such as thalamic intergeniculate leaflet (IGL). In this context, we studied possible age-related alterations in neurochemical components and retinal projections of rat IGL. For this goal, young (3 months), adult (13 months), and aged (23 months) Wistar rats were submitted to an intraocular injection of neural tracer, cholera toxin subunit b (CTb), 5 days before a tissue fixation process by paraformaldehyde perfusion. Optical density measurements and cell count were performed at digital pictures of brain tissue slices processed by immunostaining for glutamic acid decarboxylase (GAD), enkephalin (ENK), neuropeptide Y (NPY) and CTb, characteristic markers of IGL and its retinal terminals. We found a significant age-related loss in NPY immunoreactive neurons, but not in immunoreactivity to GAD and ENK. We also found a decline of retinal projections to IGL with age. We conclude aging impairs both a photic environmental clue afferent to IGL and a neurochemical expression which has an important modulatory circadian function, providing strong anatomical correlates to functional deficits of the aged biological clock.
Keywords: Aging, Intergeniculate leaflet, Circadian timing system, Immunohistochemistry, Cholera toxin subunit b, Retinal projections
Introduction
Aging is a multidimensional process which impairs many aspects of cognitive functions. This process is associated with a fragmentation of sleep-wake cycle (Pace-Schott and Spencer 2011) and increases in frequency of mood disorders (Magee and Carmin 2010; Wolkowitz et al. 2011). These functions are also regulated by the circadian timing system (CTS), an interconnected network of brain nuclei and peripheral tissues responsible for synchronization of anticipatory physiologic responses to environmental changes (see Dibner et al. 2010). In this context, several studies aim to understand the mechanisms underlying aging effects in this circadian clock in order to improve elderly health quality (Kondratova and Kondratov 2012; Farajnia et al. 2014; Fonseca Costa and Ripperger 2015).
Changes in aged circadian clock are described in many levels (see Engelberth et al. 2013). For instance, the aging process is associated with declines in biological rhythm amplitude and phase duration controlled by the clock (Satinoff et al. 1993) as well as fragmentation of locomotor activity rhythm (Valentinuzzi et al. 1997). In addition, aging leads to a reduction in central brain structures activation by light, as revealed by a decrease in expression of immediate early genes, c-Fos and nerve growth factor one A, after photic stimulation (Sutin et al. 1993). In general, an age-related disruption of CTS homeostatic function is linked to progression of neurodegenerative disorders and detrimental effects in sleep and memory (Kondratova and Kondratov 2012).
It should be noted that several age-related alterations of circadian rhythms may be due, or strongly influenced by, aging effects on central brain structures of CTS (Aujard et al. 2006). Among these nuclei of rodent circadian clock are hypothalamic suprachiasmatic nucleus (SCN) and intergeniculate leaflet (IGL) in lateral geniculate complex of thalamus. While SCN is a master pacemaker of circadian rhythms, IGL provides a major regulatory pathway to SCN function (for review see Morin 2013).
Anatomically IGL is a contralateral-predominant retinorecipient area of laminar shape, located between dorsal lateral geniculate (dLGN) and ventral lateral geniculate (vLGN) nuclei (Hickey and Spear 1976). Rat IGL is characterized by GABAergic neurons, revealed by glutamic acid decarboxylase (GAD) immunoreactivity (IR), and colocalized either with enkephalin (ENK) or neuropeptide Y (NPY), composing two neuron subpopulations in this structure (Moore and Card 1994). Interestingly, neural tracer studies using cholera toxin subunit b (CTb) reveal the retinal projections arising to NPY-IR cells are much more modest than those arising to ENK-IR ones, suggesting they have distinct functional roles (Juhl et al. 2007; Blasiak and Lewandowski 2013). Moreover, the main well-known function of IGL is to promote non-photic phase-shifting (e.g. such as behavioral arousal and food intake) due to NPY action in SCN, through geniculo-hypothalamic tract (GHT; Biello et al. 1994; Janik et al. 1995; Harrington 1997; Saderi et al. 2013). Additionally, IGL is thought to have an integrative function of photic and non-photic stimuli, since it also mediates the effects of constant light exposure to circadian system (Harrington 1997; Morin and Pace 2002; Gall et al. 2014).
Regarding morphological and neurochemical findings of aging effects on CTS, some studies report a sex-specific neuron number reduction in SCN of rats (Tsukahara et al. 2005), rhesus monkey (Roberts et al. 2012), and marmosets (Engelberth et al. 2014). Moreover, SCN have a reduced expression of two major neurochemical constituents, vasoactive intestinal polypeptide (Chee et al. 1988; Krajnak et al. 1998), and vasopressin (Roozendaal et al. 1987; Cayetanot et al. 2005), which accounts to a weakening of SCN interconnections, therefore affecting the circadian clock functionality (Engelberth et al. 2013). Current data, however, are focused solely on the aging effects on SCN, which brings a limitation to understand the whole process of aging in CTS (Yamazaki et al. 2002; Davidson et al. 2008). In view of this absence of data concerning effects of aging in IGL, we documented neurochemical and retinal afferent changes in IGL between young, adult, and aged rats based on GAD, ENK, NPY, and CTb immunoreactivity.
Methods
Animals
Male Wistar rats were divided into three groups: young (n = 5; 3 months old), adult (n = 5; 13 months old), and aged (n = 5; 23 months old). They were maintained at 22 °C, 50 % humidity in a 12:12 h light/dark (12:12 LD) cycle. Food and water were available ad libitum. All procedures were in accordance with Brazilian law number 11.794/2008 for animal experimental use. All experiments were approved by local ethics committee for animal use (CEUA-UFRN number 021/2014).
Intraocular tracer injection
Rats were anesthetized with a ketamine hydrochloride (80 mg/kg) and xylazin (20 mg/kg) intramuscular injection. Then, 18 μL of cholera toxin subunit b neural tracer (1 mg/mL; List Biological Laboratories, Campbell, CA) in a solution containing 10 % dimethylsulfoxide was injected into the vitreous chamber of one eye with a glass micropipette attached to a 10-μL Hamilton syringe. After a 5-day post-surgery period, animals were submitted to the following procedures.
Tissue fixation
All animals were submitted to an intracardiac paraformaldehyde perfusion between 5:30 and 6:30 p.m. They were deeply anesthetized with ketamine hydrochloride (80 mg/kg) and xylazin (20 mg/kg). Then, they were transcardially perfused with a 300-mL NaCl solution (0.9 %; 32 °C) followed by 300 mL paraformaldehyde (4 %) in a 0.1 M phosphate-buffered saline (PBS), pH 7.4. Following perfusion, the brains were removed, post-fixed with paraformaldehyde (4 %) overnight, and immersed in a solution containing 30 % sucrose in 0.1 M PBS, pH 7. 4 for 3 days. Brains were cut into six series of coronal sections (30 μm) collected at a 180-μm interval.
Immunohistochemistry
Four series of sections were incubated overnight with GAD, ENK, NPY, or CTb primary antibodies (see Table 1) in a dilution containing 2 % normal donkey serum with PBS (0.1 M) and 0.5 % Triton X-100. After rinsing, sections were incubated with biotinylated secondary antibodies (see Table 1) diluted with PBS (0.1 M) and 0.5 % Triton X-100. Then, sections were incubated in a 2 % avidin-biotin solution (ABC Elite kit, Vector Labs, Burlingame, CA, USA), with NaCl addition, for 120 min. The sections were placed with a 2.5 % solution of diaminobenzidine (DAB) diluted with PBS (0.1 M) for 5 min. The final reaction was performed by adding a 0.01 % H2O2 solution for 1 min, to reveal marked areas in brown colors resulting from DAB oxidation. After DAB revelation, sections were washed in PBS (0.1 M) four times and stored overnight at 4 °C. All sections were submitted simultaneously to these procedures in order to minimize background differences and ensure the same conditions to development of chromagen.
Table 1.
Characteristics of used antibodies
| Host and marked target | Source company | Catalog code | Lot number | Working dilution | |
|---|---|---|---|---|---|
| Primary antibodies | Goat anti-GAD | Santa Cruz Biotechnology, Inc, Dallas, TX, USA | sc-7512 | L0707 | 1:100 |
| Rabbit anti-ENK | Peninsula Laboratories Inc, San Carlos, CA, USA | T4290 | 010607-1 | 1:1000 | |
| Rabbit anti-NPY | Sigma-Aldrich, St Louis, MO, USA | N9528 | 057K4869 | 1:1000 | |
| Goat anti-CTb | List Biological Labs, Campbell, CA, USA | 703 | 7032A | 1:9000 | |
| Secondary antibodies | Donkey anti-goat | Jackson ImmunoResearch Labs, Westgrove, PA, USA | 705-065-003 | 71434 | 1:1000 |
| Goat anti-rabbit | 111-065-003 | 74532 | 1:1000 |
Sections were mounted in gelatinized slides, dried, dehydrated in graded ethanol solutions, cleared in xylene, and coverslipped with DPX embedding matrix. Sections in which the primary antibodies were omitted and replaced with normal serum from the same species served as controls. Under these conditions, staining was completely abolished.
Image analysis and statistics
After histochemical procedures, digital pictures of biological tissues were taken by a CCD camera (Nikon DXM-1200) connected to a light microscope (Olympus BX-41). To quantify GAD, ENK, NPY, and CTb immunoreactivities, five sections representing IGL from each brain were analyzed bilaterally using ImageJ 1.48v software, which performed optical density (OD) measurements based on gray levels of pixels. OD has been used in previous reports to quantify similar immunostainings in IGL and SCN (Saderi et al. 2013; Engelberth et al. 2014). For these measurements, the background was subtracted from the positive staining. Regarding NPY-stained sections, a cell count was also performed since well-defined NPY-IR cell bodies are observed in IGL. Quantitative data are expressed as mean ± standard error from the mean (SEM) and analyzed with one-way ANOVA followed by Tukey test for post-hoc comparisons. In all analyses, differences were considered significant at p < 0.05. Data analyses were performed using GraphPad Prism version 6.0.
Results
Age-related alterations in neurochemical content of IGL
Visually, a densely stained plexus of GAD- and ENK-IR were observed, with no differences between age groups (Fig. 1a–f). This was confirmed by one-way ANOVA, which did not reveal a significant effect of age on changes in GAD [F(2,12) = 0.6420; p = 0.5434] and ENK [F(2,12) = 1.177; p = 0.3414] OD between young, adult, and aged groups (Fig. 1g, h).
Fig. 1.
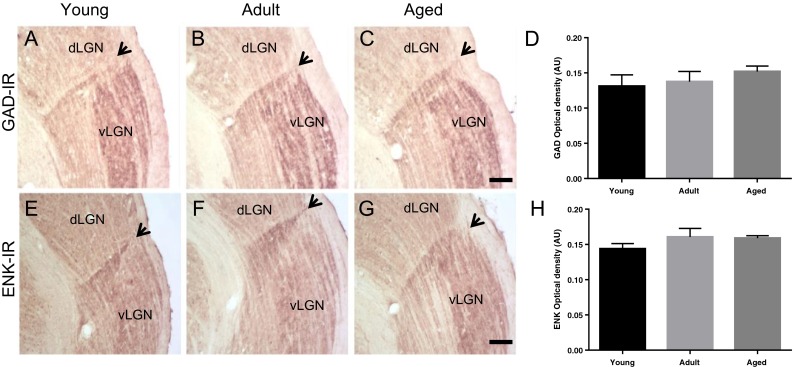
Digital images of coronal sections, at thalamic level, of glutamic acid decarboxylase (GAD) (a, b, c) and enkephalin (ENK) (e, f, g) immunoreactivity (IR) in young, adult, and aged rats. Black arrows indicate intergeniculate leaflet (IGL), stained area between dorsal geniculate (dLGN) and ventral geniculate (vLGN) lateral nuclei. Scale bar, 100 μm. GAD-IR optical density (OD) measurements in arbitrary units (A.U.) of young (n = 5), adult (n = 5), and aged (n = 5) animals (d). One-way ANOVA did not reveal a significant effect of age on changes in GAD-IR OD [F(2,12) = 0.6420; p = 0.5434] between age groups. ENK-IR OD measurements in A.U. of young (n = 5), adult (n = 5), and aged (n = 5) animals (h). One-way ANOVA did not reveal a significant effect of age on changes in ENK-IR OD [F(2,12) = 1.177; p = 0.3414] between age groups
We observed a reduction of NPY-IR between the age groups (Fig. 2a). One-way ANOVA showed a significant effect of age on alterations in cell counts [F(2,12) = 20.73; p = 0.0001] and OD [F(2,12) = 15.76; p = 0.0004] between age groups. The post hoc Tukey honest significant difference (HSD) multiple-comparison test revealed the mean cell count number (92 ± 6) and OD (0.152 ± 0.007) of the young group are higher than those of the adult group (59 ± 5 and 0.112 ± 0.011, respectively). The aged group have a reduced mean cell count number (47 ± 4) and OD (0.071 ± 0.013) when compared to both groups (Fig. 2b).
Fig. 2.
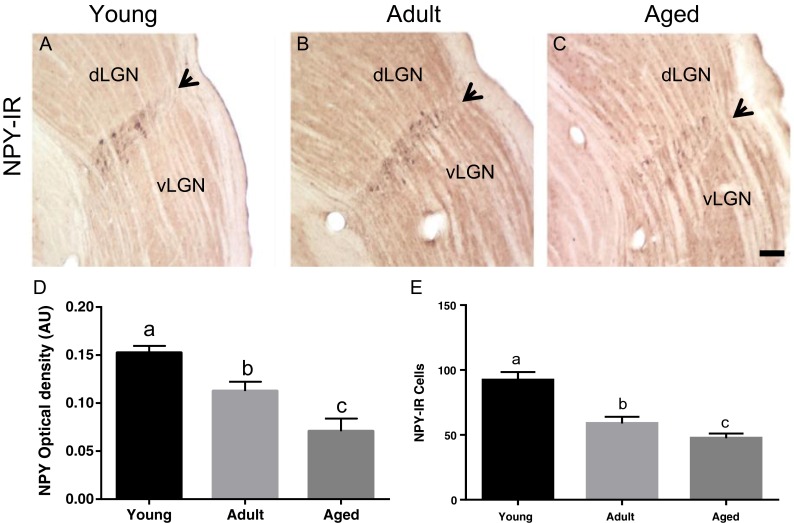
Digital images of coronal sections, at thalamic level, of neuropeptide Y (NPY) immunoreactivity (IR) in young (a), adult (b), and aged (c) rats. Black arrows indicate intergeniculate leaflet (IGL), stained area between dorsal geniculate (dLGN) and ventral geniculate (vLGN) lateral nuclei. Scale bar, 100 μm. NPY-IR optical density (OD) in arbitrary units (A.U.) (d) and cell counts (e) of young (n = 5), adult (n = 5), and aged (n = 5) animals. One-way ANOVA shows a significant effect of aging in NPY-IR reduction in both adult and aged compared to young ones [counting: F(2,12) = 20.73; p = 0.0001; OD: F(2,12) = 15.76; p = 0.0004]
Age-related alterations in retinal input to IGL
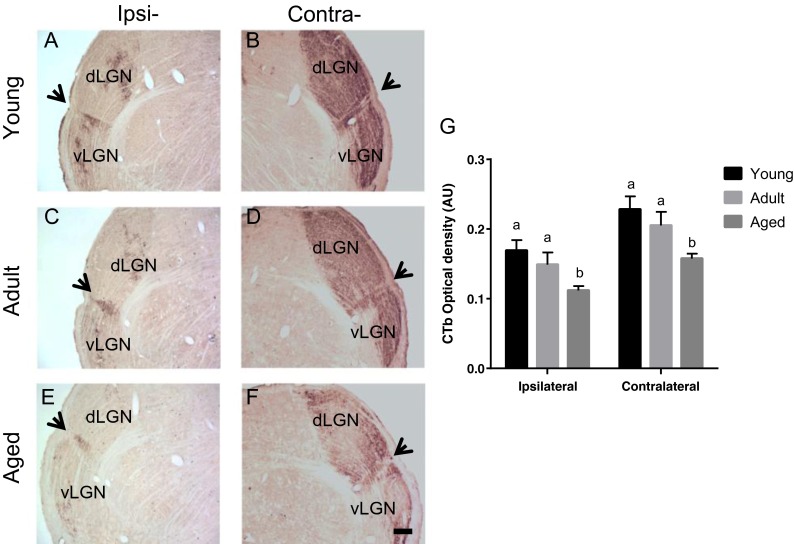
The retinal projections to IGL are reduced with age, in both ipsi- and contralateral sites to the CTb tracer injection (Fig. 3a). One-way ANOVA showed a significant effect of aging on alterations in CTb OD, at the ipsilateral [F(2,12) = 4.315; p = 0.038] and contralateral [F(2,12) = 5.011; p = 0.026] IGL between the age groups. The post hoc Tukey HSD multiple-comparison test revealed the mean of the young group (ipsilateral, 0.169 ± 0.015; contralateral, 0.228 ± 0.018) and adult group (ipsilateral, 0.148 ± 0.017; contralateral, 0.205 ± 0.019) have no significant differences compared to each other, but they are significantly higher than the mean OD of the aged group (ipsilateral, 0.111 ± 0.006; contralateral, 0.157 ± 0.007; Fig. 3b).
Fig. 3.
Digital images of coronal sections, at thalamic level, of cholera toxin b (CTb) immunoreactivity (IR) in both ipsi- and contralateral sites to tracer injection in young (a, b), adult (c, d), and aged (e, f) rats. Black arrows indicate intergeniculate leaflet (IGL), stained area between dorsal geniculate (dLGN) and ventral geniculate (vLGN) lateral nuclei. Scale bar 200 μm. CTb-IR optical density measurements in arbitrary units (A.U.) (g). One-way ANOVA shows a significant effect of aging on CTb-IR reduction in aged animals compared to young ones in both ipsi- and contralateral sites [ipsilateral: F(2,12) = 4.315; p = 0.038; OD: F(2,12) = 5.011; p = 0.026]
Discussion
A central question regarding the aging process on a circadian system perspective is to understand the mutual antagonism between aging and circadian clock homeostatic function (Kondratova and Kondratov 2012). In the present study, we describe age-related neurochemical alterations in IGL—an important modulator of circadian rhythmicity—providing a new insight for further interventions in CTS to antagonize aging effects.
We found no age-related differences in both GAD and ENK immunoreactivities. Similarly, other brain nuclei, such as the CA3 and dentate gyrus in the hippocampus, have no GAD profile alterations in adult and aged animals (Shi et al. 2004). Also, ENK-IR of several brain areas such as the hypothalamus, frontal cortex, and hippocampus (Kowalski et al. 1992) remain unaltered between age groups. Furthermore, aged hamsters have a similar GAD-IR in SCN when compared to young ones (Duncan and Wheeler 1999), which points that our data is coherent in a CTS organization perspective. Taken together, these data indicate that any deficiency the aged IGL might have in ENKergic and GABAergic physiology would not be at a cellular level, which supports further molecular studies regarding age-related alterations in GABA and ENK receptors and synaptic terminals.
Aging leads to a NPY-IR decline in IGL. This indicates either an age-related loss in NPY cell subpopulation of IGL or a decline in NPY production and expression with age. Accordingly, aging is correlated with decreases in NPY gene expression, receptor subtypes, and cell number in the arcuate nucleus, cerebral cortex, and dentate gyrus (Gruenewald et al. 1994; Cha et al. 1996; Cadacio et al. 2003; Veyrat-Durebex et al. 2013). Considering IGL have two major neurochemical subpopulations, GABA-ENK and GABA-NPY (Moore and Card 1994), the former with no direct circadian function well established so far and the latter projecting to SCN promoting non-photic phase shifting, the observed reduction in NPY-IR suggests aging impairs the non-photic adjustments IGL provides to CTS.
This observation is reinforced by evidence of loss in circadian response to non-photic stimuli (Hughes and Piggins 2012). For instance, non-photic phase-sifting induced by triazolam (Van reeth et al. 1992; 1993) and novel-wheel confinement (Mrosovsky and Biello 1994) are attenuated in old hamsters. In addition, locomotor rhythm fragmentation is observed in aged human and non-human primates (Czeisler et al. 1992; Zhdanova et al. 2011) and mice (Valentinuzzi et al. 1997; Farajnia et al. 2012). Hughes and Piggins (2012) state these alterations might be due to reduction in neurochemical mediators of circadian locomotor activity, such as NPY. Thus, our results corroborate this hypothesis.
Interestingly, locomotor activity rhythm is not simply an output of circadian function, once it provides a SCN feedback (Hughes and Piggins 2012). Therefore, age-related NPY reduction of IGL would account for locomotor activity fragmentation which jeopardizes the feedback provided to SCN, resulting in a loss of locomotor activity entrainment and establishing a disruptive loop effect (Farajnia et al. 2014). It is also noteworthy that phase advances caused by NPY administration in SCN at the middle of the day are equally observed in young and older mice indicating aged SCN do not lose its responsiveness to NPY (Biello 2009). For this reason, we suggest the reduction of NPY input to SCN, rather than the ability of NPY in activated SCN, is the main cause of age-related behavioral shortfalls influenced by IGL function in CTS.
Through CTb-IR analysis, we found a significant decline in IGL retinal afferents of aged rats. Correspondingly, Lupi et al. (2012) showed a reduction in CTb-IR and Fos expression after photic stimulation in both IGL and SCN of aged mice, indicating an age-related reduction in retinal input and light responsiveness of both nuclei. This impairment likely account for functional deficits of circadian system and may be caused by a deterioration of retinohypothalamic tract (RHT), since it forms both the retina-SCN and retina-IGL connections (Pickard 1985). Although aging diminishes the retinal input to circadian brain structures in mice and rats, Zhang et al. (1998) reported no reduction in retinal projections to SCN of elderly golden hamsters. Collectively, these data point the deleterious effect of aging in retinal input to CTS occurs in a species-specific manner.
A question formerly addressed by Lupi et al. (2012) concerns the relative importance of retinal degeneration versus age in the circadian system. Comparing wild-type mice with ocular mutants lacking rods and cones, Lupi et al. (2012) showed a higher impact of the intrinsic aging effects, rather than degeneration of photoreceptors, on IGL and SCN activation. However, they did not study this relationship with melanopsin-based photosensitive retinal ganglion cells (pRGCs), which forms RHT (Gooley et al. 2001; Hattar et al. 2002). It should be noted that we used an albino rat animal model, which has an abnormally increased predominance of contralateral retinofugal projections (Fleming et al. 2006). Thus, the reduction in both ipsi- and contralateral projections to IGL indicates aging causes a general loss of pRGCs and therefore RHT. This idea is also supported by previous reports of age-related reduction in retinal ganglion cells in both albino and pigmented rat strains (Weisse 1995).
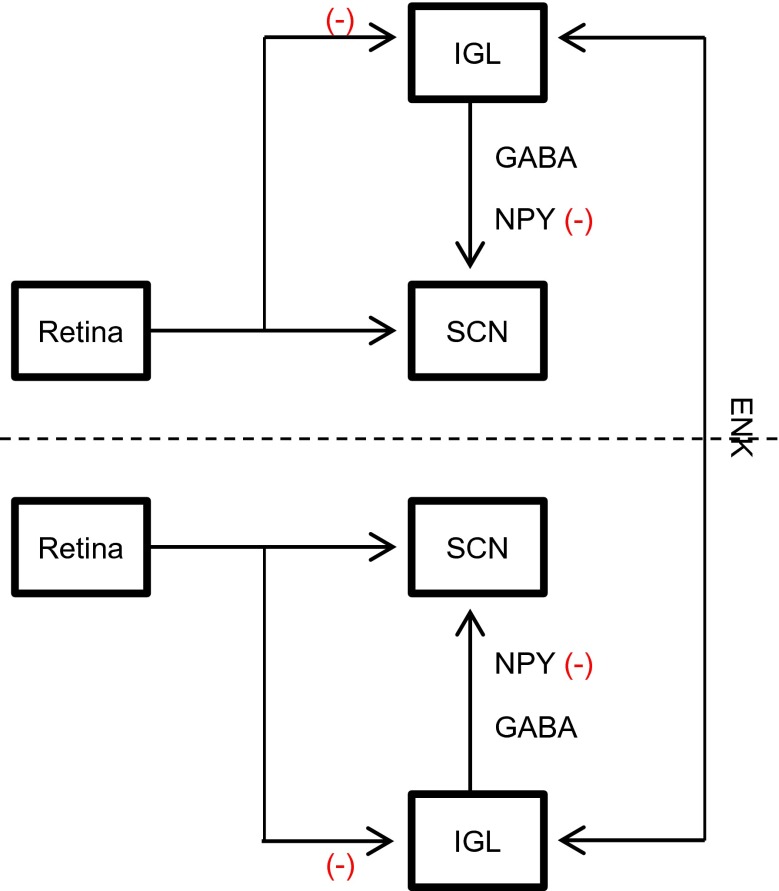
Moreover, Thankachan and Rusak (2005) characterized three classes of IGL neurons according to its activation by light and NPY presence. Among these classes, type I and type III neurons presented sustained activation by light and absence of NPY synthesis, differing in activation during darkness. Both types are likely involved in mediating IGL photic adjustments to SCN. In contrast, type II neurons presented large NPY amounts and heterogeneous response to illumination and mediate the IGL non-photic function (Thankachan and Rusak 2005). Since we found a decrease in both IGL retinal projections and NPY-IR, we believe these three types of IGL neurons are functionally disrupted within the aging process, type I and type III in its activation by light input and type II in NPY expression, which points the adjustments IGL provides to central clock, by integration of photic and non-photic clues, are hindered with aging. In a hodological perspective, two sources of retinal information to central pacemaker, a direct RHT and an indirect GHT, involved in entrainment to light-dark cycles (Moore and Lenn 1972; Pickard et al. 1987; Morin and Pace 2002) are impaired by the aging process. Based on our results, we summarize these age-related impairments in Fig. 4.
Fig. 4.
Summary of age-related alterations in rat intergeniculate leaflet (IGL) connections with retina and suprachiasmatic nucleus (SCN). Although no age-related changes were observed in gamma-aminobutyric acid (GABA) and enkephalin (ENK) neurochemical content, the aging process is correlated with losses of retinal input and neuropeptide Y (NPY) in rat IGL, which are likely involved in functional deficits of the aged circadian timing system. Minus sign represents age-related impairments
In summary, the present study is the first to demonstrate an age-related loss in NPY-IR and retinal afferents of rat IGL. Thus, IGL integrative function of photic and non-photic clues in circadian system is likely impaired in elderly rats providing strong anatomical correlates to functional deficits of aged circadian clock.
Acknowledgments
The study was supported by Brazilian funding agencies Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Conselho Nacional de Desenvolvimento Científico e Tecnológico, and Fundação de apoio à pesquisa no RN. The authors also would like to thank Dr. Ruthnaldo R.M. Lima, Dr. J. Ronaldo dos Santos, and Dr. Ramon H. Lima for the valuable comments during the manuscript preparation.
Compliance with ethical standards
All procedures were in accordance with Brazilian law number 11.794/2008 for animal experimental use. All experiments were approved by local ethics committee for animal use (CEUA-UFRN number 021/2014).
References
- Aujard F, Cayetanot F, Bentivoglio M, Perret M. Age-related effects on the biological clock and its behavioral output in a primate. Chronobiol Int. 2006;23:451–460. doi: 10.1080/07420520500482090. [DOI] [PubMed] [Google Scholar]
- Biello SM. Circadian clock resetting in the mouse changes with age. Age (Dordr) 2009;31:293–303. doi: 10.1007/s11357-009-9102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biello SM, Janik D, Mrosovsky N. Neuropeptide Y and behaviorally induced phase shifts. Neuroscience. 1994;62:273–279. doi: 10.1016/0306-4522(94)90331-X. [DOI] [PubMed] [Google Scholar]
- Blasiak T, Lewandowski MH. Differential firing pattern and response to lighting conditions of rat intergeniculate leaflet neurons projecting to suprachiasmatic nucleus or contralateral intergeniculate leaflet. Neuroscience. 2013;228:315–324. doi: 10.1016/j.neuroscience.2012.10.044. [DOI] [PubMed] [Google Scholar]
- Cadacio CL, Milner TA, Gallagher M, Pierce JP. Hilar neuropeptide Y interneuron loss in the aged rat hippocampal formation. Exp Neurol. 2003;183:147–158. doi: 10.1016/S0014-4886(03)00126-2. [DOI] [PubMed] [Google Scholar]
- Cayetanot F, Bentivoglio M, Aujard F. Arginine-vasopressin and vasointestinal polypeptide rhythms in the suprachiasmatic nucleus of the mouse lemur reveal aging-related alterations of circadian pacemaker neurons in a non-human primate. Eur J Neurosci. 2005;22:902–910. doi: 10.1111/j.1460-9568.2005.04268.x. [DOI] [PubMed] [Google Scholar]
- Cha CI, Lee YI, Park KH, Baik SH. Age-related change of neuropeptide Y-immunoreactive neurons in the cerebral cortex of aged rats. Neurosci Lett. 1996;214:37–40. doi: 10.1016/0304-3940(96)12876-7. [DOI] [PubMed] [Google Scholar]
- Chee CA, Roozendaal B, Swaab DF, et al. Vasoactive intestinal polypeptide neuron changes in the senile rat suprachiasmatic nucleus. Neurobiol Aging. 1988;9:307–312. doi: 10.1016/S0197-4580(88)80070-8. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Dumont M, Duffy JF, et al. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–936. doi: 10.1016/0140-6736(92)92817-Y. [DOI] [PubMed] [Google Scholar]
- Davidson AJ, Yamazaki S, Arble DM, et al. Resetting of central and peripheral circadian oscillators in aged rats. Neurobiol Aging. 2008;29:471–477. doi: 10.1016/j.neurobiolaging.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Wheeler DL. Aging and photoperiod regulate glutamic acid decarboxylase(67) messenger RNA expression. Brain Res Mol Brain Res. 1999;71:325–331. doi: 10.1016/S0169-328X(99)00206-5. [DOI] [PubMed] [Google Scholar]
- Engelberth RCGJ, de Pontes ALB, Fiuza FP, et al. Changes in the suprachiasmatic nucleus during aging: implications for biological rhythms. Psychol Neurosci. 2013;6:287–297. doi: 10.3922/j.psns.2013.3.07. [DOI] [Google Scholar]
- Engelberth RCGJ, de A Silva KD, de M Azevedo CV, et al. Morphological changes in the suprachiasmatic nucleus of aging female marmosets (Callithrix jacchus) BioMed Res Int. 2014;2014 doi: 10.1155/2014/243825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajnia S, Michel S, Deboer T, et al. Evidence for neuronal desynchrony in the aged suprachiasmatic nucleus clock. J Neurosci. 2012;32:5891–5899. doi: 10.1523/JNEUROSCI.0469-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajnia S, Deboer T, Rohling JHT, et al. Aging of the suprachiasmatic clock. Neuroscientist. 2014;20:44–55. doi: 10.1177/1073858413498936. [DOI] [PubMed] [Google Scholar]
- Fleming MD, Benca RM, Behan M. Retinal projections to the subcortical visual system in congenic albino and pigmented rats. Neuroscience. 2006;143:895–904. doi: 10.1016/j.neuroscience.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca Costa SS, Ripperger JA. Impact of the circadian clock on the aging process. Front Neurol. 2015;6:43. doi: 10.3389/fneur.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gall AJ, Yan L, Smale L, Nunez AA. Intergeniculate leaflet lesions result in differential activation of brain regions following the presentation of photic stimuli in Nile grass rats. Neurosci Lett. 2014;579:101–105. doi: 10.1016/j.neulet.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Chou TC, et al. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4:1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Naai MA, Marck BT, Matsumoto AM. Age-related decrease in neuropeptide-Y gene expression in the arcuate nucleus of the male rat brain is independent of testicular feedback. Endocrinology. 1994;134:2383–2389. doi: 10.1210/endo.134.6.8194464. [DOI] [PubMed] [Google Scholar]
- Harrington ME. The ventral lateral geniculate nucleus and the intergeniculate leaflet: interrelated structures in the visual and circadian systems. Neurosci Biobehav Rev. 1997;21:705–727. doi: 10.1016/S0149-7634(96)00019-X. [DOI] [PubMed] [Google Scholar]
- Hattar S, Liao HW, Takao M, et al. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey TL, Spear PD. Retinogeniculate projections in hooded and albino rats: an autoradiographic study. Exp Brain Res. 1976;24:523–529. doi: 10.1007/BF00234968. [DOI] [PubMed] [Google Scholar]
- Hughes ATL, Piggins HD. Feedback actions of locomotor activity to the circadian clock. Prog Brain Res. 2012;199:305–336. doi: 10.1016/B978-0-444-59427-3.00018-6. [DOI] [PubMed] [Google Scholar]
- Janik D, Mikkelsen JD, Mrosovsky N. Cellular colocalization of Fos and neuropeptide Y in the intergeniculate leaflet after nonphotic phase-shifting events. Brain Res. 1995;698:137–145. doi: 10.1016/0006-8993(95)00878-T. [DOI] [PubMed] [Google Scholar]
- Juhl F, Hannibal J, Fahrenkrug J. Photic induction of c-Fos in enkephalin neurons of the rat intergeniculate leaflet innervated by retinal PACAP fibres. Cell Tissue Res. 2007;329:491–502. doi: 10.1007/s00441-007-0422-6. [DOI] [PubMed] [Google Scholar]
- Kondratova AA, Kondratov RV. The circadian clock and pathology of the ageing brain. Nat Rev Neurosci. 2012;13:325–335. doi: 10.1038/nrn3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski C, Micheau J, Corder R, et al. Age-related changes in cortico-releasing factor, somatostatin, neuropeptide Y, methionine enkephalin and beta-endorphin in specific rat brain areas. Brain Res. 1992;582:38–46. doi: 10.1016/0006-8993(92)90314-Y. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM. Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci. 1998;18:4767–4774. doi: 10.1523/JNEUROSCI.18-12-04767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupi D, Semo M, Foster RG. Impact of age and retinal degeneration on the light input to circadian brain structures. Neurobiol Aging. 2012;33:383–392. doi: 10.1016/j.neurobiolaging.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Magee JC, Carmin CN. The relationship between sleep and anxiety in older adults. Curr Psychiatry Rep. 2010;12:13–19. doi: 10.1007/s11920-009-0087-9. [DOI] [PubMed] [Google Scholar]
- Moore RY, Card JP. Intergeniculate leaflet: an anatomically and functionally distinct subdivision of the lateral geniculate complex. J Comp Neurol. 1994;344:403–430. doi: 10.1002/cne.903440306. [DOI] [PubMed] [Google Scholar]
- Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J Comp Neurol. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- Morin LP. Neuroanatomy of the extended circadian rhythm system. Exp Neurol. 2013;243:4–20. doi: 10.1016/j.expneurol.2012.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin LP, Pace L. The intergeniculate leaflet, but not the visual midbrain, mediates hamster circadian rhythm response to constant light. J Biol Rhythm. 2002;17:217–226. doi: 10.1177/07430402017003005. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N, Biello SM. Nonphotic phase shifting in the old and the cold. Chronobiol Int. 1994;11:232–252. doi: 10.3109/07420529409067792. [DOI] [PubMed] [Google Scholar]
- Pace-Schott EF, Spencer RMC. Age-related changes in the cognitive function of sleep. Prog Brain Res. 2011;191:75–89. doi: 10.1016/B978-0-444-53752-2.00012-6. [DOI] [PubMed] [Google Scholar]
- Pickard GE. Bifurcating axons of retinal ganglion cells terminate in the hypothalamic suprachiasmatic nucleus and the intergeniculate leaflet of the thalamus. Neurosci Lett. 1985;55:211–217. doi: 10.1016/0304-3940(85)90022-9. [DOI] [PubMed] [Google Scholar]
- Pickard GE, Ralph MR, Menaker M. The intergeniculate leaflet partially mediates effects of light on circadian rhythms. J Biol Rhythm. 1987;2:35–56. doi: 10.1177/074873048700200104. [DOI] [PubMed] [Google Scholar]
- Roberts DE, Killiany RJ, Rosene DL. Neuron numbers in the hypothalamus of the normal aging rhesus monkey: stability across the adult lifespan and between the sexes. J Comp Neurol. 2012;520:1181–1197. doi: 10.1002/cne.22761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, van Gool WA, Swaab DF, et al. Changes in vasopressin cells of the rat suprachiasmatic nucleus with aging. Brain Res. 1987;409:259–264. doi: 10.1016/0006-8993(87)90710-4. [DOI] [PubMed] [Google Scholar]
- Saderi N, Cazarez-Márquez F, Buijs FN, et al. The NPY intergeniculate leaflet projections to the suprachiasmatic nucleus transmit metabolic conditions. Neuroscience. 2013;246:291–300. doi: 10.1016/j.neuroscience.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Satinoff E, Li H, Tcheng TK, et al. Do the suprachiasmatic nuclei oscillate in old rats as they do in young ones? Am J Physiol. 1993;265:R1216–R1222. doi: 10.1152/ajpregu.1993.265.5.R1216. [DOI] [PubMed] [Google Scholar]
- Shi L, Argenta AE, Winseck AK, Brunso-Bechtold JK. Stereological quantification of GAD-67-immunoreactive neurons and boutons in the hippocampus of middle-aged and old Fischer 344 x Brown Norway rats. J Comp Neurol. 2004;478:282–291. doi: 10.1002/cne.20303. [DOI] [PubMed] [Google Scholar]
- Sutin EL, Dement WC, Heller HC, Kilduff TS. Light-induced gene expression in the suprachiasmatic nucleus of young and aging rats. Neurobiol Aging. 1993;14:441–446. doi: 10.1016/0197-4580(93)90102-H. [DOI] [PubMed] [Google Scholar]
- Thankachan S, Rusak B. Juxtacellular recording/labeling analysis of physiological and anatomical characteristics of rat intergeniculate leaflet neurons. J Neurosci. 2005;25:9195–9204. doi: 10.1523/JNEUROSCI.2672-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara S, Tanaka S, Ishida K, et al. Age-related change and its sex differences in histoarchitecture of the hypothalamic suprachiasmatic nucleus of F344/N rats. Exp Gerontol. 2005;40:147–155. doi: 10.1016/j.exger.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol. 1997;273:R1957–R1964. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]
- Van Reeth O, Zhang Y, Zee PC, Turek FW. Aging alters feedback effects of the activity-rest cycle on the circadian clock. Am J Physiol. 1992;263:R981–R986. doi: 10.1152/ajpregu.1992.263.4.R981. [DOI] [PubMed] [Google Scholar]
- Van Reeth O, Zhang Y, Reddy A, et al. Aging alters the entraining effects of an activity-inducing stimulus on the circadian clock. Brain Res. 1993;607:286–292. doi: 10.1016/0006-8993(93)91518-W. [DOI] [PubMed] [Google Scholar]
- Veyrat-Durebex C, Quirion R, Ferland G, et al. Aging and long-term caloric restriction regulate neuropeptide Y receptor subtype densities in the rat brain. Neuropeptides. 2013;47:163–169. doi: 10.1016/j.npep.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Weisse I. Changes in the aging rat retina. Ophthalmic Res. 1995;27:154–163. doi: 10.1159/000267862. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Mellon SH. Of sound mind and body: depression, disease, and accelerated aging. Dialogues Clin Neurosci. 2011;13:25–39. doi: 10.31887/DCNS.2011.13.1/owolkowitz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Straume M, Tei H, et al. Effects of aging on central and peripheral mammalian clocks. PNAS. 2002;99:10801–10806. doi: 10.1073/pnas.152318499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brainard GC, Zee PC, et al. Effects of aging on lens transmittance and retinal input to the suprachiasmatic nucleus in golden hamsters. Neurosci Lett. 1998;258:167–170. doi: 10.1016/S0304-3940(98)00887-8. [DOI] [PubMed] [Google Scholar]
- Zhdanova IV, Masuda K, Quasarano-Kourkoulis C, et al. Aging of intrinsic circadian rhythms and sleep in a diurnal nonhuman primate, Macaca mulatta. J Biol Rhythm. 2011;26:149–159. doi: 10.1177/0748730410395849. [DOI] [PubMed] [Google Scholar]