Abstract
Ultraviolet radiations (UV) are the primary causative agent for skin aging (photoaging) and cancer, especially UV-A. The mode of action and the molecular mechanism behind the damages caused by UV-A is not well studied, in vivo. The current study was employed to investigate the impact of UV-A exposure using the model organism, Caenorhabditis elegans. Analysis of lifespan, healthspan, and other cognitive behaviors were done which was supported by the molecular mechanism. UV-A exposure on collagen damages the synthesis and functioning which has been monitored kinetically using engineered strain, col-19:: GFP. The study results suggested that UV-A accelerated the aging process in an insulin-like signaling pathway dependent manner. Mutant (daf-2)-based analysis concrete the observations of the current study. The UV-A exposure affected the usual behavior of the worms like pharyngeal movements and brood size. Quantitative PCR profile of the candidate genes during UV-A exposure suggested that continuous exposure has damaged the neural network of the worms, but the mitochondrial signaling and dietary restriction pathway remain unaffected. Western blot analysis of HSF-1 evidenced the alteration in protein homeostasis in UV-A exposed worms. Outcome of the current study supports our view that C. elegans can be used as a model to study photoaging, and the mode of action of UV-A-mediated damages can be elucidated which will pave the way for drug developments against photoaging.
Keywords: UV-A, C. elegans, Photoaging, Lifespan, IIS pathway
Introduction
Ultraviolet radiations (UV) are one of the electromagnetic radiations emitted by the Sun. The ozone layer in the atmosphere blocks most of the UV radiations. The major forms of UV include UV-A (315–400 nm), UV-B (280–315 nm), and UV-C (100–280 nm). Among these, the ozone layer completely and partially blocks UV-C and UV-B, respectively. But UV-A can penetrate the ozone layer and can reach Earth’s crust. According to the World Health Organization (WHO), 90–95 % of UV radiations that reach Earth are UV-A. Mild exposure to UV-A will help in increasing the melanin production in the skin (Watanuki et al. 2014) which enhances the innate immunity. In addition, UV-A is the major factor in producing vitamin D than UV-B (McKenzie et al. 2012).
However, continuous exposure may lead to chronic irreversible damages ranging from sun burns to skin cancer. McKenzie et al. (2009) mentioned that during summer season, there will be enough UV to photosynthesize vitamin D in 1 min in the mid-latitude regions. However, if the exposure time is further extended to 15 min, it will lead to skin damage (McKenzie et al. 2009). In addition, long-term exposure of skin against UV radiations leads to premature aging or photoaging, which is characterized by wrinkle formation, hyperpigmented lesions or age spots, and decrease in the integrity of the skin (Puglia et al. 2014). Photoaging can be characterized by fragmentation and reduced production of collagen in the skin. Lower doses of UV radiation in the skin affect the activity of collagen (Wang et al. 2014). As the level of UV exposure increases, the collagen that is already present in the extracellular matrix will breakdown, and also, the synthesis of new collagen will be hindered (Pandel et al. 2013).
Exposure to UV-B leads to the degradation of collagen followed by the upregulation of matrix metalloproteinase (MMP) and reactive oxygen species (ROS) production which will result in photoaging (Wang et al. 2014). However, the role of UV-A, in eliciting photoaging, is less studied. As UV-A is 10 to 100 times abundant in natural sunlight and because of its capacity to penetrate the skin more deeply than UV-B (Hung et al. 2015), it is necessary to focus more on UV-A-mediated photoaging process.
All age-related diseases are induced by the universal phenomenon, aging, which can be analyzed by the random but progressive accumulation of damaged cells, tissues, and organs. These are irreversible damages that depend on the genetic factors of an individual (Juckett 2010). In the last two decades, several reports suggest that there is an unexpected periodic pattern in the prevalence and mortality of age-related diseases (Juckett and Rosenberg, 1991). In the current scenario, age-related diseases are predominant in causing death. Death caused by cancers, especially skin cancer, is a major threat to mankind (Juckett 2010).
Model organisms such as mice (Kong et al. 2014) and rats (Barcelos et al. 2014) have been routinely used to study photoaging. UV-A exposure to the skin of nude mice causes pale coloring of the skin that lead to decreased blood supply and eventually to apoptosis and necrosis (Hung et al. 2015). A recent study used hen as a model to check the level of vitamin D in eggs upon exposure to UV radiation (Kuhn et al. 2015). Researchers favor these models because of the ease of identifying the physiological changes in the animals during the course of exposure. However, these studies may take an extended duration. Even though the UV-mediated response was studied in higher model organisms, the molecular mechanism behind the UV-A-mediated photoaging was not yet established.
The model nematode Caenorhabditis elegans which are widely used as a model in aging studies (Fawcett et al. 2015; Chondrogianni et al. 2015) were used to study the physiological and molecular changes in a biological system during UV-A exposure. Many natural compounds with antioxidants that have anti-aging activity have been successfully tested in this model (Zheng et al. 2014; Sonani et al. 2014). This microscopic model system is preferred due to its short life cycle and transparent cuticle, which enables to monitor the physiological changes through a microscope. Moreover, it facilitates to study the regulation of single gene, which can make a significant change in lifespan or any other physiological activity, through RNAi-mediated approach (Qian et al. 2015).
In the current study, we tried to understand the mode of action of UV-A exposure upon C. elegans by analyzing the changes in physiological and cognitive behaviors in the host, along with the molecular changes. It was observed that the lifespan and healthspan was decreased drastically upon exposure, as UV-A has triggered photoaging in C. elegans.
Materials and methods
Nematodes, reagents, and equipments
The wild type C. elegans strain (Bristol N2), col-19::GFP strain (TP12), and daf-2 mutant (CB1370) were obtained from Caenorhabditis Genetics Center, University of Minnesota, USA. All strains were grown in nematode growth medium (NGM) at 20 °C as described (Brenner 1974). The uracil auxotroph, E. coli OP50, was used as food source for C. elegans. All experiments were done in triplicates with age-synchronized young adult worms. Synchronization was done by bleaching of gravid adults (Sivamaruthi et al. 2011).
C. elegans were exposed to UV-A at a constant wavelength of 365 nm for 2, 4, and 6 h using a UV transilluminator. All other chemicals used were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Confocal microscopy
Transgenic strain of C. elegans after exposure along with control worms were washed separately using M9 buffer and placed in a drop of sodium azide solution on 2 % agarose pad and viewed through Confocal laser scanning microscope (Carl Zeiss, Germany). The intensity profile of GFP was read using the Zen software provided with the microscope (Durai et al. 2011).
Collagen quantification assay
Quantification of collagen inside the worms was done using a Sircol Collagen assay kit method. Briefly, 50 μg of total protein sample was taken and incubated with sircol dye for 30 min. Further, the proteins were collected as pellet through centrifugation, and excess dye was washed off through specific acid salt reagent. The pellet was further dissolved in alkali reagent and checked for absorbance at 555 nm, which was plotted against a standard graph.
Lifespan assay
Lifespan assay was carried out in both solid and liquid conditions as described (Sivamaruthi et al. 2011) with some modifications. In solid assay, a known number of ~20 UV-A-exposed young adults were positioned on solid NGM plates seeded with Escherichia coli OP50. Similarly, a known number of UV-A-exposed worms were maintained with M9 buffer and E. coli OP50 in a 24-well microtiter plate for liquid assay. The worms were monitored during every 24 h. The worms were transferred to fresh media on every alternate day to replenish food and also to avoid false positive data due to the presence of young ones. Worms were considered as dead when they did not respond to a gentle tap or touch with a platinum wire pick. Nematodes unexposed to UV-A were considered as control.
Pharyngeal pumping assay
To determine the pumping rate, worms (~10 young adults) after exposure to UV-A for 2, 4, or 6 h were placed on NGM plates seeded with E. coli OP50. Pharyngeal pumping was observed once at every 24-h interval using a stereomicroscope (Nikon SMZ1000, Japan) for ten consecutive seconds. Worms unexposed to UV-A were considered as control.
Egg laying assay
Similar to the pharyngeal pumping assay, worms (~10 young adults) after exposure to UV-A for 2, 4, or 6 h were placed individually on NGM plates seeded with E. coli OP50. The number of eggs laid was counted once at every 24-h interval as described (Kesika et al. 2011). Worms were transferred to new plates after every 24 h. Worms unexposed to UV-A were considered as control. The experiment was carried out in three independent trials.
Drop assay
Drop assay was performed with a method developed by Hilliard et al. (2002) with some modifications. Briefly, worms after exposure to UV-A for 2, 4, or 6 h were allowed to crawl on unseeded NGM plates. Worms unexposed to UV-A was considered as control. A drop of repellent (Glycerol) with ~5–10 nl was kept on the tail of the worm without touching it or disturbing its forward movement. The drop will reach the anterior amphid sensory organ through capillary action. Within 1–3 s, worms will move backward by sensing the repellent. If the worm continues to move forward, then the neural network is probably damaged.
Osmosensation assay
Briefly, a pinch of Bromophenol blue, which acts as an indicator, was mixed with 4 M NaCl solution. A circular ring with ~1 cm diameter was dipped in the solution and placed in the center of an unseeded NGM plate and marked, without tampering the media. A ring was formed, which was visible with the dye, and then, it was allowed to absorb by the plate for 5–10 min. UV-A-exposed worms were placed inside the ring and observed for 10 min along with control. The trial was done independently with all different exposures.
Total RNA isolation and qPCR analysis
Synchronized populations of wild type young adult worms were generated from eggs at 20 °C on the standard food source. Worms were collected from E. coli OP50 lawns in M9 buffer at room temperature and washed several times with M9 buffer. Worms were then exposed to UV-A for 2, 4, and 6 h. Worms unexposed to UV-A were kept as control. The experimental worms were washed and treated with TRIzol reagent (RNA X Press reagent, Sigma) for isolating total RNA and were reverse-transcribed using oligodT primer and MultiScribe™ Reverse Transcriptase (Applied Biosystems) enzyme. After first-strand synthesis, quantitative PCR (qPCR) was carried out to analyze the expression pattern of candidate genes that regulate lifespan and healthspan using gene-specific primers. The expression data was represented as upregulated or downregulated by normalizing the 0-h control values and internal control actin. The sequences of the primers are given in Table 1.
Table 1.
List of primers
| Gene name | Forward primer | Reverse primer |
|---|---|---|
| col-19 | CACACAAATGCTCCACCAAC | CTGGATTTCCCTTCTGTCCA |
| daf-2 | TCGAGCTCTTCCTACGGTGT | CATCTTGTCCACCACGTGTC |
| daf-16 | TGGTGGAATTCAATCGTGAA | ATGAATATGCTGCCCTCCAG |
| age-1 | ATAGAGCTCCACGGCACTTT | TGTCAAGCACGTTTTCTTCG |
| utx-1 | GCAGAACACCAGCTCATCAG | ATCAACGCCATTCTTCTCGC |
| egl-8 | CGTATCGTTGCGCTTCTCA | AGTAGTGACACAGCGGTTG |
| egl-30 | TCAGAAAGGCGGAAGTGGAT | GGTTCTCGTTGTCACACTCG |
| dgk-1 | GTTGGGGAAGTGGTGCAAAT | GCGAGCTTGGATTGGATGAG |
| goa-1 | TGTTCGATGTGGGAGGTCAA | TCGTGCATTCGGTTTGTTGT |
Western blot analysis
To monitor the changes at protein level, total proteins were isolated from the UV-A-exposed samples by following standard protocols. Sixty micrograms of each protein sample (exposed and control) was boiled to break the complex proteins and separated in 12 % SDS-PAGE. A PVDF membrane was used to transfer the proteins from the gel at a constant voltage (15 V) for 3 h. Immunodetection was performed by using specific antibodies against candidate protein. The antibodies used in the present study include rabbit polyclonal antibody raised against heat shock factor of human origin, HSF-1 (Santa Cruz Biotechnology, Inc.) at 1:1000 dilution and monoclonal anti-actin purified mouse immunoglobulin (Sigma-Aldrich) at 1:1000 dilution, followed by exposure to corresponding secondary antibody for 4 h. The membrane was developed by transferring to 1X Alkaline Phosphatase (AP) buffer containing substrate NBT and 5-bromo-4-chloro-3-indolyphosphate until intense bands were observed in the membrane (Durai et al. 2014). Further, quantification of the bands developed was done using ImageLab software.
Statistical analysis
All the experiments were done in triplicates, and one-way ANOVA (SPSS 17) was used to compare the mean values of each treatment. The data were represented as average of three independent experiments. Significant differences between the means of parameters were determined by using the Duncan’s test (p < 0.05) comparing between the groups control vs treated.
Results
UV-A induces damage in the nematode
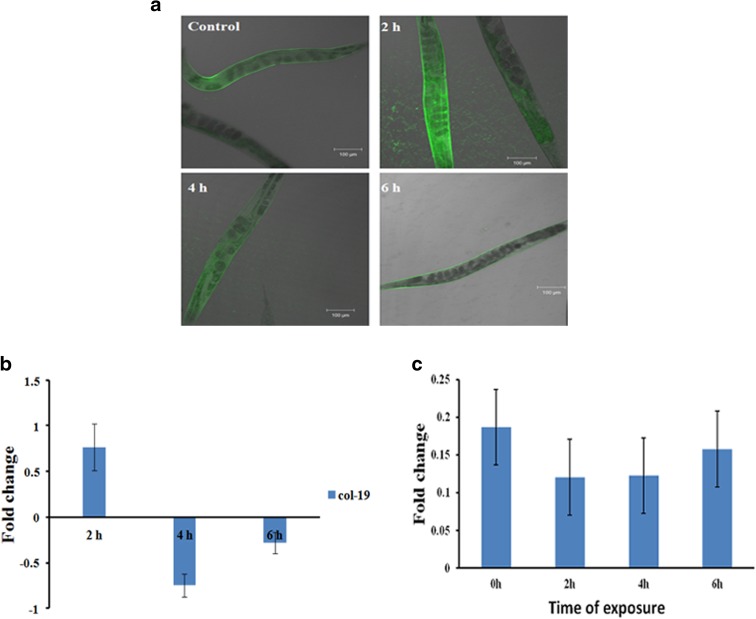
UV-A is known to cause direct impact on the outer epidermal layer of humans. Since the model host lacks a proper epidermal layer as that of humans, the preliminary aim was to confirm whether UV-A can cause any damage in the nematode. For this, transgenic strain of collagen tagged with GFP, col-19::GFP strain (TP12), was exposed to UV-A for 2, 4, and 6 h. The col-19 gene is an adult specific marker and is also essential for the normal structure of the alae in adult C. elegans (Thein et al. 2003). In this strain, the col-19 gene is fused with GFP and localized in the cuticle. After exposure, the worms were viewed and imaged using a confocal laser scanning microscope. It was observed that the level of fluorescence was altered significantly upon exposure to UV-A (Fig. 1a). This suggested that UV-A can cause damage to the cuticular layer of the worms. To further confirm this, the level of expression of col-19 in wild type nematodes during exposure was analyzed. A marked change in the level of expression of col-19 was observed (Fig. 1b). Additionally, the rate of collagen synthesis after exposure was monitored quantitatively. The reduced levels of collagen indicate that the host lost its capacity to synthesize collagen (Fig. 1c).
Fig. 1.
Effect of UV-A in nematode. a Confocal images showing differential regulation of col-19 in the transgenic strain TP12. b qPCR analysis showing altered expression of col-19 in wild type C. elegans. c Quantification of collagen inside the worms after exposure showing decrease in the level of collagen
UV-A alters lifespan of C. elegans
To understand the physiological damage caused by UV-A to the nematode, the total lifespan of wild type as well as daf-2 mutants in both solid and liquid media after UV-A exposure was analyzed. DAF-16 is the effector of DAF-2/DAF-16 pathway which regulates the expression of many aging regulating factors in C. elegans. DAF-2 phosphorylates DAF-16 and prevents it from integrating into nucleus and activating the regulatory genes. Knockdown of DAF-2 allows the activation of DAF-16, and these mutants were known for their extended lifespan (Murphy and Hu 2013; Sonani et al. 2014).
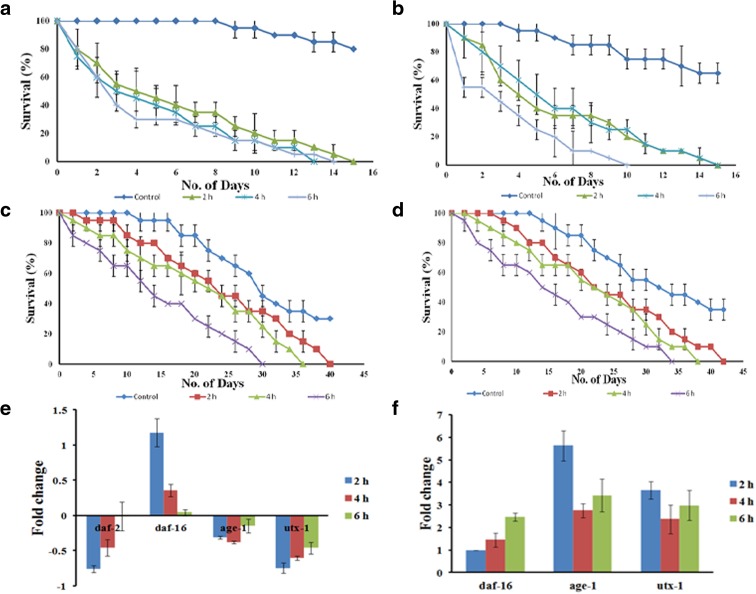
After exposure, wild type worms survived up to 15, 13, and 14 days in liquid and 15, 15, and 10 days in solid media whereas daf-2 mutants survived up to 40, 35, and 30 days in liquid and 42, 38, and 33 days in solid media, after 2, 4, and 6 h of UV exposure, respectively (Fig. 2a–d). These observations clearly indicate that UV-A significantly reduced the lifespan of the nematode. Further, to confirm the role of DAF-2/DAF-16 pathway, which is widely known as insulin-like signaling (IIS) pathway, during UV exposure, qPCR analysis of selected candidate genes (daf-2, daf-16, age-1, utx-1) were performed. It was observed that daf-2 and utx-1 were upregulated during the course of exposure whereas daf-16 was downregulated. In the case of age-1, it was found to be downregulated during the initial hours of exposure, but in 6 h exposed sample, it was slightly upregulated (Fig. 2e). In the case of daf-2 mutants, daf-16 got upregulated. But both age-1 and utx-1 had a higher fold expression than daf-16 (Fig. 2f). The altered regulation of these genes that regulate lifespan also supported our findings.
Fig. 2.
Effect of UV-A on C. elegans lifespan. a Lifespan assay of wild type C. elegans in liquid media. Worms survived up to 15, 13, and 14 days when exposed to 2, 4, and 6 h of UV-A, respectively (p < 0.05). b Lifespan assay of wild type C. elegans in solid media. Worms survived up to 15, 15, and 10 days when exposed to 2, 4, and 6 h of UV-A, respectively (p < 0.05). c Lifespan assay of daf-2 mutant worms in liquid media. Worms survived up to 40, 35, and 30 days when exposed to 2, 4, and 6 h of UV-A, respectively (p < 0.05). d Lifespan assay of daf-2 mutant worms in solid media. Worms survived up to 42, 38, and 33 days when exposed to 2, 4, and 6 h of UV-A, respectively (p < 0.05). e qPCR expression in wild type worms showing upregulation of daf-2 and utx-1 and downregulation of daf-16 (1.18, 0.36, and 0.04 folds in 2, 4, and 6 h, respectively) during the course of exposure. Altered regulation was expressed by age-1. f qPCR expression in daf-2 mutant worms showing upregulation of daf-16 (0.98, 1.46, and 2.48 folds in 2, 4, and 6 h, respectively). However, both age-1 (5.63, 2.76, and 3.43 folds in 2, 4, and 6 h, respectively) and utx-1 (3.67, 2.37, and 2.98 folds in 2, 4, and 6 h, respectively) are showing higher fold expression than daf-16
UV-A-mediated aging is independent of mitochondrial signaling and dietary restriction
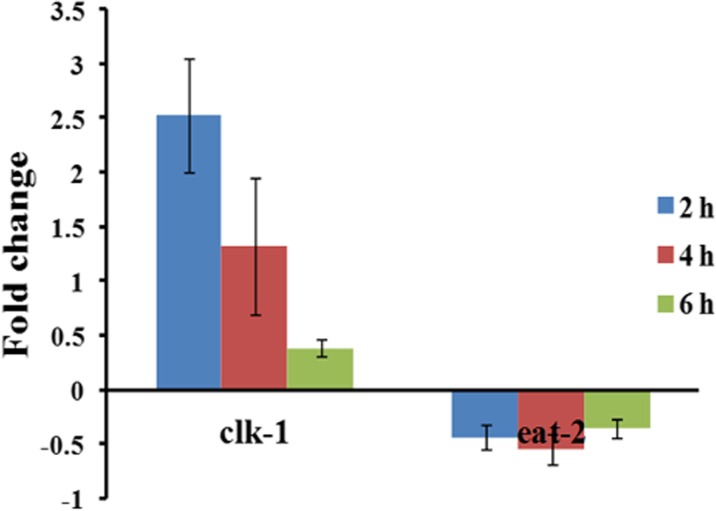
Apart from the IIS pathway, the aging process is mediated by mitochondrial signaling (Lakowski and Hekimi 1996) and calorie restriction process (Yen and Mobbs 2010). Quantitative PCR analysis of clk-1 and eat-2 which are the key regulators of these above events were performed to confirm whether the aging process initiated by UV-A is dependent on any of these pathways. It was observed that the level of expression of clk-1 was downregulated and that of eat-2 was almost constant during the course of exposure (Fig. 3).
Fig. 3.
Differential expression of clk-1 and eat-2 upon UV-A exposure in C. elegans. The expression of clk-1 was downregulated during the course of exposure whereas eat-2 was downregulated consistently
UV-A disrupts the normal healthspan of C. elegans
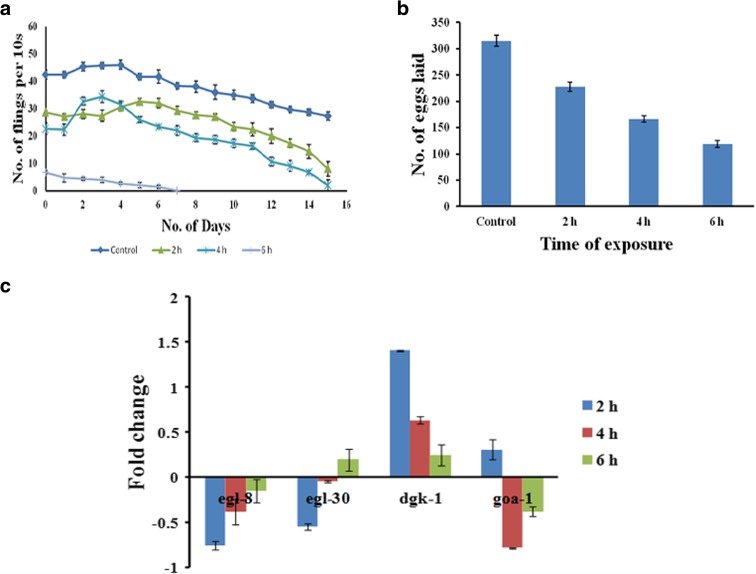
Further, pharyngeal pumping and egg laying were monitored to assess the healthspan of the nematode after exposure to UV-A. The reduced rates of both parameters suggest that UV-A has a negative impact on the normal survival and health of C. elegans. The pharyngeal pumping almost completely seized within 15 days in 2 and 4 h exposed worms whereas in the case of 6 h exposed worms, complete seizure occurred within 7 days (Fig. 4a). In the case of egg laying, the brood size drastically reduced as 227, 166, and 118 in 2, 4, and 6 h exposed worms, respectively, as compared to control which laid 314 eggs (Fig. 4b). The genes necessary for normal healthspan, egl-8, egl-30, dgk-1, and goa-1 were analyzed during exposure. It was observed that both egl-8 and egl-30 were downregulated during the initial hours of exposure, which later got slightly upregulated. In the case of dgk-1 and goa-1, the expression was upregulated during the initial hours which later on got subsided. The expression pattern indicated that UV-A has damaged the normal healthspan of the worms (Fig. 4c).
Fig. 4.
Evaluation of healthspan in C. elegans exposed to UV-A. a Pharyngeal pumping assay in C. elegans. The pharyngeal movement was reduced in worms exposed to UV-A (p < 0.05). b Brood size assay in C. elegans. The total number of eggs laid was 227, 166, and 118 in 2, 4, and 6 h exposed worms, respectively, when compared to control (314) (p < 0.05). c qPCR expression of egl-8 and egl-30 was found to be downregulated from the initial hours of exposure which showed slight upregulation during the course of exposure. In the case of dgk-1 and goa-1, it was upregulated during the initial hours which was later subsided
UV-A damages the neuronal behavior
Since all the activities of the worm are mediated by the 302 neurons in the system, the neural network was analyzed for any damage during UV-A exposure, through drop and osmosensation assays. It was observed that worms exposed to UV-A had caused significant damage to the worm’s neural network since the response of the worms towards chemical repellents was altered after exposure (Tables 2 and 3).
Table 2.
Differential activity of worms exposed to UV-A against repellent glycerol
| Time of exposure | Worms tested | Worms repelled | No response |
|---|---|---|---|
| Control | 10 | 10 | 0 |
| 2 h | 10 | 10 | 0 |
| 4 h | 10 | 8 | 2 |
| 6 h | 10 | 6 | 4 |
Table 3.
Differential activity of worms exposed to UV-A when introduced into a high osmotic barrier
| Trial 1 | Trial 2 | Trial 3 | ||||
|---|---|---|---|---|---|---|
| Worms introduced | Worms escaped | Worms introduced | Worms escaped | Worms introduced | Worms escaped | |
| Control | 10 | 0 | 10 | 0 | 10 | 0 |
| 2 h | 10 | 0 | 10 | 1 | 10 | 1 |
| 4 h | 10 | 0 | 10 | 1 | 10 | 2 |
| 6 h | 10 | 1 | 10 | 1 | 10 | 1 |
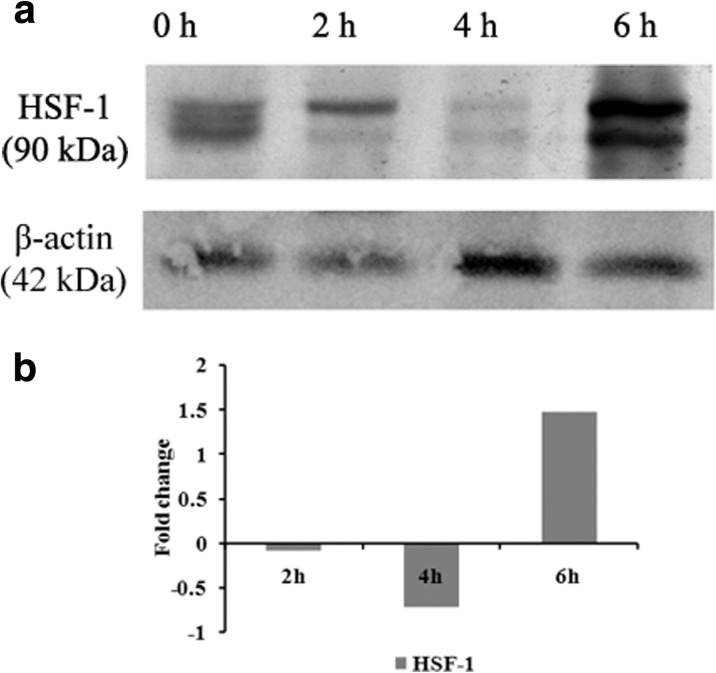
HSF-1 regulates the aging mechanism
Western blot analysis was done for HSF-1 to understand the role of DAF-16 pathway in the UV-A-mediated aging process. It was observed that HSF-1 was highly expressed in the 6 h exposed worms, whereas the expression was absent in 4 h exposed worms. In the case of 2 h exposed worms, the expression was meager as compared to control. The elevated expression suggests that UV-A radiation has altered the normal aging mechanism of the nematode (Fig. 5) which was further quantified.
Fig. 5.
Western blot analysis of HSF-1 in wild type C. elegans. a Maximum expression was observed in 6 h exposed worms, whereas the expression was absent in 4 h exposed worms. In the case of 2 h exposed worms, the expression was meager as compared to control. b Quantification of the expressed HSF-1 was done using the ImageLab software
Discussion
Persistent and continuous exposure to UV-A will cause photoaging and skin cancer (MacFarlane and Alonso 2009). Even though, UV-A is considered to be less carcinogenic as compared to UV-B, the former appears to induce photoaging by generating reactive oxygen species, superoxides and other free radicals that subsequently increases the chances of skin cancer (MacFarlane and Alonso 2009; Burke and Wei 2009; Shirai et al. 2015). Mortality rates due to skin-related diseases and cancer are on the rising side, even in this era of modern medicine (Juckett 2010). Almost all of the available sunscreens elicit protection against UV-B (Burke and Wei 2009), even though UV-A is known to reach earth predominantly than UV-B (Hung et al. 2015) and also cause detrimental effects. So there is a far cry need to find strategies to inhibit the action of UV-A on biological systems. C. elegans with its immense advantages appear to be a suitable model to study this phenomenon, though the lack of a thick epithelial layer may be considered as a hindrance. The col-19::GFP strain was used in order to understand the response of C. elegans against UV-A. In humans, collagen is necessary for the strength and elasticity of the skin. However, during aging, collagen probably get degraded and accumulated in the skin, which results in wrinkle formation. Many environmental stresses including UV radiation induces photoaging also appeared to accelerate the wrinkle formation (Chauhan and Shakya 2009) and eventually led to skin cancer (Chiarelli-Neto et al. 2014). In wild type C. elegans, the expression of col-19 begins at late L4 stage at low levels (Hada et al. 2010), and it acts like an adult specific marker (Thein et al. 2003; Li and Paik 2011). Further, it deciphers the accumulation of collagen inside the organism. The increasing accumulation of this gene directly implies that the worm has undergone photoaging upon exposure to UV-A. The qPCR analysis of this gene in wild type nematode has confirmed that UV-A has an impact on the outer epithelial layer of the model host. The collagen production assay, which estimates the level of collagen synthesis during a given period, also supported this view (Fig. 1). Since the worms had undergone aging, it might have lost its ability to synthesize collagen.
Any kind of environmental stress will have a potential impact on the lifespan of the nematode (Patananan et al. 2015). Moreover, lifespan analysis is the first and foremost indication in aging analysis (Tissenbaum 2012). The reduced survival of UV-A-exposed worms clearly indicated that worms were susceptible to continuous UV-A exposure and related induced photoaging. The DAF-2/DAF-16 pathway is otherwise called as insulin-like signaling pathway in C. elegans. This regulates the lifespan, normal metabolism, and development (Evans et al. 2008). The altered regulation of the major players in this pathway, daf-2, daf-16, and age-1 suggested that the changes in the lifespan of the host was under the influence of the above pathway. The genes daf-2 and age-1 are negative regulators of lifespan whereas daf-16 positively regulates lifespan (Sonani et al. 2014). In the present study, as the time of exposure increased, the level of expression of daf-2 and age-1 were also increased; in turn, the expression of daf-16 decreased. The above data clearly indicate that UV-A radiation had altered the aging mechanism of the host system (Fig. 2a–b, e).
To further confirm the role of this pathway, lifespan assay was carried out in daf-2 mutants after exposure. DAF-2 is the major player which phosphorylates DAF-16 in cytoplasm and prevents its integration into nucleus. When DAF-2 is mutated, DAF-16 will integrate into the nucleus leading to the activation of many genes responsible for lifespan and stress resistance (Murphy and Hu 2013). The decreased survival in mutant worms due to exposure indicates that this pathway was also affected. The qPCR data suggested upregulation of daf-16 which is expected since daf-2 is mutated. But age-1 had a higher fold expression than daf-16 which suppressed the lifespan of the mutant worms (Fig. 2c–d, f).
UTX-1 constitutes one of the histone demethylases which is conserved in all mammalian species (Swigut and Wysocka 2007). Previous reports stated that RNAi of utx-1 in C. elegans extends lifespan up to 30 % in a DAF-16 dependent manner. The expression of utx-1 was followed by the increased expression of daf-2 and other aging mechanisms (Jin et al. 2011) which subsequently regulate the aging process in somatic cells and independent of germ cells (Maures et al. 2011). The increased expression of utx-1 in wild type worms after exposure suggests that the aging process was accelerated due to exposure whereas in the case of daf-2 mutants, the regulation seems to be almost constant because of the absence of daf-2 to mediate the aging process (Fig. 2e-f).
Apart from the IIS pathway, the mitochondrial genes (clk-1) (Lakowski and Hekimi 1996) and dietary restriction genes (eat-2) (Yen and Mobbs 2010) are known to play a role in the aging process. In C. elegans, mutation in clk-1 gene causes extension of adult lifespan (Hekimi et al. 1998). Mutation of eat-2 causes reduced pharyngeal pumping and food intake. This leads to calorie restriction and lifespan extension which is proved in many mammalian systems (Yen and Mobbs 2010). When exposed to UV-A, the level of expression of clk-1 was found to be decreasing and that of eat-2 was almost constant during the course of exposure in wild type worms. These results suggested that the aging process accelerated by UV-A is not dependent on mitochondrial signaling or dietary restriction process (Fig. 3).
Similar to lifespan, it is necessary to know the healthspan of the worm during its survival. Healthspan can be defined as the healthy and productive time of an organism that will decline during aging (Tissenbaum 2012). In the current scenario, extending lifespan without extending health will not fulfill the goal of aging research. Some of the recent studies show that phycoeryhtrin isolated from a marine cyanobacterium (Sonani et al. 2014) and cranberry extract (Guha et al. 2013) can extend the healthspan of nematode along with extending lifespan. Here, we have used pharyngeal pumping and egg laying ability of C. elegans as the phenotypic readouts to measure healthspan. As the worms undergo aging, the rate of pharyngeal pumping reduced and consequently seized prior to its death (Collins et al. 2008). Here, upon UV-A exposure, the level of pharyngeal pumping has reduced considerably, which indicates that the radiation has accelerated the aging process inside the worms. Similarly, the movement of worms after exposure was altered (data not shown). This may be due to the degradation of muscles that help in movement, which is common during aging (Herndon et al. 2002). Already we have stated that UV-A radiation accumulates degraded collagen in the worm’s outer layer. In addition, any kind of stress altered the reproductive ability of most of the living organisms including human (Cizmeli et al. 2013; Duan et al. 2015). In a recent study, it was proved that exposure of C. elegans to copper reduced the number of eggs laid (Song et al. 2014). In our study also, the rate of egg laying was reduced which implies that the UV-A irradiation has altered the normal functions taking place inside the model organism (Fig. 4).
Similarly, the expressions of orthologs of Go ligands, (egl-8, egl-30, goa-1, and dgk-1) that are essential for chemotaxis apart from regulation of healthspan of the nematode (Hofer 2005) also substantiate our findings. An ortholog of the heterotrimeric G protein alpha subunit Gq, egl-30, (Wang and Wadsworth 2002) and its downstream player, egl-8, (Ziegler et al. 2009) in C. elegans plays a pivotal role in regulating pharyngeal pumping, egg laying and locomotion (Govorunova et al. 2010). Previous reports suggest that mutation in goa-1 has a negative impact on egg laying, locomotion, and other normal behaviors of the worm (Matsuki et al. 2006). With the downstream activator dgk-1, GOA-1 will inhibit the egl-30-mediated DAG pathway (Matsuki et al. 2006; Avery and You 2012). During the initial hours of exposure (2 h), egl-8, egl-30 were downregulated, and dgk-1, goa-1 were upregulated. Alterations in these genes might have affected both pharyngeal pumping and brood size. Even though the regulation was altered in during the later hours of exposure (4 and 6 h), worms were not able to regain the normal health as UV-A had already triggered photoaging processes in C. elegans (Fig. 4).
C. elegans has 302 neurons that control, co-ordinate, and monitor the activities inside the system. The decreased level of insulin signaling via daf-2 RNAi have shown to improve the mechanosensation and alter many key neuronal activities of the host, C. elegans (Scerbak et al. 2014). The present study found daf-2 to be upregulated during UV-A exposure (Fig. 2e) and that led us to observe the changes in their neuronal behavior of the nematodes after UV-A-mediated photoaging. Chemotaxis is a behavior of the worm with which it responds to a particular chemical stimulus. In C. elegans benzaldehyde is usually used as a positive control for chemotaxis (Rabinowitch et al. 2014). In a recent study, it was proved that worms show no chemotaxis towards ethanol (Patananan et al. 2015). Our group has previously reported that C. elegans avoid Vibrio alginolyticus (Durai et al. 2011) whereas it gets attracted to Cronobacter sakazakii (Sivamaruthi et al. 2011), which may be by the alteration of serotonin transporter (Sivamaruthi et al. 2015a). When nematodes where exposed to UV-A, there was no significant change in the rate of chemotaxis behavior (data not shown). Hence, neural behaviors of the system were analyzed using drop and osmosensation assays.
ASH neurons in the amphid regions of C. elegans play a pivotal role against osmotic stress, volatile chemicals, and also mechanosensation by reversing its movement (Sambongi et al. 1999; Tobin and Bargmann 2004). Hilliard et al. (2002) stated that sensation of a repellent placed on the tail region of the worm and subsequent reversal be mediated by a head-to-tail spatial map. When the worms exposed to UV-A, this network system have partially damaged as the time of exposure increased. This was attributed to the depletion of the activity of ASH neurons (Sambongi et al. 1999) or the increased activity of PHA and PHB phasmid neurons (Hilliard et al. 2002). The osmosensation assay results also suggest the damage in neural network, since it is also chiefly mediated by ASH neurons (Srinivasan et al. 2008).
In nematodes, the transcription factor HSF-1 activates several proteins which are needed to maintain protein homeostasis during thermal stress (Kenyon 2010). Moreover, this DAF-16 dependent protein plays a pivotal role against oxidative stress (Honda and Honda 1999), invading pathogens and their subcellular components (Sivamaruthi et al. 2015b; JebaMercy et al. 2013), and heat shock (Volovik et al. 2014). All these factors help HSF-1 to play a role in the lifespan extension of the nematode (Kenyon et al. 1993; Antebi 2007). However, the activities of the DAF-16 were downregulated by the IIS pathway receptor, DAF-2 by phosphorylating DAF-16 (Henderson and Johnson 2001) and DDL-1 that is required by HSF-1 for its cellular localization (Chiang et al. 2012). Since both daf-16 and hsf-1 are important for the lifespan of the nematode, Western blot analysis of hsf-1 was done. Both 2 and 4 h exposed worms showed significant downregulation that probably led the reduced survival of the same. The elevated expression suggests that DAF-2 appears to be upregulated during UV-A exposure, which probably suppressed both DAF-16 and HSF-1 (Fig. 5).
Conclusion
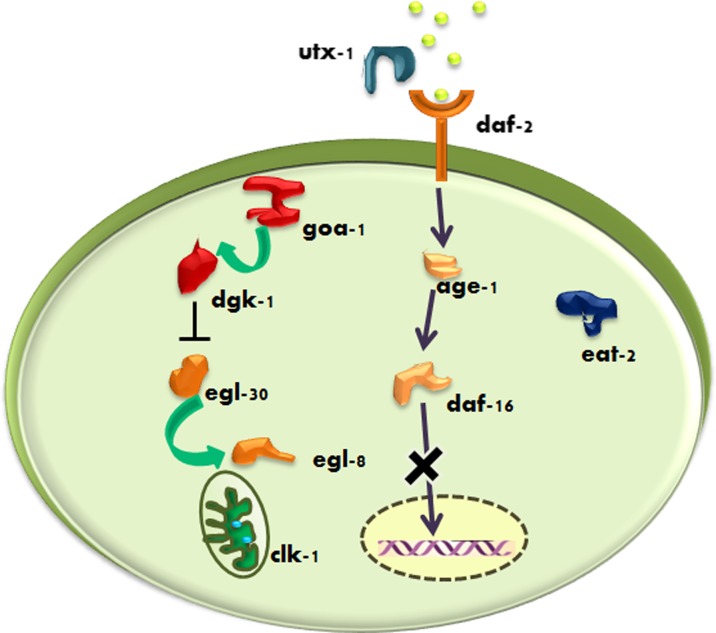
This is the first report that UV-A can cause detrimental effects on the model nematode. This was suggested by the expression of collagen inside the body of C. elegans. Our results emphasize that UV-A radiations not only decreases lifespan but also damages its healthspan. This response was independent of mitochondrial and dietary restriction pathways (Fig. 6). The damaged neural network system suggests the level of damage induced by the radiation. The studies using daf-2 mutants confirmed that IIS pathway appears to be regulated during exposure. Apart from this, the qPCR analysis of candidate genes and Western blot analysis of HSF-1 shows that heat shock factor was also regulated. More proteomic analyses are required to identify the players involved in the process which will pave the way for drug discovery against UV-A-mediated damages.
Fig. 6.
Schematic representation of initiation of photoaging by UV-A. Exposure to UV-A regulates the pivotal players of the IIS pathway, such as daf-2 (which is mediated by utx-1) along with age-1, which in turn mediates the phosphorylation and eventually blocking daf-16 from integrating into the nucleus by which lifespan was reduced. Also, the candidate players of the DAG pathway were differentially regulated, by which a reduction in healthspan was also observed. However, the other mediators of lifespan regulation, mitochondrial gene clk-1 and dietery restriction gene eat-2, were found unaffected during the course of exposure
Acknowledgments
We thank Caenorhabditis Genetics Center, which is funded by the National Institute of Health, National Center for Research Resources for providing C. elegans N2 WT, mutant strains and E. coli OP50. KB thankfully acknowledges the ITC India Ltd., Department of Biotechnology (DBT), University Grants Commission (UGC), Indian Council of Medical Research (ICMR) and Council of Scientific and Industrial Research (CSIR), Department of Science and Technology (DST), Government of India, New Delhi, India, for the financial assistances. PMI wishes to thank ITC and CSIR, India, for the financial assistance (AU-ITC JRF & CSIR-SRF). The authors also gratefully acknowledge the use of the Bioinformatics Infrastructure Facility, Alagappa University funded by the Department of Biotechnology, Ministry of Science and Technology, Government of India (No. BT/BI/25/015/2012), the Instrumentation Facility provided by DST, Government of India through PURSE [Grant No.SR/S9Z-23/2010/42(G)] & FIST (Grant No.SR-FST/LSI-087/2008) and UGC, New Delhi through SAP-DRS1 [Grant No.F.3-28/2011(SAP-II)].
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- Antebi A. Genetics of aging in Caenorhabditis elegans. PLoS Genet. 2007;3(9):1565–1571. doi: 10.1371/journal.pgen.0030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery L, You YJ. C. elegans Feeding. WormBook. 2012;21:1–23. doi: 10.1895/wormbook.1.150.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcelos RC, Vey LT, Segat HJ, et al. Cross-generational trans fat intake exacerbates UV radiation-induced damage in rat skin. Food Chem Toxicol. 2014;69:38–45. doi: 10.1016/j.fct.2014.03.031. [DOI] [PubMed] [Google Scholar]
- Burke KE, Wei H. Synergistic damage by UVA radiation and pollutants. Toxicol Ind Health. 2009;25(4–5):219–224. doi: 10.1177/0748233709106067. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of C. elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan P, Shakya M. Modeling signaling pathways leading to wrinkle formation: identification of the skin aging target. Indian J Dermatol Venereol Leprol. 2009;75(5):463–468. doi: 10.4103/0378-6323.55388. [DOI] [PubMed] [Google Scholar]
- Chiarelli-Neto O, Ferreira AS, Martins WK (2014) Melanin photosensitization and the effect of visible light on epithelial cells. PLoS One 18;9(11):e113266. doi:10.1371/journal.pone.0113266. [DOI] [PMC free article] [PubMed]
- Chiang WC, Ching TT, Lee HC, Mousigian C, Hsu AL. HSF-1 regulators DDL-1/2 link insulin-like signaling to heat-shock responses and modulation of longevity. Cell. 2012;148:322–334. doi: 10.1016/j.cell.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chondrogianni N, Georgila K, Kourtis N, Tavernarakis N, Gonos ES. 20S proteasome activation promotes life span extension and resistance to proteotoxicity in Caenorhabditis elegans. FASEB J. 2015;29(2):611–622. doi: 10.1096/fj.14-252189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizmeli C, Lobel M, Franasiak J, Pastore LM. Levels and associations among self-esteem, fertility distress, coping, and reaction to potentially being a genetic carrier in women with diminished ovarian reserve. Fertil Steril. 2013;99(7):2037–2044. doi: 10.1016/j.fertnstert.2013.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JJ, Huang C, Hughes S, Kornfeld K. The measurement and analysis of age-related changes in Caenorhabditis elegans. WormBook. 2008;24:1–21. doi: 10.1895/wormbook.1.137.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan X, Wang QC, Chen KL, Zhu CC, Liu J, Sun SC (2015) Acrylamide toxic effects on mouse oocyte quality and fertility in vivo. Sci Rep 25;5:11562. [DOI] [PMC free article] [PubMed]
- Durai S, Pandian SK, Balamurugan K. Establishment of a Caenorhabditis elegans infection model for Vibrio alginolyticus. J Basic Microbiol. 2011;51(3):243–252. doi: 10.1002/jobm.201000303. [DOI] [PubMed] [Google Scholar]
- Durai S, Singh N, Kundu S, Balamurugan K. Proteomic investigation of Vibrio alginolyticus challenged Caenorhabditis elegans revealed regulation of cellular homeostasis proteins and their role in supporting innate immune system. Proteomics. 2014;14(15):1820–1832. doi: 10.1002/pmic.201300374. [DOI] [PubMed] [Google Scholar]
- Evans EA, Chen WC, Tan MW. The DAF-2 insulin-like signaling pathway independently regulates aging and immunity in C. elegans. Aging Cell. 2008;7(6):879–893. doi: 10.1111/j.1474-9726.2008.00435.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett EM, Hoyt JM, Johnson JK, Miller DL. Hypoxia disrupts proteostasis in Caenorhabditis elegans. Aging Cell. 2015;14(1):92–101. doi: 10.1111/acel.12301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha S, Cao M, Kane RM, Savino AM, Zou S, Dong Y. The longevity effect of cranberry extract in Caenorhabditis elegans is modulated by daf-16 and osr-1. Age (Dordr) 2013;35(5):1559–1574. doi: 10.1007/s11357-012-9459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govorunova EG, Moussaif M, Kullyev A, Nguyen KC, McDonald TV, Hall DH, Sze JY (2010) A homolog of FHM2 is involved in modulation of excitatory neurotransmission by serotonin in C. elegans. PLoS One 5(4):e10368. doi:10.1371/journal.pone.0010368. [DOI] [PMC free article] [PubMed]
- Hada K, Asahina M, Hasegawa H, Kanaho Y, Slack FJ, Niwa R. The nuclear receptor gene nhr-25 plays multiple roles in the Caenorhabditis elegans heterochronic gene network to control the larva-to-adult transition. Dev Biol. 2010;344(2):1100–1109. doi: 10.1016/j.ydbio.2010.05.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hekimi S, Lakowski B, Barnes TM, Ewbank JJ. Molecular genetics of life span in C. elegans: how much does it teach us? Trends Genet. 1998;14(1):14–20. doi: 10.1016/S0168-9525(97)01299-7. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11:1975–1980. doi: 10.1016/S0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Herndon LA, Schmeissner PA, Dudaronek JM. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature. 2002;419(6909):808–814. doi: 10.1038/nature01135. [DOI] [PubMed] [Google Scholar]
- Hilliard MA, Bargmann CI, Bazzicalupo P. C. elegans responds to chemical repellents by integrating sensory inputs from the head and the tail. Curr Biol. 2002;12(9):730–734. doi: 10.1016/S0960-9822(02)00813-8. [DOI] [PubMed] [Google Scholar]
- Hofer AM. Another dimension to calcium signaling: a look at extracellular calcium. J Cell Sci. 2005;118(Pt 5):855–862. doi: 10.1242/jcs.01705. [DOI] [PubMed] [Google Scholar]
- Honda Y, Honda S. The daf-2 gene network for longevity regulates oxidative stress resistance and Mn-superoxide dismutase gene expression in Caenorhabditis elegans. FASEB J. 1999;13:1385–1393. [PubMed] [Google Scholar]
- Hung CF, Chen WY, Aljuffali IA, Lin YK, Shih HC, Fang JY. Skin aging modulates percutaneous drug absorption: the impact of ultraviolet irradiation and ovariectomy. Age (Dordr) 2015;37(2):9757. doi: 10.1007/s11357-015-9757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JebaMercy G, Vigneshwari L, Balamurugan K. A MAP kinase pathway in Caenorhabditis elegans is required for defense against infection by opportunistic Proteus species. Microbes Infect. 2013;15(8–9):550–568. doi: 10.1016/j.micinf.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Jin C, Li J, Green CD, et al. Histone demethylase UTX-1 regulates C. elegans life span by targeting the insulin/IGF-1 signaling pathway. Cell Metab. 2011;14(2):161–172. doi: 10.1016/j.cmet.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Juckett DA. What determines age-related disease: do we know all the right questions? Age (Dordr) 2010;32(2):155–160. doi: 10.1007/s11357-009-9120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckett DA, Rosenberg B. An unexpected periodicity among the prevalences of human age-related, mortal diseases. Mech Ageing Dev. 1991;59(1–2):139–152. doi: 10.1016/0047-6374(91)90080-J. [DOI] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nature. 2010;464(7288):504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kesika P, Pandian SK, Balamurugan K. Analysis of Shigella flexneri mediated infections in model organism Caenorhabditis elegans. Scand J Infect Dis. 2011;43(4):286–295. doi: 10.3109/00365548.2010.548400. [DOI] [PubMed] [Google Scholar]
- Kong S, Chen H, Yu X, et al. The protective effect of 18β-glycyrrhetinic acid against UV irradiation induced photoaging in mice. Exp Gerontol. 2014;61C:147–155. doi: 10.1016/j.exger.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Kuhn J, Schutkowski A, Hirche F, Baur AC, Mielenz N, Stangl GI. Non-linear increase of vitamin D content in eggs from chicks treated with increasing exposure times of ultraviolet light. J Steroid Biochem Mol Biol. 2015;148:7–13. doi: 10.1016/j.jsbmb.2014.10.015. [DOI] [PubMed] [Google Scholar]
- Lakowski B, Hekimi S. Determination of life-span in Caenorhabditis elegans by four clock genes. Science. 1996;272(5264):1010–1013. doi: 10.1126/science.272.5264.1010. [DOI] [PubMed] [Google Scholar]
- Li Y, Paik YK. A potential role for fatty acid biosynthesis genes during molting and cuticle formation in Caenorhabditis elegans. BMB Rep. 2011;44(4):285–290. doi: 10.5483/BMBRep.2011.44.4.285. [DOI] [PubMed] [Google Scholar]
- Matsuki M, Kunitomo H, Iino Y. Goalpha regulates olfactory adaptation by antagonizing Gqalpha-DAG signaling in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2006;103(4):1112–1117. doi: 10.1073/pnas.0506954103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane DF, Alonso CA. Occurrence of nonmelanoma skin cancers on the hands after UV nail light exposure. Arch Dermatol. 2009;145(4):447–449. doi: 10.1001/archdermatol.2008.622. [DOI] [PubMed] [Google Scholar]
- Maures TJ, Greer EL, Hauswirth AG, Brunet A. The H3K27 demethylase UTX-1 regulates C. elegans lifespan in a germline-independent, insulin-dependent manner. Aging Cell. 2011;10(6):980–990. doi: 10.1111/j.1474-9726.2011.00738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie RL, Liley JB, Bjorn LO. UV radiation: balancing risks and benefits. Photochem Photobiol. 2009;85(1):88–98. doi: 10.1111/j.1751-1097.2008.00400.x. [DOI] [PubMed] [Google Scholar]
- McKenzie R, Scragg R, Liley B, Johnston P, Wishart J, Stewart A, Prematunga R. Serum 25-hydroxy vitamin-D responses to multiple UV exposures from solaria: inferences for exposure to sunlight. Photochem Photobiol Sci. 2012;11(7):1174–1185. doi: 10.1039/c2pp05403e. [DOI] [PubMed] [Google Scholar]
- Murphy CT, Hu PJ. Insulin/insulin-like growth factor signaling in C. elegans. WormBook. 2013;26:1–43. doi: 10.1895/wormbook.1.164.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandel R, Poljsak B, Godic A, Dahmane R. Skin photoaging and the role of antioxidants in its prevention. ISRN Dermatol. 2013;2013:930164. doi: 10.1155/2013/930164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patananan AN, Budenholzer LM, Eskin A, Torres ER, Clarke SG. Ethanol-induced differential gene expression and acetyl-CoA metabolism in a longevity model of the nematode Caenorhabditis elegans. Exp Gerontol. 2015;61:20–30. doi: 10.1016/j.exger.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglia C, Offerta A, Saija A, Trombetta D, Venera C. Protective effect of red orange extract supplementation against UV-induced skin damages: photoaging and solar lentigines. J Cosmet Dermatol. 2014;13(2):151–157. doi: 10.1111/jocd.12083. [DOI] [PubMed] [Google Scholar]
- Qian H, Xu X, Niklason LE. PCH-2 regulates Caenorhabditis elegans lifespan. Aging (Albany NY) 2015;7(1):1–13. doi: 10.18632/aging.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitch I, Chatzigeorgiou M, Zhao B, Treinin M, Schafer WR (2014) Rewiring neural circuits by the insertion of ectopic electrical synapses in transgenic C. elegans. Nat Commun 5:4442. [DOI] [PMC free article] [PubMed]
- Sambongi Y, Nagae T, Liu Y, Yoshimizu T, Takeda K, Wada Y, Futai M. Sensing of cadmium and copper ions by externally exposed ADL, ASE, and ASH neurons elicits avoidance response in Caenorhabditis elegans. Neuroreport. 1999;10:753–757. doi: 10.1097/00001756-199903170-00017. [DOI] [PubMed] [Google Scholar]
- Scerbak C, Vayndorf EM, Parker JA, Neri C, Driscoll M, Taylor BE. Insulin signaling in the aging of healthy and proteotoxically stressed mechanosensory neurons. Front Genet. 2014;5:212. doi: 10.3389/fgene.2014.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai A, Onitsuka M, Maseda H, Omasa T. Effect of polyphenols on reactive oxygen species production and cell growth of human dermal fibroblasts after irradiation with ultraviolet-A light. Biocontrol Sci. 2015;20(1):27–33. doi: 10.4265/bio.20.27. [DOI] [PubMed] [Google Scholar]
- Sivamaruthi BS, Ganguli A, Kumar M, Bhaviya S, Pandian SK, Balamurugan K. Caenorhabditis elegans as a model for studying Cronobacter sakazakii ATCC BAA-894 pathogenesis. J Basic Microbiol. 2011;51(5):540–549. doi: 10.1002/jobm.201000377. [DOI] [PubMed] [Google Scholar]
- Sivamaruthi BS, Madhumita R, Balamurugan K, Rajan KE. Cronobacter sakazakii infection alters serotonin transporter and improved fear memory retention in the rat. Front Pharmacol. 2015;4(6):188. doi: 10.3389/fphar.2015.00188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivamaruthi BS, Prasanth MI, Balamurugan K. Alterations in Caenorhabditis elegans and Cronobacter sakazakii lipopolysaccharide during interaction. Arch Microbiol. 2015;197(2):327–337. doi: 10.1007/s00203-014-1064-1. [DOI] [PubMed] [Google Scholar]
- Sonani RR, Singh NK, Awasthi A, Prasad B, Kumar J, Madamwar D. Phycoerythrin extends lifespan and healthspan of Caenorhabditis elegans. Age (Dordr) 2014;36(5):9717. doi: 10.1007/s11357-014-9717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Guo Y, Zhang X, Zhang X, Zhang J, Ma E. Changes to cuticle surface ultrastructure and some biological functions in the nematode Caenorhabditis elegans exposed to excessive copper. Arch Environ Contam Toxicol. 2014;66(3):390–399. doi: 10.1007/s00244-013-9991-4. [DOI] [PubMed] [Google Scholar]
- Srinivasan J, Durak O, Sternberg PW. Evolution of a polymodal sensory response network. BMC Biol. 2008;6:52. doi: 10.1186/1741-7007-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swigut T, Wysocka J. H3K27 demethylases, at long last. Cell. 2007;131(1):29–32. doi: 10.1016/j.cell.2007.09.026. [DOI] [PubMed] [Google Scholar]
- Thein MC, McCormack G, Winter AD, Johnstone IL, Shoemaker CB, Page AP. Caenorhabditis elegans exoskeleton collagen COL-19: an adult-specific marker for collagen modification and assembly, and the analysis of organismal morphology. Dev Dyn. 2003;226(3):523–539. doi: 10.1002/dvdy.10259. [DOI] [PubMed] [Google Scholar]
- Tissenbaum HA. Genetics, lifespan, healthspan, and the aging process in Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci. 2012;67(5):503–510. doi: 10.1093/gerona/gls088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin DM, Bargmann CI. Invertebrate nociception: behaviors, neurons and molecules. J Neurobiol. 2004;61(1):161–174. doi: 10.1002/neu.20082. [DOI] [PubMed] [Google Scholar]
- Volovik Y, Moll L, Marques FC, Maman M, Bejerano-Sagie M, Cohen E. Differential regulation of the heat shock factor 1 and DAF-16 by neuronal nhl-1 in the nematode C. elegans. Cell Rep. 2014;9(6):2192–2205. doi: 10.1016/j.celrep.2014.11.028. [DOI] [PubMed] [Google Scholar]
- Wang F, Smith NR, Tran BA, Kang S, Voorhees JJ, Fisher GJ. Dermal damage promoted by repeated low-level UV-A1 exposure despite tanning response in human skin. JAMA Dermatol. 2014;150(4):401–406. doi: 10.1001/jamadermatol.2013.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Wadsworth WG. The C domain of netrin UNC-6 silences calcium/calmodulin-dependent protein kinase- and diacylglycerol-dependent axon branching in Caenorhabditis elegans. J Neurosci. 2002;22(6):2274–2282. doi: 10.1523/JNEUROSCI.22-06-02274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanuki Y, Kageyama K, Takayasu S, Matsuzaki Y, Iwasaki Y, Daimon M. Ultraviolet B radiation-stimulated urocortin 1 is involved in tyrosinase-related protein 1 production in human melanoma HMV-II cells. Peptides. 2014;61C:93–97. doi: 10.1016/j.peptides.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Yen K, Mobbs CV. Evidence for only two independent pathways for decreasing senescence in Caenorhabditis elegans. Age (Dordr) 2010;32(1):39–49. doi: 10.1007/s11357-009-9110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng S, Liao S, Zou Y, Qu Z, Shen W, Shi Y. Mulberry leaf polyphenols delay aging and regulate fat metabolism via the germline signaling pathway in Caenorhabditis elegans. Age (Dordr) 2014;36(6):9719. doi: 10.1007/s11357-014-9719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K, Kurz CL, Cypowyj S, Couillault C, Pophillat M, Pujol N, Ewbank JJ. Antifungal innate immunity in C. elegans: PKCdelta links G protein signaling and a conserved p38 MAPK cascade. Cell Host Microbe. 2009;5(4):341–352. doi: 10.1016/j.chom.2009.03.006. [DOI] [PubMed] [Google Scholar]