Abstract
Physical activity recommendations for public health include typically muscle-strengthening activities for a minimum of 2 days a week. The range of inter-individual variation in responses to resistance training (RT) aiming to improve health and well-being requires to be investigated. The purpose of this study was to quantify high and low responders for RT-induced changes in muscle size and strength and to examine possible effects of age and sex on these responses. Previously collected data of untrained healthy men and women (age 19 to 78 years, n = 287 with 72 controls) were pooled for the present study. Muscle size and strength changed during RT are 4.8 ± 6.1 % (range from −11 to 30 %) and 21.1 ± 11.5 % (range from −8 to 60 %) compared to pre-RT, respectively. Age and sex did not affect to the RT responses. Fourteen percent and 12 % of the subjects were defined as high responders (>1 standard deviation (SD) from the group mean) for the RT-induced changes in muscle size and strength, respectively. When taking into account the results of non-training controls (upper 95 % CI), 29 and 7 % of the subjects were defined as low responders for the RT-induced changes in muscle size and strength, respectively. The muscle size and strength responses varied extensively between the subjects regardless of subject’s age and sex. Whether these changes are associated with, e.g., functional capacity and metabolic health improvements due to RT requires further studies.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-015-9870-1) contains supplementary material, which is available to authorized users.
Keywords: Individual variation, Muscle hypertrophy, Responders, Aging
Introduction
It is well documented that long-term systematic resistance training (RT) causes increased skeletal muscle size and strength in both men and women of different ages. RT-induced gains in muscle size and strength, however, are highly variable between individuals. The robust study of individual responses to unilateral upper arm RT by Hubal et al. (2005) showed that of 585 subjects, approximately 6 % showed practically no gains in muscle size. Also, other RT studies have reported that, in some subjects, muscle size gains are either minimal or non-existent following a training period (Bamman et al. 2007; Davidsen et al. 2011; Raue et al. 2012; Mitchell et al. 2013; Phillips et al. 2013). Similarly to muscle size responses, gains in muscle strength during RT are also highly individual (Hubal et al. 2005; Erskine et al. 2010). However, the range of individual responses to RT in people of different ages has not yet been elucidated. This is particularly relevant considering how people respond to a RT program based on physical activity recommendations for health.
Muscular adaptations to RT are important to manage conditions where muscle weakness may limit function, such as muscle wasting disorders, prolonged bed rest, aging, and rehabilitation. Low muscle strength is related to lower functional capacity, and muscle strength has been shown to be inversely associated with risk of mortality and cardiovascular diseases (Timpka et al. 2014). Similarly to muscle strength, muscle mass per se is an important factor for health and physical performance. Loss of muscle mass leads to a reduction in metabolic rate, and consequently, gains in body fat. These detrimental changes in body composition are associated with adverse metabolic conditions and several diseases, especially in older age (Westcott 2012). Skeletal muscle is a primary target for impacting insulin resistance since it constitutes 40 % of body mass and is the main tissue for glucose disposal. Thus, the significance of an individual’s ability to increase muscle size due to RT may ultimately influence multiple risk factors by enhanced insulin sensitivity and to counteract deteriorating effects of sarcopenia (Westcott 2012; Mann et al. 2014).
Understanding an individual’s sensitivity to a certain type of RT may enable individually tailored exercise training programs to optimally improve or maintain healthy muscle function throughout the lifespan. Furthermore, identifying responders and non-responders can aid in defining mechanisms controlling muscle growth. The aim of the present study was to quantify the range of human muscle responses to 20–24 weeks systematic and supervised RT with regard to changes in muscle size and strength in previously untrained younger and older individuals. Specifically, the present data allowed us to investigate possible age and sex differences in muscle size and strength responses to RT. Moreover, we aimed to quantify for the first time the proportion of subjects who were high and low responders for gains in muscle size and strength due to RT in a large study set. Therefore, the results of non-training controls were taken into account as a normal variation within the same time period in defining low responders.
Methods
Subjects
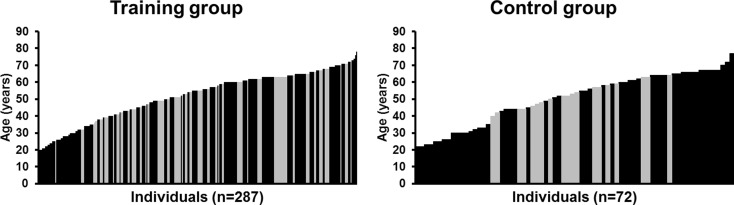
Ten 20–24 weeks RT interventions conducted in our laboratory during 1996–2011 were included into a retrospective analysis (see Supporting Information 1, Table 1). Research projects were grouped into four clusters depending on the timing and methodologies used to determine muscle size. All subjects whose muscle size was determined before and after the intervention as mentioned below were included from the original data in the current study. Within the particular experiments, the subjects were randomly divided to the RT or non-training control groups. The final study groups of the present investigation consisted of 287 subjects (men, n = 183; women, n = 104) in the training group and 72 subjects (men, n = 53; women, n = 19) in the control group in total. The age of the subjects varied from 19 to 78 years in trainees and from 22 to 77 years in controls (Fig. 1). Those subjects randomized into the training group were divided into three age groups with similar group sizes and gender distribution for the current analyses: below 45 years (younger adults), 45–60 years (middle-aged), and over 60 years (older people) (Table 1). Subjects were healthy volunteers and not experienced in RT. All subjects underwent a similar recruitment process (for details see Supporting Information 2, Methods). Subjects were carefully informed about the design of the study with special information on possible risks and benefits both verbally and in writing, and they signed a written consent form before participation in the study. The studies were conducted according to the Declaration of Helsinki and were approved by the Ethics Committee of the University of Jyväskylä, Finland, and/or by the Ethics Committee of the Central Finland Health Care District.
Table 1.
Subject characteristics and muscle strength of the leg extensors at baseline and after the intervention period (mean ± SD)
| Gender | Groups | Age (years) | Height (cm) | Body mass (kg) | BMI | 1RM (kg) | 1RM/body mass | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||||
| Men | <45 (n = 61) | 31.2 | 179.8# | 78.0 | 79.0* | 24.1# | 24.4* | 169.3# | 200.5* | 2.2#,§ | 2.6* |
| (7.3) | (5.8) | (11.6) | (11.4) | (3.1) | (3.1) | (29.5) | (32.2) | (0.4) | (0.4) | ||
| 45–60 (n = 55) | 53.8 | 177.5 | 81.0 | 80.7 | 25.7 | 25.6 | 161.7# | 192.2* | 2.0# | 2.4* | |
| (5.0) | (6.7) | (10.3) | (9.8) | (3.2) | (2.9) | (29.0) | (32.2) | (0.3) | (0.4) | ||
| >60 (n = 67) | 66.1 | 175.7 | 79.2 | 79.3 | 25.7 | 25.7 | 140.4 | 167.0* | 1.8 | 2.1* | |
| (4.0) | (5.7) | (8.3) | (8.7) | (2.7) | (2.8) | (28.3) | (34.6) | (0.3) | (0.4) | ||
| Controls (n = 53) | 49.8 | 177.7 | 79.0 | 78.8 | 24.9 | 24.9 | 155.1 | 161.7* | 2.0 | 2.0 | |
| (16.9) | (6.7) | (11.7) | (11.8) | (2.8) | (2.8) | (28.3) | (29.5) | (0.4) | (0.5) | ||
| Women | <45 (n = 27) | 38.7 | 164.6 | 64.1 | 64.8* | 23.7# | 23.9* | 107.1# | 135.2* | 1.7# | 2.1* |
| (4.6) | (5.6) | (8.6) | (8.7) | (3.0) | (3.0) | (17.8) | (24.2) | (0.3) | (0.4) | ||
| 45–60 (n = 41) | 53.0 | 163.4 | 67.3 | 66.6* | 25.2 | 24.9* | 105.5 | 126.3* | 1.6# | 1.9* | |
| (4.5) | (6.3) | (11.7) | (11.4) | (3.8) | (3.8) | (21.7) | (27.4) | (0.3) | (0.4) | ||
| >60 (n = 36) | 65.2 | 161.1 | 68.6 | 67.9 | 26.4 | 26.1 | 93.6 | 117.9* | 1.4 | 1.7* | |
| (3.1) | (5.3) | (6.8) | (6.4) | (2.0) | (2.1) | (18.2) | (21.2) | (0.3) | (0.3) | ||
| Controls (n = 19) | 52.3 | 166.0 | 66.9 | 66.6* | 24.2 | 24.1* | 91.6 | 92.8 | 1.4 | 1.4 | |
| (7.4) | (5.7) | (7.6) | (7.7) | (2.3) | (2.3) | (12.1) | (12.8) | (0.1) | (0.1) | ||
BMI body mass index, 1RM one repetition maximum
*p < 0.05 compared to the value measured before the resistance training period (Pre-RT); # p < 0.05 compared to the corresponding older group (>60 years) at baseline; § p < 0.05 compared to the corresponding middle-aged group (45–60 years) at baseline
Fig. 1.
Age distribution of training and control subjects. Black bars denote men (n = 183 in the training group and n = 53 in the control group), while grey bars denote women (n = 104 in the training group and n = 19 in the control group)
Resistance training program
The subjects participated in a 20–24-week periodized RT program (for details see Supporting Information 2, Methods). Total body RT sessions were carried out twice a week with at least 2 days of rest between the training sessions. Hence, the present RT program was in line with physical activity recommendations based on the available evidence of the dose–response relationship between exercise and health. These recommendations typically include muscle-strengthening activities for a minimum of 2 days a week (Oja and Titze 2011). All training sessions were supervised by the research team to ensure that proper techniques and high loading effort were used in each exercise. The RT program was designed to increase muscle size and strength extensively throughout the training period. Both overall intensity and volume of training increased progressively throughout the training period. Regarding to the present study, high adherence to RT (99 ± 2 %) has been previously reported (Karavirta et al. 2011b). Moreover, all subjects were required to complete a minimum of 90 % of total training sessions.
The subjects randomized to the control group were instructed to continue their normal low-frequency and low-intensity recreational physical activities and refrain from any resistance-type exercise training throughout the intervention period.
Anthropometry
Body height was measured by an inelastic tape measure with the subjects standing barefoot. Body mass was measured by a scale with a resolution of 0.1 kg with the subjects in their underwear. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters (kg/m2).
Muscle size
Depending of the methods selected at that time, different methodologies were used over the years to determine the changes in leg muscle size (see Supporting Information 1, Table 1). Only the data of the changes in m.quadriceps femoris muscle cross-sectional area (CSA) (by ultrasound (US) and magnetic resonance imaging (MRI)), m.vastus lateralis (VL) muscle thickness (by US) or leg lean mass (by dual-energy X-ray absorptiometry (DXA)) were included in the analyses. All measurements were preceded by at least 2 days of rest from physical activity. All the data within the studies were analyzed by the same experienced researcher.
Study cluster 1
The CSA of the quadriceps femoris (QF) muscle group (rectus femoris, VL, vastus medialis, and vastus intermedialis) was measured with a compound ultrasonic scanner (model SSD-190, Aloka Fansonic) and a 5-MHz convex transducer (Häkkinen et al. 1998). The CSA was measured at the lower one third portion between the greater trochanter and lateral joint line of the knee. Two consecutive measurements were taken from the right thigh and then averaged for further analyses. The CSA of the QF was then calculated from the image by the computerized system of the apparatus. In a previous study from our laboratory, a coefficient of variation (CV) of the repeated measurement over consecutive days was 4.3 % for the CSA of QF (Sipilä and Suominen 1991).
Study clusters 2 and 3
The muscle CSA of the right QF muscle was determined using MRI (GE Signa Exite HD 1.5 T or Philips Gyroscan ACS-NT Scanner, 1.5 T). During the measurement, the thighs of both legs were kept parallel to the MRI table and the feet were strapped together with a belt and a special cast to prevent rotation. Great care was taken to reproduce the same axial plane MRI scans before and after RT by using the appropriate anatomical landmarks. Several axial scans were obtained from the level of femur length for subsequent analyses. Using OsiriX image analysis software, CSA was determined by tracing manually along the border of each muscle of the QF. Correlation of two repeated MRI measurements (n = 8) over a 2-week control period was r > 0.96 (Hulmi et al. 2009). Further analyses of that data showed the one-way random, single measure model of intraclass correlation coefficient (ICC1,1) to be 0.957, CV of 2.1 %, and technical error of measurement of 2.2 %.
Study cluster 3
The muscle thickness of VL from the midpoint of the lateral knee joint surface and trochanter major of the right leg was measured with a compound US (Aloka SSD-2000, Aloka Co, Tokyo, Japan) and a 7.5-MHz linear array transducer. The scanning head was coated with water-soluble transmission gel to provide acoustic contact without depressing the dermal surface. The ultrasonography measurement site was tattooed to ensure that the same site was used both before and after training. From the scanning image, the distance between the subcutaneous adipose tissue–muscle interface and intramuscular interface (i. e., aponeurosis between m.vastus intermedius and m.vastus lateralis) was defined as muscle thickness. At each ultrasonography measurement, two consecutive measurements were taken and then averaged for further analyses. The ICC1,1 of two repeated US muscle thickness measurements for thigh muscles has been shown to be 0.92 (Sillanpää et al. 2008).
Study clusters 3 and 4
DXA was used to assess whole-body composition (LUNAR Prodigy Advance with enCORE software version 9.30, GE Medical Systems, Madison, USA). The legs were secured together by non-elastic straps about the knees and ankles to prevent movement during scanning. The lean mass of the lower limbs were assessed according to software-generated regions with manual adjustments. In a previous study from our laboratory, the CV of two repeated DXA measurements was 1.0 % for lean tissue mass (Xu et al. 2010).
In study cluster 3, different methodologies were used concurrently within the experiments. If several muscle size measurements were available, MRI was preferred over DXA or US data, and if the DXA data was available, that was used instead of US data.
Maximal muscle strength
Of the total group of subjects whose muscle size data was available, maximal muscle strength data was available from 283 subjects in the training group and of 66 subjects from the control group. The same horizontal leg press device (David 210, David Health Solutions Ltd., Helsinki, Finland) was used in all studies to measure maximal bilateral concentric strength (i.e., one repetition maximum, 1RM) of the hip and knee extensors and plantar flexors (for details see Supporting Information 2, Methods). The 1RM results were subsequently divided by the subject’s body mass (1RM/body mass ratio) to determine normalized strength. Regarding the part of the present data, the ICC1,1 of 1RM strength over a 2-week control period before RT has ranged between 0.9 and 1.0 (Holviala et al. 2006). Furthermore, in a previous study in our laboratory, inter-day reliability values for the 1RM measurements were 0.981 and 3.1 % for ICC1,1 and CV, respectively (Walker et al. 2015).
Statistics
All data are presented as mean ± standard deviation (SD). Statistical analyses were carried out using SPSS 22.0 software for Windows (SPSS, Inc., Chicago, IL). The Kolmogorov–Smirnov test was used to test normality, and the Levene’s test was used to analyze the homogeneity of variances. Due to random violations in the normal distribution assumption, relative changes of muscle size were reciprocally transformed before the between-group testing. The untransformed data is presented throughout the report. Differences between the age and sex groups at baseline and training response in muscle size and strength were assessed using Univariate GLM with Bonferroni post hoc test. In muscle strength analyses, the pre-training value was used as a covariate. The changes between pre- and post-training values within the experimental groups (age/sex) were analyzed by the paired samples t test. A Pearson product–moment correlation coefficient was used to determine the association between the relative changes in 1RM and muscle size. In general, training responses can be influenced by age, sex, and baseline physical fitness level (Garber et al. 2011). To assess the determinants of the RT-induced change in muscle size, linear regression analysis and principal component analysis were conducted. Age, sex, and baseline values of BMI, 1RM, and 1RM/body mass ratio, as well as training-induced changes in BMI, 1RM, and 1RM/body mass ratio, were investigated as potential predictors. Individuals were defined as low responders when taking into account the results of non-training controls. The confidence intervals (CI) of changes in muscle size and strength in the control group were determined by Univariate GLM, and the upper 95 % CI was used as the lower limit for a significant individual training-induced change in the training group (Hopkins 2000; Karavirta et al. 2011b). Individuals with gains in muscle size and strength beyond 1 SD from the mean of the training group were defined as high responders (Erskine et al. 2010). Statistical significance was accepted when p ≤ 0.05.
Results
Muscle strength
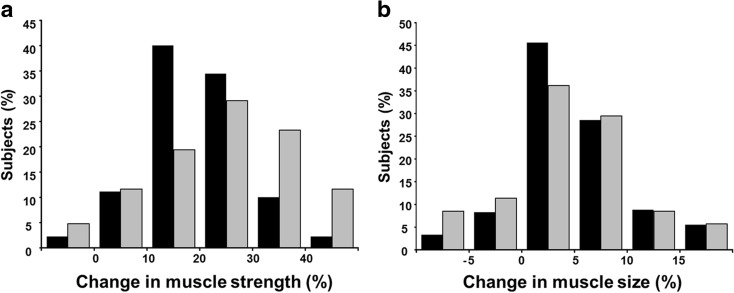
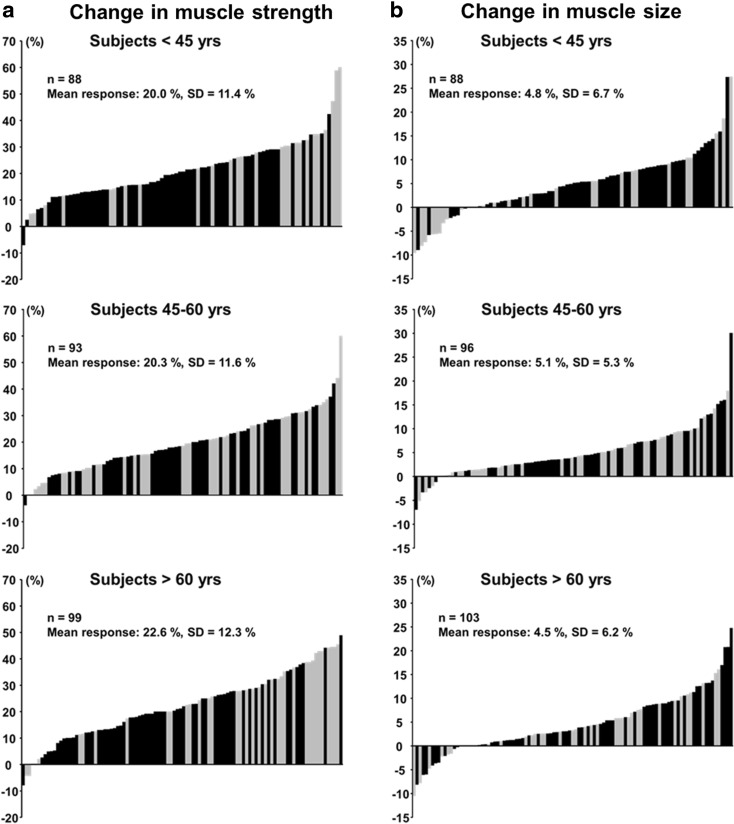
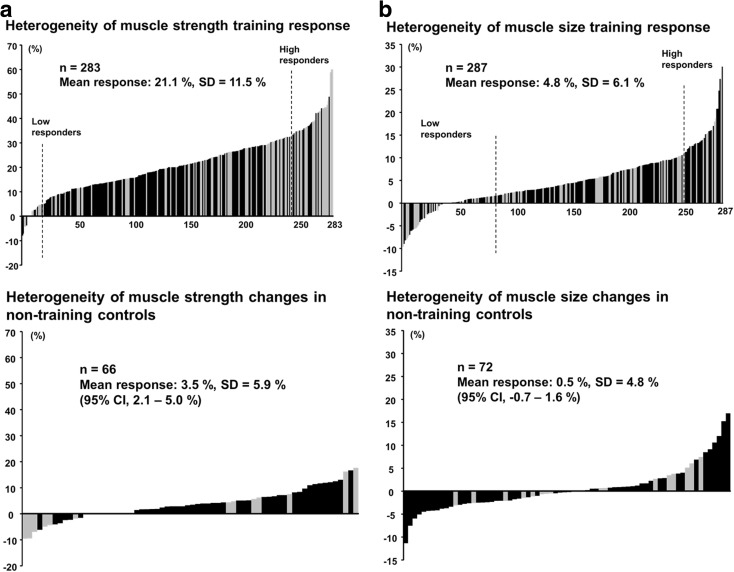
At baseline, 1RM and 1RM/body mass were greater (p < 0.05) in men and women below 45 years compared to the corresponding group of the men and women over 65 years (Table 1). 1RM/body mass ratio was greater in men below 45 than in men 45–60 years. From pre- to post-RT, and compared to the changes in the control group, 1RM and 1RM/body mass ratio increased significantly (p < 0.05) in all age groups of trainees in both men and women. When all age groups were combined, relative change in 1RM was higher (p < 0.05) in women (24.2 ± 13.8 %) compared to men (19.4 ± 9.5 %), but the absolute change in 1RM did not differ statistically significantly between men (28.3 ± 15.7 kg) and women (25.9 ± 17.1 kg). The greatest proportion of men gained 10–20 % of muscle strength while the greatest proportion of women gained 20–30 % (Fig. 2a). 1RM increased during RT 19.0 ± 8.6 %, 19.4 ± 9.0 %, and 19.7 ± 10.8 % in men and 26.7 ± 13.9 %, 20.0 ± 12.7 %, and 27.0 ± 14.1 % in women in age groups of below 45, 45–60, and over 65 years, respectively (Fig. 3a). There were no statistically significant interactions between sex group (F1, 276 = 1.2; p = 0.274), age group (F2, 276 = 1.74; p = 0.178), or sex × age group (F2, 276 = 1.803; p = 0.167) and in the absolute change of 1RM. Relative changes in 1RM varied from −8 to 60 % in the training group and from −10 to 18 % in the control group (Fig. 4a).
Fig. 2.
Histogram of muscle strength (a) and size (b) changes (relative to baseline) in men and women in the training group. Black bars denote responses of men, while grey bars denote responses of women
Fig. 3.
Heterogeneity of muscle strength (a) and size (b) training responses in relation to the baseline value of different age groups. Black bars denote responses of men, while grey bars denote responses of women
Fig. 4.
Heterogeneity of muscle strength (a) and size (b) training responses in relation to the baseline value in the training and control groups. High and low responders in the training group were denoted by the vertical dotted lines. Individuals with training response below the upper 95 % CI of control group were defined as low responders. Individuals with training response beyond 1 SD from the mean of the training group were defined as high responders. Black bars denote responses of men, while grey bars denote responses of women
When taking into account the results of non-training controls (the upper 95 % CI; 5.0 %), 19 from a total of 283 subjects (6.7 %) were defined as low responders for muscle strength gains. From the total group of 283 subjects, the response of 39 subjects (13.8 %) were greater than 1 SD (>32.60 %) from the mean of the training group and were defined as high responders.
Muscle size
The range of the relative changes in muscle size of trainees varied from −7 to 14 % (mean ± SD: 2.0 ± 3.6 %, n = 95), from −11 to 27 % (5.3 ± 5.7 %, n = 135), from −9 to 27 % (6.2 ± 7.4 %, n = 37), and from 2 to 30 % (11.6 ± 7.8 %, n = 20) when analyzed by DXA, MRI, US CSA, and US muscle thickness, respectively. In the control group, the relative changes in muscle size varied from −8 to 7 % (−0.4 ± 3.1 %, n = 53), from −4 to 4 % (−1.2 ± 2.7 %, n = 7), and from −11 to 17 % (5.4 ± 8.2 %, n = 12) when analyzed by DXA, MRI, and US muscle thickness, respectively.
Muscle size increased during the RT significantly (p < 0.05) in men (5.1 ± 5.9 %) and in women (4.2 ± 6.3 %) with no differences between men and women (p = 0.21). In 71 % of the whole group of trainees, the gains in muscle size were between 0 and 10 % (Fig. 2b). During RT, muscle size increased by 5.5 ± 5.6 %, 5.2 ± 5.7 %, and 4.7 ± 6.4 % in men and by 3.3 ± 8.6 %, 4.9 ± 4.6 %, and 4.1 ± 5.9 % in women in the age groups of below 45 years, 45–60 years, and over 65 years, respectively (all significant p < 0.05, except women below 45 years.) (Fig. 3b). No statistically significant interactions were observed between sex group (F1, 281 = 2.772; p = 0.097), age group (F2, 281 = 0.758; p = 0.470), or sex × age group (F2, 281 = 1.191; p = 0.305) and muscle size responses to RT. The relative change in muscle size varied from −11 to 30 % in the training group and from −11 to 17 % in the control group (Fig. 4b).
When taking into account the results of non-training controls (the upper 95 % CI; 1.6 %), 84 from 287 subjects (29.3 %) were defined as low responders for muscle size gains. In total, 39 subjects from 287 (14.6 %) showed a 0 % or negative response to RT. From the total group of 287 training subjects, the response of 35 subjects (12.2 %) were greater than 1 SD (>10.85 %) from the mean of the training group and were defined as high responders. In total, 9 subjects (3.2 %) were defined as low responders for both muscle size and strength. The linear regression analysis and principal component analysis revealed that none of the determinants investigated (age, sex, and baseline and training-induced changes in BMI, 1RM, and 1RM/body mass ratio) predict RT-induced change in muscle size.
Relationship between responses in muscle size and strength
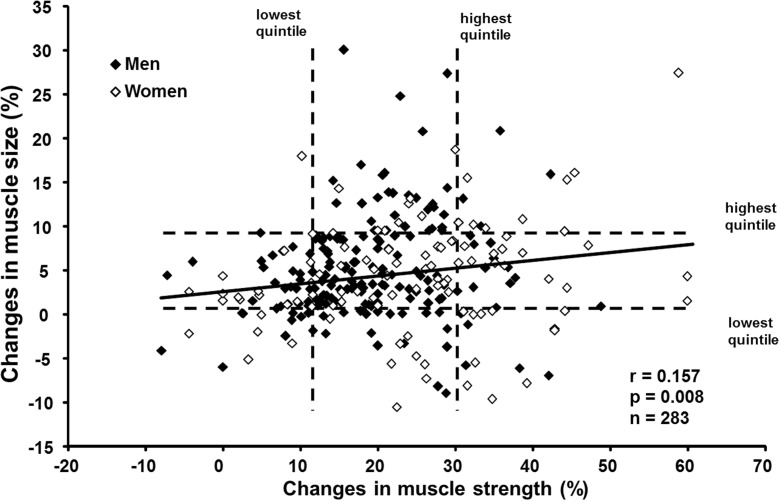
In the training group, relative RT-induced changes in muscle size and strength correlated at a statistically significant level (r = 0.157, p < 0.01) (Fig. 5). To further compare individual RT-induced changes in muscle size and strength, the training responses were divided into quintiles. Overall, 15 subjects (5 men, 10 women) were in the highest quintile in both muscle size (>9.1 %) and strength (>30.3 %) responses. In total, 15 subjects (10 men, 5 women) were in the lowest quintile in both muscle size (<0.6 %) and strength (<11.7 %) responses, and 6 of them (4 men, 2 women; i.e., 2.1 % of the total group of subjects) were defined as low responders for both muscle size and strength. Two subjects (1 man, 1 woman) responded negatively in both muscle size and strength.
Fig. 5.
The association between the relative changes in muscle size and strength in the training group. Dashed lines represent the lowest and highest quintiles in changes of muscle size and strength
Discussion
The present study quantified the continuum of heterogeneity of muscle size and strength responses of healthy and previously non-strength-trained men and women to long-term RT using a pooled data of several earlier research projects conducted in our laboratory. The novel aspect of the present study was to utilize the data of non-trained peers in determination of low responders to RT. The present findings demonstrate that the response to the same RT stimulus varies noticeably between previously untrained men and women at different ages. However, the present RT-induced responses in muscle size and strength did not differ between men and women or between the age groups. The present study further established that in some subjects, exceptionally large training responses were observed. On the other hand, approximately 30 and 7 % of the subjects showed only minor gains or no gains at all in muscle size and strength, respectively, although they had performed the same RT program. Most of the subjects were not, however, low or high responders in both muscle size and strength as highlighted in Fig. 5.
There are several strengths of this study. One of the strengths was the relatively large group of non-training control subjects to which trainees can be compared. This allows us to take into account measurement accuracy, as well as biological and methodological (e.g., unintended lifestyle changes and learning effect due to intervention) variability, in current measures. Second, the present RT program was in line with the ACSM position stand recommendations of RT programming for healthy adults (Garber et al. 2011). Third, the RT program was almost identical in all studies, and fourth, all subjects were healthy volunteers, Caucasian, and lived in the same geographical area. Moreover, the subjects did not have previous experience in RT before the intervention; all training sessions were supervised, and compliance and adherence to RT were very high. Thereby, including separate studies in this investigation was justifiable.
The RT program focused on lower extremity muscles since the role of these muscles is important in daily ambulatory activities, such as walking and stair climbing, particularly in the aged population. Moreover, the measurements of muscle size and strength gains were obtained from the lower extremity muscles. It should be noted that the present RT program was not designed only to maximize gains in size and strength of leg muscles but also to improve general muscle strength in all main muscle groups of the body. Thereby, local muscle endurance and muscle power exercises were included in the program to a considerable extent. Thus, the aim of the present RT program was to improve muscular fitness for health and well-being of previously untrained men and women at different ages with the intention that it is feasible to continue following the experimental period as a part of healthy lifestyle. Nevertheless, considerable gains in muscle size and strength were achieved in leg muscles during the present chronic RT in the majority, but not in all subjects.
A very large variation (from −8 to 60 %) was observed in maximal muscle strength gain in the present trainees with no actual differences between men and women. Similar inter-individual variation has been observed also previously in muscle strength responses to RT (Erskine et al. 2010). Additionally, aging did not influence the present changes in muscle strength although a sarcopenic phenotype (i.e., lower muscle strength per body mass, see Table 1) was observed in older subjects. The data of non-training controls revealed that considerable periodical variation occurs in maximal muscle strength in untrained subjects. This variation may be due to day-to-day differences in the capacity to produce maximal neuromuscular performance, psychological confounds, and/or a seasonal variations in daily physical activities. Interestingly, despite systematic RT, almost 7 % of the subjects did not demonstrate gains in maximal muscle strength during the present 5–6-month training period when taking into account the “normal” variation in maximal muscle strength in non-training controls. As expected, a statistically significant correlation was observed between the changes in muscle size and strength. However, the association was low and muscle size changes appear to minimally explain changes in maximal muscle strength. Thus, maximal muscle strength gains may be mediated largely by adaptations in the nervous system during the first months of RT. Indeed, this is supported by previous findings in the part of the present subjects showing significant RT-induced increases in the m.vastus lateralis and/or medialis electromyography activity (Häkkinen et al. 1998; Häkkinen et al. 2000a, b; Häkkinen et al. 2001a, b; Holviala et al. 2010; Karavirta et al. 2011a; Mikkola et al. 2012; Holviala et al. 2012; Walker et al. 2014). Interestingly, some of the present subjects respond poorly in hypertrophy (i.e., ranked to lowest quintile in muscle size changes in Fig. 5) but respond well in strength (highest quintile in muscle strength changes) indicating that they had exceptionally high contributions of neural adaptations in RT-induced muscle strength gains. It should be noted, however, that present measures of muscle size do not take into account possible architectural changes of trained muscles that may also have effect on muscle size–strength relationship. According to the present data, only ~2 % of the subjects were defined as low responders for both muscle size and strength. The majority of the low responders for muscle size or strength were, therefore, considered a responder for the other trait. Although not yet scientifically proven, it might be that RT-induced gains in muscle mass and/or strength and improvements in some health factors (insulin sensitivity, blood pressure, blood lipids, etc.) are not causally linked between each other. Thus, it is likely that actually all individuals may get some benefit from RT and it can be recommended also to individuals whose muscular characteristics somehow respond poorly to RT.
The observed variation in changes of muscle size due to RT was large, ranging from −10.6 to 30.0 % with a mean response of 4.8 %. Also, other studies have found a similar range of changes in muscle size due to RT (Phillips et al. 2013). Muscle size responses to RT were similar between the sex and age groups. It seems that, at least in the initial phase of RT, muscle size responses to the present type of 2 days-a-week RT program is practically similar in men and women of different ages. In addition, age, sex, body composition, and baseline muscle strength or its response to training did not predict muscle size response to RT in the present study. It should be noted that the degree of variation in muscle size in the control group during the intervention period was also large. This finding indicates that muscle size can normally change, or fluctuate over time, probably due to temporal variations in daily physical activity levels and nutritional status. When these were taken into account, approximately 30 % of the present trainees were low responders for RT-induced muscle size gains. As indicated by Fig. 3, age and sex distribution in these subjects are more or less the same. Contrary to what might have been expected, these findings clearly demonstrate that some individuals do not respond to the present type of RT. It is not known, however, whether the present low responders may have expressed a better training response if an alternative training design had been adopted (e.g., longer training period, whole-body training vs. split-body training program, different recovery period between the training sessions, or different training periodization).
Several aspects may potentially have an effect on RT-induced muscular responses. Firstly, nutrition may play a significant role in the amount of an individual’s muscle size, as well as its adaptation to RT. In some of the experiments within this investigation, dietary intake of the subjects was registered by dietary diaries and analyzed for overall nutritional status and daily macronutrient intake. The mean protein intake per body mass varied in younger subjects between 1.3 and 1.7 g/kg/day (Hulmi et al. 2009; Ahtiainen et al. 2011; Mero et al. 2013) and in middle-aged and older subjects between 0.9 and 1.3 g/kg/day (Sallinen et al. 2007; Hulmi et al. 2009; Ahtiainen et al. 2009; Sillanpää et al. 2010; Ahtiainen et al. 2011; Mero et al. 2013). Hence, the protein intake in the majority of these subjects were above the recommended dietary allowance (RDA) of 0.8 g/kg/day and, therefore, adequate to meet the requirements for RT-induced muscular adaptations also in older adults (Campbell and Leidy 2007). Moreover, the habitual dietary intake, when adequate, appears not to be a major determinant of muscle growth during the initial months of RT in previously untrained individuals (Thalacker-Mercer et al. 2009). Secondly, physical activity other than assigned for the present RT may have affected muscular responses. During the intervention period, the subjects continued taking part in low-volume and intensity daily physical activities, such as a short commute by walking or biking in a manner similar to what they were accustomed to before the study. However, the daily physical activity was not determined objectively in the studies included in this investigation. Although the effects of nutrition and daily physical activity on the present results cannot be ruled out; they likely play only a minor role in the present findings of heterogeneous muscle size and strength responses to RT.
The reasons for inter-individual variations in adaptations of muscle size to RT are still poorly known. In previous studies with healthy human subjects, e.g., several genetic variations (Riechman et al. 2004; Devaney et al. 2009; Walsh et al. 2012; Van Deveire et al. 2012), differences in skeletal muscle gene (Bamman et al. 2007; Raue et al. 2012; Phillips et al. 2013) and microRNA expression (Davidsen et al. 2011), phosphorylation status of signaling proteins (Mayhew et al. 2011; Mitchell et al. 2013), androgen receptor concentrations (Ahtiainen et al. 2011; Mitchell et al. 2013), and satellite cell count (Petrella et al. 2008) have been suggested to segregate high and low responders to RT-induced muscle hypertrophy. The physiological aspects of individual variation in phenotype responses to RT are apparently very complex phenomena and more studies specifically focused on high and low responders are required to reveal unambiguously the mechanisms of individual differences in RT-induced adaptations.
A main limitation of the present investigation was that different methods were applied to the analyses of muscle size changes. Therefore, only relative pre- to post-training changes were applicable for the analyses of training-induced responses and group differences. It should be also recognized that the magnitude of the relative changes in muscle size may differ to a minor degree between analyzing methods utilized in the present study. However, all methods to assess muscle size gave approximately similar ranges of responses during the intervention and demonstrate inter-individual adaptability to RT. Therefore, these methods are considered to be comparable between each other enabling present data pooling and retrospective analyses. It should be also noted that current findings can be generalized only to lower extremity muscles and to the present type of RT in healthy men and women of different ages who have not engaged to RT previously.
Conclusions
The aim of the present RT program was to improve muscular fitness for health and well-being of previously untrained men and women of different ages. The present RT resulted in approximately 5 % mean increase in muscle size and 21 % mean increase in muscle strength. However, considerable inter-individual variation was observed in both muscle size and strength adaptations. While in some subjects exceptionally large training-induced adaptations were observed, nearly 30 % of the subjects were low responders in muscle size and almost 7 % were low responders in muscle strength. However, only 9 from 283 subjects were low responders for both muscle size and strength and, thus, it is likely that nearly all individuals will get some benefit from RT. The present study further showed that heterogeneity of muscle size and strength responses were similar between men and women and subjects at different ages. Inter-individual variation in RT responses should be acknowledged and taken account when designing RT programs and interpretation of RT outcomes by trainers. Since all individuals will not obtain equal benefits of RT, the present recommendations for health-related muscle-strengthening activities should be reconsidered. In some individuals, training-induced improvements in muscular characteristics may be elicited with different training dosage (i.e., training volume, intensity, and/or frequency) than typically generalized in physical activity recommendations (Oja and Titze 2011). Also, the underpinning physiological determinants of individual training response to RT are not yet well known and require further studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 641 kb)
(DOC 19.4 kb)
(PDF 20 kb)
Acknowledgments
Studies included in the present investigation were funded by the following foundations: The Finnish Ministry of Culture and Education; Finnish Cultural Foundation; Ellen and Artturi Nyyssönen Foundation, Finland; Polar Electro Oy; Juho Vainio Foundation, Finland; Central Finland Health Care District, Jyväskylä, Finland; Sport Institute Foundation, Finland; Yrjö Jahnsson Foundation, Finland; and National Doctoral Programme of Musculoskeletal Disorders and Biomaterials, Finland.
Compliance with ethical standards
Subjects were carefully informed about the design of the study with special information on possible risks and benefits both verbally and in writing, and they signed a written consent form before participation in the study. The studies were conducted according to the Declaration of Helsinki and were approved by the Ethics Committee of the University of Jyväskylä, Finland, and/or by the Ethics Committee of the Central Finland Health Care District.
Conflict of interest
The authors declare that they have no competing interests.
References
- Ahtiainen JP, Hulmi JJ, Kraemer WJ, Lehti M, Pakarinen A, Mero AA, Karavirta L, Sillanpää E, Selänne H, Alen M, et al. Strength, [corrected] endurance or combined training elicit diverse skeletal muscle myosin heavy chain isoform proportion but unaltered androgen receptor concentration in older men. Int J Sports Med. 2009;30:879–887. doi: 10.1055/s-0029-1238290. [DOI] [PubMed] [Google Scholar]
- Ahtiainen JP, Hulmi JJ, Kraemer WJ, Lehti M, Nyman K, Selänne H, Alen M, Pakarinen A, Komulainen J, Kovanen V, et al. Heavy resistance exercise training and skeletal muscle androgen receptor expression in younger and older men. Steroids. 2011;76:183–192. doi: 10.1016/j.steroids.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Bamman MM, Petrella JK, Kim JS, Mayhew DL, Cross JM. Cluster analysis tests the importance of myogenic gene expression during myofiber hypertrophy in humans. J Appl Physiol (1985) 2007;102:2232–2239. doi: 10.1152/japplphysiol.00024.2007. [DOI] [PubMed] [Google Scholar]
- Campbell WW, Leidy HJ. Dietary protein and resistance training effects on muscle and body composition in older persons. J Am Coll Nutr. 2007;26:696S–703S. doi: 10.1080/07315724.2007.10719650. [DOI] [PubMed] [Google Scholar]
- Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F, Helge JW, Timmons JA, Phillips SM. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol (1985) 2011;110:309–317. doi: 10.1152/japplphysiol.00901.2010. [DOI] [PubMed] [Google Scholar]
- Devaney JM, Tosi LL, Fritz DT, Gordish-Dressman HA, Jiang S, Orkunoglu-Suer FE, Gordon AH, Harmon BT, Thompson PD, Clarkson PM, et al. Differences in fat and muscle mass associated with a functional human polymorphism in a post-transcriptional BMP2 gene regulatory element. J Cell Biochem. 2009;107:1073–1082. doi: 10.1002/jcb.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine RM, Jones DA, Williams AG, Stewart CE, Degens H. Inter-individual variability in the adaptation of human muscle specific tension to progressive resistance training. Eur J Appl Physiol. 2010;110:1117–1125. doi: 10.1007/s00421-010-1601-9. [DOI] [PubMed] [Google Scholar]
- Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP, American College of Sports Medicine American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- Häkkinen K, Kallinen M, Izquierdo M, Jokelainen K, Lassila H, Mälkiä E, Kraemer WJ, Newton RU, Alen M. Changes in agonist-antagonist EMG, muscle CSA, and force during strength training in middle-aged and older people. J Appl Physiol (1985) 1998;84:1341–1349. doi: 10.1152/jappl.1998.84.4.1341. [DOI] [PubMed] [Google Scholar]
- Häkkinen K, Pakarinen A, Kraemer WJ, Häkkinen A, Valkeinen H, Alen M. Selective muscle hypertrophy, changes in EMG and force, and serum hormones during strength training in older women. J Appl Physiol 1985. 2001;91:569–580. doi: 10.1152/jappl.2001.91.2.569. [DOI] [PubMed] [Google Scholar]
- Häkkinen K, Alen M, Kallinen M, Newton RU, Kraemer WJ. Neuromuscular adaptation during prolonged strength training, detraining and re-strength-training in middle-aged and elderly people. Eur J Appl Physiol. 2000;83:51–62. doi: 10.1007/s004210000248. [DOI] [PubMed] [Google Scholar]
- Häkkinen K, Pakarinen A, Kraemer WJ, Newton RU, Alen M. Basal concentrations and acute responses of serum hormones and strength development during heavy resistance training in middle-aged and elderly men and women. J Gerontol A Biol Sci Med Sci. 2000;55:B95–B105. doi: 10.1093/gerona/55.2.B95. [DOI] [PubMed] [Google Scholar]
- Häkkinen K, Kraemer WJ, Newton RU, Alen M. Changes in electromyographic activity, muscle fibre and force production characteristics during heavy resistance/power strength training in middle-aged and older men and women. Acta Physiol Scand. 2001;171:51–62. doi: 10.1046/j.1365-201X.2001.00781.x. [DOI] [PubMed] [Google Scholar]
- Holviala JH, Sallinen JM, Kraemer WJ, Alen MJ, Häkkinen KK. Effects of strength training on muscle strength characteristics, functional capabilities, and balance in middle-aged and older women. J Strength Cond Res. 2006;20:336–344. doi: 10.1519/R-17885.1. [DOI] [PubMed] [Google Scholar]
- Holviala J, Häkkinen A, Karavirta L, Nyman K, Izquierdo M, Gorostiaga EM, Avela J, Korhonen J, Knuutila VP, Kraemer WJ, et al. Effects of combined strength and endurance training on treadmill load carrying walking performance in aging men. J Strength Cond Res. 2010;24:1584–1595. doi: 10.1519/JSC.0b013e3181dba178. [DOI] [PubMed] [Google Scholar]
- Holviala J, Kraemer WJ, Sillanpää E, Karppinen H, Avela J, Kauhanen A, Häkkinen A, Häkkinen K. Effects of strength, endurance and combined training on muscle strength, walking speed and dynamic balance in aging men. Eur J Appl Physiol. 2012;112:1335–1347. doi: 10.1007/s00421-011-2089-7. [DOI] [PubMed] [Google Scholar]
- Hopkins WG. Measures of reliability in sports medicine and science. Sports Med. 2000;30:1–15. doi: 10.2165/00007256-200030010-00001. [DOI] [PubMed] [Google Scholar]
- Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, et al. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc. 2005;37:964–972. doi: 10.1097/00005768-200505001-00881. [DOI] [PubMed] [Google Scholar]
- Hulmi JJ, Kovanen V, Selänne H, Kraemer WJ, Häkkinen K, Mero AA. Acute and long-term effects of resistance exercise with or without protein ingestion on muscle hypertrophy and gene expression. Amino Acids. 2009;37:297–308. doi: 10.1007/s00726-008-0150-6. [DOI] [PubMed] [Google Scholar]
- Karavirta L, Häkkinen A, Sillanpää E, Garcia-Lopez D, Kauhanen A, Haapasaari A, Alen M, Pakarinen A, Kraemer WJ, Izquierdo M, et al. Effects of combined endurance and strength training on muscle strength, power and hypertrophy in 40-67-year-old men. Scand J Med Sci Sports. 2011;21:402–411. doi: 10.1111/j.1600-0838.2009.01059.x. [DOI] [PubMed] [Google Scholar]
- Karavirta L, Häkkinen K, Kauhanen A, Arija-Blazquez A, Sillanpää E, Rinkinen N, Häkkinen A. Individual responses to combined endurance and strength training in older adults. Med Sci Sports Exerc. 2011;43:484–490. doi: 10.1249/MSS.0b013e3181f1bf0d. [DOI] [PubMed] [Google Scholar]
- Mann S, Beedie C, Balducci S, Zanuso S, Allgrove J, Bertiato F, Jimenez A. Changes in insulin sensitivity in response to different modalities of exercise: a review of the evidence. Diabetes Metab Res Rev. 2014;30:257–268. doi: 10.1002/dmrr.2488. [DOI] [PubMed] [Google Scholar]
- Mayhew DL, Hornberger TA, Lincoln HC, Bamman MM. Eukaryotic initiation factor 2B epsilon induces cap-dependent translation and skeletal muscle hypertrophy. J Physiol. 2011;589:3023–3037. doi: 10.1113/jphysiol.2010.202432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mero AA, Hulmi JJ, Salmijärvi H, Katajavuori M, Haverinen M, Holviala J, Ridanpää T, Häkkinen K, Kovanen V, Ahtiainen JP, et al. Resistance training induced increase in muscle fiber size in young and older men. Eur J Appl Physiol. 2013;113:641–650. doi: 10.1007/s00421-012-2466-x. [DOI] [PubMed] [Google Scholar]
- Mikkola J, Rusko H, Izquierdo M, Gorostiaga EM, Häkkinen K. Neuromuscular and cardiovascular adaptations during concurrent strength and endurance training in untrained men. Int J Sports Med. 2012;33:702–710. doi: 10.1055/s-0031-1295475. [DOI] [PubMed] [Google Scholar]
- Mitchell CJ, Churchward-Venne TA, Bellamy L, Parise G, Baker SK, Phillips SM. Muscular and systemic correlates of resistance training-induced muscle hypertrophy. PLoS One. 2013;8:e78636. doi: 10.1371/journal.pone.0078636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oja P, Titze S. Physical activity recommendations for public health: development and policy context. EPMA J. 2011;2:253–259. doi: 10.1007/s13167-011-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrella JK, Kim JS, Mayhew DL, Cross JM, Bamman MM. Potent myofiber hypertrophy during resistance training in humans is associated with satellite cell-mediated myonuclear addition: a cluster analysis. J Appl Physiol (1985) 2008;104:1736–1742. doi: 10.1152/japplphysiol.01215.2007. [DOI] [PubMed] [Google Scholar]
- Phillips BE, Williams JP, Gustafsson T, Bouchard C, Rankinen T, Knudsen S, Smith K, Timmons JA, Atherton PJ. Molecular networks of human muscle adaptation to exercise and age. PLoS Genet. 2013;9:e1003389. doi: 10.1371/journal.pgen.1003389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC, Trappe S. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol (1985) 2012;112:1625–1636. doi: 10.1152/japplphysiol.00435.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riechman SE, Balasekaran G, Roth SM, Ferrell RE. Association of interleukin-15 protein and interleukin-15 receptor genetic variation with resistance exercise training responses. J Appl Physiol (1985) 2004;97:2214–2219. doi: 10.1152/japplphysiol.00491.2004. [DOI] [PubMed] [Google Scholar]
- Sallinen J, Pakarinen A, Fogelholm M, Alen M, Volek JS, Kraemer WJ, Häkkinen K. Dietary intake, serum hormones, muscle mass and strength during strength training in 49–73-year-old men. Int J Sports Med. 2007;28:1070–1076. doi: 10.1055/s-2007-965003. [DOI] [PubMed] [Google Scholar]
- Sillanpää E, Häkkinen A, Nyman K, Mattila M, Cheng S, Karavirta L, Laaksonen DE, Huuhka N, Kraemer WJ, Häkkinen K. Body composition and fitness during strength and/or endurance training in older men. Med Sci Sports Exerc. 2008;40:950–958. doi: 10.1249/MSS.0b013e318165c854. [DOI] [PubMed] [Google Scholar]
- Sillanpää E, Häkkinen A, Laaksonen DE, Karavirta L, Kraemer WJ, Häkkinen K. Serum basal hormone concentrations, nutrition and physical fitness during strength and/or endurance training in 39-64-year-old women. Int J Sports Med. 2010;31:110–117. doi: 10.1055/s-0029-1242811. [DOI] [PubMed] [Google Scholar]
- Sipilä S, Suominen H. Ultrasound imaging of the quadriceps muscle in elderly athletes and untrained men. Muscle Nerve. 1991;14:527–533. doi: 10.1002/mus.880140607. [DOI] [PubMed] [Google Scholar]
- Thalacker-Mercer AE, Petrella JK, Bamman MM. Does habitual dietary intake influence myofiber hypertrophy in response to resistance training? A cluster analysis. Appl Physiol Nutr Metab. 2009;34:632–639. doi: 10.1139/H09-038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpka S, Petersson IF, Zhou C, Englund M. Muscle strength in adolescent men and risk of cardiovascular disease events and mortality in middle age: a prospective cohort study. BMC Med. 2014;12:62. doi: 10.1186/1741-7015-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Deveire KN, Scranton SK, Kostek MA, Angelopoulos TJ, Clarkson PM, Gordon PM, Moyna NM, Visich PS, Zoeller RF, Thompson PD, et al. Variants of the ankyrin repeat domain 6 gene (ANKRD6) and muscle and physical activity phenotypes among European-derived American adults. J Strength Cond Res. 2012;26:1740–1748. doi: 10.1519/JSC.0b013e31825c2bef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker S, Peltonen H, Sautel J, Scaramella C, Kraemer WJ, Avela J, Häkkinen K. Neuromuscular adaptations to constant vs. variable resistance training in older men. Int J Sports Med. 2014;35:69–74. doi: 10.1055/s-0033-1343404. [DOI] [PubMed] [Google Scholar]
- Walker S, Peltonen H, Hakkinen K. Medium-intensity, high-volume “hypertrophic” resistance training did not induce improvements in rapid force production in healthy older men. Age (Dordr) 2015;37:9786. doi: 10.1007/s11357-015-9786-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh S, Haddad CJ, Kostek MA, Angelopoulos TJ, Clarkson PM, Gordon PM, Moyna NM, Visich PS, Zoeller RF, Seip RL, et al. Leptin and leptin receptor genetic variants associate with habitual physical activity and the arm body composition response to resistance training. Gene. 2012;510:66–70. doi: 10.1016/j.gene.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westcott WL. Resistance training is medicine: effects of strength training on health. Curr Sports Med Rep. 2012;11:209–216. doi: 10.1249/JSR.0b013e31825dabb8. [DOI] [PubMed] [Google Scholar]
- Xu L, Nicholson P, Wang QJ, Wang Q, Alen M, Cheng S. Fat mass accumulation compromises bone adaptation to load in Finnish women: a cross-sectional study spanning three generations. J Bone Miner Res. 2010;25:2341–2349. doi: 10.1002/jbmr.136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 641 kb)
(DOC 19.4 kb)
(PDF 20 kb)