Abstract
Sarcopenia is defined as age-related lean tissue mass (LTM) loss resulting in reduced muscular strength, physical function, and mobility. Up to 33 % of older adults currently are sarcopenic, with likely many more undiagnosed. The purpose of this investigation was to predict sarcopenia status from easily accessible functional measures of community-dwelling older adults. Forty-three community-dwelling older adults (n = 32 females and n = 11 males) participated in the present investigation. Inclusion criteria included ≥65 years of age, mini-mental state examination score ≥24, and no falls within previous 12 months. All subjects completed their appendicular skeletal mass (ASM) assessment via dual-energy X-ray absorptiometry (DXA) and were categorized as either sarcopenic or non-sarcopenic. Physical assessments included 10-m usual walk, hand-grip (HG) strength, 6-min walk, 8-ft up-and-go, 30-s chair stand, 30-s arm curl, and sit-to-stand muscular power. A forward, stepwise multiple regression analysis revealed that age, sex, weight, height, 10-m walk, HG, and sit-to-stand muscular power account for 96.1 % of the variance in ASM. The area under the curve was 0.92 for correctly identifying sarcopenic participants compared to their actual classification. This is the first prediction model used to identify sarcopenia based on parameters of demographic and functional fitness measures in community-dwelling older adults. The ability to accurately identify sarcopenia in older adults is imperative to their quality of life and ability to perform activities of daily living.
Keywords: Lean tissue mass, Sarcopenia, Physical function
Introduction
In the USA, there are 40 million older adults with an expected increase of more than twofold by 2050 (Centers for Disease Control and Prevention 2013). With this anticipated rise in the older adult population also comes an increased burden on the health-care system. It is estimated that older adults contribute $555 billion to annual health-care costs (Centers for Disease Control and Prevention 2013) with nearly $19 billion directly related to declining lean tissue mass (LTM) (Janssen et al. 2004). Termed sarcopenia, age-related LTM reductions affect approximately 24 % of adults over 65 years with the prevalence reaching 50 % after 80 (Baumgartner et al. 1998). LTM declines as much as 1.0 % per year after 20 years and accelerates more drastically after age 50 (Doherty 2003). While total-body LTM loss is detrimental to the health of older adults, the more important are the reductions in appendicular skeletal mass (ASM) (Visser et al. 2000, 2002). A loss of ASM is attributed to reduced muscular strength (Goodpaster et al. 2006), functional capacity, and ability to perform activities of daily living (ADLs) (Janssen et al. 2002).
Reduced LTM may remain undetected due to a lack of accessibility to body composition assessment. Detecting sarcopenia requires a reliable method of LTM determination such as a dual-energy X-ray absorptiometry (DXA) or computed topography (CT). These types of equipment are not widely available in clinics, nor commonly used as LTM assessment when performed in health-care settings (Cesari et al. 2012). Thus, other sarcopenia classification measures are needed in the absence of DXA (or CT) to assess LTM. Prediction models exist that estimate LTM from functional parameters; however, these existing models use bioelectrical impedance analysis (BIA) to quantify LTM (Campbell and Vallis 2014; Ishii et al. 2014). BIA algorithms are dependent on many factors that are more variable among older adults when compared to younger populations (Batsis et al. 2013). Compared to DXA, LTM differs up to 24 % when measured with BIA (Kim and Kim 2013; Kyle et al. 2003; Sergi et al. 2014). In addition, BIA reports only total-body LTM and not appendicular LTM, a more important factor when performing ADLs (Visser et al. 2000, 2002). Thus, prediction models using LTM ascertained from DXA will potentially be more precise and reduce error when classifying sarcopenic status. Therefore, the primary aim of this investigation was to determine which assessments most closely predicted low lean mass among community-dwelling older adults by measuring LTM via DXA.
Methods
Study population
Subjects were recruited from a retirement community via advertisements, flyers, and word of mouth. Preliminary screening included physician clearance, mini-mental state examination (MMSE) (Cockrell and Folstein 2002), and health history questionnaire (HHQ). Participant inclusion criteria included ≥65 years of age, MMSE score ≥24, no self-reported falls within the previous 12 months, and absence of unstable or unmanaged cardiovascular disease, diabetes, or hypertension. This project was approved by the Institutional Review Board of Land-Grant Midwest University, and informed consent was obtained from all interested participants prior to any physical assessments.
Body composition assessment
Height and body weight were measured using a Detecto Physician’s Scale (Webb City, MO); measures were recorded to the nearest 0.1 cm and 0.1 kg, respectively. Body mass index (BMI) was calculated as the body mass (kg) divided by the height (m2). Dual energy X-ray absorptiometry (DXA; GE, Medical Systems, Madison, WI) was used to assess total-body composition, including lean tissue and fat mass. For consistency, all scans were analyzed by the same investigator. Appendicular skeletal mass (ASM) was calculated using the sum of LTM from the arms and legs. ASM was expressed relative to BMI in order to account for body mass (ASM/BMI) and was used to determine sarcopenic status. Sarcopenia was defined as <0.512 and 0.789 for women and men, respectively (Studenski et al. 2014). This value was chosen due to its strong association with mortality among older adults (Cheung et al. 2016).
Physical parameters
Muscular power
Lower body muscular power was assessed using the Tendo Weightlifting Analyzer (Slovak Republic) (Glenn et al. 2015). Each participant sat in a standard chair (seat height = 0.43 m) with the Tendo attached to their waist. With their arms crossed over their chest, each participant stood up as quickly as possible. Five chair stand trials were completed with 60-s rest between each trial. The average and peak muscular power for each stand was recorded. The average of the trials was computed and used in the analyses. Relative muscular power was calculated for each individual (W/kg) to account for differences in body weight. Chair stand power is a valid and reliable measure of lower-body muscular power assessment for older adults (Gray and Paulson 2014).
Functional assessments
Parameters of functional fitness were assessed using arm curl (upper-body strength), chair stand (CS; lower-body strength), 8-ft up-and-go (UPGO; agility), and 6-min walk (6-MW; aerobic fitness). Each test was completed based on the previously established protocols by Rikli and Jones (2001). The assessments have high previously established test-retest reliability (0.93–0.97) for all measures (Hesseberg et al. 2015). The 10-m walk is a reliable (Adell et al. 2013) and valid assessment of functional fitness (Peters et al. 2013) among community-dwelling older adults. Walking velocity was performed over a 20-m total distance in a well-lit hallway. Each participant began at a cone placed 5 m behind the first timing gate (Lafayette Instruments, Lafayette, IN) and was instructed to walk to a second cone positioned 5 m beyond the second timing gate. The middle 10 m was used in the analyses to calculate gait velocity. The 5 m distance at each end guarded against potential acceleration and deceleration associated with the assessment. For walking speed conditions, participants were instructed to walk the entire 20 m distance at their usual pace or as quickly, but as safely possible for the habitual and maximal walking speeds, respectively. For each condition, two trials were completed and averaged. Ten-meter usual walking speed reliability is 0.93 (Peters et al. 2013); test-retest reliability is 0.86 for maximum walking speed (Adell et al. 2013).
Muscular strength
Hand-grip (HG) testing determined muscular strength (Lauretani et al. 2003). All HG measurements were administered by a trained technician and measured in kilograms using a handheld, digital grip strength dynamometer (Takei Scientific Instruments, Niigata City, Japan). All measurements were performed on the preferred hand with the subject standing, arm down at the side, wrist in neutral position, and interphalangeal joint of the index finger maintained at 90°. Participants maximally squeezed the handle for a minimum of 3 s; standard encouragement was provided. The test was repeated three times on the preferred hand with 60-s rest between trials. The greatest of the three trials was used as the final strength measurement. High test-retest reliability for the HG strength test has been previously recorded (ICC = 0.95) (Bohannon and Schaubert 2005).
Data analysis
Values are presented as mean ± SD. A one-way ANOVA was performed to determine differences between sexes. Potential multicollinearity issues between the predictor variables were detected using variance inflation factor (VIF). Multicollinearity (>10) between average and peak muscular power was detected; thus, average muscular power was removed from the analysis (due to peak muscular power’s greater association with lean mass). Forward, stepwise multiple regression analysis was conducted on the prediction group by adding one variable at a time to the model, provided the F statistic was significant (entry criteria α = 0.05). The predictors included in the analysis are as follows: sex, age, height, body weight, HG, 10-m maximal walk, and peak muscular power, and these variables were utilized to develop prediction equations for ASM. The forward stepwise multiple regression analysis was also performed using the following variables: sex, age, height, body weight, HG, and 10-m maximal walk. Using the predicted ASM values, ASM (ASM/BMI) was computed. Differences in measured and predicted ASM/BMI were determined using a paired-samples t test and a Pearson correlation coefficient to determine the strength of the association between these two variables. A receiver operating characteristic (ROC) analysis assessed the area under the curve and determined the prediction model’s ability to correctly discriminate sarcopenia status along with sensitivity and specificity. All analyses were computed using Statistical Package for Social Sciences (SPSS, version 22, Chicago, IL).
Results
Table 1 includes the group characteristics for all independent variables. Based on the definition by Studenski et al. (2014), 10 % of the current samples had muscle mass (ASM/BMI) below the reference population and were characterized as sarcopenic. Statistical differences between the two groups were noted for hand-grip strength (p = 0.04); individuals with sarcopenia had on average 40 % less hand-grip strength compared to their non-sarcopenic counterparts. No other differences existed between sarcopenic groups. Sex differences are presented in Table 2. Men scored higher on all variables with the exception of 10-m maximal walk.
Table 1.
Subject demographic characteristics
| Variables | Total | Sarcopenic | Non-sarcopenic | p value |
|---|---|---|---|---|
| n = 43 | n = 4 | n = 39 | ||
| Age (years) | 77.2 (6.2) | 72.8 (9.4) | 77.8 (5.7) | 0.13 |
| Female (%) | 74 | 100 | 72 | |
| Height (cm) | 165.66 (9.92) | 156.75 (5.06) | 166.58 (9.88) | 0.06 |
| Body weight (kg) | 72.59 (15.81) | 66.93 (7.57) | 73.17 (16.37) | 0.46 |
| BMI (kg/m2) | 26.21 (3.84) | 27.26 (3.23) | 26.10 (3.92) | 0.57 |
| ASM (kg) | 6.45 (1.24) | 5.32 (0.64) | 6.84 (1.15) | 0.06 |
| HG strength (kg) | 25.82 (10.00) | 16.07 (1.24) | 26.82 (9.97) | 0.04 |
| Peak muscular power (W) | 674.35 (211.22) | 464.00 (53.00) | 585.67 (218.34) | 0.28 |
| Maximal walk (s) | 5.70 (1.33) | 5.51 (0.80) | 5.72 (1.38) | 0.77 |
Differences between group was determined by one-way ANOVA; α = 0.05
ASM appendicular skeletal mass, HG hand-grip, BMI body mass index
Table 2.
Sex differences
| Variables | Total | Women | Men | p value |
|---|---|---|---|---|
| n = 43 | n = 32 | n = 11 | ||
| Age (years) | 77.2 (6.2) | 77.5 (6.5) | 76.3 (5.4) | 0.57 |
| Height (cm) | 165.66 (9.92) | 161.14 (5.66) | 178.82 (7.64) | 0.001 |
| Body weight (kg) | 72.59 (15.81) | 66.30 (11.59) | 90.87 (11.76) | 0.001 |
| BMI (kg/m2) | 26.21 (3.84) | 25.46 (3.83) | 28.41 (3.08) | 0.03 |
| ASM/ht2 (kg/m2) | 6.45 (1.24) | 5.92 (0.90) | 7.99 (0.67) | 0.001 |
| HG strength (kg) | 25.82 (10.00) | 21.19 (5.16) | 39.29 (8.34) | 0.001 |
| Peak muscular power (W) | 574.35 (211.22) | 501.31 (175.47) | 864.82 (158.73) | 0.001 |
| Maximal walk (s) | 5.70 (1.33) | 5.80 (1.42) | 5.40 (1.02) | 0.40 |
Differences between group was determined by one-way ANOVA; α = 0.05
ASM appendicular skeletal mass, HG hand-grip, BMI body mass index
For the stepwise multiple regression procedure, seven variables remained in the analysis (Table 3). The regression analysis revealed that 96.1 % of the variance in ASM was accounted for by the following variables: age, sex (0 = male; 1 = female), body weight (kg), height (cm), hand-grip (HG) strength (kg), maximal walking time (s), and peak sit-to-stand muscular power (W) (Eq. 1). The correlation between the estimated ASM and measured ASM is 0.98. When removing lower-body muscular power from the analysis, the remaining six variables accounted for 95.5 % of the variance in ASM (Eq. 2).
| 1 |
| 2 |
Table 3.
Statistical results of the forward stepwise regression prediction model
| Variable | Parameter estimate | Standard error | Β value | p value |
|---|---|---|---|---|
| Intercept | −1.390 | 0.250 | 0.000 | |
| Age (years) | −0.006 | 0.001 | 0.243 | <0.001 |
| Sex | −0.146 | 0.032 | −0.395 | <0.001 |
| Body weight (kg) | −0.006 | 0.001 | −0.617 | <0.001 |
| Height (cm) | 0.012 | 0.001 | −0.757 | <0.001 |
| 10-m max walk (s) | −0.014 | 0.007 | −0.113 | 0.04 |
| Hand-grip strength (kg) | 0.003 | 0.001 | 0.192 | 0.02 |
| Peak muscular power (W) | 0.000 | 0.000 | 0.181 | 0.03 |
Sex = 0 for males and 1 for females
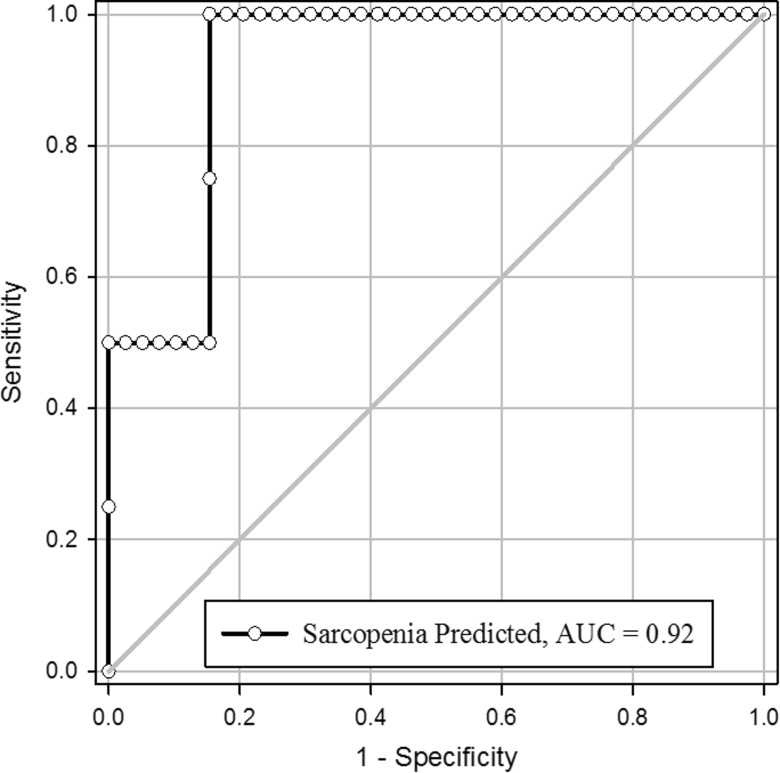
Using the predicted ASM values from the prediction model, we calculated relative appendicular skeletal muscle mass (ASM/BMI). Correlation analysis revealed a strong statistically significant correlation between the measured and predicted values (r = 0.97; Fig. 1). We used the ROC curve to determine the ability of the prediction model to appropriately classify the participants as sarcopenic or non-sarcopenic. The area under the curve (AUC) using this procedure was 0.92 (Fig. 2) with a sensitivity of 50 % and a specificity of 95 %.
Fig. 1.
Correlation analysis between the measured and predicted ASM/BMI
Fig. 2.
ROC analysis for determination of sarcopenia status
Discussion
Low relative muscle mass identification is difficult in many clinics, as valid and reliable LTM measures are costly and often not readily available in many clinics. Thus, it is imperative to determine alternative measures that appropriately estimate LTM leading to accurate sarcopenia classification. Thus, the purpose of the current investigation was to identify alternative measures for estimating ASM to correctly classify sarcopenic status among community-dwelling older adults. In the present investigation, commonly accessible measurements (sex, age, height, body weight, HG strength, and maximal walking time) predicted nearly 96 % of the variance in ASM among community-dwelling older adults. In addition, results from the ROC analysis revealed a near-excellent ability to predict sarcopenia status from the aforementioned variables (Fig. 2).
Currently, models exist that estimate sarcopenia (Campbell and Vallis 2014; Kim and Kim 2013; Kyle et al. 2003; McIntosh et al. 2013; Sergi et al. 2014); however, sarcopenic classification requires an accurate LTM measure, which is difficult to obtain in many clinics. More recently, researchers have attempted to identify alternative measures that adequately predict sarcopenic status using various physical function assessments. However, the only other known prediction models use bioelectrical impedance to estimate LTM (Kim and Kim 2013; Kyle et al. 2003; McIntosh et al. 2013), which is problematic for older adults (Batsis et al. 2013). When assessing LTM, bioelectrical impedance varies from DXA measurements up to 24 % (Kim and Kim 2013; Kyle et al. 2003; Sergi et al. 2014). BIA has been suggested to be a good portable alternative to DXA (Cruz-Jentoft et al. 2010); however, BIA makes several assumptions in its algorithms that increase its variable and decrease its accuracy among older adults (Sillanpää et al. 2014). These inherent issues warrant limited use when more precise alternatives are available. The more important is the ability of an assessment to differentiate between appendicular LTM and total-body LTM (Sergi et al. 2014). In the present investigation, the ROC produced a higher AUC value when compared to other researchers predicting low LTM among older adults. Rossi and colleagues (2014) found that the AUC for their older adult sample was 0.74; however, their body composition method was BIA. Our method produced AUC values of 0.92 with the DXA utilized as the body composition method in addition to using similar functional measures.
In the newest models defining sarcopenia, physical function measures are included in addition to LTM (Cruz-Jentoft et al. 2010; Studenski et al. 2014). In the current investigation, HG strength, walk time, and lower-body muscular power were identified as significant physical ASM contributors, together accounting for more than 96 % of the variance. It should be noted that HG strength alone accounted for nearly 70 % of the variance in ASM, making it a strong independent predictor of ASM. Other investigations have identified HG strength as an influential measure related to physical functioning of older adults (Martin-Ponce et al. 2014), and the newest guidelines have suggested HG be included as an inclusion variable along with LTM assessment (Studenski et al. 2014). However, other investigations have found as little as 22 % of the variance in total-body LTM relative to height accounted for by HG strength (Campbell and Vallis 2014). Thus, further investigations are needed to determine the influence of HG strength on ASM among older adults.
One limitation of the current investigation is the sole use of community-dwelling older adults. While the sarcopenia prevalence was similar to other studies (Baumgartner et al. 1998; Rolland et al. 2008), the majority of the present sample was highly functioning so caution should be used when extrapolating results to lower functioning older adults. Another limiting factor is the majority of the participants were female (74 %). While it is well known that men have higher LTM when compared to women (Baumgartner et al. 1998), sex was used as an individual variable in the prediction equation to control for this potential confounding variable. If more equitable sex groups had been available, the predictors may have changed; therefore, further research should be performed highlighting specific predictors in males. In addition, care should be taken when extrapolating these results to changes in LTM. LTM and physical function change at different rates; therefore, future studies should focus on the strength of the predictor variables with changes in LTM over time. Although the use of DXA to assess LTM is one of the most used methodological tools (Toombs et al. 2012), it does come with inherent limitations. DXA is not a portable device and, thus, must be used in a laboratory setting greatly limiting its use in many clinics. In addition, although low and typically perceived as harmless, DXA uses radiation technology. It is well known that ionizing radiation exposure is additive and could potentially exceed yearly limits (Chen et al. 2010). Finally, the sample size is relatively small, potentially overestimating the prediction values. Thus, validation of this particular prediction model with a larger sample is warranted.
We conclude that by using less expensive and more practical LTM assessment methods, physicians and other health-care professionals will be more inclined to assess sarcopenic status among older adults. Early identification can result in faster treatment and potentially fewer complications that arise from sarcopenia.
References
- Adell E, Wehmohorner S, Rydwik E. The test-retest reliability of 10 meters maximal walking speed in older people living in a residential care unit. J Geriatr Phys Ther. 2013;36:74–77. doi: 10.1519/JPT.0b013e318264b8ed. [DOI] [PubMed] [Google Scholar]
- Batsis JA, Barre LK, Mackenzie TA, Pratt SI, Lopez-Jimenez F, Bartels SJ. Variation in the prevalence of sarcopenia and sarcopenic obesity in older adults associated with different research definitions: dual-energy X-ray absorptiometry data from the National Health and Nutrition Examination Survey 1999–2004. J Am Geriatr Soc. 2013;61:974–980. doi: 10.1111/jgs.12260. [DOI] [PubMed] [Google Scholar]
- Baumgartner RN, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Schaubert KL. Test-retest reliability of grip-strength measures obtained over a 12-week interval from community-dwelling elders. J Hand Ther. 2005;18:426–427. doi: 10.1197/j.jht.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Campbell T, Vallis L. Predicting fat-free mass index and sarcopenia in assisted-living older adults. Age. 2014;36:1–13. doi: 10.1007/s11357-013-9536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2013) The state of aging and health in America in 2013. Atlanta, GA
- Cesari M, et al. Biomarkers of sarcopenia in clinical trials-recommendations from the International Working Group on Sarcopenia. J Cachex Sarcopenia Muscle. 2012;3:181–190. doi: 10.1007/s13539-012-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, et al. Cumulative exposure to ionizing radiation from diagnostic and therapeutic cardiac imaging procedures: a population-based analysis. J Am Coll Cardiol. 2010;56:702–711. doi: 10.1016/j.jacc.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CL, Lam KS, Cheung BM. Evaluation of cutpoints for low lean mass and slow gait speed in predicting death in the National Health and Nutrition Examination Survey 1999-2004. J Gerontol A Biol Sci Med Sci. 2016;71:90–95. doi: 10.1093/gerona/glv112. [DOI] [PubMed] [Google Scholar]
- Cockrell JR, Folstein MF. Principles and practice of geriatric psychiatry. In: Copeland JRM, Abou-Saleh MT, Blazer DG, editors. Principles and practice of geriatric psychiatry. New York: Wiley; 2002. pp. 147–158. [Google Scholar]
- Cruz-Jentoft AJ, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol (1985) 2003;95:1717–1727. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Glenn JM, Gray M, Binns A (2015) The effects of loaded and unloaded high-velocity resistance training on functional fitness among community-dwelling older adults. Age & Ageing. doi:10.1093/ageing/afv081 [DOI] [PubMed]
- Goodpaster BH, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol Ser A Biol Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- Gray M, Paulson S. Developing a measure of muscular power during a functional task for older adults. BMC Geriatr. 2014;14:145–150. doi: 10.1186/1471-2318-14-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesseberg K, Bentzen H, Bergland A. Reliability of the senior fitness test in community-dwelling older people with cognitive impairment. Physiother Res Int. 2015;20:37–44. doi: 10.1002/pri.1594. [DOI] [PubMed] [Google Scholar]
- Ishii S, et al. Development of a simple screening test for sarcopenia in older adults. Geriatr Gerontol Int. 2014;14(Suppl 1):93–101. doi: 10.1111/ggi.12197. [DOI] [PubMed] [Google Scholar]
- Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52:80–85. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- Kim M, Kim H. Accuracy of segmental multi-frequency bioelectrical impedance analysis for assessing whole-body and appendicular fat mass and lean soft tissue mass in frail women aged 75 years and older. Eur J Clin Nutr. 2013;67:395–400. doi: 10.1038/ejcn.2013.9. [DOI] [PubMed] [Google Scholar]
- Kyle UG, Genton L, Hans D, Pichard C. Validation of a bioelectrical impedance analysis equation to predict appendicular skeletal muscle mass (ASMM) Clin Nutr. 2003;22:537–543. doi: 10.1016/S0261-5614(03)00048-7. [DOI] [PubMed] [Google Scholar]
- Lauretani F, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–1860. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- Martin-Ponce E, Hernandez-Betancor I, Gonzalez-Reimers E, Hernandez-Luis R, Martinez-Riera A, Santolaria F. Prognostic value of physical function tests: hand grip strength and six-minute walking test in elderly hospitalized patients. Sci Rep. 2014;4:7530. doi: 10.1038/srep07530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh EI, Smale KB, Vallis LA. Predicting fat-free mass index and sarcopenia: a pilot study in community-dwelling older adults. Age (Dordr) 2013;35:2423–2434. doi: 10.1007/s11357-012-9505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters DM, Fritz SL, Krotish DE. Assessing the reliability and validity of a shorter walk test compared with the 10-meter walk test for measurements of gait speed in healthy, older adults. J Geriatr Phys Ther. 2013;36:24–30. doi: 10.1519/JPT.0b013e318248e20d. [DOI] [PubMed] [Google Scholar]
- Rikli RE, Jones CJ. Senior fitness test manual. Champaign: Human Kinetics; 2001. [Google Scholar]
- Rolland Y, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–450. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi AP, et al. Identifying sarcopenia in acute care setting patients. J Am Med Dir Assoc. 2014;15:303.e307–303.e312. doi: 10.1016/j.jamda.2013.11.018. [DOI] [PubMed] [Google Scholar]
- Sergi G et al. (2014) Assessing appendicular skeletal muscle mass with bioelectrical impedance analysis in free-living Caucasian older adults Clinical nutrition (Edinburgh, Scotland) doi: 10.1016/j.clnu.2014.07.010. [DOI] [PubMed]
- Sillanpää E, et al. Body composition in 18- to 88-year-old adults—comparison of multifrequency bioimpedance and dual-energy X-ray absorptiometry. Obesity. 2014;22:101–109. doi: 10.1002/oby.20583. [DOI] [PubMed] [Google Scholar]
- Studenski SA, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol Ser A Biol Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toombs RJ, Ducher G, Shepherd JA, De Souza MJ. The impact of recent technological advances on the trueness and precision of DXA to assess body composition. Obesity. 2012;20:30–39. doi: 10.1038/oby.2011.211. [DOI] [PubMed] [Google Scholar]
- Visser M, Deeg DJH, Lips P, Harris TB, Bouter LM. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J Am Geriatr Soc. 2000;48:381–386. doi: 10.1111/j.1532-5415.2000.tb04694.x. [DOI] [PubMed] [Google Scholar]
- Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc. 2002;50:897–904. doi: 10.1046/j.1532-5415.2002.50217.x. [DOI] [PubMed] [Google Scholar]