Abstract
Recent work has found that older adults with obesity and systemic inflammation have associated metabolic dysfunction but do not have associated lower lean mass or strength. However, this lean mass estimate may be inflated with obesity, given that 15 % of adipose tissue is composed of fat-free tissue. The primary purpose of this study was to investigate, in a nationally representative sample of adults, whether obese adults with chronic systemic inflammation (unhealthy) have differences in lean mass, muscle strength, and insulin resistance when compared to normal weight individuals without elevated levels of systemic inflammation (healthy). A secondary objective was to determine whether these potential differences were moderated by physical activity and to determine if these groups had a differential risk for all-cause mortality. Our findings suggests that the unhealthy group was associated with higher upper body lean mass (β = 823; 95 % confidence interval (CI) 637–1010; P < 0.001), lower body lean mass (β = 2724; 95 % CI 2291–3158; P < 0.001), and strength (β = 34.6; 95 % CI 13.5–55.7; P = 0.003) compared to the healthy group despite having systemic inflammation and correcting for fat-free adipose tissue. However, the unhealthy group was associated with insulin resistance (odds ratio (OR) = 16.1; 95 % CI 2.7–96.1; P = 0.005) although this finding was attenuated in those physically active (OR = 8.5; 95 % CI 2.43–30.15; P = 0.003). Despite this metabolic dysfunction, there was no difference in all-cause mortality risk between groups (hazard ratio (HR) = 1.16 (95 % CI 0.69–1.96; P = 0.54)) suggesting that higher amounts of lean mass and strength may be protective of premature mortality.
Keywords: Obesity, Muscle function, Muscle size
Introduction
The maintenance of skeletal muscle across a life span is important from both a functional and metabolic perspective (Booth et al. 2002). Skeletal muscle is highly plastic and is capable of responding to a variety of stimuli, however, some data suggests that obesity may alter the anabolic response through chronic inflammation and insulin resistance (Barzilay et al. 2001; Schaap et al. 2006; Yudkin et al. 1999). Recently, a study among 11 obese and 15 healthy weight older males found that obese men with chronic inflammation had a blunting of muscle protein synthesis but that this was offset by a decrease in leg protein breakdown (Murton et al. 2015). In support of this, they found similar levels of leg lean mass and strength between those who were obese and of a healthy bodyweight. Although these authors did not observe a difference in leg lean mass, they did note a decrease in leg glucose disposal suggesting a decrease in muscle metabolic quality. However, this lean mass estimate may be inflated in the obese, given that 15 % of adipose tissue is composed of fat-free tissue (Heymsfield et al. 2002). Abe et al. (2015) recently found that fat-free adipose tissue may falsely inflate the dual-energy X-ray absorptiometry (DXA)-derived lean mass measurements in individuals with a relatively high amount of adipose tissue. Thus, the primary purpose of this study was to investigate, in a nationally representative sample of adults in the USA, whether obese adults with chronic systemic inflammation have differences in lean mass, muscle strength, and insulin resistance when compared to normal-weight individuals without elevated levels of systemic inflammation. A secondary objective was to determine whether these potential differences were moderated by physical activity status and to also determine if these two groups (i.e., obese with inflammation vs. normal weight without inflammation) had a differential risk for all-cause mortality.
Methods
Study design
Data were extracted from the 1999–2000 National Health and Nutrition Examination Survey (NHANES). NHANES evaluates a representative sample of non-institutionalized US civilians, selected by a complex, multistage probability design. NHANES is conducted by the National Center for Health Statistics (NCHS), and all procedures for data collection were approved by the NCHS ethics review board, with written informed consent obtained from all participants prior to data collection.
Assessment of mortality status
Data from participants in the 1999–2000 NHANES were linked to death certificate data from the National Death Index. Person-months of follow-up were calculated from the date of the interview until date of death or censoring on December 31, 2011, whichever came first.
Measurement of peak knee extensor muscle strength
A Kin Com MP dynamometer (Chattanooga Group, Inc.) was used to assess isokinetic knee extensor strength (IKES) at peak force in newtons (at a speed of 60°/s). A total of six measurements of muscle strength of the right quadriceps were taken: three warm-up trial measurements followed by three outcome measurements. If a participant completed four to six measures, the highest peak force was selected from trials 4 to 6; however, if a participant completed fewer than four measures, the highest peak force from the warm-up trials was selected. Notably, only 1.6 % of the highest peak force trials came from one of the warm-up trials. When these trials were excluded from the analyses, results were unchanged (data not shown). All values were gravity-corrected for limb and lever arm weight.
Measurement of lean mass
Upper and lower extremity lean mass was estimated from whole-body DXA scans using the Hologic QDR 4500A fan beam X-ray bone densitometer (Hologic, Inc, Bedford, MA). Details on generating estimates from the multiple imputed DXA data are provided elsewhere (Loprinzi et al. 2015). Lower extremity lean mass was calculated by summing the lower extremity lean mass (excluding bone mineral content) of the left and right legs, with upper extremity lean mass calculated as the sum of the upper extremity lean mass (excluding bone mineral content) of the right and left arms. Corrections for fat-free adipose tissue was calculated according to methods described by Heymsfield et al. (2002) where they reported that 85 % of adipose tissue is fat and 15 % of adipose tissue is the remaining calculated fat-free component. Fat-free adipose tissue can then be calculated as fat-free adipose tissue = fat mass ÷ 0.85 × 0.15.
Weight status and inflammatory groups
Two mutually exclusive groups were created, including (1) those who had evidence of obesity and elevated systemic inflammation (hereafter “unhealthy”) and (2) those without elevated systemic inflammation and of normal weight (hereafter “healthy”).
Obesity was defined as a measured body mass index (BMI) of 30 kg/m2 or higher; normal weight is 18.5–24.9 kg/m2. We also considered higher thresholds of obesity (e.g., obese class II of 35 kg/m2), but results were in the same direction as the 30 kg/m2 threshold, so we chose to retain the obese class I threshold (30 kg/m2). Further, we considered the overweight BMI threshold (25–29.9 kg/m2), but results were unchanged using this threshold. Elevated systemic inflammation was defined as a C-reactive protein (CRP) level >0.3 mg/dL (Chew et al. 2001). Blood samples were obtained to assess high-sensitivity CRP, using latex-enhanced nephelometry. Both strength and CRP measurements were taken during the participant’s visit to the Mobile Examination Center. CRP measurements were taken prior to the strength assessments. The coefficients of variation (CV) ranged from 3.1 to 9.9 %. The coefficient of variation is in reference to different Calibrator Lots. Within-day variation was assessed, and this was done with a higher or lower dilution when the initial CRP result was outside the range of the standard curve.
Measurement of physical activity
Participants were asked open-ended questions about participation in leisure time physical activity over the past 30 days. Data was coded into 48 activities, including 16 sports-related activities, 14 exercise-related activities, and 18 recreational-related activities; these individual physical activities are published elsewhere (Ham et al. 2009).
For each of the 48 activities where participants reported moderate or vigorous intensity for the respective activity, they were asked to report the number of times they engaged in that activity over the past 30 days and the average duration they engaged in that activity. For each of the 48 physical activities, MET-min-month (MET = metabolic equivalent) was calculated by multiplying the number of days, by the mean duration, by the respective MET level (MET-min-month = days * duration * MET level). The MET levels for each activity are provided elsewhere (Ainsworth et al. 2011).
Measurement of insulin resistance
The Homeostasis Model Assessment (HOMA) was used to evaluate insulin resistance using the following formula: fasting serum insulin (uU/mL) × fasting plasma glucose (mmol/L) / 22.5 (Matthews et al. 1985). A cut-point of 2.6 was used to denote insulin resistance (>2.6) from insulin sensitivity (≤2.6) (Ascaso et al. 2003). Notably, only a subsample of participants were eligible for the fasting insulin and glucose assessments, so the analyses with objectively measured insulin resistance are among this subsample of participants.
Covariates
Covariates included age, gender, race–ethnicity, moderate to vigorous physical activity (MVPA) MET-min-month, self-reported smoking status, statin medication use, measured hypertension (≥140/90 mmHg), and physician diagnosis of the following conditions: diabetes, coronary artery disease, cancer, and arthritis.
Statistical analysis
All analyses were computed in Stata (v. 12) and accounted for the complex survey design employed in NHANES. As noted in the “Results” section, multivariate linear, logistic, and Cox proportional hazard models were used to examine interrelationships between the healthy/unhealthy groups and lean mass, strength, insulin resistance, and mortality. Statistical significance was established as P < 0.05.
Results
The sample consisted of 482 adults, ranging between 50 and 85 years. Among these 482 adults, 220 were in the healthy group with 262 in the unhealthy group. Among the 482 participants, 287 (59.5 %) were female (115 in healthy group and 172 in unhealthy group), 266 (55.2 %) were non-Hispanic white (132 in the healthy group and 134 in the unhealthy group), and the unweighted mean age was 64.6 (65.8 in the healthy group and 63.6 in the unhealthy group). The unweighted mean IKES was 272.5 (255.8 in the healthy group and 286.5 in the unhealthy group). The unweighted MVPA MET-min-month was 2940.1 (3492.0 in the healthy group and 2476.7 in the unhealthy group). Lastly, the unweighted mean HOMA was 3.79 (2.29 in the healthy group and 5.06 in the unhealthy group).
Among the 262 unhealthy participants (currently obese and inflammation), 1 year prior, 223 were considered obese (85 %) and, 10 years prior, 134 of these 262 adults were obese (51 %); prior obesity is defined as self-reported BMI 30 or higher. Results for regression models were not significantly changed when controlling for BMI from 1 or 10 years prior (data not shown).
Lean mass across healthy and unhealthy groups
The healthy (normal weight no inflammation) group had a higher relative strength (strength/leg lean body mass) when compared to the unhealthy (obese with inflammation) group: 0.021 (SE 0.0003) vs. 0.018 (P < 0.001). After correcting for fat-free adipose tissue, the relative strength was still statistically higher in the healthy group (0.022 vs. 0.021, P = 0.0045) although this difference is unlikely meaningful. Absolute strength, however, was lower among the healthy group compared to that among the unhealthy group (Fig. 1, P < 0.001).
Fig. 1.
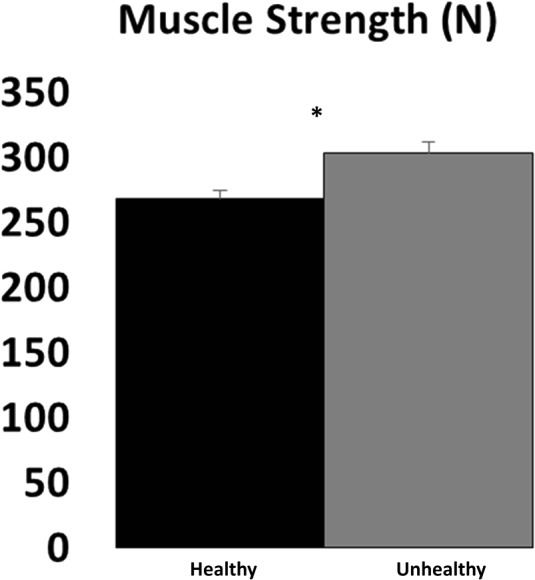
Isokinetic strength in healthy weight and obese (unhealthy) individuals. Values represent mean (standard error) for isokinetic strength. Asterisk denotes significant (P < 0.05) differences between healthy (black bar) and unhealthy (grey bar) individuals
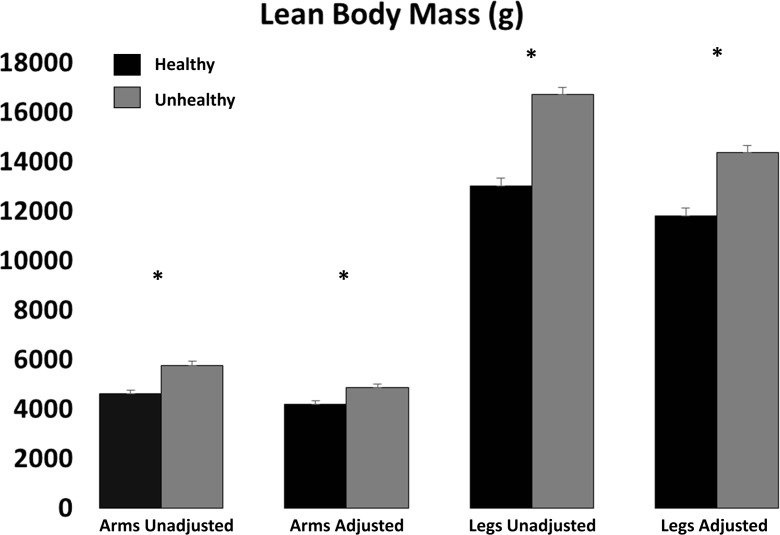
Upper and lower body lean mass was lower among the healthy group compared to that among the unhealthy group before and after correcting for fat-free adipose tissue (Fig. 2, P < 0.001).
Fig. 2.
Lean body mass in healthy weight and obese (unhealthy) individuals. Values represent mean (standard error) for lean body mass. Asterisk denotes significant (P < 0.05) differences between healthy (black bar) and unhealthy (grey bar) individuals before and after adjusting for fat-free adipose tissue
Adjusted lean mass regression results
In a multivariate linear regression model adjusting for age, gender, race–ethnicity, diabetes status (physician diagnosis), hypertension, coronary artery disease, cancer, arthritis, smoking status, medication use, and MVPA MET-min-month, those in the unhealthy group had greater lower body lean mass before (β = 3803; 95 % confidence interval (CI) 3337–4270; P < 0.001) and after (β = 2724; 95 % CI 2291–3158; P < 0.001) correcting for fat-free adipose tissue. Similarly, after adjustment, those in the unhealthy group had greater upper body lean mass before (β = 1284; 95 % CI 1083–1485; P < 0.001) and after (β = 823; 95 % CI 637–1010; P < 0.001) correcting for fat-free adipose tissue.
Lastly, after adjustment, those in the unhealthy group had a higher absolute IKES (β = 34.6; 95 % CI 13.5–55.7; P = 0.003). When made relative to leg lean body mass, the healthy group had greater strength (β = −0.002; 95 % CI −0.003, −0.001; P < 0.001). After correcting for fat-free adipose tissue, the relative strength still statistically favored the healthy group (β = −0.002; 95 % CI −0.003, −0.0004; P < 0.001), although the magnitude of this difference is unlikely to be meaningful.
Adjusted insulin resistance results
The sample size for the insulin resistance results was reduced due to only a subsample of participants participating in the fasting morning blood sample. Among the 482 adults, 230 provided fasting data for HOMA assessment.
After adjustments, and among those meeting MVPA guidelines (i.e., MVPA MET-min-month ≥2000), the unhealthy group (vs. healthy group) had an 8.5-fold increased odds (odds ratio (OR) = 8.5; 95 % CI 2.43–30.15; P = 0.003) of having insulin resistance (HOMA >2.6). Those not meeting MVPA guidelines, however, had a much greater odds of having insulin resistance: After adjustments, and among those not meeting MVPA guidelines (MVPA MET-min-month < 2000), the unhealthy group (vs. healthy group) had a 16.1-fold increased odds (OR = 16.1; 95 % CI 2.7–96.1; P = 0.005) of having insulin resistance (HOMA >2.6).
Adjusted mortality results
Among the 482 adults, 137 died over the mean follow-up period of 124.0 months (10.3 years). Among the 137 that died, 72 were in the healthy group at baseline and 65 were in the unhealthy group at baseline. In the sample, 59,773 person-months occurred with an incidence rate of 2.29 per 1000 person-months.
In a Cox proportional hazard model adjusting for the same covariates as noted above, there was no difference in mortality risk between the two groups: The hazard rate for the unhealthy group versus healthy group was HR = 1.16 (95 % CI 0.69–1.96; P = 0.54). Results were similar when excluding participants (N = 4) who died within the first year of follow-up (HR = 1.19; 95 % CI 0.68–2.07; P = 0.50). Results were not significant when stratified by men (HR = 1.50; 95 % CI 0.89–2.55; P = 0.12) or women (HR = 0.74; 95 % CI 0.33–1.62; P = 0.43). The proportional hazards assumption was not violated (P = 0.48), and the Harrell’s C concordance statistic was 0.78.
In the entire sample, 30.5 % meet MVPA guidelines. Among those considered healthy in our sample, 37.7 % met MVPA guidelines, and among those defined as unhealthy in our sample, 24.4 % met MVPA guidelines. Notably, our mortality analysis controlled for physical activity (MET-min-month) when expressed as a continuous variable. When we recomputed this analysis while controlling for whether they met MVPA guidelines, results were unchanged (HR changed from 1.16, P = 0.54, to HR of 1.14, P = 0.61).
Discussion
The present study suggests that those with obesity and systemic inflammation are associated with higher amounts of lean body mass and strength compared to those of a healthy weight despite having systemic inflammation and correcting for fat-free adipose tissue. Although no decrements were observed in lean mass or muscle strength, the obese group was associated with insulin resistance although this finding was attenuated in those who met the physical activity guidelines. Despite this apparent metabolic dysfunction, there was no difference in premature all-cause mortality risk between the obese and healthy weight groups suggesting that higher amounts of lean mass and strength may be playing an important role in this phenomenon termed the “obesity paradox,” which is in alignment with some studies suggesting that obesity, compared to their non-obese counterparts, has a similar or perhaps lower mortality risk (Gruberg et al. 2002; Mohebi et al. 2015; Romero-Corral et al. 2006). Given that most of the studies that confirm the obesity paradox are completed in the elderly, it has been suggested that this paradox may be largely an effect of elderly who exhibit only late onset obesity, meaning the health risks and comorbidities associated with obesity have not been able to manifest (Hainer and Aldhoon-Hainerova 2013). Interestingly, 85 % of our sample was obese for at least 1 year prior and 51 % were obese for at least 10 years suggesting that duration of obesity may not explain this paradox entirely. Regardless of these findings being in potential support of the obesity paradox, the greater strength and lean mass among these obese individuals have important implications for their health, as increased strength and lean mass are associated with greater mobility function, which in turn is linked with greater quality of life. It could be argued that there was muscle dysfunction with the unhealthy group given that relative strength was higher with the healthy group; however, we do not feel the statistical differences were meaningful following adjustments for co-variates or correction for fat-free adipose tissue.
This analysis was modeled on a recent study that suggested that lean body mass and strength was not decreased in the obese compared to the healthy weight group, despite having a blunting of muscle protein synthesis in response to amino acids and a lower disposal of glucose (Murton et al. 2015). One limitation noted by the authors was that they were unable to account for the influence of daily physical activity, although they noted that “overt traits of muscle deconditioning were evident in the obese volunteers.” We provide epidemiological evidence that appears to confirm their findings, even after controlling for physical activity status. Further, the greater lean mass in the obese group remained after applying an important, yet often overlooked, additional correction to lean body mass given recent data that suggests an overestimation of lean body mass in the obese due to fat-free adipose tissue (Abe et al. 2015). It is noted that the odds of insulin resistance were cut in half if participants met the physical activity guidelines, despite being obese and having systemic inflammation. Although the obese group was associated with insulin resistance, this did not appear to increase their risk for premature all-cause mortality. This finding, which necessitates replication, suggests that the maintenance of lean mass and strength may contribute to this obesity paradox, which suggests that obesity may provide a survival benefit under certain situations (Hainer and Aldhoon-Hainerova 2013). Our finding that the obese were associated with greater lean mass and strength is in agreement with previous research and is thought to be the result of the added mechanical work performed by the obese during activities of daily living (James et al. 1978; Murton et al. 2015). Although some have suggested that this obesity paradox is specific to men (Mohebi et al. 2015; Migaj et al. 2015), we observed no sex differences with this effect.
Limitations of this study include the cross-sectional design and the subjective measure of physical activity. In addition, we were only able to measure HOMA-IR on a sub section of our sample. It is also acknowledged that HOMA-IR is not a gold standard measurement of insulin sensitivity; however, estimating insulin resistance through the hyperinsulinemic euglycemic glucose clamp technique is impractical for epidemiological research. Regardless, major strengths of this investigation include employing a national sample of US adults, and utilizing objective measures of lean body mass and knee extension strength.
Based on our findings, we wish to suggest the following: (1) When compared to normal-weight individuals, obesity in combination with systemic inflammation is not associated with lower lean body mass or strength but is associated with insulin resistance; (2) meeting the physical activity guidelines does appear to reduce odds of insulin resistance, even if obese with systemic inflammation; and (3) the greater lean body mass and strength found in the obese may provide a protective benefit and may contribute to the obesity paradox previously observed in the literature.
Acknowledgments
No funding was used to prepare this manuscript. The authors’ contributions were as follows. JPL: study design, analysis and interpretation of the data, and drafting of the manuscript; and PDL: study design, performed statistical analysis, interpretation of the data, and the drafting of the manuscript. JPL and PDL are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors have no conflicts of interest to declare.
References
- Abe T, Patterson KM, Stover CD, Young KC. Influence of adipose tissue mass on DXA-derived lean soft tissue mass in middle-aged and older women. Age (Dordr) 2015;37:9741. doi: 10.1007/s11357-014-9741-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R. Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care. 2003;26:3320–3325. doi: 10.2337/diacare.26.12.3320. [DOI] [PubMed] [Google Scholar]
- Barzilay JI, Abraham L, Heckbert SR, Cushman M, Kuller LH, Resnick HE, Tracy RP. The relation of markers of inflammation to the development of glucose disorders in the elderly: the Cardiovascular Health Study. Diabetes. 2001;50:2384–2389. doi: 10.2337/diabetes.50.10.2384. [DOI] [PubMed] [Google Scholar]
- Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol. 2002;93:3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- Chew DP, Bhatt DL, Robbins MA, Penn MS, Schneider JP, Lauer MS, Topol EJ, Ellis SG. Incremental prognostic value of elevated baseline C-reactive protein among established markers of risk in percutaneous coronary intervention. Circulation. 2001;104:992–997. doi: 10.1161/hc3401.095074. [DOI] [PubMed] [Google Scholar]
- Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, Ahmed LM, Kent KM, Pichard AD, Suddath WO, Satler LF, Lindsay J., Jr The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39:578–584. doi: 10.1016/S0735-1097(01)01802-2. [DOI] [PubMed] [Google Scholar]
- Hainer V, Aldhoon-Hainerova I. Obesity paradox does exist. Diabetes Care. 2013;36(Suppl 2):S276–S281. doi: 10.2337/dcS13-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham SA, Kruger J, Tudor-Locke C. Participation by US adults in sports, exercise, and recreational physical activities. J Phys Act Health. 2009;6:6–14. doi: 10.1123/jpah.6.1.6. [DOI] [PubMed] [Google Scholar]
- Heymsfield SB, Gallagher D, Kotler DP, Wang Z, Allison DB, Heshka S. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am J Physiol Endocrinol Metab. 2002;282:E132–E138. doi: 10.1152/ajpendo.2002.282.1.E132. [DOI] [PubMed] [Google Scholar]
- James WP, Davies HL, Bailes J, Dauncey MJ. Elevated metabolic rates in obesity. Lancet. 1978;1:1122–1125. doi: 10.1016/S0140-6736(78)90300-8. [DOI] [PubMed] [Google Scholar]
- Loprinzi PD, Cardinal BJ, Lee H, Tudor-Locke C. Markers of adiposity among children and adolescents: implications of the isotemporal substitution paradigm with sedentary behavior and physical activity patterns. J Diabetes Metab Disord. 2015;14:46. doi: 10.1186/s40200-015-0175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Migaj J, Prokop E, Straburzynska-Migaj E, Lesiak M, Grajek S, Mitkowski P (2015) Does influence of obesity on prognosis differ in men and women? A study of obesity paradox in patients with acute coronary syndrome. Kardiol Pol [DOI] [PubMed]
- Mohebi R, Simforoosh A, Tohidi M, Azizi F, Hadaegh F. Obesity paradox and risk of mortality events in chronic kidney disease patients: a decade of follow-up in Tehran lipid and glucose study. J Ren Nutr. 2015;25:345–350. doi: 10.1053/j.jrn.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Murton AJ, Marimuthu K, Mallinson JE, Selby AL, Smith K, Rennie MJ, Greenhaff PL (2015) Obesity appears to be associated with altered muscle protein synthetic and breakdown responses to increased nutrient delivery in older men, but not reduced muscle mass or contractile function. Diabetes [DOI] [PubMed]
- Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, Mookadam F, Lopez-Jimenez F. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–678. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SM, Deeg DJ, Visser M (2006) Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 119(6):526.e9–17 [DOI] [PubMed]
- Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.ATV.19.4.972. [DOI] [PubMed] [Google Scholar]